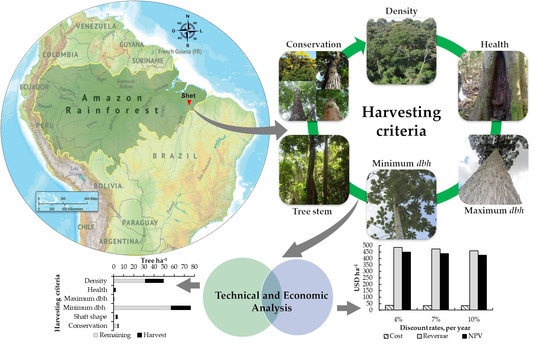
Harvesting Criteria Application as a Technical and Financial Alternative for Management of Degraded Tropical Forests: A Case Study from Brazilian Amazon
Abstract
:1. Introduction
2. Material and methods
2.1. Study Area
2.2. Harvesting Criteria
2.3. Cost–Benefit and Sensitivity Analysis
3. Results
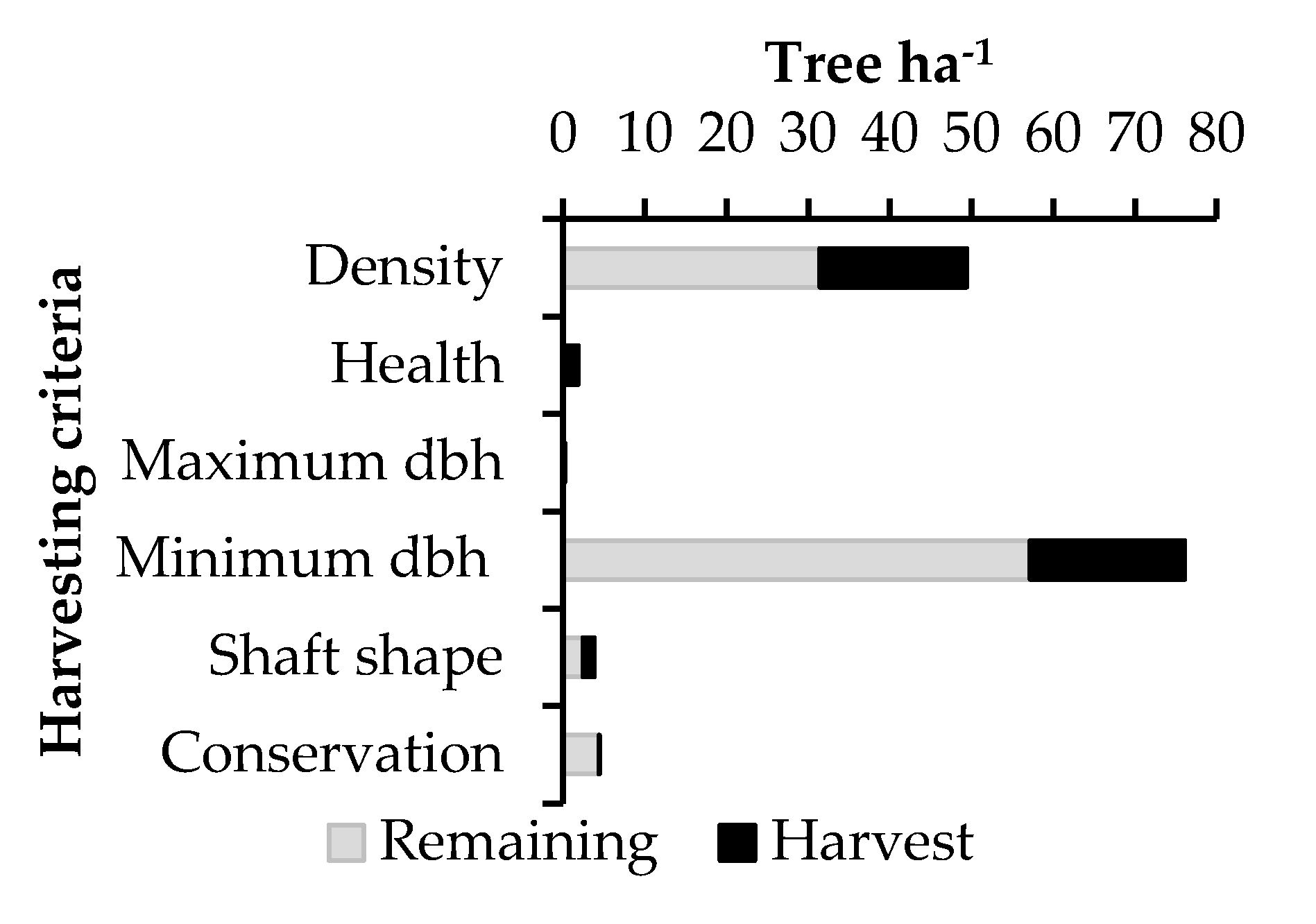
3.1. Technical Analysis
3.2. Cost–Benefit and Sensitivity Analysis
4. Discussion
4.1. Technical Analysis
4.2. Cost-Benefit and Sensitivity Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garrido Filha, I. Manejo florestal: Questões econômico-financeiras e ambientais. Estud. Av. 2002, 16, 91–106. [Google Scholar] [CrossRef]
- Edwards, D.P.; Tobias, J.A.; Sheil, D.; Meijaard, E.; Laurance, W.F. Maintaining ecosystem function and services in logged tropical forests. Trends Ecol. Evol. 2014, 29, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, G.; Peña-Claros, M.J.; Lopes, C.A.J.; Mohrena, G.M.J.; Kanashiro, M. Mid-term effects of reduced-impact logging on the regeneration of seven tree commercial species in the Eastern Amazon. For. Ecol. Manag. 2012, 274, 116–125. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Carter, D.R.; Schulze, M.; Vidal, E. The sustainability of timber production from Eastern Amazonian forests. Land Use Policy 2012, 29, 339–350. [Google Scholar] [CrossRef]
- Braz, E.M.; Schneider, P.R.; de Mattos, P.P.; Thaines, F.; Selle, G.L.; de Oliveira, M.F.; Oliveira, L.C. Manejo da estrutura diamétrica remanescente de florestas tropicais. Cienc. Florest. 2012, 22, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C.G.C.; da Silva, M.L.; Torres, C.M.M.E.; Ruschel, A.R.; da Silva, L.F.; de Andrade, D.F.C.; Reis, L.P. Crescimento diamétrico e tempo de passagem de Minquartia guianensis após manejo na Floresta Nacional do Tapajós. Pesqui. Florest. Bras. 2017, 37, 299–309. [Google Scholar] [CrossRef] [Green Version]
