Urochloa Grasses Swap Nitrogen Source When Grown in Association with Legumes in Tropical Pastures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Plant and Soil Sampling
2.3. Chemical and Isotopic Analysis of Plants and Soil
2.4. Legume N Derived From the Atmosphere
2.5. Data Analysis
3. Results
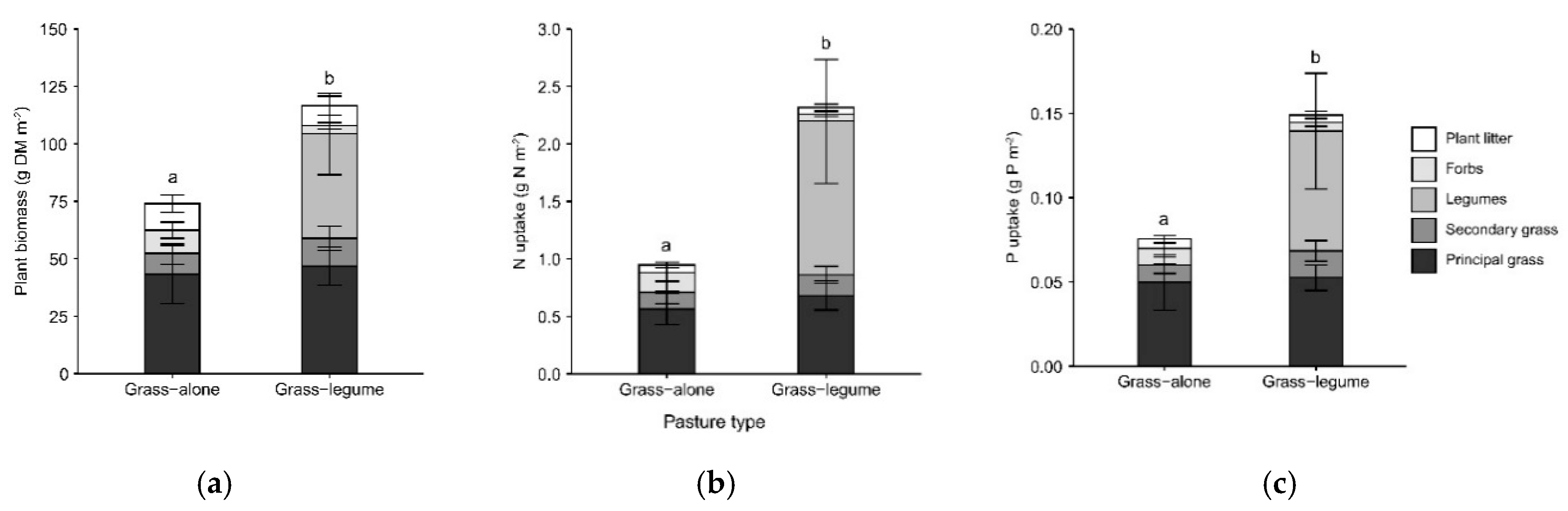
3.1. Dry Matter Productivity and Nutrient Uptake
3.2. Legume-N derived from the atmosphere
3.3. δ15N and δ13C Isotopic Signature of Pasture Components
4. Discussion
4.1. Legumes Improve Pasture Productivity and Nutrient Uptake
4.2. What N Sources are Exploited by Grasses in Each Pasture Type
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asner, G.P.; Townsend, A.R.; Bustamante, M.M.C.; Nardoto, G.B.; Olander, L.P. Pasture degradation in the central Amazon: Linking changes in carbon and nutrient cycling with remote sensing. Glob. Chang. Biol. 2004, 10, 844–862. [Google Scholar] [CrossRef]
- Jank, L.; Barrios, S.C.; Do Valle, C.B.; Simeáo, R.M.; Alves, G.F. The value of improved pastures to Brazilian beef production. Crop Pasture Sci. 2014, 65, 1132–1137. [Google Scholar] [CrossRef]
- Wassenaar, T.; Gerber, P.; Verburg, P.H.; Rosales, M.; Ibrahim, M.; Steinfeld, H. Projecting land use changes in the Neotropics: The geography of pasture expansion into forest. Glob. Environ. Chang. Hum. Policy Dimens. 2007, 17, 86–104. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Lal, R. Mechanisms of C sequestration in soils of Latin America. Crit. Rev. Plant Sci. 2006, 25, 337–365. [Google Scholar] [CrossRef] [Green Version]
- Boddey, R.M.; Macedo, R.; Tarre, R.M.; Ferreira, E.; de Oliveira, O.C.; Rezende, C.D.; Cantarutti, R.B.; Pereira, J.M.; Alves, B.J.R.; Urquiaga, S. Nitrogen cycling in Brachiaria pastures: The key to understanding the process of pasture decline. Agric. Ecosyst. Environ. 2004, 103, 389–403. [Google Scholar] [CrossRef]
- De Oliveira, O.C.; de Oliveira, I.P.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Chemical and biological indicators of decline/degradation of Brachiaria pastures in the Brazilian Cerrado. Agric. Ecosyst. Environ. 2004, 103, 289–300. [Google Scholar] [CrossRef]
- Nesper, M.; Buenemann, E.K.; Fonte, S.J.; Rao, I.M.; Velasquez, J.E.; Ramirez, B.; Hegglin, D.; Frossard, E.; Oberson, A. Pasture degradation decreases organic P content of tropical soils due to soil structural decline. Geoderma 2015, 257, 123–133. [Google Scholar] [CrossRef]
- Steinfield, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; FAO: Rome, Italy, 2006. [Google Scholar]
- FAO. Country Pasture/Forage Resource Profiles Brazil. 2006. Available online: http://www.fao.org/ag/agp/AGPC/doc/Counprof/Brazil/brazil2.htm (accessed on 10 June 2020).
