3.1. Archaeal Isolates
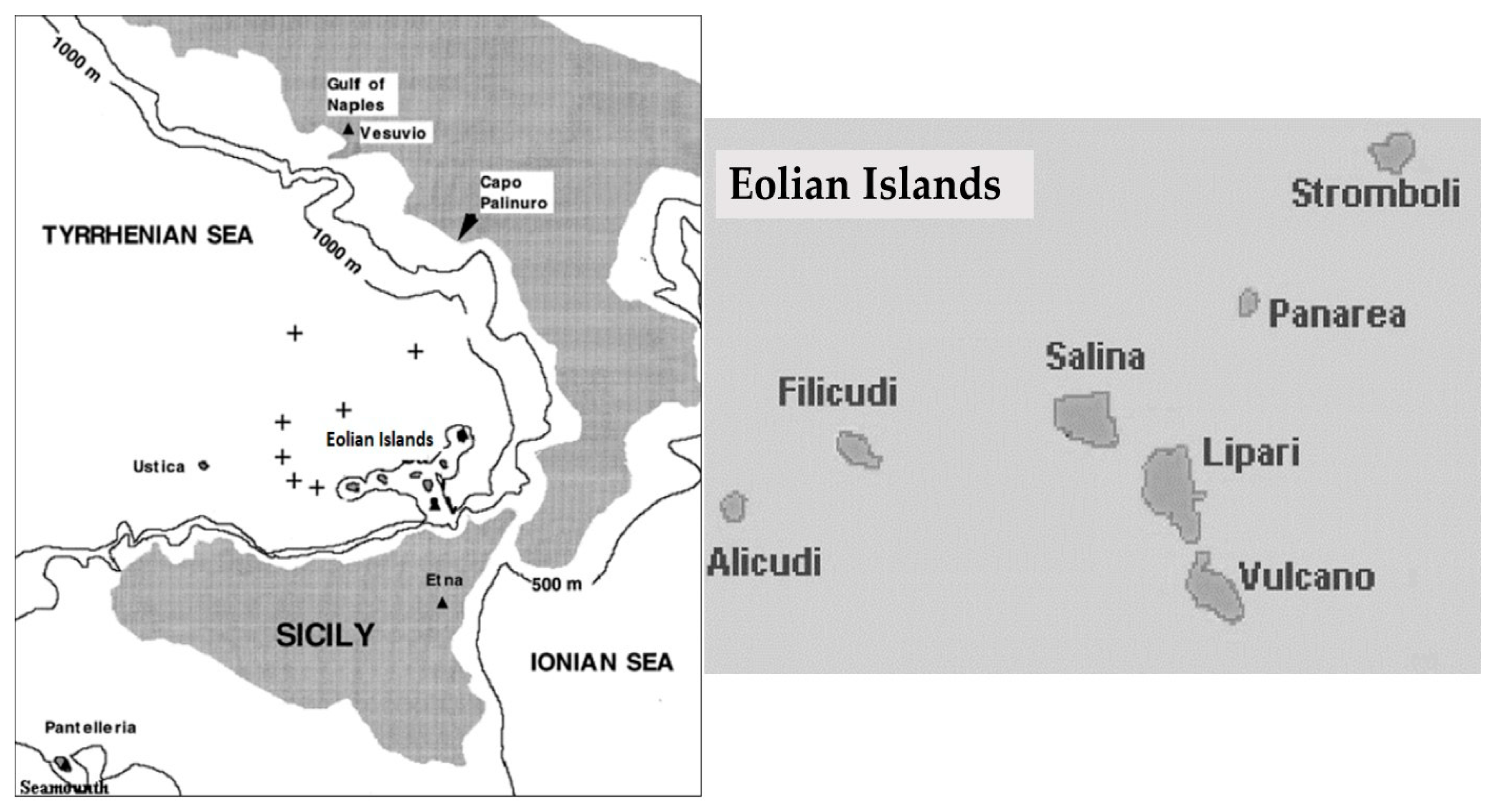
The thermal springs in the Levante harbor of Vulcano Island host dozens of aerobic and anaerobic, thermophilic, and hyperthermophilic microorganisms belonging to bacteria and archaea domains [
13]. Thermophilic and hyperthermophilic archaea were isolated from the thermal springs of Vulcano Island, some of them are of interest in pure and applied research (
Table 4).