- David, H.C.; Carvalho, J.O.P.; Piresb, I.P.; Santosa, L.S.; Barbosa, E.S.; Braga, N.S. A 20-year tree liberation experiment in the Amazon: Highlights for diameter growth rates and species-specific management. For. Ecol. Manag. 2019, 453, 117584. [Google Scholar] [CrossRef]
- Ferreira, T.M.C.; de Carvalho, J.O.P.; Emmert, F.; Ruschel, A.R.; Nascimento, R.G.M. How long does the Amazon rainforest take to grow commercially sized trees? An estimation methodology for Manilkara elata (Allemão ex Miq.) Monach. For. Ecol. Manag. 2020, 473, 118333. [Google Scholar] [CrossRef]
- Free, C.M.; Matthew Landis, R.; Grogan, J.; Schulze, M.D.; Lentini, M.; Dünisch, O. Management implications of long-term tree growth and mortality rates: A modeling study of big-leaf mahogany (Swietenia macrophylla) in the Brazilian Amazon. For. Ecol. Manag. 2014, 330, 46–54. [Google Scholar] [CrossRef]
- Vinson, C.C.; Kanashiro, M.; Sebbenn, A.M.; Williams, T.C.; Harris, S.A.; Boshier, D.H. Long-term impacts of selective logging on two Amazonian tree species with contrasting ecological and reproductive characteristics: Inferences from Eco-gene model simulations. Heredity 2014, 115, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Putz, F.E.; Zuidema, P.A.; Synnott, T.; Peña-Claros, M.; Pinard, M.A.; Sheil, D.; Vanclay, J.K. Sustaining conservation values in selectively logged tropical forests: The attained and the attainable. Conserv. Lett. 2012, 5, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Institui o Código Florestal. Diário Oficial da República Federativa do Brasil; Ministério do Meio Ambiente (MMA): Brasília, Brazil, 1965.
