Ontogenetic Habitat Usage of Juvenile Carnivorous Fish Among Seagrass-Coral Mosaic Habitats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Habitat Structure
2.3. Fish Surveys
2.4. Diet Analysis
2.5. Invertebrate Prey Abundance and Prey Availability
2.6. Data Analysis
3. Results
3.1. Habitat Structure Features
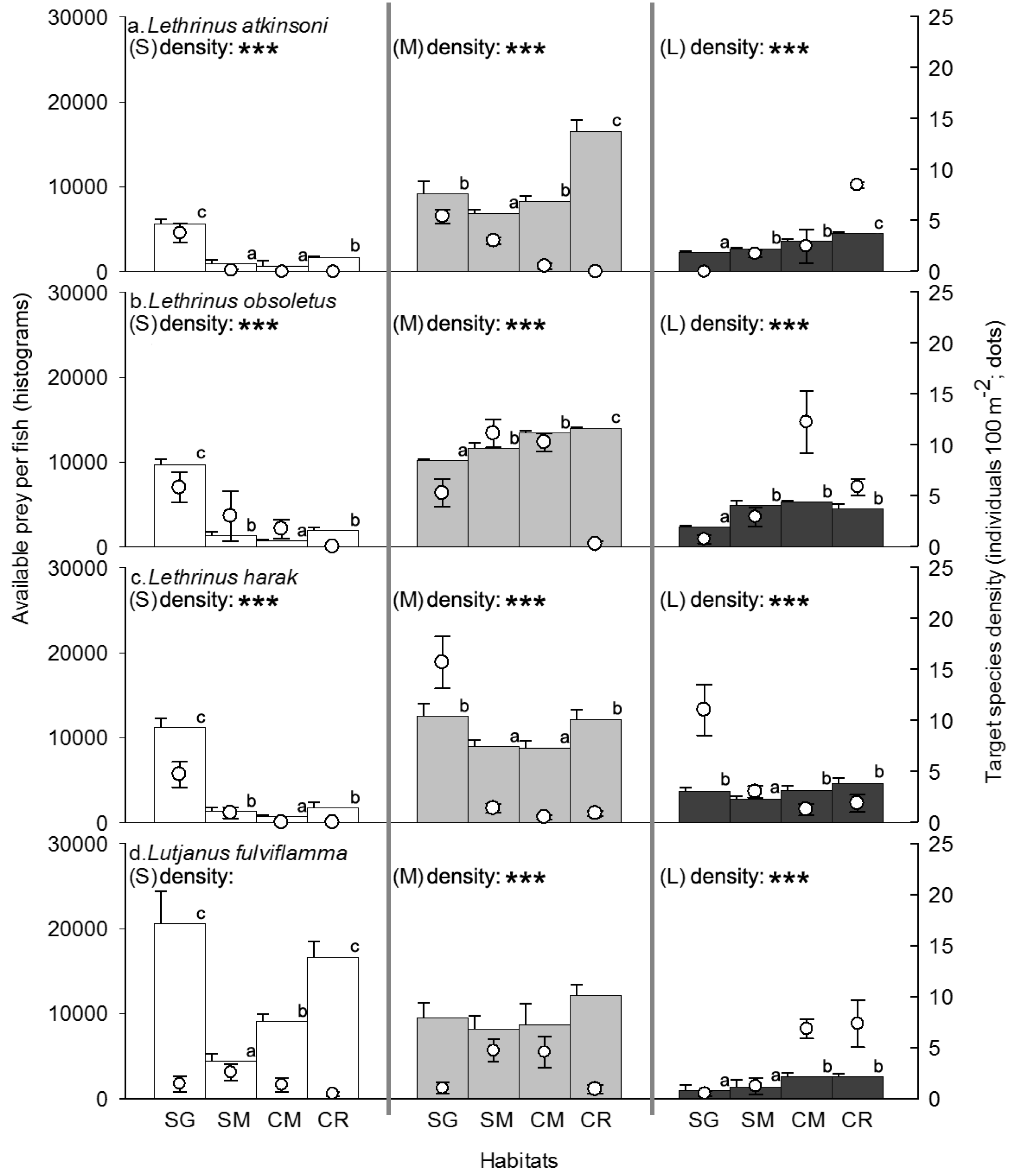
3.2. Competitor, Food Availability, and Habitat Usage Pattern of the Target Fish Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olds, A.D.; Connolly, R.M.; Pitt, K.A.; Maxwell, P.S. Primacy of seascape connectivity effects in structuring reef fish assemblages. Mar. Ecol. Prog. Ser. 2012, 462, 191–203. [Google Scholar] [CrossRef]
- Berkström, C.; Lindborg, R.; Thyresson, M.; Gullström, M. Assessing connectivity in a tropical embayment: Fish migrations and seascape ecology. Biol. Conserv. 2013, 166, 43–53. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Sheaves, M.; Baker, R.; Connolly, R.M. The seascape nursery: A novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fish. 2015, 16, 362–371. [Google Scholar] [CrossRef]
- Eggertsen, L.; Ferreira, C.E.L.; Fontoura, L.; Kautsky, N.; Gullström, M.; Berkström, C. Seaweed beds support more juvenile reef fish than seagrass beds in a south-western Atlantic tropical seascape. Estuar. Coast. Shelf Sci. 2017, 196, 97–108. [Google Scholar] [CrossRef]
- Tano, S.A.; Eggertsen, M.; Wikström, S.A.; Berkström, C.; Buriyo, A.S.; Halling, C. Tropical seaweed beds as important habitats for juvenile fish. Mar. Freshwater Res. 2017, 68, 1921–1934. [Google Scholar] [CrossRef]
- Nagelkerken, I. Evaluation of nursery function of mangroves and seagrass beds for tropical decapods and reef fishes: And underlying mechanisms. In Ecological Connectivity among Tropical Coastal Ecosystems; Nagelkerken, I., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 357–399. [Google Scholar]
- Nyström, M.; Folke, C. Spatial resilience of coral reefs. Ecosystems 2001, 4, 406–417. [Google Scholar] [CrossRef]
- Mumby, P.J.; Hastings, A. The impact of ecosystem connectivity on coral reef resilience. J. Appl. Ecol. 2008, 45, 854–862. [Google Scholar] [CrossRef]
- McCook, L.J.; Almany, G.R.; Berumen, M.L.; Day, J.C.; Green, A.L.; Jones, G.P.; Leis, J.M.; Planes, S.; Russ, G.R.; Sale, P.F.; et al. Management under uncertainty: Guide-lines for incorporating connectivity into the protection of coral reefs. Coral Reefs 2009, 28, 353–366. [Google Scholar] [CrossRef]
- Dahlgren, C.P.; Eggleston, D.B. Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology 2000, 81, 2227–2240. [Google Scholar] [CrossRef]