The first isolated organism at temperatures above 103 °C was the sulfur-reducing archaeon
Pyrodictium occultum [
65] with optimal growth temperature of 105 °C. The furious fireball,
Pyrococcus furiosus (optimal growth temperature of 100 °C) [
72], is the source of a very thermostable, commercially available DNA polymerase.
Archaeoglobus fulgidus still holds the record as the highest temperature marine sulfate reducer, able to oxidize H
2 [
68], and
Ferroglobus placidus (65–95 °C) couples nitrate reduction with iron oxidation [
69].
Several Eolian isolates were closely related to those isolated from deep-sea locations. Among them,
Methanococcus aeolicus PL-15/H, a CO
2-reducing methanogen isolated from Eolian marine sediments [
70], producing three different restriction enzymes, was closely related to two strains from sediments taken from the Nankai Trough near the coast of Japan. The new species
Palaeococcus helgesonii, one of the rare oxygen tolerant hyperthermophiles, was isolated from a geothermal well of Vulcano Island [
71]. This species belongs to the same genus first isolated from a deep-sea hydrothermal vent chimney in Japan [
78]. All these species make the Levante harbor of Vulcano Island the “type locality” for cultivated hyperthermophiles.
From Panarea submarine vents (20 m depth, fluid temperature 80 °C), Amend and colleagues (personal communication) isolated Thermococcus barossii, T. celer, T. peptonophilus, T. profundus, and T. stetteri, and two strains closely related to Thermococcus retrieved in Loihi Seamount (Hawaii).
Due to the frequent isolation of archaea from Eolian shallow hydrothermal vents, it was assumed that archaea could dominate prokaryotic communities of these sites.
3.2. Archaeal Community Composition by Culture-Independent Approach
With advances in culture-independent approaches, which mainly involve techniques based on 16S rRNA genes, an unpredictable, high diversity of microbial community was observed. Archaeal communities at shallow hydrothermal vents are generally found to be less abundant and diverse than the coexisting bacterial communities [
17,
79]. The location and nature of substrata could greatly influence the composition of the archaeal community. As demonstrated by culture-independent 16S rRNA gene surveys, thermophiles and hyperthermophiles appear segregated into specific vents, to higher temperature sediments, or internal chimney habitats [
80,
81]. In spite of the great variety of archaea previously isolated by several authors from Vulcano Island, a quite different picture was revealed when molecular methods were used. Samples from hydrothermal seeps of Vulcano Island, investigated by fluorescent in situ hybridization (FISH) using new probes, reported that the diversity of thermophiles at Vulcano was far greater than that represented by cultivated strains [
82]. Sequences of DNA extracted from water and sediment samples, and from a geothermal well (Pozzo Istmo), detected a dozen of crenarchaeal, euryarchaeal, and korarchaeal lineages belonging to phylogenetic groups that have no cultured representatives at all [
83]. However, one of the sequences from Pozzo Istmo was nearly identical (99% similarity) to
Palaeococcus helgesonii, the euryarchaeon previously isolated from this well [
71].
The diversity of both bacteria and archaea thriving at Eolian SHS has been investigated by a fingerprinting method, the denaturing gradient gel electrophoresis (DGGE) [
12,
13,
21]. This method is considered one of the most reliable tools for screening and analyzing the microbial community of complex ecosystems. Since this approach allows for the detection of microorganisms only if their proportion is greater than 1% of the total community, the microbial community diversity is expressed in terms of dominant phylotypes [
84]. Fragments resolved by PCR/DGGE of 16S rRNA from bacteria and archaea from sediment samples collected at two Vulcano vents (VS1 and VS2), characterized by different temperatures (35 and 60 °C, respectively), indicated that the richness of archaea was lower than that of bacteria [
21]. The dominant phylotypes retrieved at these vents were referred to uncultivated clones of Euryarchaeota, with the only exception of one sequence affiliated with
Natronorubrum thiooxidans (Halobacteriaceae), a hyperhalophilic archaeon currently detected only in other extreme habitats [
85].