- Institui o novo Código Florestal. Diário Oficial da República Federativa do Brasil; Ministério do Meio Ambiente (MMA): Brasília, Brazil, 2012.
- Institui o Código Florestal. Diário Oficial da República Federativa do Brasil; Ministério do Meio Ambiente (MMA): Brasília, Brazil, 2006.
- Conselho Nacional de Meio Ambiente (CONAMA). Diário Oficial da República Federativa; Conselho Nacional de Meio Ambiente: Brasília, Brazil, 2009. [Google Scholar]
- Brasília Ambiental Ministry of Environment. Dispõe Sobre as Sanções Penais e Administrativas Derivadas de Condutas e Atividades Lesivas ao Meio Ambiente, e dá Outras Providências; Diário Oficial da República Federativa: Brasília, Brazil, 1998. [Google Scholar]
- Cardoso, D.; Särkinen, T.; Alexander, S.; Amorim, A.M.; Bittrich, V.; Celis, M.; Daly, D.C. Amazon plant diversity revealed by a taxonomically verified species list. Proc. Natl. Acad. Sci. USA 2017, 114, 10695–10700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Assis, R.L.; Wittmann, F. Forest structure and tree species composition of the understory of two central Amazonian várzea forests of contrasting flood heights. Flora 2011, 206, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Condé, T.M.; Tonini, H. Fitossociologia de uma Floresta Ombrófila Densa na Amazônia Setentrional, Roraima, Brasil. Acta Amaz. 2013, 43, 247–259. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Veiga Carim, M.; da Silva Guimarães, J.R.; de Cassia Leoncio Tostes, L.; Takiyama, L.R.; Wittmann, F. Composition, structure and floristic diversity in dense rain forest in the Eastern Amazon, Amapá, Brazil. Acta Sci. 2015, 37, 419–426. [Google Scholar]
- Pereira, P.C.G. Potencial Silvicultural das Espécies do Gênero Cecropia na Amazônia. Master’s Thesis, Universidade Federal Rural da Amazônia, Belém, Brasil, 2015. [Google Scholar]
- De Avila, A.L.; Ruschel, A.R.; de Carvalho, J.O.P.; Mazzei, L.; Silva, J.N.M.; do Carmo Lopes, J.; Araujo, M.M.; Dormann, C.F.; Bauhus, J. Medium-term dynamics of tree species composition in response to silvicultural intervention intensities in a tropical rain forest. Biol. Conserv. 2015, 191, 577–586. [Google Scholar] [CrossRef]
- De Avila, A.L.; Schwartz, G.; Ruschel, A.R.; do Carmo Lopes, J.; Silva, J.N.M.; de Carvalho, J.O.P.; Dormann, C.F.; Mazzei, L.; Soares, M.H.M.; Bauhus, J. Recruitment, growth and recovery of commercial tree species over 30 years following logging and thinning in a tropical rain forest. For. Ecol. Manag. 2017, 385, 225–235. [Google Scholar] [CrossRef]
- Dionisio, L.F.S.; Schwartz, G.; do Carmo Lopes, J.; de Assis Oliveira, F. Growth, mortality, and recruitment of tree species in an Amazonian rainforest over 13 years of reduced impact logging. For. Ecol. Manag. 2018, 430, 150–156. [Google Scholar] [CrossRef]
- Dionisio, L.F.S.; de Carvalho, J.O.P.; Schwartz, G.; Leão, F.; Castro, T.C. Incremento, recrutamento e mortalidade pós-colheita de Duguetia spp. na Floresta Nacional do Tapajós, Pará. Sci. For. 2018, 46. [Google Scholar] [CrossRef]
- Inpe. Instituto Nacional de Pesquisas Espaciais. Degradação florestal de 2007 a 2016. Available online: http://www.obt.inpe.br/OBT/assuntos/programas/amazonia/degrad (accessed on 17 November 2019).