- Navarrete, D.; Sitch, S.; Aragão, L.E.O.C.; Pedroni, L. Conversion from forests to pastures in the Colombian Amazon leads to contrasting soil carbon dynamics depending on land management practices. Glob. Chang. Biol. 2016, 22, 3503–3517. [Google Scholar] [CrossRef] [Green Version]
- Dias-Filho, M.B.; Davidson, E.A.; de Carvalho, C.J.R. Linking biochemical cycles to cattle pasture management and sustainability in the Amazon Basin. In The Biogeochemistry of the Amazon Basin; McClain, M.E., Victoris, R.L., Richery, J.E., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 84–105. [Google Scholar]
- Fisher, M.J.; Braz, S.P.; Dos Santos, R.S.M.; Urquiaga, S.; Alves, B.J.R.; Bodddey, R.M. Another dimension to grazing systems: Soil carbon. Trop. Grassl. 2007, 41, 65–83. [Google Scholar]
- Neill, C.; Piccolo, M.C.; Melillo, J.M.; Steudler, P.A.; Cerri, C.C. Nitrogen dynamics in Amazon forest and pasture soils measured by N-15 pool dilution. Soil Biol. Biochem. 1999, 31, 567–572. [Google Scholar] [CrossRef]
- Miles, J.W.; Do Valle, C.B.; Rao, I.M.; Euclides, V.P.B. Brachiaria grasses. In Warm Season (C4) Grasses; ASA, CSSA, SSSA: Madison, WI, USA, 2004; Volume Monograph No. 45, p. 39. [Google Scholar]
- Rao, I.M. Adapting tropical forages to low-fertility soils. In Proceedings of the Xix International Grassland Congress, São Pedro, São Paulo, Brazil, 11–21 February 2001; pp. 247–254. [Google Scholar]
- Fisher, M.J.; Kerridge, P.C. The agronomy and physiology of Brachiaria species. In Brachiaria: Biology, Agronomy, and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; Centro International de Agricultura Tropical: Cali, Colombia, 1996; pp. 43–52. [Google Scholar]
- Schultze-Kraft, R.; Rao, I.M.; Peters, M.; Clements, R.J.; Bai, C.; Liu, G. Tropical forage legumes for environmental benefits: An overview. Trop. Grassl. Forrajes Trop. 2018, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cadisch, G.; Sylvester-Bradley, R.; Nösberger, J. 15N-based estimation of nitrogen fixation by eight tropical forage-legumes at two levels of P:K supply. Field Crops Res. 1989, 22, 181–194. [Google Scholar] [CrossRef]
- Thomas, R.J. The role of the legume in the nitrogen cycle of productive and sustainable pastures. Grass Forage Sci. 1992, 47, 133–142. [Google Scholar] [CrossRef]
- Lüscher, A.; Mueller-Harvey, I.; Soussana, J.F.; Rees, R.M.; Peyraud, J.L. Potential of legume-based grassland-livestock systems in Europe: A review. Grass Forage Sci. 2014, 69, 206–228. [Google Scholar] [CrossRef]
- Cipagauta, M.; Velásquez, J.; Pulido, J.I. Producción de leche en tres pasturas del Piedemonte Amazonico del Caquetá, Colombia. Pasturas Trop. 1998, 20, 2–10. [Google Scholar]
- Boddey, R.M.; Casagrande, D.R.; Homem, B.G.C.; Alves, B.J.R. Forage legumes in grass pastures in tropical Brazil and likely impacts on greenhouse gas emissions: A review. Grass Forage Sci. 2020. [Google Scholar] [CrossRef]
- Rao, I.; Peters, M.; Castro, A.; Schultze-Kraft, R.; White, D.; Fisher, M.; Miles, J.; Lascano, C.; Blümmel, M.; Bungenstab, D.; et al. LivestockPlus—The sustainable intensification of forage-based agricultural systems to improve livelihoods and ecosystem services in the tropics. Trop. Grassl. Forrajes Trop. 2015, 3, 59–82. [Google Scholar] [CrossRef]
- Gaviria-Uribe, X.; Naranjo-Ramírez, J.F.; Bolívar-Vergara, D.M.; Barahona-Rosales, R. Intake and digestibility of nutrients in zebu steers grazing in intensive silvopastoral system. Arch. Zootec. 2015, 64, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Fonte, S.J.; Nesper, M.; Hegglin, D.; Velásquez, J.E.; Ramirez, B.; Rao, I.M.; Bernasconi, S.M.; Bünemann, E.K.; Frossard, E.; Oberson, A. Pasture degradation impacts soil phosphorus storage via changes to aggregate-associated soil organic matter in highly weathered tropical soils. Soil Biol. Biochem. 2014, 68, 150–157. [Google Scholar] [CrossRef]
