Abstract
We describe and name a new species of Noblella Barbour, 1930 (Strabomantidae) from southern Peru. Key diagnostic characteristics of the new species include the presence of a short, oblique fold-like tubercle on the ventral part of the tarsal region, two phalanges on finger IV, and an evident tympanum. The elevational distribution of the new species spans 1250 m (240–1490 m) from lowland Amazon rainforest to montane forest on the eastern slopes of the Andes.
1. Introduction
The terrestrial-breeding frog genus Noblella Barbour, 1930 [1] (Strabomantidae) is distributed in the Andes–Amazon region of Ecuador, Peru, Brazil, and Bolivia and currently includes 14 species: N. carrascoicola (De la Riva and Köhler, 1998) [2], N. coloma Guayasamin and Terán-Valdez, 2009 [3], N. duellmani (Lehr, Aguilar, and Lundberg, 2004) [4], N. heyeri (Lynch, 1986) [5], N. lochites (Lynch, 1976) [6], N. lynchi (Duellman, 1991) [7], N. madreselva Catenazzi, Uscapi, and von May, 2015 [8], N. myrmecoides (Lynch, 1976) [6], N. naturetrekii Reyes-Puig et al. [9], N. personina Harvey, Almendáriz, Brito-M., and Batallas-R., 2013 [10], N. peruviana (Noble, 1921) [11], N. pygmaea Lehr and Catenazzi, 2009 [12], N. ritarasquinae (Köhler, 2000) [13], and N. thiuni Catenazzi and Ttito, 2019 [14]. Most of these species inhabit montane forests above 1000 m and are morphologically very similar to those in the genus Psychrophrynella Hedges, Duellman, and Heinicke 2008 [15]. Recent analyses indicate that there is uncertainty regarding the relationships among species of Noblella and Psychrophrynella [14,16]. Hedges et al. [15] assigned N. peruviana and P. bagrecito, respectively, as type species of the two genera. However, the lack of DNA sequences for both N. peruviana and P. bagrecito has prevented researchers from resolving the phylogenetic relationships between Noblella and Psychrophrynella. Additionally, recent studies have inferred the non-monophyly of the genus Noblella [14] and researchers identified a “northern clade” and a “southern clade” containing species ascribed to Noblella [9,14]. This taxonomic issue will only be properly resolved when sequences from N. peruviana become available. In the meantime, the description of new species will continue advancing our knowledge of the diversity of these small terrestrial-breeding frogs. Here, we describe and name a new species of Noblella on the basis of specimens collected in the lowland Amazon forest and the montane forest of the Amazonian Andes in southern Peru.
2. Materials and Methods
2.1. Fieldwork and Data Collection
We conducted fieldwork at Los Amigos Biological Station (12°34′07″ S, 70°05′57″ W, 250 m a.s.l.), located in the Madre de Dios region, Peru, and at various sites along the Kosñipata Valley, located in the Cusco region, Peru [17]. Specimens were euthanized by immersion in benzocaine hydrochloride solution (250 mg/L), where animals were kept for 10 to 20 min, until movement ceased, or by application of 20% benzocaine paste to the ventral region. After euthanasia, tissue samples (e.g., liver, muscle) were taken from the animals and preserved in 2 mL cryogenic tubes filled with RNAlater or 95% ethanol. Following tissue collection, specimens were fixed in 10% formalin, and permanently stored in 70% ethanol, except for specimens collected in 2018, which were fixed in 95% ethanol and stored in 70% ethanol. Sex and maturity of specimens were determined by observing sexual characters and gonads through dissections. Photographs were taken by R. von May, R. Santa Cruz, C. Whitcher, and A. Catenazzi, and were used for descriptions of coloration in life. We were unable to record calls of the new species.
Use of vertebrate animals was approved by the Animal Care and Use committees of the University of California (ACUC #R278-0412, R278-0413, and R278-0314), the University of Michigan (PRO00008306), Florida International University (IACUC #18-009), and Southern Illinois University (IACUC protocol #16-006).
2.2. Morphological Characters
We followed Duellman and Lehr [18] and Lynch and Duellman [19] for formats of diagnosis and description, except for using the term “dentigerous processes of vomers” instead of “vomerine odontophores” [20]. For taxonomy we follow Padial et al. [21] and Heinicke et al. [22]. We measured the following variables to the nearest 0.1 mm with digital calipers under a stereomicroscope: snout-vent length (SVL), tibia length (TL), foot length (FL, distance from proximal margin of inner metatarsal tubercle to tip of Toe IV), head length (HL, from angle of jaw to tip of snout), head width (HW, at level of angle of jaw), eye diameter (ED), tympanum diameter (TY), interorbital distance (IOD), upper eyelid width (EW), internarial distance (IND), eye–nostril distance (E–N, straight line distance between anterior corner of orbit and posterior margin of external nares), eye to tympanum distance (E-TY), forearm length (ForL), hand length (HaL), finger I length (FIL), finger II length (FIIL), toe I length (TIL), and toe II length (TIIL). Fingers and toes are numbered preaxially to postaxially from I–IV and I–V, respectively. We determined comparative lengths of toes III and V by adpressing both toes against toe IV; lengths of fingers I and II were determined by adpressing the fingers against each other. We compared the new taxon with all described species. Specimens examined are listed in Appendix A; codes of collections are: CORBIDI = Centro de Ornitología y Biodiversidad, Lima, Peru; MUBI = Museo de Biodiversidad del Peru, Cusco, Peru; MUSM = Museo de Historia Natural Universidad Nacional Mayor de San Marcos, Lima, Peru; MUSA = Museo de Historia Natural de la Universidad Nacional de San Agustín, Arequipa, Peru; UMMZ = University of Michigan Museum of Zoology, Ann Arbor, MI, USA.
Additionally, we used the morphological data to examine if body size and body shape vary across elevations. We used principal components analysis (PCA) to examine morphological variation between males and females, and between low (<300 m) and high elevation (1200–1490 m) populations. To remove the possible confounding effect of body size, we performed a body size-correction in which all variables were divided by SVL. We used generalized least-squares (GLS) regression to examine the relationship between SVL and elevation, and fitted a regression line separately for males and females. Subsequently, we calculated PCA using the corrected morphological data. We projected the first two PC axes on a morphospace using the function princomp in R and displayed differences between male and females taking into account elevation.
2.3. Micro-Computed Tomography
We obtained X-ray micro-computed tomography (μCT) images from two specimens to examine the skull and skeleton of the new species. We scanned two voucher specimens (paratypes) stored in 70% ethanol in the Micro-CT Core facility at the University of Michigan. We placed these specimens inside a glass vial, which in turn was placed in a 34 mm diameter specimen holder, prior to scanning. We used a microCT system, µCT100 Scanco Medical (Bassersdorf, Switzerland), and scan settings were as follows: voxel size 11.4 µm, 55 kVp, 145 µA, 0.5 mm AL filter, 1000 projections around 180°, integration time of 1000 ms, and average data of 3 replicates. We used Scanco’s proprietary software to export data to DICOM files. We used the Amira-Avizo software to obtain three-dimensional renderings based on isosurface representations.
The main purpose of the CT-scans was to examine the condition of the tympanic middle ear (inspect if columella was present or not) and the number and shape of phalanges. Both of these characters are key for the diagnosis of species of Noblella and Psychrophrynella. Thus, images presented here will facilitate osteological comparisons with other species once additional CT-scan data become available.
2.4. Molecular Phylogenetic Analysis
We determined the phylogenetic position of the new species with respect to other closely related taxa through analysis of DNA sequences. Our analysis included sequences obtained from tissue samples collected from type specimens (holotype and paratypes) as well as legacy data from GenBank (Table S1). Sequence data included a fragment of the 16S rRNA gene (16S), a fragment of the 12S rRNA gene (12S), the protein-coding gene cytochrome c oxidase subunit I (COI), the nuclear protein-coding gene recombination-activating protein 1 (RAG1), and the tyrosinase precursor (Tyr). We used Phrynopus peruanus to root the tree. We followed previously reported procedures [14,23] for lab work and sequencing, and we deposited new sequences in GenBank (Table S1).
We aligned the sequences with Geneious R6, v. 6.1.8 [24], using the built-in Geneious Aligner program. Subsequently, we used PartitionFinder, v. 1.1.1 [25] to select the appropriate models of nucleotide evolution. We determined the best partitioning scheme and substitution model for each gene using the Bayesian information criterion (BIC). The best partitioning scheme included six subsets (best fitting substitution model in parentheses) as follows. A first partition subset including both 12S and 16S sequences (GTR + I + G). For COI, the best partitioning scheme included three sets of sites (substitution models in parentheses): the first set with first codon position (K80 + G), the second set with second codon position (F81 + I), and the third set with the third codon position (HKY + G). The next subset included the first and second codon positions of RAG together with the first and third codon positions of Tyr (HKY + G). The last subset included the third codon position of RAG together with the second codon position of Tyr (K80 + G).
We used MrBayes, v. 3.2.0 [26] to infer a molecular phylogeny. Our analysis included 36 terminals and a 2571 bp concatenated partitioned dataset (16S, 12S, COI, RAG1, Tyr). We performed an MCMC Bayesian analysis that included two simultaneous runs of 10 million generations, sampled once every 1000 generations. Each run had one “cold” chain and three heated chains, and the burn-in was set to discard 25% samples from the cold chain. The average standard deviation of split frequencies at the end of the runs was 0.001661. Subsequently, we used Tracer version 1.5 [27] to examine the effective sample sizes (ESS), to verify convergence, and to verify that the runs reached stationarity. The observed effective sample sizes were sufficient for all parameters (ESS > 200). Lastly, we used FigTree v. 1.4.2 [28] to visualize the majority-rule consensus tree and assess node support (based on posterior probability values).
2.5. Registration of New Nomenclatural Acts
According to the International Commission on Zoological Nomenclature (ICZN), which produces the International Code of Zoological Nomenclature, the electronic publication of this article in portable document format (PDF) represents a published work. Therefore, the new species name contained in the PDF is effectively published under the International Code of Zoological Nomenclature from the electronic edition alone. This publication and the nomenclatural acts contained in it have been registered in ZooBank, the online official register for the ICZN. The ZooBank Life Science Identifiers (LSIDs) can be accessed and viewed through standard web browsers by appending the LSID to the prefix http://zoobank.org/. The online version of this work is archived and available from the following digital repositories: Diversity, CLOCKSS, and e-Helvetica.