- Fearnside, F.P. Manejo Florestal na Amazônia: Necessidade de novos critérios na avaliação de opções de desenvolvimento. In Pará Desenvolvimento; Departamento de Ecologia do Instituto Nacional de Pesquisas da Amazônia-INPA: Manaus, Brazil, 1989; p. 25. [Google Scholar]
- Domingues, M.S.; Bermann, C. O arco de desflorestamento na Amazônia: Da pecuária à soja. Ambiente Soc. 2012, 15, 1–22. [Google Scholar] [CrossRef]
- Anderson, L.O.; Rojas, E.H.M.; Shimabukuro, Y.E. Avanço da soja sobre os ecossistemas cerrado e floresta no estado do Mato Grosso. In SBSR 05-10 Abril 2003; INPE: Belo Horizonte, Brasil, 2003; Volume 11, pp. 19–25. [Google Scholar]
- Sales, A.; Gonzáles, D.G.E.; Martins, T.G.V.; Silva, G.C.C.; Spletozer, A.G.; de Almeida Telles, L.A.; Siviero, M.A.; Lorenzon, A.S. Optimization of Skid Trails and Log Yards on the Amazon Forest. Forests 2019, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Melo, L.E.L. Caracterização do Câmbio e do Lenho de Árvores de Schizolobium parahyba var. amazonicum (Huber ex Ducke) Barneby de Plantação em Clareira da Amazônia. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, MG, Brazil, 2017. [Google Scholar]
- Machado, J.F.; Hillig, É.; Watzlawic, L.F.; Bednarczuk, E.; Tavares, E.L. Production of plywood panel for exterior use with paricá and embaúba timbers. Rev. Árvore 2018, 42, e420406. [Google Scholar] [CrossRef]
- Schwartz, G.; Pereira, P.C.G.; Siviero, M.A.; Pereira, J.F.; Ruschel, A.R.; Yared, J.A.G. Enrichment planting in logging gaps with Schizolobium parahyba var. amazonicum (Huber ex Ducke) Barneby: A financially profitable alternative for degraded tropical forests in the Amazon. For. Ecol. Manag. 2017, 390, 166–172. [Google Scholar] [CrossRef]
- Sales, A.; Siviero, M.A.; Pereira, P.C.G.; Vieira, S.B.; Berberian, G.A.; Miranda, B.M. Estimation of the commercial height of trees with laser meter: A viable alternative for forest management in the Brazilian Amazon. Ecol. Evol. 2020, 10, 3578–3583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siviero, M.A.; Yared, J.A.G.; Ruschel, A.R.; Vieira, S.B.; Sales, A.; Pereira, J.F.; Aguiar, O.J.R.; Brienza Junior, S.; Pereira, P.C.G.; Berberian, G.A.; et al. Manejo de florestas naturais degradadas na Amazônia: Estudo de caso sobre critérios de colheita. Cienc. Florest. 2020, 30. [Google Scholar] [CrossRef] [Green Version]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Metz 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Instituto de Desenvolvimento Econômico, Social e Ambiental do Pará. Estatística Municipal de Dom Eliseu; Prefeitura de Dom Eliseu: Pará, Brazil, 2014; p. 47. [Google Scholar]
- Martorano, L.G.; Monteiro, D.C.A.; Brienza Junior, S.; Lisboa, L.S.; Espírito Santo, J.M.; Almeida, R.F. Top-bioclimate conditions associated to natural occurrence of two Amazonian native tree species for sustainable reforestation in the State of Para. In Ecosystems and Sustainable Development VIII; Villacampa, Y., Brebbia, C.A., Eds.; Universidad de Alicant: Valencia, Spain, 2011; Volume 144, pp. 111–122. [Google Scholar]