More recently, investigations were carried out along a pH gradient at increasing distance from the primary vent (PV) in the Levante Harbor [
62]. DGGE results showed that archaeal richness and diversity increased the narrowing PV site. Archaeal sequences were affiliated with Euryarchaeota and Crenarchaeota and all phylotypes were related to archaea retrieved from extreme environments, most of which were characterized by high temperatures. Sequences were affiliated with members assigned to hyperthermophilic Euryarcheota belonging to the genus
Thermococcus (Thermococci) and to the thermophilic genus
Methanobrevibacter (Methanobacteria). Norteworthy, more sequences were retrieved within Crenarcheota and related to different members of the class Thermoprotei, with the hyperthermophilic genera
Desulfurococcus, Vulcanisaeta and
Aeropyrum. Hyperthermophilic Thermococci (
Ferroglobus, Palaeococcus, Pyrococcus and
Thermococcus) and Thermoprotei (
Staphylothermus) were demonstrated using DGGE in sediments from two extremely hot (100°C) shallow hydrothermal vents of Vulcano Island by Antranikian et al. [
86]. Moreover, as revealed using enrichment cultures, the high dominance of
Thermococcus and
Palaeococcus in the two vents was mainly dependent on the carbon source available, rather than the site temperature [
86].
The DGGE technique, applied in samples collected from three different vents off Panarea Island (Bottaro1, Campo 7, and Black Point), showed that archaeal populations from the vents were more homogenously distributed in sediments than in fluids, due to the fact that changes in chemical properties and gas composition in fluids were more evident. Phylogenetic analysis of archaeal DGGE 16S rRNA gene sequences revealed that most of sequences were referred to Euryarchaeota, followed by Crenarchaeota. Almost all sequences were affiliated with uncultured clones of archaea mainly retrieved from hot springs and hydrothermal vents of different geographical zones (
Table 5)
Some sequences were only moderately related (<95% similarity) to database entries and therefore they could represent new archaeal taxa. All retrieved uncultered clones were related to Archaea detected at extreme environments, most of which were characterized by high temperature. Only two sequences were related to
Palaeococcus helgesonii, the euryarchaeon isolated from a Vulcano Island well [
68], demonstrating that Panarea and Vulcano hydrothermal systems may host similar members of archaeal populations. Sequences related to an uncultured
Ferroglobus sp. (Archaeoglobi), reported from deep-sea fluids [
87], were also retrieved.
3.3. Data from Next Generation Sequencing Technologies
To analyze the real microbial diversity, powerful sequencing technologies have recently been applied to marine samples from various deep-sea hydrothermal vents [
39,
88,
89,
90] and in shallow-water hydrothermal vents [
14,
17,
91,
92,
93].
High-throughput sequencing techniques represent powerful tools for studing microbial diversity, since the increased numbers (millions) of operational taxonomic units (OTUs) offer the opportunity of revealing simultaneously a large number of individuals and their taxonomic affiliation [
14,
94]. To gain a better understanding of microbial diversity associated with Panarea vents, we applied Illumina high-throughput amplicon sequencing of the V3 region of the 16S rRNA gene for bacteria and archaea to samples collected from Black Point [
14,
95] and Hot Lake sites [
15]. This tool enabled us to detect and enumerate also microorganisms occurring at very low relative abundance (lower than 0.01%). As resulted by the analysis of high-quality reads, archaea represented a minor component of the prokaryotic community at Black Point (from 0.03% to 1.6% of high-quality reads), and Hot Lake (from 0.05% to 3.5%) sites, at both low- and high-temperature conditions.
In fluids emitted at Black Point sites, Euryarchaeota dominated the archaeal community at high (74 °C, BPF74) and low (27 °C, BPF27) temperatures, Halobacteria were prevalent (
Figure 4) and were affiliated with several genera (
Haloarcula, Halobacterium, Halobiforma, Halomicrobium, Haloplanus, Halorubrum, and Natronomonas) (
Table 6).