- Almany, G.R. Differential effects of habitat complexity, predators and competitors on abundance of juvenile and adult coral reef fishes. Oecologia 2004, 141, 105–113. [Google Scholar] [CrossRef]
- Kimirei, I.A.; Nagelkerken, I.; Trommelen, M.; Blankers, P.; Van Hoytema, N.; Hoeijmakers, D.; Huijbers, C.M.; Mgaya, Y.D.; Rypel, A.L. What drives ontogenetic niche shifts of fishes in coral reef ecosystems? Ecosystems 2013, 16, 783–796. [Google Scholar] [CrossRef]
- Wen, C.K.; Almany, G.; Williamson, D.; Pratchett, M.; Jones, G. Evaluating the effects of marine reserves on diet, prey availability and prey selection by juvenile predatory fishes. Mar. Ecol. Prog. Ser. 2012, 469, 133–144. [Google Scholar] [CrossRef]
- Wilson, S.K.; Depczynski, M.; Holmes, T.H.; Noble, M.M.; Radford, B.T.; Tinkler, P.; Fulton, C.J. Climatic conditions and nursery habitat quality provide indicators of reef fish recruitment strength. Limnol. Oceanogr. 2017, 62, 1868–1880. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sano, M. Comparison of invertebrate abundance in a seagrass bed and adjacent coral and sand areas at Amitori Bay, Iriomote Island, Japan. Fish. Sci. 2005, 71, 543–550. [Google Scholar] [CrossRef]
- Enochs, I.C. Motile cryptofauna associated with live and dead coral substrates: Implications for coral mortality and framework erosion. Mar. Biol. 2012, 159, 709–722. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sano, M. Overlaps in habitat use of fishes between a seagrass bed and adjacent coral and sand areas at Amitori Bay, Iriomote Island, Japan: Importance of the seagrass bed as juvenile habitat. Fish. Sci. 2004, 70, 788–803. [Google Scholar] [CrossRef]
- Dorenbosch, M.; Grol, M.G.G.; Nagelkerken, I.; van der Velde, G. Distribution of coral reef fishes along a coral reef-seagrass gradient: Edge effects and habitat segregation. Mar. Ecol. Prog. Ser. 2005, 299, 277–288. [Google Scholar] [CrossRef]
- Lee, C.L.; Lin, H.J. Ontogenetic habitat utilization patterns of juvenile reef fish in low predation habitats. Mar. Biol. 2015, 162, 1799–1811. [Google Scholar] [CrossRef]
- Grol, M.G.G.; Dorenbosch, M.; Kokkelmans, E.M.; Nagelkerken, I. Mangroves and seagrass beds do not enhance growth of early juveniles of a coral reef fish. Mar. Ecol. Prog. Ser. 2008, 366, 137–146. [Google Scholar] [CrossRef]
- Grol, M.G.G.; Nagelkerken, I.; Rypel, A.L.; Layman, C.A. Simple ecological trade-offs give rise to emergent cross-ecosystem distributions of a coral reef fish. Oecologia 2011, 165, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Hirota, K.; Shibuno, T.; Watanabe, Y. Variability in nursery function of tropical seagrass beds during fish ontogeny: Timing of ontogenetic habitat shift. Mar. Biol. 2012, 159, 1305–1315. [Google Scholar] [CrossRef]