- IBGE. Instituto Brasileiro de Geografia e Estatística. Manual Técnico da Vegetação Brasileira: Sistema Fitogeográfico, Inventário das Formações Florestais e Campestres, Técnicas e Manejo de Coleções Botânicas, Procedimentos para Mapeamentos; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2012; p. 92. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo; Embrapa Solos: Rio de Janeiro, Brazil, 2017; p. 574. [Google Scholar]
- Siviero, M.A. É possível inovar no manejo de florestas naturais? Ref. Florest. 2011, 120, 94–96. [Google Scholar]
- Sabogal, C.; Silva, J.N.M.; Zweede, J.; Júnior, R.; Barreto, P.; Guerreiro, C.A. Diretrizes Técnicas Para a Exploração de Impacto Reduzido em Operações Florestais de Terra Firme na Amazônia Brasileira; Embrapa Amazônia Oriental: Belém, Pará, Brazil, 2009; p. 51. [Google Scholar]
- De Souza, A.L.; Soares, C.P.B. Florestas Nativas: Estrutura, Dinâmica e Manejo, 2nd ed.; Universidade Federal de Viçosa: Minas Gerais, Brazil, 2013; p. 322. [Google Scholar]
- Rezende, J.L.P.; Oliveira, A.D. Análise Econômica e Social de Projetos Florestais; Universidade Federal de Viçosa: Viçosa, MG, Brazil, 2013; p. 385. [Google Scholar]
- Virgens, A.P.D.; Freitas, L.C.; Leite, A.M.P. Análise econômica e de sensibilidade em um povoamento implantado no sudoeste da Bahia. Floresta Ambient. 2016, 23, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Peña-Claros, M.; Peters, E.M.; Justiniano, M.J.; Bongers, F.; Blate, G.M.; Fredericksen, T.S.; Putz, F.E. Regeneration of commercial tree species following silvicultural treatments in a moist tropical forest. For. Ecol. Manag. 2008, 255, 1283–1293. [Google Scholar] [CrossRef]
- De Araujo, H.J.B. Inventário florestal a 100% em pequenas áreas sob manejo florestal madeireiro. Acta Amaz. 2006, 36, 447–464. [Google Scholar] [CrossRef] [Green Version]
- Sist, P.; Ferreira, F.N. Sustainability of reduced-impact logging in the Eastern Amazon. For. Ecol. Manag. 2007, 243, 199–209. [Google Scholar] [CrossRef]
- De Barros Cavalcanti, F.J.; do Amaral Machado, S.; Osokawa, R.T.; da Cunha, U.S. Comparação dos valores estimados por amostragem na caracterização da estrutura de uma área de floresta na Amazônia com as informações registradas no censo florestal. Rev. Árvore 2011, 35, 1061–1068. [Google Scholar] [CrossRef]
- Iwakiri, S.; Zeller, F.; Pinto, J.A.; Ramirez, M.G.L.; Souza, M.M.; Seixas, R. Avaliação do potencial de utilização da madeira de Schizolobium amazonicum “Paricá” e Cecropia hololeuca “Embaúba” para produção de painéis aglomerados. Acta Amaz. 2010, 40, 303–308. [Google Scholar] [CrossRef]
- Braga, A.J.T.; Griffith, J.J.; de Paiva, H.N.; Meira Neto, J.A.A. Composição do banco de sementes de uma floresta semidecidual secundária considerando o seu potencial de uso para recuperação ambiental. Rev. Árvore 2008, 32, 1089–1098. [Google Scholar] [CrossRef] [Green Version]
- Garzón, G.; Paola, L. Conocimiento tradicional sobre la plantas medicinales de yarumo (Cecropia sciadophylla), carambolo (Averrhoa carambola) y uña de gato (Uncaria tomentosa) en el resguardo indígena de Macedonia, Amazonas. Luna Azul 2016, 43, 386–414. [Google Scholar] [CrossRef]