Sequences referred to class Methanobacteria, with the genus
Methanobrevibacter were more abundant in the low-temperature fluid (BPF27), while those affiliated class Methanomicrobia, with the genus
Methanosarcina, were prevalent in the high-temperature fluid (BPF74) (
Table 6). Sequences affiliated with the Thermococci class, felting into the genus
Palaeococcus, were more abundant in the high-than in the low-temperature fluid. All crenarchaeotal sequences were related to hyperthermophilic members of the Thermoprotei class. Among them, the most abundant genus was
Staphylothermus (
Table 6) of which the type strain
Staphylothermus marinus, a strictly anaerobic, heterotrophic, and S°-dependent archaeon was isolated from the heated submarine sediments at Vulcano Island [
66]. Very few sequences were referred to the genus
Thermocladium, firstly isolated from solfataric muds in Japan, and reported as inhabitant of acidic, extremely thermophilic (i.e., 65–80 °C), terrestrial hot spring area [
96].
A high number of archaeal sequences from sediments collected from BP and HL remained unclassified at the phylum and the class level. Archaeal community composition in the sediment samples from BP and those from HL vents greatly differed, determining distinct communities at the different temperature conditions (
Figure 5).
Members of Euryarchaeota dominated the archaeal community associated with sediments at Hot Lake, while Crenarchaeota (Thermoprotei class) prevailed at BP120. The archaeal community composition from the two sites was distinct, since some groups retrieved at BP were not found at HL and
vice versa (
Table 6).
Sequences referred to Methanococcus and Methanothermococcus (Methanococci) were found only in sediments from Black Point, while those referred to Halococcus, Methanosphaera, and Methanohalophilus were only retrieved from Hot Lake, whereas Methanobrevibacter (Methanobacteria) were in both sites. Among Methanomicrobia class, the genus Methanococcoides was present in samples (BP74, BP120, and HL94) with higher temperatures. Sample BP120 was the richest in hyperthermophilic genera within Thermococci (Palaeococcus, Pyrococcus, and Thermococcus) and Thermoprotei (Ignicoccus, Ignisphaera, Staphylothermus, Thermodiscus, Thermocladium, Thermofilum, and Vulcanisaeta). The single genus of Korarchaeota, related to “Candidatus Korarchaeum”, was retrieved in samples from both Black Point (BPF74 and BP120) and Hot Lake (HL94 and HL28) sites.