- Dorenbosch, M.; Grol, M.G.G.; Groene, A.; van der Velde, G.; Nagelkerken, I. Piscivore assemblages and predation pressure affect relative safety of some back-reef habitats for juvenile fish in a Caribbean bay. Mar. Ecol. Prog. Ser. 2009, 379, 181–196. [Google Scholar] [CrossRef]
- Sheaves, M. Consequences of ecological connectivity: The coastal ecosystem mosaic. Mar. Ecol. Prog. Ser. 2009, 391, 107–115. [Google Scholar] [CrossRef]
- Ries, L.; Fletcher, R.J.; Battin, J.; Sisk, T.D. Ecological responses to habitat edges: Mechanisms, models, and variability explained. Ann. Rev. Ecol. Syst. 2004, 35, 491–522. [Google Scholar] [CrossRef]
- Dorenbosch, M.; Grol, M.G.G.; Nagelkerken, I.; van der Velde, G. Different surrounding landscapes may result in different fish assemblages in East African seagrass beds. Hydrobiologia 2006, 563, 45–60. [Google Scholar] [CrossRef]
- Tuya, F.; Vanderklift, M.A.; Wernberg, T.; Thomsen, M.S. Gradients in the number of species at reef-seagrass ecotones explained by gradients in abundance. PLoS ONE 2011, 6, e20190. [Google Scholar] [CrossRef] [PubMed]
- Hylkema, A.; Vogelaar, W.; Meesters, H.W.G.; Nagelkerken, I.; Debrot, A.O. Fish species utilization of contrasting sub-habitats distributed along an ocean-to-land environmental gradient in a tropical mangrove and seagrass lagoon. Estuar. Coast 2015, 38, 1448–1465. [Google Scholar] [CrossRef]
- Lin, H.J.; Hsieh, L.Y.; Liu, P.J. Seagrasses of Tongsha Island, with descriptions of four new records to Taiwan. Bot. Bull. Acad. Sin. 2005, 46, 163–168. [Google Scholar]
- Lee, C.L.; Huang, Y.H.; Chung, C.Y.; Lin, H.J. Tidal variation in fish assemblages and trophic structures in tropical Indo-Pacific seagrass beds. Zool. Stud. 2014, 53, 56. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lee, C.L.; Chung, C.Y.; Hsiao, S.C.; Lin, H.J. Carbon budgets of multispecies seagrass beds at Dongsha Island in the South China Sea. Mar. Environ. Res. 2015, 106, 92–102. [Google Scholar] [CrossRef]
- Aioi, K.; Pollard, P.C. Biomass, leaf growth and loss rate of the seagrass Syringodium isoetifolium on Dravuni Island, Fiji. Aquat. Bot. 1993, 46, 283–292. [Google Scholar] [CrossRef]
- Gratwicke, B.; Speight, M.R. The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J. Fish Biol. 2005, 66, 650–667. [Google Scholar] [CrossRef]
- Ebisawa, A.; Ozawa, T. Life-history traits of eight Lethrinus species from two local populations in waters off the Ryukyu Islands. Fish. Sci. 2009, 75, 553–566. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web electronic publication, version 2012. Available online: www.fishbase.org (accessed on 1 June 2012).