3. Results
3.1. Molecular Phylogenetic Analysis
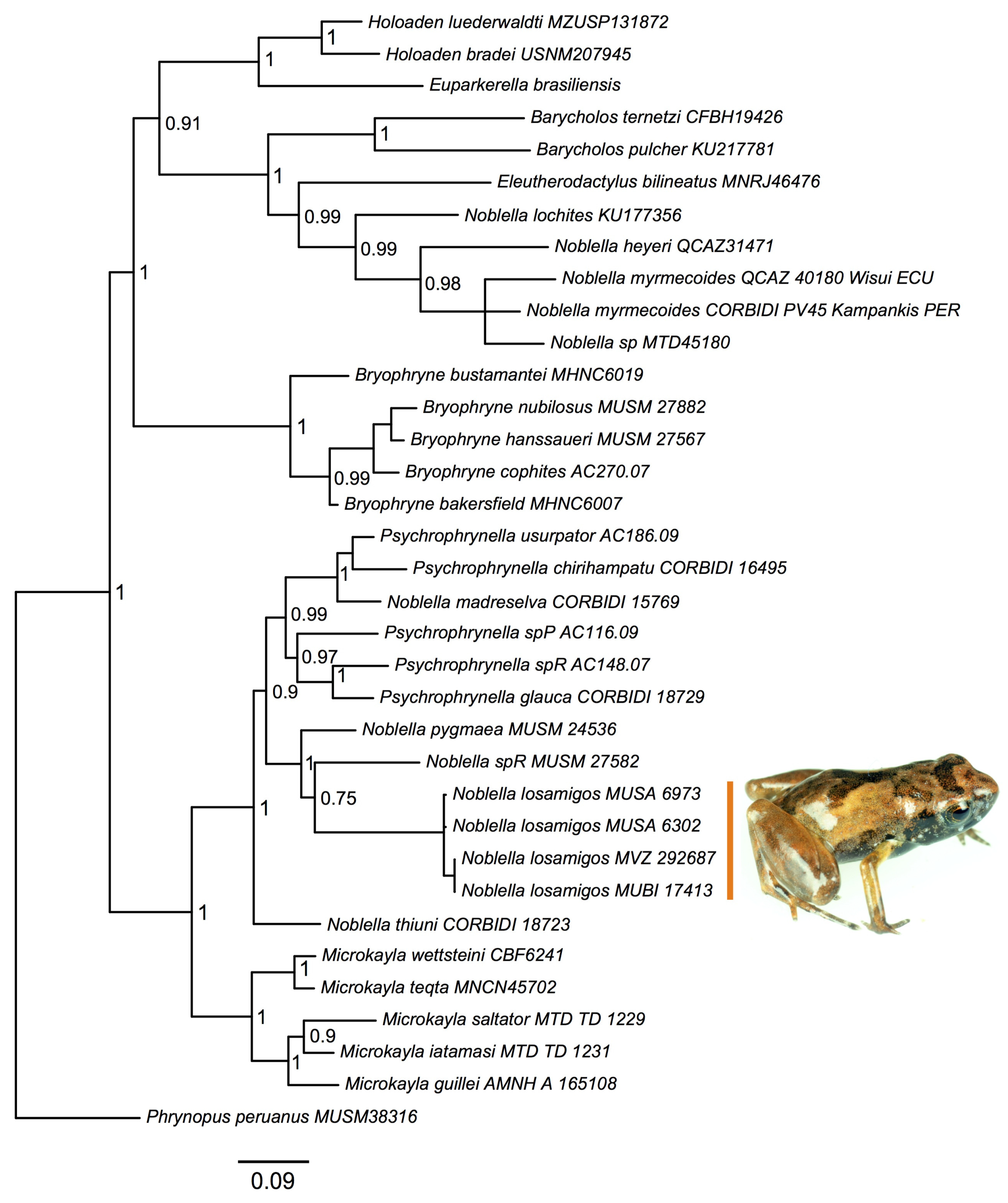
We recovered a phylogenetic tree (Figure 1) that was congruent with previous analyses [16,21], and supported a unique history of divergence of the new species. There was strong support for placing the new species in a clade containing species of Noblella distributed in southern Peru as well as several species of Psychrophrynella. According to this analysis, the new species is most closely related to Noblella pygmaea and to an undescribed species (Noblella sp. R in [23]).
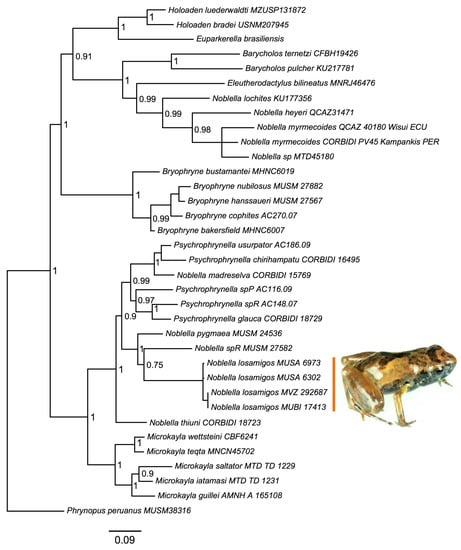
Figure 1.
Phylogeny. Bayesian maximum clade-credibility tree for species included in this study based on a 2571 bp concatenated partitioned dataset analyzed in MrBayes (posterior probabilities indicated at each node).
3.2. Taxonomy
Noblella losamigos sp. n. MUSA 6973 (Field number: RvM 3.12)
Phyllonastes myrmecoides Heyer 1977—Rodríguez and Cadle 1990 [29]: p. 413, Table 22.1; Rodríguez 1992 [30]: p. 162, 172, 174, Table I, Table V; Morales and McDiarmid 1996 [31]: p. 511, Table 2; Doan and Arizabal 2002 [32]: p. 114, Appendix 1
Noblella myrmecoides (Lynch 1976)—von May et al. 2009 [33]: p. 18, Table 1; von May et al. 2010a [34]: p. 513, 519, Figure 3, Appendix 1; von May et al. 2010 [35]: p. 10, Figures 193–194 Catenazzi et al. 2013 [17]: p. 274, 280, Table 1; von May et al. 2017 [23]: p. 3261, 3262, Figures 2 and 3, Supplementary Tables S2–S3, Supplementary Figures S2–S4; Whitworth et al. 2016 [36]: Appendices D–E; Villacampa et al. 2017 [37]: p. 4, 58; von May et al. 2018 [38]: Appendix A, Table A1, p. 8, 10–11; von May et al. 2019 [39]: Figures 1 and 2, Figures S1 and S3, Supplementary Information Tables 1 and 2.
Noblella cf. myrmecoides (Lynch 1976)—Catenazzi et al. 2013 [17]: p. 274, Table 1.
Noblella sp. SP—Catenazzi and Ttito 2019 [14]: p. 6, 8, Table 1, Figure 3, Appendix 2.
3.2.1. Holotype
MUSA 6973 (Field number: RvM 3.12) (Figure 2), an adult female from 12°34′13.84″ S, 70°04′54.66″ W (Datum WGS 84), Los Amigos Biological Station, 245 m a.s.l., Manu District, Manu Province, Madre de Dios Region, Peru, collected by R. von May and R. Santa Cruz on 16 January 2012.

Figure 2.
Photographs of preserved specimen of Noblella losamigos sp. n.: Adult female holotype MUSA 6973 (SVL 10.7 mm): (A) Dorsal view, (B) ventral view, (C) lateral view, (D) right hand, and (E) right toe. Photographs by Rudolf von May.
3.2.2. Paratypes
A total of 27 specimens: Three adult males (MUSM 37355, MUSM 37357, UMMZ 246569) and an adult female (MUSM 37356) (Figure 3 and Figure 4); from Los Amigos Biological Station, collected by R. von May and R. Santa Cruz on 26 November 2016; an adult female (UMMZ 246570) from Los Amigos Biological Station, collected by R. von May and R. Santa Cruz on 29 November 2016; three adults (sex unknown) (UMMZ 244945, MUSM 33247, MUSA 6302) from Los Amigos Biological Station, collected by R. von May and R. Santa Cruz on 14–19 May 2014; three specimens male/male/adult (MUSA 6974, MUSM 24219, MUSM 24251) from Los Amigos Biological Station, collected with the holotype by R. von May and R. Santa Cruz on 16 January 2012. Seven males: MUBI 17412, CORBIDI 17521, from San Pedro, Kosñipata Valley (13.04514S, 71.52922W, 1274 m), collected on 7 April 2018 by A. Catenazzi and M. I. Diaz; CORBIDI 17520, from San Pedro, Kosñipata Valley (13.04833S, 71.53178W, 1341 m), collected on 7 April 2018 by A. Catenazzi and M. I. Diaz; CORBIDI 17524 from San Pedro, Kosñipata Valley (13.03724S, 71.52798W, 1200 m), collected on 26 January 2009 by A. Catenazzi; MVZ:Herp:292685, MVZ:Herp:292686 from San Pedro, Kosñipata Valley (13.0541S, 71.54632W, 1370 m), collected on 24 January 2009 by A. Catenazzi; MVZ:Herp:292687, from San Pedro, Kosñipata Valley (13.05093S, 71.53711W, 1375 m), collected on 28 January 2009 by A. Catenazzi. One juvenile: MUBI 17413, from San Pedro, Kosñipata Valley (13.04514S, 71.52922W, 1274 m), collected on 7 April 2018 by A. Catenazzi and M. I. Diaz. Eight females: MUSM 30429, from San Pedro, Kosñipata Valley (13.04335S, 71.53027W, 1234 m), collected on 26 January 2009 by A. Catenazzi; CORBIDI 17523, from San Pedro, Kosñipata Valley (13.05044S, 71.53373W, 1342 m), collected on 26 January 2009 by A. Catenazzi; MUSM 27578, from San Pedro, Kosñipata Valley (13.05686S, 71.54081W, 1369 m), collected on 2 February 2008 by A. Catenazzi; MUSM 30426, MUSM 30427, MUSM 30428, MVZ:Herp:292684, from San Pedro, Kosñipata Valley (13.05193S, 71.5376W, 1376 m), collected on 24 January 2009 by A. Catenazzi; CORBIDI 17522, from San Pedro, Kosñipata Valley (13.05704S, 71.54778W, 1412 m), collected on 7 February 2008 by A. Catenazzi. Dorsal and ventral views of five paratypes (MUSA 6974, MUSM-37355, UMMZ 246569, MUSM 37356, UMMZ 246570), as well as the holotype, are presented in Figure 5. Three-dimensional reconstructions based on μCT data, from the skull and skeleton of two paratypes (MUSM 37355, MUSM 37356) are presented in Figure 6.