- De Barros Francez, L.M.; de Carvalho, J.O.P.; da Sliva Jardim, F.C.; Quanz, B.; Pinheiro, K.A.O. Efeito de duas intensidades de exploração de madeira na estrutura de uma floresta natural na região de Paragominas, Pará. Acta Amaz. 2009, 39, 851–863. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, T.; Carvalho, J. Dinâmica da população de Manilkara huberi (Ducke) A. Chev. durante 26 anos após a exploração florestal em uma área de terra firme na Amazônia brasileira. Ciênc. Florest. 2014, 24, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Grogan, J.; Landis, R.M.; Free, C.M.; Schulze, M.D.; Lentini, M.; Ashton, M.S. Big-leaf mahogany Swietenia macrophylla population dynamics and implications for sustainable management. J. Appl. Ecol. 2014, 51, 664–674. [Google Scholar] [CrossRef]
- Siviero, M.A. Nossa indústria madeireira rumo à sustentabilidade. Ref. Florest. 2009, 98, 72–73. [Google Scholar]
- Schwartz, G.; Lopes, J.C.A.; Mohren, G.M.J.; Peña-Claros, M. Post-harvesting silvicultural treatments in logging gaps: A comparison between enrichment planting and tending of natural regeneration. Ecol. Manag. 2013, 293, 57–64. [Google Scholar] [CrossRef]
- Gomes, J.M.; da Silva, J.C.F.; Vieira, S.B.; de Carvalho, J.O.P.; Oliveira, L.C.L.Q.; de Queiroz, W.T. Schizolobium parahyba var. amazonicum (Huber ex Ducke) Barneby pode ser utilizada em enriquecimento de clareiras de exploração florestal na Amazônia. Cienc. Florest. 2019, 29, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Araujo, H.J.B.; Correia, M.F. Sobrevivência e causas da debilidade das mudas em enriquecimento de clareiras em florestas no Acre. In Proceedings of the XII Congresso de Ecologia do Brasil 2015, Embrapa Acre, Rio Branco, Acre, Brazil, 24 September 2015; p. 3. [Google Scholar]
- Vieira, S.B.; de Carvalho, J.O.P.; Gomes, J.M.; da Silva, J.C.F.; Ruschel, A.R. Cedrela odorata L. tem potencial para ser utilizada na silvicultura pós-colheita na Amazônia brasileira? Cienc. Florest. 2018, 28, 1230–1238. [Google Scholar] [CrossRef] [Green Version]
- Fontes, C.G. Revelando as Causas e a Distribuição Temporal da Mortalidade Arbórea em uma Floresta de Terra-Firme na Amazônia Central. Master’s Thesis, Mestrado em Ciências Florestais, Instituto Nacional de Pesquisa na Amazônia, INPA, Manaus, Amazonas, Brazil, 2012. [Google Scholar]
- Poggiani, F. Florestas para fins energéticos e ciclagem de nutrientes. Série Técnica IPEF 1980, 1, D1–D11. [Google Scholar]
- Garcia, L.C.; Sousa, S.G.A.; Lima, R.B.M. Seleção de Matrizes, Coleta e Manejo de Sementes Florestais Nativas da Amazônia; Documentos 89; Embrapa Amazônia Ocidental: Manaus, Amazonas, Brazil, 2011; p. 20. [Google Scholar]
- Dos Santos Guedes, J.; Kruped, R.A. Características ecológicas e fitossanidade de espécies arbóreas em um fragmento de Floresta Ombrófila Densa da região sudeste do estado de São Paulo. Ambiência 2017, 13, 311–324. [Google Scholar]
- Serviço Florestal Brasileiro. Inventário Florestal Nacional: Principais Resultados: Paraná Recurso Eletrônico/Serviço Florestal Brasileiro; Série Relatórios Técnicos—IFN; MMA: Brasília, Brazil, 2018; p. 84. ISBN 978-85-7738-402-0. (algumas color). Available online: http://www.florestal.gov.br/publicacoes (accessed on 22 May 2020).