- Hyslop, E.J. Stomach contents analysis: A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Schoener, T.W. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Sala, E.; Ballesteros, E. Partitioning of space and food by three fish of the genus Diplodus (Sparidae) in a Mediterranean rocky infralittoral ecosystem. Mar. Ecol. Prog. Ser. 1997, 152, 273–283. [Google Scholar] [CrossRef]
- Nagelkerken, I.; van der Velde, G.; Verberk, W.C.E.P.; Dorenbosch, M. Segregation along multiple resource axes in a tropical seagrass fish community. Mar. Ecol. Prog. Ser. 2006, 308, 79–89. [Google Scholar] [CrossRef]
- Almany, G.R.; Webster, M.S. The predation gauntlet: Early post-settlement mortality in reef fishes. Coral Reefs 2006, 25, 19–22. [Google Scholar] [CrossRef]
- Feeney, W.E.; Lönnstedt, O.M.; Bosiger, Y.; Martin, J.; Jones, G.P.; Rowe, R.J.; McCormick, M.I. High rate of prey consumption in a small predatory fish on coral reefs. Coral Reefs 2012, 31, 909–918. [Google Scholar] [CrossRef]
- Kramer, M.J.; Bellwood, D.R.; Bellwood, O. Benthic Crustacea on coral reefs: A quantitative survey. Mar. Ecol. Prog. Ser. 2014, 511, 105–116. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Enochs, I.C.; Toth, L.T.; Brandtneris, V.W.; Afflerbach, J.C.; Manello, D.P. Environmental determinants of motile cryptofauna on an eastern Pacific coral reef. Mar. Ecol. Prog. Ser. 2011, 438, 105–118. [Google Scholar] [CrossRef]
- Glynn, P.W.; Enochs, I.C. Invertebrates and their roles in coral reef ecosystems. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 273–325. [Google Scholar]
- Peduzzi, P.; Herndl, G.J. Decomposition and significance of seagrass leaf litter (Cymodocea nodosa) for the microbial food web in coastal waters (Gulf of Trieste, Northern Adriatic Sea). Mar. Ecol. Prog. Ser. 1991, 71, 163–174. [Google Scholar] [CrossRef]
- Lepoint, G.; Cox, A.S.; Dauby, P.; Poulicek, M.; Gobert, S. Food sources of two detritivore amphipods associated with the seagrass Posidonia oceanica leaf litter. Mar. Biol. Res. 2006, 2, 355–365. [Google Scholar] [CrossRef]
- Parrish, J.D. Fish communities of interacting shallow-water habitats in tropical oceanic regions. Mar. Ecol. Prog. Ser. 1989, 58, 143–160. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Hindell, J.S.; Keough, M.J.; Jenkins, G.P.; Connolly, R.M. Resource distribution influences positive edge effects in a seagrass fish. Ecology 2010, 91, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Dimech, M.; Borg, J.A.; Schembri, P.J. Motile macroinvertebrate assemblages associated with submerged Posidonia oceanica litter accumulations. Biol. Mar. Medit. 2006, 13, 130–133. [Google Scholar]
- Ritchie, E.G.; Johnson, C.N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 2009, 12, 982–998. [Google Scholar] [CrossRef]
- Hixon, M.A.; Jones, G.P. Competition, predation, and density-dependent mortality in demersal marine fishes. Ecology 2005, 86, 2847–2859. [Google Scholar] [CrossRef]
- Nanami, A.; Shimose, T. Interspecific differences in prey items in relation to morphological characteristics among four lutjanid species (Lutjanus decussatus, L. fulviflamma, L. fulvus and L. gibbus). Environ. Biol. Fishes 2013, 96, 591–602. [Google Scholar] [CrossRef]