Figure 3.
Photographs of live specimen of Noblella losamigos sp. n. (A–C) Adult male paratype MUSM 37355 (SVL 9.5 mm). Photographs by Rudolf von May.

Figure 4.
Photographs of live specimens of Noblella losamigos sp. n. (A,B) Adult male paratype MUSM 37357 (SVL 10.3 mm). (C,D) Adult female paratype MUSM 37356 (SVL 9.7 mm). Photographs by Roy Santa-Cruz.

Figure 5.
Photographs of preserved specimens of Noblella losamigos sp. n. (A,B) Adult female holotype MUSA 6973 (SVL 10.7 mm); (C,D) adult male paratype MUSA 6974 (SVL 9.8 mm); (E,F) adult male paratype MUSM 37355 (SVL 9.5 mm); (G,H) adult male paratype UMMZ 246569 (SVL 9.6 mm); (I,J): adult female paratype MUSM 37356 (SVL 9.7 mm); and (K,L) adult female paratype UMMZ 246570 (SVL 11.3 mm). Photographs by Rudolf von May, Roy Santa-Cruz, and Courtney Whitcher.
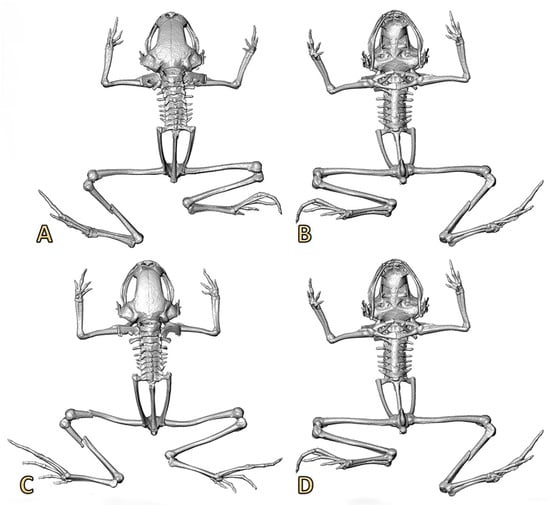
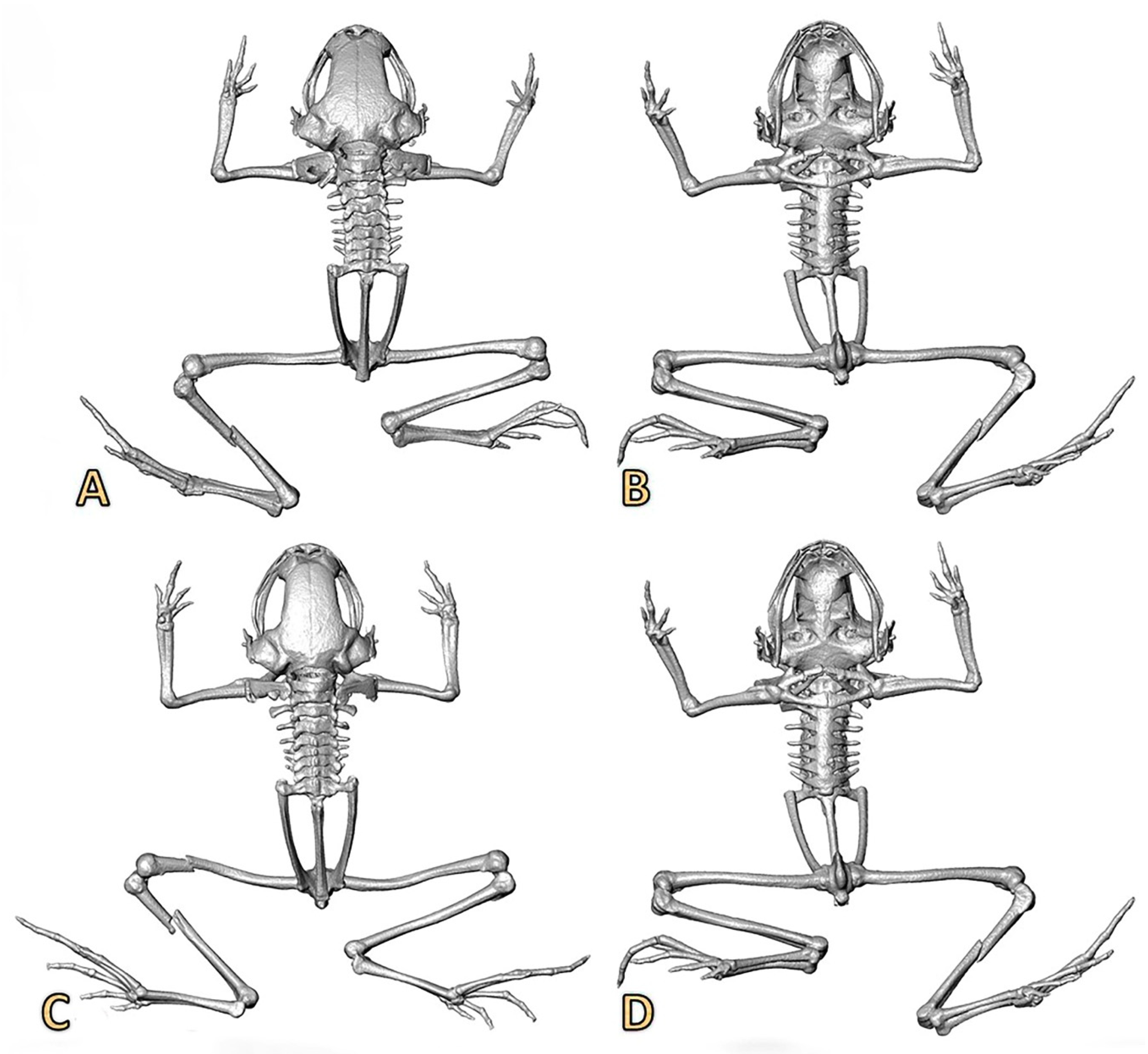
Figure 6.
Three-dimensional reconstructions based on μCT data, from the skull and skeleton of two specimens of Noblella losamigos sp. n. (A,B) Dorsal and ventral views of specimen MUSM 37355 (SVL 9.6 mm). (C,D) Dorsal and ventral views of specimen MUSM 37356 (SVL 9.7 mm).
3.2.3. Generic Placement
We assign this species to the genus Noblella based on its overall morphological resemblance with other species of Noblella, including the presence of T-shaped and pointed terminal phalanges (especially in toes). The genus Noblella Barbour, 1930 can be recognized by the following characters [3,7,15,40,41]: tympanic membrane differentiated (except in N. duellmani and N. madreselva); head narrower than body; cranial crests absent; dentigerous processes of vomers absent; finger I shorter than, or equal in length to, finger II; toe III shorter than toe V; tips of at least toes III–IV pointed; subarticular tubercles not protruding; conspicuous tarsal tubercle; dark inguinal spots present (except in N. duellmani); small body size (SVL < 22 mm). Additionally, our phylogenetic analysis indicates that the new species is closely related to other species of Noblella distributed in southern Peru (Figure 1), but also to several species of Psychrophrynella. However, given that N. peruviana and P. bagrecito (the type species of Noblella and Psychrophrynella, respectively) have not been included in phylogenetic analyses, the assignment remains tentative.
3.2.4. Diagnosis
A new species of Noblella characterized by (1) skin on dorsum smooth to finely shagreen, skin on belly smooth, discoidal fold absent, dorsolateral folds absent; (2) tympanic annulus visible below skin, with the upper portion (1/4) covered by a supratympanic fold; tympanic membrane evident; columella present (Figure 6); (3) snout short, rounded in dorsal view and bluntly rounded to subtruncate in profile; (4) upper eyelid with minute tubercles, cranial crests absent; (5) dentigerous process of vomers absent; (6) vocal slits present; nuptial pads absent; (7) finger I shorter than finger II; tips of digits rounded, distally ending in papillae; Finger IV having two phalanges (Figure 2 and Figure 7); (8) fingers with narrow lateral fringes; (9) ulnar tubercles absent; (10) short, oblique fold-like tubercle on the ventral part of tarsal region (Figure 2E); (11) no other tubercles on heel and tarsus; (12) inner metatarsal tubercle oval, of higher relief and about one and a half times the size of conical, rounded outer metatarsal tubercle; supernumerary plantar tubercles absent; (13) toes bearing narrow lateral fringes; webbing absent; toe V shorter than toe III; tips of digits weakly acuminate distally and expanded slightly in the digits II, III and IV; tips of the digits II, III, IV and V with discs slightly expanded, elongately acuminate, grooves present distally with papillae; (14) facial mask and lateral band dark brown with cream spots interrupted, extending from tip of snout along the flanks, almost reaching the point of insertion of thighs; (15) dorsum ocher gold to copper brown with or without irregularly-shaped middorsal dark brown marks; some specimens present a clear and slightly evident middorsal line that extends from middle of body to cloaca; dark brown suprainguinal stripes; interorbital bar present or absent; black or gray clear venter, always with irregular white markings; irregular white markings also present on the neck, thighs, and toes; (16) mean SVL 12.18 mm in females (range 9.74–13.60, n = 9), 10.08 mm in males (range 9.16–11.40, n = 12). Mean values and ranges of other morphological characters are provided in Table 1.