- Parisi, J.J.D.; dos Santos, A.F.; Barbedo, C.J.; Medina, P.F. Patologia de Sementes Florestais: Danos, Detecção e Controle, uma revisão. Summa Phytopathol. 2010, 45, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Steege, H.T.; Nigel, C.A.; Sabatier, D.; Baraloto, C.; Salomao, R.P.; Guevara, J.E.; Phillips, O.L.; Castilho, C.V.; Magnusson, W.E.; Molino, J.; et al. Hyperdominance in the Amazonian Tree Flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef] [Green Version]
- Procópio, L.C.; Gayot, M.; Sist, P.; Ferraz, I.D.K. As espécies de tauari (Lecythidaceae) em florestas de terra firme da Amazônia: Padrões de distribuição geográfica, abundâncias e implicações para a conservação. Acta Bot. Bras. 2010, 24, 883–897. [Google Scholar] [CrossRef] [Green Version]
- Thomas, H. Senescence, ageing and death of the whole plant. New Phytol. 2013, 197, 696–711. [Google Scholar] [CrossRef]


| Group 1 |
| Angelim-pedra (Hymenolobium petraeum Ducke); cedro (Cedrela odorata L.); copaíba (Copaifera Ducke); cumaru (Dipteryx odorata (Aubl.) Willd.); freijó-cinza (Cordia goeldiana Huber); ipê-amarelo (Handrohanthus serratifolius (Vahl) S. Grose); jatobá (Hymenaea courbaril L.); jatobá-curuba (Hymenaea parvifolia Huber); louro-canela (Nectandra sp.); maçaranduba (Manilkara elata (Allemão ex Miq.) Monach.); muiracatiara (Astronium lecointei Ducke); roxinho (Peltogyne lecointei Ducke); and tatajuba (Bagassa guianensis Aubl.). |
| Group 2 |
| Amapá (Brosimum guianense (Aubl.) Huber); amarelão (Apuleia leiocarpa (Vogel) J. F. Macbr.); amescla/breu (Trattinnickia burseraefolia Mart. Willd.); amesclão (Trattinnickia rhoifolia Willd.); amesclinha (Protium altissimum (Aubl.)) Marchand); angico/timborana (Pseudopiptadenia suaveolens (Miq.) J. W. Grimes); caju (Anacardium giganteum W. Hancock ex Engl.); caneleiro (Cenostigma tocantinum Ducke); casca seca (Licania sp. Aubl.); catuaba (Lacmellea aculeata (Ducke) Monach.); cedrorana (Vochysia maxima Ducke); coco-pau (Coupeia robusta Huber); cupiúba (Goupia glabra Aubl.); axixá/envira-quiabo (Sterculia pruriens (Aubl.) K. Schum.); envira/envira-preta (Guatteria punctata (Aubl.) R. A. Howard); escorrega-macaco (Albizia pedicellaris (DC.) L. Rico); estopeiro/tauari (Couratari sp. Aubl.); farinha-seca (Ampelocera edentula Kuhlm.); faveira (Parkia multijuga Benth.); goiabão (Pouteria bilocularis (H. K. A. Winkl.) Baehni); inharé (Helicostylis pedunculata Benoist); jarana (Lecythis lurida (Miers) S.A. Mori); louro-pimenta (Ocotea sp.); louro-vermelho (Sextonia rubra (Mez) van der Werff); mandiocão/morototó (Didymopanax morototoni (Aubl.) Decne. & Planch.); marupá (Simarouba amara Aubl.); orelha-de-macaco (Enterolobium schomburgkii (Benth.) Benth.); paricá (Schizolobium parahyba var. amazonicum (Huber ex Ducke) Barneby); pau-santo (Zollernia paraensis Huber); pequiá (Caryocar vilosum (Aubl.) Pers.); pequiarana (Caryocar glabrum (Aubl.) Pers.); quina (Geissospermum sericeum Miers); quina-rosa (Quiina amazonica A.C.Sm.); sapucaia (Lecythis pisonis Cambess.); seringarana (Ecclinusa guianensis Eyma); sumaúma (Ceiba pentandra (L.) Gaertn.); tanibuca (Terminalia tanibouca Rich.); itaúba (Mezilaurus itauba (Meisn.) Taub. ex Mez); tauari (Couratari ssp./Eschweilera coriacea (DC.) S. A. Mori) and uxi (Endopleura uchi (Huber) Cuatrec.). |
| Group 3 |