- Dorenbosch, M.; Verweij, M.C.; Nagelkerken, I.; Jiddawi, N.; van der Velde, G. Homing and daytime tidal movements of juvenile snappers (Lutjanidae) between shallow-water nursery habitats in Zanzibar, western Indian Ocean. Environ. Biol. Fishes 2004, 70, 203–209. [Google Scholar] [CrossRef]




| Habitat Type | Side View | Top View | Seagrass Cover (%) | Hard Substrate Cover (%) |
|---|---|---|---|---|
| Seagrass beds (SG) |  |  | 100% | 0% |
| Seagrass-dominated mosaic habitats (SM) |  |  | 50% | 50% (live coral: 20–30%) |
| Coral-dominated mosaic habitats (CM) |  |  | 10% | 80% (live coral: 20–40%) |
| Coral reefs (CR) |  |  | 0% | 80% (live coral: 20–50%) |
| Variable | F Statistic | Post-hoc Differences |
|---|---|---|
| Seagrass density | 52327.24 *** | SG > SM > CM > CR |
| Seagrass leaf area index (LAI) | 858.80 *** | SG, SM > CM > CR |
| Seagrass cover | 22088.20 *** | SG > SM > CM > CR |
| Live coral cover | 437.05 *** | CR, CM > SM > SG |
| Structure height | 2433.20 *** | CR > CM > SM, SG |
| Structure rugosity | 438.92 *** | CR > CM > SM > SG |
| Large crustacean density | 67.75 *** | CM > SG, SM, CR |
| Small crustacean density | 0.55 | None |
| Large piscivore density | 4.32 * | CR > SG, SM, CM |
| Small piscivore density | 84.31 *** | SM, CM > CR > SG |
| Potential competitor density | 415.36 *** | CM > SM, CR > SG |
| Species | n | Fi | De | Is | Os | Am | Ta | Co | Ga | Bi | Po | Ec | Dt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lethrinus atkinsoni | |||||||||||||
| Small | 26 | 1 | 3 | 27 | 7 | 60 | 2 | ||||||
| Medium | 11 | 83 | 2 | 9 | 1 | 2 | 2 | 1 | |||||
| Large | 22 | 88 | 1 | 7 | 2 | 1 | 1 | ||||||
| Lethrinus obsoletus | |||||||||||||
| Small | 28 | 1 | 2 | 6 | 13 | 77 | 1 | ||||||
| Medium | 30 | 3 | 78 | 1 | 6 | 4 | 1 | 1 | 5 | 1 | |||
| Large | 19 | 8 | 84 | 1 | 1 | 1 | 2 | 3 | |||||
| Lethrinus harak | |||||||||||||
| Small | 32 | 6 | 3 | 15 | 38 | 37 | 1 | ||||||
| Medium | 33 | 87 | 1 | 1 | 2 | 2 | 6 | 1 | |||||
| Large | 22 | 6 | 87 | 1 | 1 | 1 | 3 | 1 | |||||
| Lutjanus fulviflamma | |||||||||||||
| Small | 24 | 1 | 26 | 1 | 34 | 14 | 21 | 2 | |||||
| Medium | 19 | 4 | 74 | 1 | 1 | 7 | 2 | 10 | 1 | 1 | |||
| Large | 21 | 21 | 77 | 1 | 1 |
| Family | Species | La-S | La-M | La-L | Lo-S | Lo-M | Lo-L | Lh-S | Lh-M | Lh-L | LJf-S | LJf-M | LJf-L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lethrinidae | Lethrinus atkinsoni (La-S) | ||||||||||||
| L. atkinsoni (La-M) | |||||||||||||
| L. atkinsoni (La-L) | 0.89 | ||||||||||||
| Lethrinus obsoletus (Lo-S) | 0.76 | ||||||||||||
| L. obsoletus (Lo-M) | 0.89 | 0.83 | |||||||||||
| L. obsoletus (Lo-L) | 0.88 | 0.89 | |||||||||||
| Lethrinus harak (Lh-S) | |||||||||||||
| L. harak (Lh-M) | 0.89 | 0.93 | 0.88 | 0.91 | |||||||||
| L. harak (Lh-L) | 0.86 | 0.91 | 0.87 | 0.96 | 0.93 | ||||||||
| Lutjanidae | Lutjanus fulviflamma (LJf-S) | ||||||||||||
| L. fulviflamma (LJf-M) | 0.75 | 0.75 | 0.77 | 0.78 | 0.76 | 0.78 | |||||||
| L. fulviflamma (LJf-L) | 0.77 | 0.81 | 0.87 | 0.79 | 0.86 | 0.74 | |||||||
| Apogonidae | Ostorhinchus spp. | 0.72 | 0.87 | 0.60 | |||||||||
| Cheilodipterus quinquelineatus | 0.63 | 0.78 | |||||||||||
| Labridae | Stethojulis strigiventer | 0.86 | 0.71 | 0.69 | |||||||||