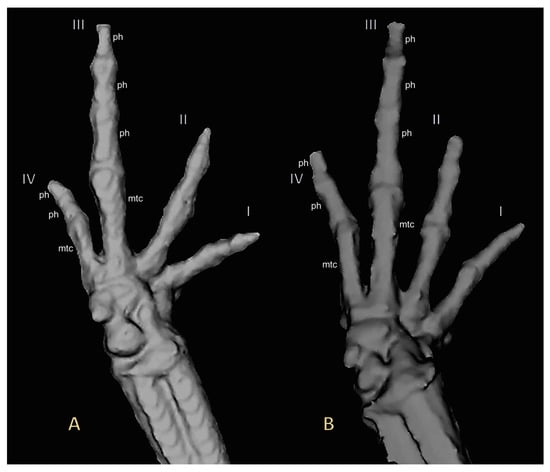
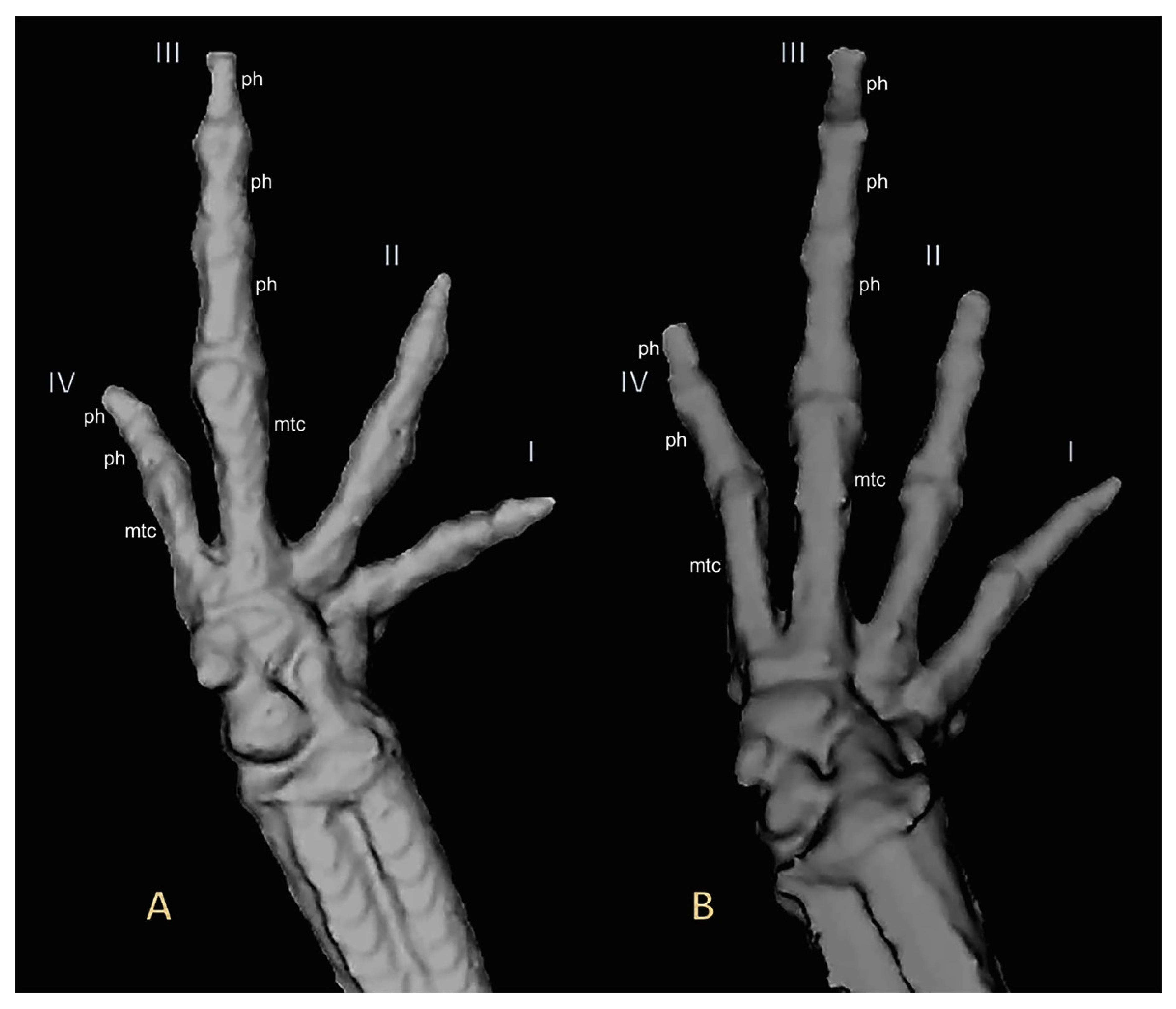
Figure 7.
Three-dimensional reconstructions based on μCT data, from (A) adult right hand paratype UMMZ 246570 of Noblella losamigos sp. n. and (B) adult right hand Noblella myrmecoides from Loreto UMMZ 246571. Finger phalanges (ph) and metacarpalia (mtc) are noted for fingers III and IV.

Table 1.
Measurements (in mm) of males and females of the type series of Noblella losamigos sp. n. See section on morphological characters for definition of each character. Abbreviations: SD = standard deviation, SVL = snout-vent length, TL = tibia length, FL = foot length, HL = head length, HW = head width, ED = eye diameter, TY = tympanum diameter, IOD = interorbital distance, EW = upper eyelid width, IND = internarial distance, E–N = and eye–nostril distance, E-TY = eye to tympanum distance, ForL = forearm length, HaL = hand length, FIL = finger I length, FIIL = finger II length, TIL = toe I length, TIIL = toe II length. Ranges are included in parentheses.
3.2.5. Comparisons with Described Species
Noblella losamigos sp. n. has two phalanges in Finger IV like N. carrascoicola, N. lochites, N. myrmecoides, and N. ritarasquinae. In contrast, Noblella losamigos differs from the following species, which have three phalanges in Finger IV: N. coloma, N. duellmani, N. heyeri, N. lynchi, N. madreselva, N. pearsonina, N. peruviana, N. pygmaea and N. thiuni. Externally, N. losamigos has an evident tympanum (absent in N. carrascoicola, N. duellmani and N. ritarasquinae; barely visible below skin in N. madreselva, N. thiuni, P. bagrecito, P. chirihampatu, P. glauca and P. usurpator). N. losamigos sp. n. is similar to N. myrmecoides and N. ritarasquinae by presence of papillae on the tips of the toes [6,12]. N. losamigos sp. n. has a short, oblique fold-like tubercle on the ventral part of tarsal region, similar to N. ritarasquinae and P. glauca (N. peruviana and N. heyeri have a prominent tubercle; N. lochites and N. myrmecoides have a tubercle transversely oriented; N. coloma, N. lynchi, N. personina, N. thiuni, P. chirihampatu, P. usurpator have a elongate tarsal tubercle; N. carrascoicola with a poorly marked tubercle; P. bagrecito have a smaller and sickle-shaped tubercle; N. duellmani, N. madreselva and N. pygmaea do not have a tubercle or tarsal fold. N. losamigos sp. n. is similar to N. madreselva, N. myrmecoides, N. thiuni, P. chirihampatu, P. glauca in that it has a heel lacking tubercles (N. pygmaea present a heel with one minute, round tubercle). The skin on the dorsum of N. losamigos is smooth to finely shagreen, similar to N. carrascoicola, N. coloma, N. heyeri, N. lochites, N. myrmecoides, N. thiuni, P. bagrecito, P. chirihampatu, P. glauca and P. usurpator (skin on the dorsum with small tubercles or pustules in N. duellmani, N. madreselva, N. lynchi, N. personina and N. pygmaea). N. losamigos presents suprainguinal spots similar to N. carrascoicola, N. coloma, N. heyeri, N. lynchi, N. myrmecoides, N. ritarasquinae, N. thiuni (diffuse suprainguinal stripes in N. madreselva or poorly defined in N. personina, longitudinal stripes in P. chirihampatu and P. glauca, absent in N. duellmani, N. pygmaea, and P. usurpator). N. losamigos has facial mask and lateral band of dark brown with creams spots interrupted and extending from the tip of the snout along the flanks, almost reaching the point of insertion of thighs (N. duellmani has a narrow dark brown post orbital stripe; N. pygmaea has a broad gray dorsolateral stripe that extends from upper eyelid to insertion of thigh; N. personina has a facial mask but lack a lateral dark band extending to the inguinal region [4,9,11]. The new species is larger in SVL (largest known female 13.60 mm, largest known male 11.40 mm) than N. pygmaea (largest known female 12.4 mm); it is similar in size to N. myrmecoides (largest known female 13.6 mm); and it is smaller than N. coloma (largest known female 16.03 mm, largest known male 14.55 mm), N. duellmani (largest known female 20.00 mm), N. heyeri (largest known female 15.90 mm, largest known male 14.10 mm), N. lynchi (largest known female 20.20 mm), N. lochites (largest known female 19.4 mm), N. madreselva (largest known female 17.6 mm, largest known male 15.6 mm), N. personina (largest known female 17.90 mm, largest known male 16.30 mm), P. bagrecito (largest known female 18.60 mm, largest known male 16.30 mm), P. glauca (largest known female 19.80 mm), P. chirihampatu (largest known female 25.80 mm, largest known male 21.70 mm), P. usurpator (largest known female 24.1 mm, largest known male 20.3 mm).