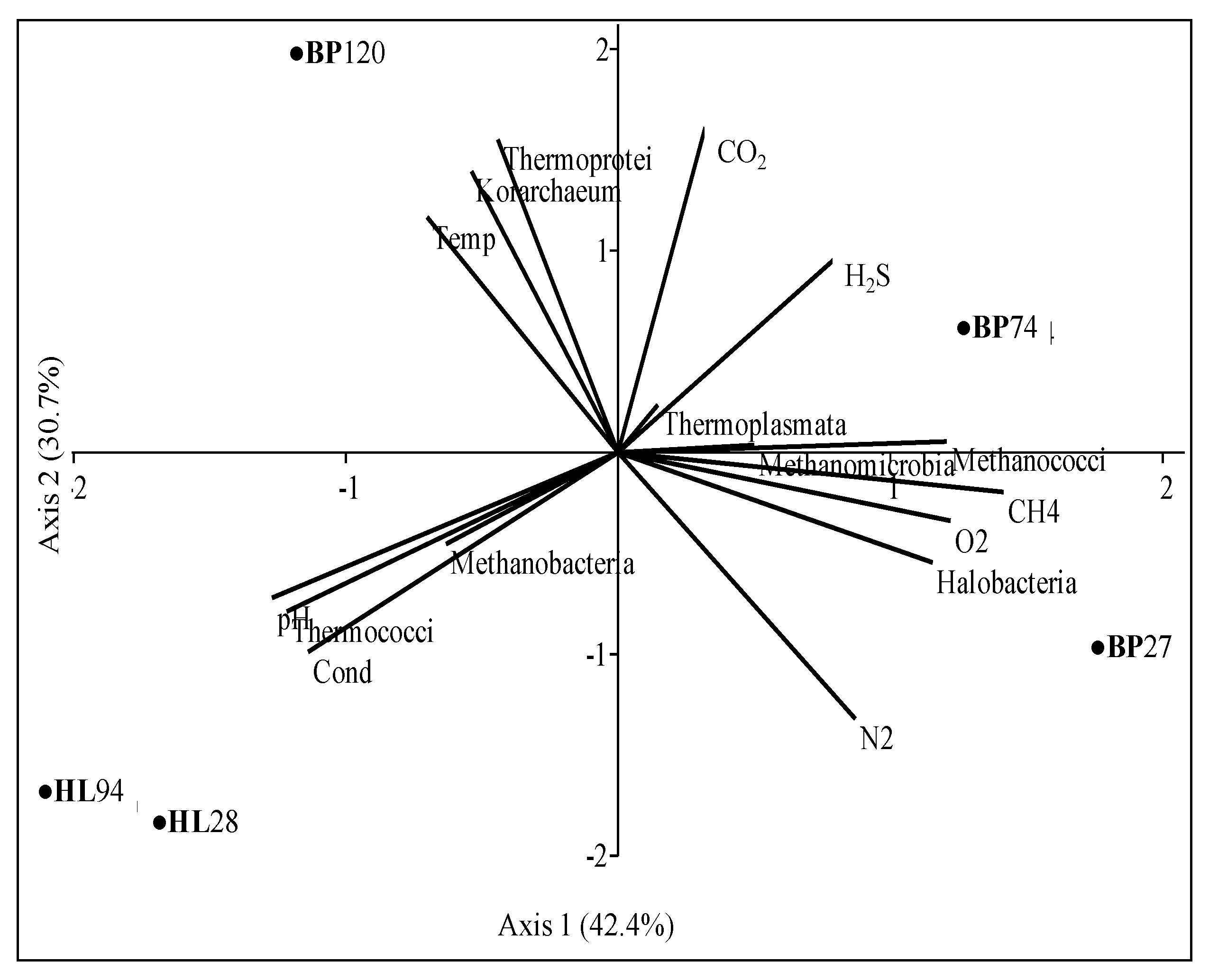
Differences in archaeal communities from Black Point and Hot Lake sites emerged more clearly when abundances of archaeal classes were analyzed together with some physical and chemical properties (
Table 3) (
Figure 6).
As expected, Halobacteria and Methanococci greatly depended on O
2 and CH
4, respectively, whereas Thermoprotei (Crenarchaeota) and Korarchaeum by temperature. Halobacteria were exclusive in fluids, whereas Methanococci in sediments at BP sites, even if each sample was related to different members at the genus level (
Table 6).
Archaeal communities in samples from BP were distinguished from each other. The hottest sample (BP120) was associated with Thermoprotei, and Korarchaeum abundances. This finding distinguished the sample from those previously analyzed from Eolian SHS, but makes it similar to deep-sea hydrothermal locations [
97]. Sample BP74 was linked with CO
2, H
2S, and Thermoplasmata, mainly represented by the moderately thermophilic
Thermogymnomonas genus. Members of methanogens, strictly linked to CH
4, distinguished BP74 communities from those of the BP27 sample, since abundances of Methanococci were linked to BP74, whereas those of mesophilic Methanomicrobia to BP27.
Despite the great difference in temperature, samples from HL sites grouped together, characterized by higher salinity and pH values (4.5–4.7) and less CO
2 content than BP sites. HL28 and HL94 were strictly associated with Thermococci (mainly represented by
Palaeococcus and
Thermococcus) whereas Methanobacteria (with genera
Methanobrevibacter and
Methanosphaera) were distinctive of the coldest site HL28 (
Table 6).
3.4. Comparison with Archaeal Communities from Other Hydrothermal Vents
After the definition of the Vulcano Island shallow hydrothermal system as the site with the most isolated hyperthemophiles, more insights on the archaeal community composition of Eolian SHS have been obtained by applying molecular culture-independent techniques. As resolved by Illumina sequencing in Vulcano and Panarea sites, the retrieved archaeal communities appeared remarkably diverse, especially when observed at class level, from each to other, and also from those of different SHS, examined by next generation methods (
Table 7).