| Halichoeres spp. | 0.78 | 0.86 | 0.68 | ||||||||||
| Choerodon anchorago | 0.65 | 0.65 | 0.68 | 0.64 | |||||||||
| Nemipteridae | Scolopsis lineata (S) | 0.94 | 0.73 | 0.68 | |||||||||
| S. lineata (M) | 0.82 | 0.70 | 0.70 | ||||||||||
| Pomacentridae | Abudefduf spp. | 0.63 | 0.67 | ||||||||||
| Chrysiptera spp. | 0.73 | 0.60 | 0.61 | ||||||||||
| Dischistodus prosopotaenia | 0.68 | 0.74 |
| Species/Size | Best Fit Model | AIC |
|---|---|---|
| Lethrinus atkinsoni | ||
| Small | prey density + food availability + piscivore density | 42.92 |
| Medium | habitat type + prey density | 78.68 |
| Large | habitat type + prey density + competitor density + food availability | 104.5 |
| Lethrinus obsoletus | ||
| Small | habitat type + piscivores density | 99.06 |
| Medium | prey density + competitor density + food availability | 119.24 |
| Large | habitat type + prey density + competitor density + food availability | 139.02 |
| Lethrinus harak | ||
| Small | habitat type + prey density + competitor density + food availability + piscivores density | 62.42 |
| Medium | habitat type + prey density + food availability | 105.03 |
| Large | prey density + competitor density + food availability | 119.24 |
| Lutjanus fulviflamma | ||
| Small | habitat type | 105.12 |
| Medium | habitat type + prey density + competitor density + food availability | 139.73 |
| Large | habitat type + prey density + food availability | 139.69 |
| Habitat Type | Sand | Seagrass | Seagrass-Coral Mixed | Rubble | Live Coral | Reference |
|---|---|---|---|---|---|---|
| Iriomote, Ryukyu Islands | SC: 6500 LC: 0 All: 11000 | SC: 10000 LC: 180 All: 24000 | SC: 4000 LC: 400 All: 12000 | [15] | ||
| Ishigaki, Ryukyu Islands | SC: 20000 LC: 350 | SC: 20000 LC: 200 | [21] | |||
| Dakwan, Southern Taiwan | SC: <10 LC: <5 All: 10 | SC: 30 LC: 10 All: 50 | SC: 80 LC: 18 All: 120 | [25] | ||
| Dongsha Island, South China Sea | SC: 100 LC: <5 | SC: 4000 LC: 20 | SC: 5000–7000 LC: 40–50 | SC: 4000–8000 LC: 20–30 | SC: 5000–7000 LC: 20 | this study |
| Lizard Island, GBR | AC: 5000 | AC: 230000 | AC: 66 | [47] | ||
| Kunduchi, Tanzania | SC: 20 LC: <10 | SC: 40 LC: 50 | [12] | |||
| Playa Larga Reef, Panama | All: 200-500 | All: 400–1600 | [17] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Wen, C.K.C.; Huang, Y.-H.; Chung, C.-Y.; Lin, H.-J. Ontogenetic Habitat Usage of Juvenile Carnivorous Fish Among Seagrass-Coral Mosaic Habitats. Diversity 2019, 11, 25. https://doi.org/10.3390/d11020025
Lee C-L, Wen CKC, Huang Y-H, Chung C-Y, Lin H-J. Ontogenetic Habitat Usage of Juvenile Carnivorous Fish Among Seagrass-Coral Mosaic Habitats. Diversity. 2019; 11(2):25. https://doi.org/10.3390/d11020025
Chicago/Turabian StyleLee, Chen-Lu, Colin K.C. Wen, Yen-Hsun Huang, Chia-Yun Chung, and Hsing-Juh Lin. 2019. "Ontogenetic Habitat Usage of Juvenile Carnivorous Fish Among Seagrass-Coral Mosaic Habitats" Diversity 11, no. 2: 25. https://doi.org/10.3390/d11020025
APA StyleLee, C.-L., Wen, C. K. C., Huang, Y.-H., Chung, C.-Y., & Lin, H.-J. (2019). Ontogenetic Habitat Usage of Juvenile Carnivorous Fish Among Seagrass-Coral Mosaic Habitats. Diversity, 11(2), 25. https://doi.org/10.3390/d11020025