3.2.6. Description of Holotype
Adult female (10.7 mm SVL); head narrower than body; head length 29% of SVL; head slightly wider than longer; head width 33% of SVL; snout short, rounded in dorsal view, subtruncate in lateral view (Figure 2); eye large, 46% of head length, its diameter 1.8 times as large as its distance from the nostril; nostrils not protuberant, situated close to snout; canthus rostralis slightly curved in dorsal view, rounded in profile; lores flat; lips rounded; dorsal surface of head and upper eyelids with small tubercles; upper eyelid width 62% of inter-orbital distance; supratympanic fold short; tympanic annulus visible below skin, tympanic membrane evident; postrictal tubercles absent. Choanae round, very small, positioned far anteriorly and laterally, widely separated from each other, slightly concealed by palatal shelf of maxilla; dentigerous process of vomer and vomerine teeth absent; tongue long and narrow; skin on dorsum finely shagreen; discoidal fold absent, dorsolateral folds absent; skin on flanks smooth; skin on ventral surfaces and gular regions smooth to finely areolate; pectoral fold poorly visible, discoidal fold not evident; cloaca protuberant; cloacal region bearing several small tubercles. Outer surface of forearm without tubercles; palmar tubercle flat and oval, approximately twice the size of elongate, thenar tubercle; tarsal tubercle small; supernumerary palmar tubercles present; subarticular tubercles like calluses, flat in ventral and lateral view, largest at the base of fingers; fingers without narrow lateral fringes; Finger IV with two phalanges; when adpressed, Finger 3 > 2 > 4 > 1 (Figure 2); tips of digits rounded, with distal grooves and papillae (Figure 2); forearm lacking tubercles. Hindlimb length moderate, tibia length 51% of SVL; foot length 43% of SVL; upper and posterior surfaces of hindlimbs without tubercles; heel without tubercles; outer surface of tarsus without tubercles; inner metatarsal tubercle, oval, of higher relief and about one time the size of conical, rounded outer metatarsal tubercle; low plantar supernumerary tubercles present; subarticular tubercles not evident in dorsal view; toes bearing narrow lateral fringes, basal webbing absent; tips of digits II, III, IV and V with discs slightly expanded, elongately acuminate, grooves present distally with papillae; digital tip of Toe V smaller than tips of Toes III—IV; when adpressed, relative lengths of toes: 4 > 3 > 5 > 2 > 1 (Figure 2).
Measurements of the holotype (in mm): SVL = 10.72, tibia length (TL) = 5.45, foot length (FL) = 4.60, head length (HL) = 3.12, head width (HW) = 3.55, interorbital distance (IOD) = 1.29, upper eyelid width (EW) = 1.00, internarial distance (IND) = 1.09, eye to nostril distance (E-N) = 0.78, snout to nostril distance (SND) = 0.50, eye diameter (ED) = 1.43, tympanum diameter (TD) = 0.45, eye to tympanum distance (ETD) = 0.19, forearm length (ForL) = 2.75, hand length (HaL) = 1.93, finger I length (FIL) = 0.91, finger II length (FIIL) = 1.30, toe I length (TIL) = 1.15, toe II length (TIIL) = 2.20.
3.2.7. Coloration of Holotype
In alcohol, the dorsal surface of the head (from the interorbital region) and body (to the cloaca) have dark brown marks of irregular shape; the dorsal surface of forelimbs is cream with a dark brown transverse bar in the shape of a wristband. The cloacal and suprainguinal regions present dark brown marks in dorsal view; those of the suprainguinal region are circular. The dorsal surfaces of the hind limbs do not have transverse dark bars, although small irregular dark bars are present in some specimens. The facial mask and lateral band are dark brown with creams spots. The lower part of the flank is dark brown. The iris is dark gray. All ventral surfaces are dark gray with minute cream spots (Figure 2). The coloration of the holotype in life is unknown.
3.2.8. Variation
The description of the coloration in life is based on notes taken in the field and photographs from multiple individuals (Figure 3, Figure 4, Figure 8, Figure 9 and Figure 10). The dorsum varies from ocher gold to light brown. Most individuals have an irregularly-shaped dark brown dorsal mark, and an interorbital bar. In some individuals, a light middorsal line extending from the middle of the body to the cloaca replaces the brown dorsal mark. Ventral coloration varies from transparent gray to black. Although all individuals have minute irregular cream flecks on venter, throat, thighs, hands, and feet, the sizes and concentration of these flecks are variable. Some males have black coloration extending from throat to mid venter.

Figure 8.
Photographs of live specimens of Noblella losamigos sp. n. (A) Dorsal view and (B) ventral view of MUBI 17413, adult male paratype (SVL 9.6 mm); (C) dorsal view and (D) ventral view of CORBIDI 17520, adult male paratype (SVL 11.1 mm); (E) dorsal view and (F) ventral view of CORBIDI 17521, adult male paratype (SVL 9.6 mm). Photographs by A. Catenazzi.

Figure 9.
Photographs of live specimens of Noblella losamigos sp. n. (A) Dorsal view and (B) ventral view of MUSM 30427, adult female paratype (SVL 11.6 mm); (C) dorsal view and (D) ventral view of MVZ:Herp:292684, adult female paratype (SVL 13.2 mm); (E) dorsal view and (F) ventral view of CORBIDI 17522, adult female paratype (SVL 13.6 mm). Photographs by A. Catenazzi.

Figure 10.
Photographs of live specimens of Noblella losamigos sp. n. (A) Dorsal view and (B) ventral view of CORBIDI 17524, adult male paratype (SVL 9.8 mm); (C) dorsal view and (D) ventral view of MUSM 30426, adult female paratype (SVL 13.1 mm); (E) dorsal view and (F) ventral view of MUBI 17412, juvenile paratype (SVL 7.1 mm).
Several individuals have evident dark brown circular suprainguinal marks. The forelimb pattern varies from speckled to some individuals having a dark brown transverse bar forming a wristband. A dark facial mask is present in most individuals, though variable in shape and extent. In most individuals, the upper lips have between one and three cream flecks; if present, one of these spots is below the eyes. In some specimens, the facial mask merges with a dark lateral line that extends from the tip of the snout and almost reaches to the point of insertion of the thighs. If present, the lateral line is often broken into blotches by interruptions of the lighter flank color.
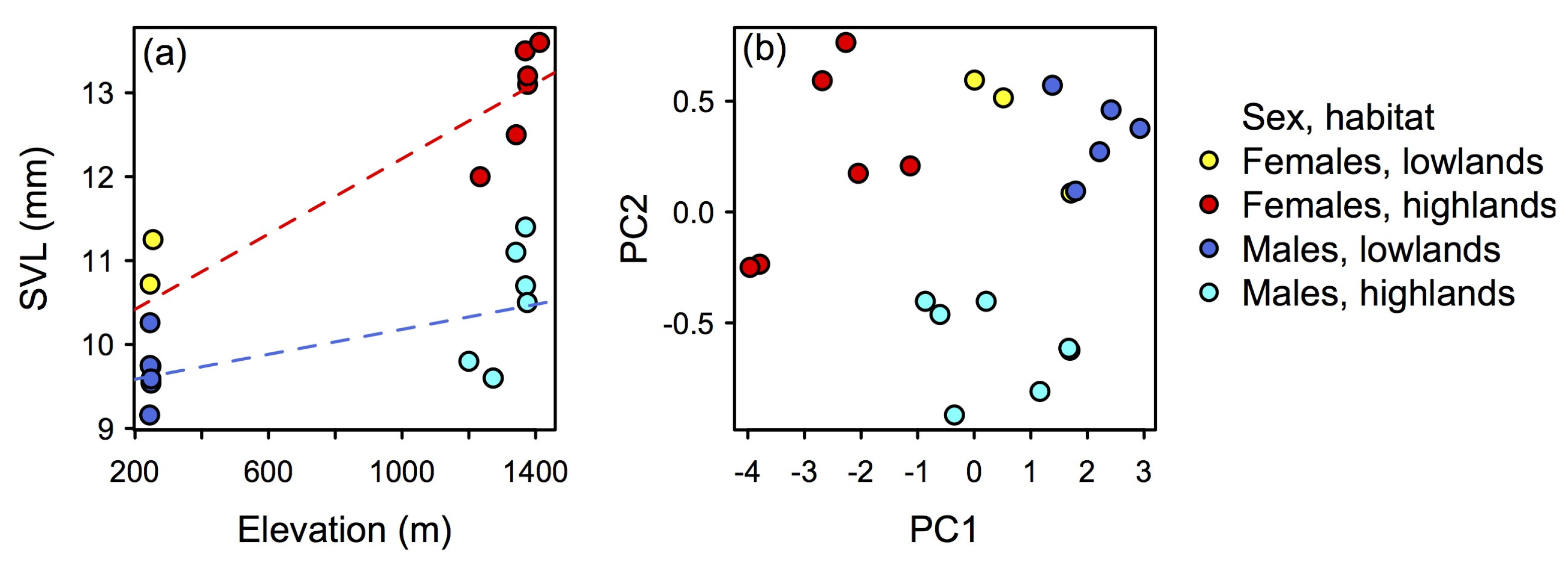
Our morphological data indicate that body size and shape of N. losamigos sp. n. vary with elevation. Body size in both males and females increases with increasing elevation (Figure 11a; OLS regression model, females R2 = 0.81, df = 7, P < 0.001; OLS regression model, males R2 = 0.28, df = 7, P = 0.045). In addition, the PCA projection of body size-corrected data indicates that body shapes of males and females vary with elevation (Figure 11b). Males and females found in the lowlands occupy a morphological space that is smaller and does not overlap with the highland population, whilst individuals found in the highlands occupy a larger and more variable morphological space.
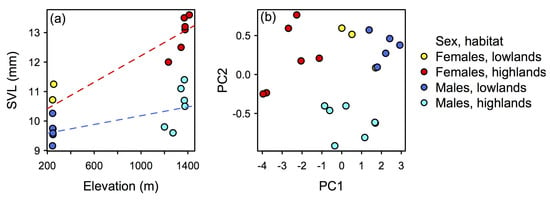
Figure 11.
Multi-panel plot displaying variation of body size and body shape in Noblella losamigos sp. n. across elevations. (a) Body size in females and males tends to increase with increasing elevation. (b) Projection of Principal Component Analysis based on body-size-corrected data; body shape differs between females and males and varies with respect to elevation.
3.2.9. Etymology
The specific epithet is a toponym used in apposition and it refers to the type locality. Los Amigos Biological Station is located next to Los Amigos Conservation Concession, on the lower Los Amigos River watershed. Both the station and the conservation concession were established by the Amazon Conservation Association, which is a nonprofit organization that (along with its Peruvian counterpart, Conservación Amazónica—ACCA) promotes scientific research, education, and conservation in the western Amazon.