Archaeal communities of extremely hot hydrothermal vents, with temperatures above 100 °C, are composed by well-known hyperthermophyles. Archaeal communities from two shallow vents at Vulcano were dominated by members of class Thermoprotei and Thermococci [
80], with the highly abundant genera
Staphylothermus,
Thermococcus, Aeropyrum, and
Pyrodictium, most of them already isolated from the same area in the past (
Table 4).
Differences in samples are also quite distinct among samples from Black Point sites, mainly referred to the occurrence of Crenarchaeota (Thermoprotei) and hyperthermophilic members of Euryarchaeota (Archaeoglobi and Thermococci) at the hottest BP120 site. Although Crenarchaeota are reported as more abundant than Euryarchaeota in cold deep-sea sites [
98], and at seamounts in the Tyrrhenian Sea [
99], they were generally less abundant than Euryarchaeota at different SHS [
16]. Thermoprotei from BP120 were referred to several genera, among them
Thermodiscus was the most commonly retrieved genus in samples from both Black Point and Hot Lake sites. Euryarchaeotal Archaeoglobi, mainly referred to the genus
Ferroglobus, and Thermocococci, with
Palaeococcus and
Thermococcus genera, were also reported as the dominant archaeal groups at the RP site of Palaeochori Bay (Milos Island, Greece) [
17]. Moreover, also the genus
Pyrococcus (Thermocococci) was only retrieved at the hottest site BP120. Our results suggest that the differences observed in the major archaeal populations at Black Point and Palaeochori Bay sites are mainly due to the different temperatures and pH values, rather than the salinity conditions. The moderately acidophilic and thermophilic, euryarchaeotal genus
Thermogymnomonas (Thermoplasmata) was commonly retrieved from samples at Black Point and Hot Lake sites, but not in the RP site of Palaeochori Bay (Milos Island), where indeed it was dominant at the less saline (W) site. The presence at Black Point sites of several strictly anaerobic thermophilic archaeal groups, such as Methanococci (including the genera
Methanococcus and
Methanothermococcus), Thermococci, and Archaeoglobi indicates that these vents are seeded by subseafloor communities.
Hyperthermophilic members of Thermococci (
Palaeococcus and
Thermococcus) were also predominant at sites from the Hot Lake thermal brine pool, under high-temperature conditions (HL94). Thermococci have been also reported as dominant in deep-sea high-temperature locations, where they act as prominent degraders of organic matter within marine hot-water ecosystems [
78]. The genus
Palaeococcus was retrieved as the most abundant genus in all the examined sites of Panarea Island. The isolation and occurrence of sequences referred to
Paleococcus hegelsonii indicate that the respiration and/or S°- fermentation of complex organic compounds may represent the metabolic key pathways at Eolian Island vents, as well as in other hydrothermal systems [
12,
21,
71,
83]. The decreasing temperature registered at Hot Lake site was accompanied by a concomitant increasing of diversity (Shannon and evenness indices,
Table 7) and a marked shift in the archaeal community composition [
15], since the majority of hyperthermophilic members retrieved at high temperature (HL94) was successively substituted by more specific mesophilic archaea of the classes Halobacteria, Methanomicrobia, and Methanobacteria [
15].
The occurrence of sequences affiliated to Halobacteria, as demonstrated by different molecular sequencing techniques, confirms that they represent dominant archaeal populations at Panarea system, as well as in the shallow system Kueishan Island, Taiwan [
100].
As recently evaluated by 16S rRNA gene clone library sequencing, archaeal communities in microbial mats collected from low-temperature, hydrothermal fields of Basiluzzo Islet (Eolian Islands) at different depths (from 26 to 211 m), were dominated by Thaumarcheota and Woesearchaeota [
92]. These results suggest that other than temperature, depth, and salinity, different geochemical factors are essential to constraining the archaeal community associated with Eolian hydrothermal sites.
The single genus of the Korarchaeota phylum, related to “Candidatus Korarchaeum” has been commonly detected in different terrestrial and marine hydrothermal habitats, including vents of Vulcano hydrothermal system [
83], sites of Palaeochori Bay (Milos Island) [
17], and at hot springs of Yellowstone Park [
83,
97], indicating a broad diffusion in different hydrothermal systems.