| Amarelinho (Neoraputia paraensis (Ducke) Emmerich ex Kallunki); andirobarana (Guarea kunthiana A. Juss.); ata (Annona sp.); atraca (Ficus sp.); baço-de-boi (Myrocarpus venezuelensis Rudd); bicuíba/ucuúba-da-terra-firme (Virola michelii Heckel); Buranju (Neea floribunda Poepp. & Endl.); Cacau (Theobroma speciosa Willd. ex Spreng.); canafístula (Senna multijuga (Rich.) H. S. Irwin & Barneby); capa-bode (Bauhinia acreana Harms.); conduru (Cynometra bauhiniifolia Benth.); cravinho/goiabarana (Myrcia paivae O.Berg); embaúba (Cecropia distachya Huber./C. sciadophylla Mart./C. palmata Willd.; Pourouma guianensis Aubl.); freijó-branco (Cordia alliodora (Ruiz & Pav.) Cham.); Gabiroba (Campomanesia grandiflora (Aubl.) Sagot); gema-de-ovo (Amphiodon effusus HuberPoecilanthe); goiabinha (Eugenia lambertiana DC.); inajarana (Quararibea guianensis Aubl.); ingá (Inga spp.; Inga alba (Sw.) Willd.; jaca-braba (Abarema campestres (Spruce ex Benth.) Barneby & J. W. Grimes); jambo/muúba (Bellucia grossularioides (L.) Triana); jiboião/matamatá-preto (Eschweilera grandiflora (Aubl.) Sandwith); jurema (Senna polyphylla (Jacq.) H. S. Irwin & Barneby); juruparana (Gustavia augusta L.); limãozinho (Zanthoxylum rhoifolia Lam/Z. ekmanii (Urb.) Alain); mangaba/abiu-mangabarana (Micropholis guyanensis (A. DC.) Pierre); mangue (Buchenavia capitata (Vahl) Eichler); maria-preta (Ziziphus cinnamomum Triana & Planch.); matamata/matamata-jibóia (Eschweilera ovata (Cambess.) Mart. ex Miers); mirindiba (Glycydendron amazonicum Ducke); moreira (Maclura tinctoria (L.) D. Don ex Steud.); mutamba (Guazuma umifolia Lam.); pele de sapo (Pausandra trianae (Müll.Arg.) Baill.); pitomba (Talisia sp.); seringueira (Hevea brasiliensis (Willd. ex A Juss) Mull. Arg.); tamburil (Enterolobium maximum Ducke); taxi/taxi-branco (Tachigali vulgaris L. G. Silva & H. C. Lima/Tachigali glauca Tul.) and tuturubá/abiurana (Pouteria guianensis Aubl./Pouteria venosa subsp. amazonica T. D. Penn) |
| Production for Harvest (m3 ha−1) | Market Value Group | Cash Flow (USD ha−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Production (m3 ha−1) | Price (USD m−3) | Cost | Revenue | Balance | |||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| 29.99 | 0.46 | 19.77 | 9.77 | 43.04 | 17.39 | 14.58 | 37.82 | 506.00 | 468.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siviero, M.A.; Ruschel, A.R.; Yared, J.A.G.; de Aguiar, O.J.R.; Pereira, P.C.G.; Vieira, S.B.; Sales, A. Harvesting Criteria Application as a Technical and Financial Alternative for Management of Degraded Tropical Forests: A Case Study from Brazilian Amazon. Diversity 2020, 12, 373. https://doi.org/10.3390/d12100373
Siviero MA, Ruschel AR, Yared JAG, de Aguiar OJR, Pereira PCG, Vieira SB, Sales A. Harvesting Criteria Application as a Technical and Financial Alternative for Management of Degraded Tropical Forests: A Case Study from Brazilian Amazon. Diversity. 2020; 12(10):373. https://doi.org/10.3390/d12100373
Chicago/Turabian StyleSiviero, Marco A., Ademir R. Ruschel, Jorge A. G. Yared, Osmar J. R. de Aguiar, Paulo C. G. Pereira, Sabrina B. Vieira, and Agust Sales. 2020. "Harvesting Criteria Application as a Technical and Financial Alternative for Management of Degraded Tropical Forests: A Case Study from Brazilian Amazon" Diversity 12, no. 10: 373. https://doi.org/10.3390/d12100373
APA StyleSiviero, M. A., Ruschel, A. R., Yared, J. A. G., de Aguiar, O. J. R., Pereira, P. C. G., Vieira, S. B., & Sales, A. (2020). Harvesting Criteria Application as a Technical and Financial Alternative for Management of Degraded Tropical Forests: A Case Study from Brazilian Amazon. Diversity, 12(10), 373. https://doi.org/10.3390/d12100373