3.3. Distribution, Natural History, and Threats
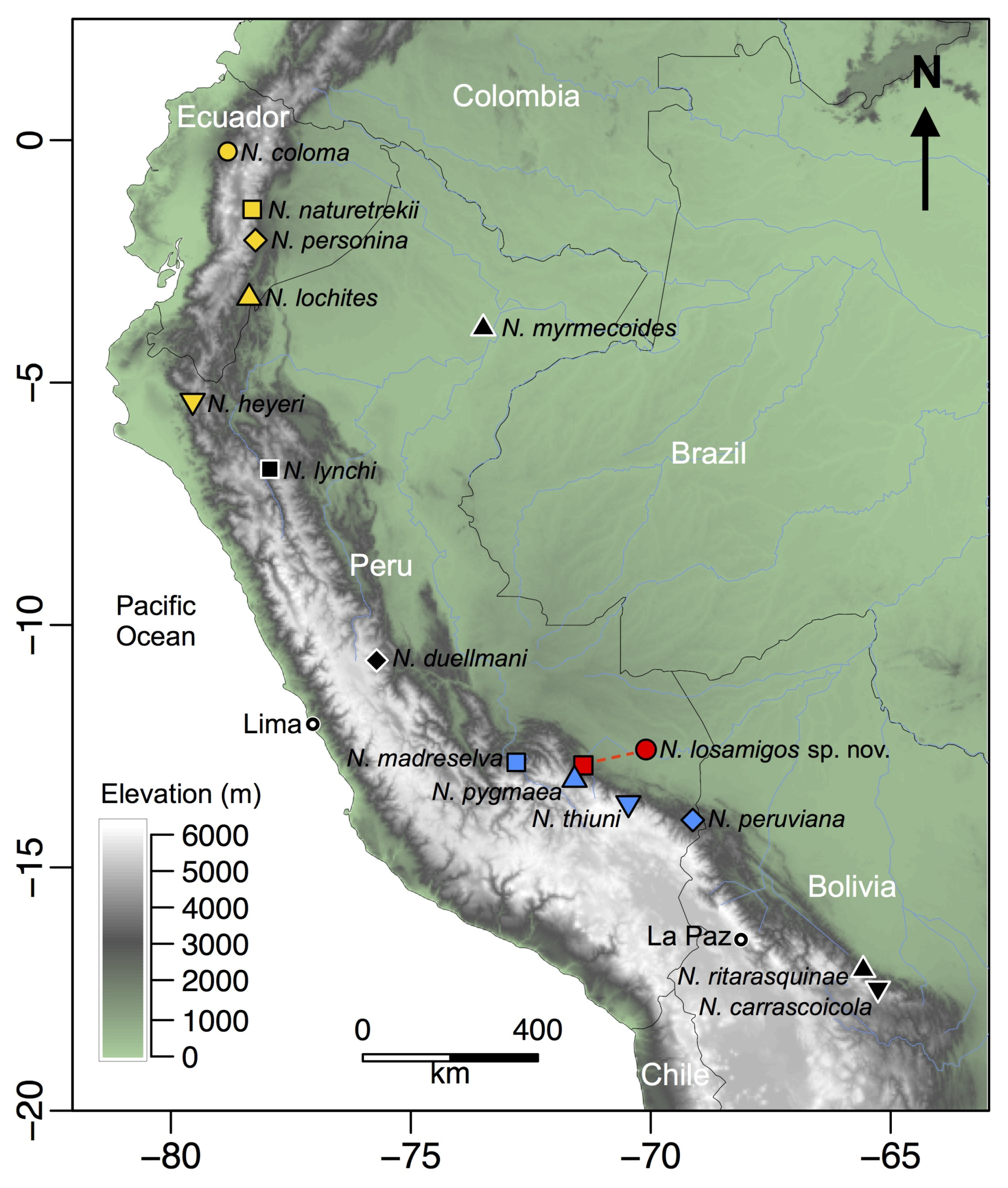
Noblella losamigos sp. n. is one of five species in the genus Noblella distributed in southern Peru (Figure 12). We found the new species in the leaf litter during surveys conducted from 2003 to 2018 at Los Amigos [33,34,42] and in the Kosñipata Valley [17,43]. The species is known to occur at Cocha Cashu Biological Station and Pakitza in the lowlands of Manu National Park [29,31], and near the Manu Learning Centre in the Andean piedmont [37]. Additionally, the species has been recorded at several lowland sites in the Tambopata Province [32].
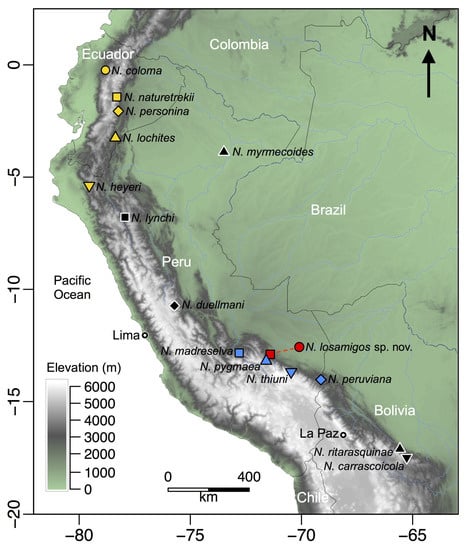
Figure 12.
Map of northwestern South America showing the location of the type localities of species in the genus Noblella. The red circle indicates the type locality of Noblella losamigos sp. n. and the red square indicates the collecting site of paratypes in San Pedro, Kosñipata Valley.
The elevational distribution of N. losamigos sp. n. thus spans 1250 m (240–1490 m) from lowland Amazon rainforest to montane forest on the eastern slopes of the Andes. At the type locality in the Amazon lowlands, field notes indicate that the species is more common in the floodplain forest. The species is also present in other forest types including terra firme, bamboo, and palm swamp [29,34]. Sympatric species of leaf-litter frogs include Adenomera andreae, Amazophrynella javierbustamantei, Ameerega hahneli, Chiasmocleis royi, Engystomops freibergi, Hamptophryne boliviana, Leptodactylus didymus, and Pristimantis carvalhoi. Additionally, several species of gymnophthalmid lizards (Gymnophthalmidae) including Cercosaura argulus, Cercosaura oshaughnessyi, Pseudogonatodes guianensis, and Ptychoglossus brevifrontalis are common in the leaf litter in the floodplain forest. At the other localities, including the premontane forest (~450 m; [37]) and montane forest (1200–1485 m) in the Kosñipata Valley, N. losamigos sp. n. inhabits the leaf litter of both pristine and secondary forests, including bamboo forest patches. Sympatric leaf litter herptiles in the montane forest include the frogs Adenomera andreae, Ameerega simulans, Noblella sp. R, Oreobates granulosus, Pristimantis danae, P. reichlei, P. toftae, P. salaputium, Rhinella leptoscelis, R. margaritifera, and the lizard Cercosaura argulus.
The geographic range of Noblella losamigos sp. n. overlaps with several natural protected areas including Manu National Park, Amarakaeri Communal Reserve, and Tambopata National Reserve. The species is present in both pristine and secondary forests. The main threats faced by N. losamigos sp. n. are habitat loss and modification associated with informal logging and mining activities in the region. According to the IUCN Red List criteria and categories [44], we suggest placing N. losamigos sp. n. in the “least concern” category.
4. Discussion
In recent decades, field research conducted in the Andes–Amazon region has uncovered dozens of new species of terrestrial-breeding frogs [12,16,40,45,46,47], and most of these species have small geographic distributions. Prior to this study, the only species of Noblella previously known to occur in lowland rainforest was N. myrmecoides and it was assumed that it had a broad geographic distribution across western Amazonia [48]. Our findings indicate that populations of Noblella from lowland and montane forest in southern Peru previously ascribed to N. myrmecoides represent a new species. Furthermore, our phylogenetic analyses indicate that N. myrmecoides belongs to a different clade than the clade including N. losamigos sp. n., other species of Noblella from southern Peru, and species of Psychrophrynella.
Noblella losamigos sp. n. is the only species in the genus Noblella that inhabits both lowland and montane rainforest, covering an elevational range of 1250 m, and the only species in the “southern clade” of Noblella (likely to represent Noblella sensu stricto) that inhabits the lowland Amazon rainforest. Previous research has shown that terrestrial breeding frogs distributed at high-elevations tend to have larger body size and different body shape than species found at lower elevations [49,50,51,52,53]. Our data supported this prediction (Figure 11). It has been unclear as to whether ectotherms follow Bergmann’s rule, in which individuals of a species tend to be larger in colder environments and smaller in warmer environments [54,55], though our data suggest that N. losamigos sp. n. appears to follow Bergmann’s rule, as body size increases with elevation. Our data also suggest that body shape of N. losamigos sp. n. varies across elevation, with lowland populations occupying a smaller morphological space than highland populations.
Regardless of the variation in body size and shape exhibited by N. losamigos sp. n., all members of the clade containing Noblella and Psychrophrynella are miniaturized. It is thought that miniaturization may allow species to access microhabitats and food sources that are not available to larger taxa, and may be particularly advantageous to species inhabiting the leaf litter of wet, tropical rainforests [56,57,58,59]. However, a smaller body size might lead to higher exposure to water loss resulting from higher surface area-to-volume ratio [59,60]. A further inquiry into the potential effect of ecological factors correlated with elevation (e.g., resource availability, competition) will develop our understanding of the ecomorphology of Noblella and Psychrophrynella.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/11/9/145/s1, Table S1: Genbank accession numbers for the taxa sampled in this study.
Author Contributions
Conceptualization, R.S.-C., R.v.M. and A.C.; methodology, R.S.-C., R.v.M. and A.C.; software, R.v.M. and D.L.R.; validation, R.S.-C., R.v.M. and A.C.; formal analysis, R.S.-C. and R.v.M.; investigation, R.S.-C., R.v.M., A.C., C.W., E.L.T. and D.L.R.; resources, R.v.M., A.C., E.L.T. and D.L.R.; data curation, R.S.-C., R.v.M., A.C. and C.W.; writing—original draft preparation, R.S.-C. and R.v.M.; writing—review and editing, R.S.-C., R.v.M., A.C., C.W., E.L.T. and D.L.R.; visualization, R.S.-C., R.v.M. and C.W.; supervision, R.v.M. and D.L.R.; project administration, R.S.-C. and R.v.M.; funding acquisition, R.v.M., A.C. and D.L.R.
Funding
R.S.-C. thanks UNSAINVESTIGA for providing support (“PP-0120-2016” Internship). R.v.M. thanks the National Science Foundation (Postdoctoral Research Fellowship DBI-1103087), the American Philosophical Society, the National Geographic Society Committee for Research and Exploration (Grant # 9191-12), and the Amazon Conservation Association. A.C. was funded with grants from the Mohamed bin Zayed Species Conservation Fund, the Disney Worldwide Conservation Fund, the Foundation Matthey-Dupraz, the Andrew Sabin Family Foundation, and the Amazon Conservation Association. Research was supported in part by a fellowship from the David and Lucile Packard Foundation to D.L.R.
Acknowledgments
We thank Cesar Aguilar (MUSM) and Greg Schneider (UMMZ) for supporting our access to specimens in the MUSM and UMMZ museum collections, respectively. We thank the Amazon Conservation Association and the staffs at Los Amigos Biological Stations and Los Amigos Conservation Concession for facilitating our work at their field stations. We thank Michelle Lynch and Erin Westeen for their help in generating μCT images included in this paper. We thank the Servicio Nacional Forestal y de Fauna Silvestre, Peru (SERFOR) for providing collecting permits (R.D.G. N° 120-2012-AG-DGFFS-DGEFFS, N° 064-2013-AG-DGFFS-DGEFFS, N° 0146-2013-AG-DGFFS-DGEFFS, N° 292-2014-AG-DGFFS-DGEFFS, N° 029-2016-SERFOR-DGGSPFFS, N° 405-2016-SERFOR-DGGSPFFS, and Contrato de Acceso Marco a Recursos Genéticos N° 359-2013-MINAGRI-DGFFS-DGEFFS). R.S.-C. thanks Julieta Cabrera for her help and patience in the coordination and use of research and training funds, and Amaranta Canazas for her support in advancing this manuscript. A.C. also thanks Perú Verde for permission to work at their biological station in San Pedro, and within their protected area (Área de Conservación Privada Bosque Nublado).
Conflicts of Interest
The authors declare no conflict of interest. The funding organizations that provided support for this work had no role in the design of the study, data collection, analyses, or interpretation of data, writing of the manuscript, or the decision to publish the results.
Appendix A
Specimens examined
Noblella duellmani (2 specimens): PERU: Pasco: Santa Bárbara, KU 315004–05.
Noblella heyeri (3 specimens): PERU: Piura: 33 km SW Huancabamba, KU 196529 (holotype), 196530–31 (paratypes).
Noblella lochites (2 specimens): ECUADOR: Morona-Santiago: Río Piuntza, KU
147070 (holotype); ECUADOR: Pastaza: Mera, KU 177356.
Noblella myrmecoides (5 specimens): PERU: Loreto: lower Río Napo region, E bank Río Yanayacu, ca. 90 km N Iquitos, KU 206120; Quebrada Oran, ca. 5 km N Río Amazonas, 85 km NE Iquitos, KU 206121; Quebrada Vásquez, N side of lower Río Tahuayo, KU 220577, 220578, 220579. PERU: Amazonas: Pongo Chinim, Kampankis, CORBIDI 9384.
Noblella pygmaea (15 specimens): PERU: Cusco: Provincia Paucartambo, Kosñipata, MHNG 2725.29–30, MUSM 24535–36, 26306–7, 26318–20, 30423–24, 30453–54, MTD 47286–87.
Noblella thiuni (holotype): PERU: Puno: Provincia Carabaya: Distrito Ollachea, Thiuni, CORBIDI 18723.
Psychrophrynella bagrecito (14 specimens): PERU: Cusco: Quispicanchis: Marcapata, Río Marcapata, below Marcapata, ca. 2740 m, KU 196512 (holotype), KU 196513–18, 196520–21, 196523–25 (all paratypes); La Convención: Hacienda Huyro between Huayopata and Quillabamba, 1830 m, KU 196527–28. (Note: specimens KU 196527–28 from La Convención might not be P. bagrecito).
Psychrophrynella chirihampatu (27 specimens): PERU: CUSCO: Provincia Paucartambo, Área de Conservación Privada (ACP) Ukumari Llaqta, Comunidad Campesina de Japu, 2730–3000 m, CORBIDI 16495–16499, CORBIDI 16501–16509, CORBIDI 16696, MHNC 14656, MHNC 14658, MHNC 14661–14662, MHNC 14664, MHNC 14666–14672.
Psychrophrynella glauca (4 specimens): PERU: PUNO: Thiuni, Ollachea, CORBIDI 18729 (holotype), CORBIDI 18730, 16322, 16323 (paratopotypes).
Psychrophrynella usurpator (78 specimens): PERU: Cusco: Provincia Paucartambo, Kosñipata, MUSM 20011, 20873–81, 20896–20913, 20925–33, 20946–47, 20955–57, 21012–18, 26272–73, 26278–79, 26308, 27592, 27906, 27950, 28033–28047, 30303, 30305, 30396–30400, 30405–30409, 30471–30474.
References
- Barbour, T. A list of Antillean reptiles and amphibians. Zoologica 1930, 11, 61–116. [Google Scholar]
- De la Riva, I.; Köhler, J. A new minute leptodactylid frog, genus Phyllonastes, from humid montane forests of Bolivia. J. Herpetol. 1998, 32, 325–329. [Google Scholar] [CrossRef]
- Guayasamin, J.M.; Terán-Valdez, A. A new species of Noblella (Amphibia: Strabomantidae) from the western slopes of the Andes of Ecuador. Zootaxa 2009, 47–59. [Google Scholar] [CrossRef]
- Lehr, E.; Aguilar, C.; Lundberg, M. A new species of Phyllonastes from Peru (Amphibia, Anura, Leptodactylidae). J. Herpetol. 2004, 38, 214–218. [Google Scholar] [CrossRef]
- Lynch, J.D. New species of minute leptodactylid frogs from the Andes of Ecuador and Peru. J. Herpetol. 1986, 20, 423–431. [Google Scholar] [CrossRef]
- Lynch, J.D. Two new species of frogs of the genus Euparkerella (Amphibia: Leptodactylidae) from Ecuador and Perú. Herpetologica 1976, 32, 48–53. [Google Scholar]
- Duellman, W.E. A new species of leptodactylid frog, genus Phyllonastes, from Peru. Herpetologica 1991, 47, 9–13. [Google Scholar]
- Catenazzi, A.; Uscapi, V.; von May, R. A new species of Noblella (Amphibia, Anura, Craugastoridae) from the humid montane forests of Cusco, Peru. ZooKeys 2015, 516, 71–84. [Google Scholar] [CrossRef]
- Reyes-Puig, J.P.; Reyes-Puig, C.; Ron, S.; Ortega, J.A.; Guayasamin, J.M.; Goodrum, M.; Recalde, F.; Vieira, J.J.; Koch, C.; Yánez-Muñoz, M.H. A new species of terrestrial frog of the genus Noblella Barbour, 1930 (Amphibia: Strabomantidae) from the Llanganates-Sangay Ecological Corridor, Tungurahua, Ecuador. PeerJ 2019, 7, e7405. [Google Scholar] [CrossRef]
- Harvey, M.B.; Almendáriz, A.; Brito, M.J.; Batallas, R.D. A new species of Noblella (Anura: Craugastoridae) from the Amazonian slopes of the Ecuadorian Andes with comments on Noblella lochites (Lynch). Zootaxa 2013, 3635, 1–14. [Google Scholar] [CrossRef]
- Noble, G.K. Five new species of Salientia from South America. Am. Mus. Novit. 1921, 29, 1–7. [Google Scholar]
- Lehr, E.; Catenazzi, A. A new species of minute Noblella (Anura: Strabomantidae) from southern Peru: The smallest frog of the Andes. Copeia 2009, 148–156. [Google Scholar] [CrossRef]
- Köhler, J. A new species of Phyllonastes Heyer from the Chapare region of Bolivia, with notes on Phyllonastes carrascoicola. Spixiana 2000, 23, 47–53. [Google Scholar]
- Catenazzi, A.; Ttito, A. Noblella thiuni sp. n., a new (singleton) species of minute terrestrial-breeding frog (Amphibia, Anura, Strabomantidae) from the montane forest of the Amazonian Andes of Puno, Peru. PeerJ 2019, 7, e6780. [Google Scholar] [CrossRef] [PubMed]
- Hedges, S.B.; Duellman, W.E.; Heinicke, M.P. New World direct-developing frogs (Anura: Terrarana): Molecular phylogeny, classification, biogeography, and conservation. Zootaxa 2008, 1737, 1–182. [Google Scholar] [CrossRef]
- De la Riva, I.; Chaparro, J.C.; Castroviejo-Fisher, S.; Padial, J.M. Underestimated anuran radiations in the high Andes: Five new species and a new genus of Holoadeninae, and their phylogenetic relationships (Anura: Craugastoridae). Zool. J. Linn. Soc. 2017, 182, 129–172. [Google Scholar] [CrossRef]
- Catenazzi, A.; Lehr, E.; von May, R. The amphibians and reptiles of Manu National Park and its buffer zone, Amazon basin and eastern slopes of the Andes, Peru. Biota Neotropica 2013, 13, 269–283. [Google Scholar] [CrossRef]
- Duellman, W.E.; Lehr, E. Terrestrial-breeding frogs (Strabomantidae) in Peru; Natur und Tier: Münster, Germany, 2009; p. 382. [Google Scholar]
- Lynch, J.D.; Duellman, W.E. Frogs of the genus Eleutherodactylus in western Ecuador: Systematics, ecology, and biogeography. Univ. Kans. Spec. Publ. 1997, 23, 1–236. [Google Scholar]
- Duellman, W.E.; Lehr, E.; Venegas, P.J. Two new species of Eleutherodactylus (Anura: Leptodactylidae) from the Andes of northern Peru. Zootaxa 2006, 1285, 51–64. [Google Scholar]
- Padial, J.M.; Grant, T.; Frost, D.R. Molecular systematics of terraranas (Anura: Brachycephaloidea) with an assessment of the effects of alignment and optimality criteria. Zootaxa 2014, 3825, 1–132. [Google Scholar] [CrossRef]
- Heinicke, M.P.; Lemmon, A.R.; Lemmon, E.M.; McGrathc, K.; Hedges, S.B. Phylogenomic support for evolutionary relationships of New World direct-developing frogs (Anura: Terraranae). Mol. Phylogenetics Evol. 2018, 118, 145–155. [Google Scholar] [CrossRef]
- von May, R.; Catenazzi, A.; Corl, A.; Santa-Cruz, R.; Carnaval, A.C.; Moritz, C. Divergence of thermal physiological traits in terrestrial breeding frogs along a tropical elevational gradient. Ecol. Evol. 2017, 7, 3257–3267. [Google Scholar] [CrossRef]
- Biomatters. Geneious R6, version 6.1.5. 2013. Available online: http://www.geneious.com/ (accessed on 31 May 2019).
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J. Tracer. Version 1.5. 2007. Available online: http://tree.bio.ed.ac.uk/ software/tracer/ (accessed on 1 June 2019).
- Rambaut, A. FigTree, Version 1.4.2. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 1 June 2019).
- Rodríguez, L.O.; Cadle, J.E. A preliminary overview of the herpetofauna of Cocha Cashu, Manu National Park, Peru. In Four Neotropical Rainforests; Gentry, A.H., Ed.; Yale University Press: New Haven, CT, USA, 1990; pp. 410–425. [Google Scholar]
- Rodríguez, L.O. Structure et organization du peuplement d’anoures de Cocha Cashu, Parc National Manu, Amazonie Péruvienne. Rev. De Ecol. 1992, 47, 151–197. [Google Scholar]
- Morales, V.R.; McDiarmid, R.W. Annotated checklist of the amphibians and reptiles of Pakitza, Manu National Park Reserve Zone, with comments on the herpetofauna of Madre de Dios, Peru. In Manu: The Biodiversity of Southeastern Peru; Wilson, D.E., Sandoval, A., Eds.; Smithsonian Institution: Lima, Peru, 1996; pp. 503–522. [Google Scholar]
- Doan, T.M.; Arizabal, W. Microgeographic variation in species composition of the herpetofaunal communities of Tambopata Region, Peru. Biotropica 2002, 34, 101–117. [Google Scholar] [CrossRef]
- von May, R.; Siu-Ting, K.; Jacobs, J.M.; Medina-Müller, M.; Gagliardi, G.; Rodríguez, L.O.; Donnelly, M.A. Species diversity and conservation status of amphibians in Madre de Dios, Peru. Herpetol. Conserv. Biol. 2009, 4, 14–29. [Google Scholar]
- von May, R.; Jacobs, J.M.; Santa-Cruz, R.; Valdivia, J.; Huamán, J.; Donnelly, M.A. Amphibian community structure as a function of forest type in Amazonian Peru. J. Trop. Ecol. 2010, 26, 509–519. [Google Scholar] [CrossRef]
- von May, R.; Jacobs, J.M.; Jennings, R.D.; Catenazzi, A.; Rodríguez, L.O. Anfibios de Los Amigos, Manu y Tambopata, Perú; Rapid Color Guide # 236; The Field Museum: Chicago, IL, USA, 2010; p. 12. [Google Scholar]
- Whitworth, A.; Downie, R.; von May, R.; Villacampa, J.; McLeod, R. How much potential biodiversity and conservation value can a regenerating rainforest provide? A “best-case scenario” approach from the Peruvian Amazon. Trop. Conserv. Sci. 2016, 9, 224–245. [Google Scholar] [CrossRef]
- Villacampa, J.; Serrano-Rojas, S.; Whitworth, A. Amphibians of the Manu Learning Centre and Other Areas of the Manu Region; The Crees Foundation: Cusco, Peru, 2017; p. 282. [Google Scholar]
- von May, R.; Catenazzi, A.; Santa-Cruz, R.; Kosch, T.A.; Vredenburg, V.T. Microhabitat temperatures and prevalence of the pathogenic fungus Batrachochytrium dendrobatidis in lowland Amazonian frogs. Trop. Conserv. Sci. 2018, 11, 1–13. [Google Scholar] [CrossRef]
- von May, R.; Catenazzi, A.; Santa-Cruz, R.; Gutiérrez, A.; Moritz, C.; Rabosky, D.L. Thermal physiological traits in tropical lowland amphibians: Vulnerability to climate warming and cooling. PLoS ONE 2019, 14, e0219759. [Google Scholar] [CrossRef]
- De la Riva, I.; Chaparro, J.C.; Padial, J.M. A new, long-standing misidentified species of Psychrophrynella Hedges, Duellman and Heinicke from Departamento Cusco, Peru (Anura: Strabomantidae). Zootaxa 2008, 1823, 42–50. [Google Scholar] [CrossRef]
- De la Riva, I.; Chaparro, J.C.; Padial, J.M. The taxonomic status of Phyllonastes Heyer and Phrynopus peruvianus (Noble) (Lissamphibia, Anura): Resurrection of Noblella Barbour. Zootaxa 2008, 1685, 67–68. [Google Scholar] [CrossRef]
- von May, R.; Donnelly, M.A. Do trails affect relative abundance estimates of rainforest frogs and lizards? Austral Ecol. 2009, 34, 613–620. [Google Scholar] [CrossRef]
- Catenazzi, A.; Lehr, E.; Vredenburg, V.T. Thermal physiology, disease and amphibian declines in the eastern slopes of the Andes. Conserv. Biol. 2014, 28, 509–517. [Google Scholar] [CrossRef]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria—Version 10.1. Prepared by the Standards and Petitions Subcommittee. 2013. Available online: http://www. iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 5 April 2015).
- Padial, J.M.; De la Riva, I. Integrative taxonomy reveals cryptic Amazonian species of Pristimantis (Anura: Strabomantidae). Zool. J. Linn. Soc. 2009, 155, 97–122. [Google Scholar] [CrossRef]
- Lehr, E.; von May, R. New species of Pristimantis (Anura: Strabomantidae) from the Amazonian lowlands of southern Peru. J. Herpetol. 2009, 43, 485–494. [Google Scholar] [CrossRef]
- Shepack, A.; von May, R.; Ttito, A.; Catenazzi, A. A new species of Pristimantis (Amphibia, Anura, Craugastoridae) from the foothills of the Andes in Manu National Park, southeastern Peru. ZooKeys 2016, 594, 143–164. [Google Scholar]
- Jungfer, K.-H.; Hoogmoed, M.; Angulo, A.; Reynolds, R.; Icochea, J.; Azevedo-Ramos, C. Noblella Myrmecoides. The IUCN Red List of Threatened Species 2010: e.T57235A11606716. 2010. Available online: http://dx.doi.org/10.2305/IUCN.UK.2010-2.RLTS.T57235A11606716.en (accessed on 29 May 2019).
- Hedges, S.B. Distribution patterns of amphibians in the West Indies. In Regional Patterns of Amphibian Distribution: A Global Perspective; Duellman, W.E., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1999; pp. 211–254. [Google Scholar]
- González-Voyer, A.; Padial, J.M.; Castroviejo-Fisher, S.; De la Riva, I.; Vilà, C. Correlates of species richness in the largest Neotropical amphibian radiation. J. Evol. Biol. 2011, 24, 931–942. [Google Scholar] [CrossRef]
- Narins, P.M.; Meenderink, S.W.F. Climate change and frog calls: Long-term correlations along a tropical altitudinal gradient. Proc. R. Soc. B 2014, 281, 20140401. [Google Scholar] [CrossRef]
- Lehr, E.; von May, R. A new species of terrestrial-breeding frog (Amphibia, Craugastoridae, Pristimantis) from high elevations of the Pui Pui Protected Forest in central Peru. ZooKeys 2017, 660, 17–42. [Google Scholar] [CrossRef]
- von May, R.; Lehr, E.; Rabosky, D.L. Evolutionary radiation of earless frogs in the Andes: Molecular phylogenetics and habitat shifts in high-elevation terrestrial breeding frogs. PeerJ 2018, 6, e4313. [Google Scholar] [CrossRef]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe. Göttingen Stud. 1847, 1, 595–708. [Google Scholar]
- Mayr, E. Geographic character gradients and climatic adaptation. Evolution 1956, 10, 105–108. [Google Scholar] [CrossRef]
- Lehr, E.; Coloma, L.A. A minute new Ecuadorian Andean frog (Anura: Strabomantidae, Pristimantis). Herpetologica 2008, 64, 354–367. [Google Scholar] [CrossRef]
- Kraus, F. At the lower size limit for tetrapods, two new species of the miniaturized frog genus Paedo-phryne (Anura, Microhylidae). ZooKeys 2011, 154, 71–88. [Google Scholar] [CrossRef]
- Rittmeyer, E.N.; Allison, A.; Gründler, M.C.; Thompson, D.K.; Austin, C.C. Ecological guild evolution and the discovery of the world’s smallest vertebrate. PLoS ONE 2012, 7, e29797. [Google Scholar] [CrossRef]
- Scherz, M.D.; Hutter, C.R.; Rakotoarison, A.; Riemann, J.C.; Rödel, M.-O.; Ndriantsoa, S.H.; Glos, J.; Roberts, S.H.; Crottini, A.; Vences, M.; et al. Morphological and ecological convergence at the lower size limit for vertebrates highlighted by five new miniaturised microhylid frog species from three different Madagascan genera. PLoS ONE 2019, 14, e0213314. [Google Scholar] [CrossRef]
- Tracy, C.R.; Christian, K.A.; Tracy, C.R. Not just small, wet, and cold: Effects of body size and skin resistance on thermoregulation and arboreality of frogs. Ecology 2010, 91, 1477–1484. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).