- Nakamura, S.; Saliou, P.S.; Takahashi, M.; Ando, Y.; Subbarao, G.V. The contribution of root turnover on biological nitrification inhibition and its impact on the ammonia-oxidizing archaea under Brachiaria cultivations. Agronomy 2020, 10, 1003. [Google Scholar] [CrossRef]
- Hammelehle, A.; Oberson, A.; Luscher, A.; Mader, P.; Mayer, J. Above- and belowground nitrogen distribution of a red clover-perennial ryegrass sward along a soil nutrient availability gradient established by organic and conventional cropping systems. Plant Soil 2018, 425, 507–525. [Google Scholar] [CrossRef]
- Oberson, A.; Frossard, E.; Bühlmann, C.; Mayer, J.; Mäder, P.; Lüscher, A. Nitrogen fixation and transfer in grass-clover leys under organic and conventional cropping systems. Plant Soil 2013, 371, 237–255. [Google Scholar] [CrossRef] [Green Version]
- Trannin, W.S.; Urquiaga, S.; Guerra, G.; Ibijbijen, J.; Cadisch, G. Interspecies competition and N transfer in a tropical grass-legume mixture. Biol. Fertil. Soils 2000, 32, 441–448. [Google Scholar] [CrossRef]
- Karwat, H.; Egenolf, K.; Nuñez, J.; Rao, I.; Rasche, F.; Arango, J.; Moreta, D.; Arevalo, A.; Cadisch, G. Low 15N natural abundance in shoot tissue of Brachiaria humidicola is an indicator of reduced N losses due to biological nitrification inhibition (BNI). Front. Microbiol. 2018, 9, 2383. [Google Scholar] [CrossRef]
- Boddey, R.; Victoria, R. Estimation of biological nitrogen fixation associated with Brachiaria and Paspalum grasses using 15N labelled organic matter and fertilizer. In Nitrogen Fixation with Non-Legumes; Springer: Dordrecht, The Netherlands, 1986; pp. 265–292. [Google Scholar]
- Reis, V.M.; dos Reis, F.B.; Quesada, D.M.; de Oliveira, O.C.A.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Biological nitrogen fixation associated with tropical pasture grasses. Aust. J. Plant Physiol. 2001, 28, 837–844. [Google Scholar] [CrossRef]
- Nunez, J.; Arevalo, A.; Karwat, H.; Egenolf, K.; Miles, J.; Chirinda, N.; Cadisch, G.; Rasche, F.; Rao, I.; Subbarao, G.; et al. Biological nitrification inhibition activity in a soil-grown biparental population of the forage grass, Brachiaria humidicola. Plant Soil 2018, 426, 401–411. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [Green Version]
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Lüscher, A. Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric. Ecosyst. Environ. 2011, 140, 155–163. [Google Scholar] [CrossRef]
- Instituto de Hidrología Meteorología y Estudios Ambientales (IDEAM). Atlas Climatológico, Radiación y de Viento. Available online: http://www.ideam.gov.co/web/tiempo-y-clima/ (accessed on 7 August 2020).
- Instituto Geográfico Agustín Codazzi (IGAC). Estudio General de Suelos y Zonificación de Tierras: Departamento de Caquetá. Escala 1:100.000; Instituto Geográfico Agustín Codazzi (IGAC): Bogotá, Colombia, 2014. [Google Scholar]
- Rosas-Patiño, G.; Puentes-Páramo, Y.J.; Menjivar-Flores, J.C. Liming effect on macronutrient intake for cacao (Theobroma cacao L.) in the Colombian Amazon. Cienc. Tecnol. Agropecu. 2019, 20, 5–28. [Google Scholar]
- Borrero Tamayo, G.; Jiménez, J.; Ricaurte Oyola, J.J.; Rivera, M.; Polanía Perdomo, J.A.; Núñez, J.; Barbosa, N.; Arango, J.; Cardoso, J.; Rao, I.M. Manual de protocolos. In Nutrición y Fisiología de Plantas-Forrajes y Fríjol; Centro Internacional de Agricultura Tropical (CIAT): Cali, Colombia, 2017. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Instituto de Hidrología Meteorología y Estudios Ambientales (IDEAM). Geoportal de datos hidrometeorológicos DHIME. Available online: http://www.dhime.ideam.gov.co/ (accessed on 13 August 2020).
- Gómez, M.; Velásquez, J.; Miles, J.; Rayo, F. Adaptation of Brachiaria to the Amazonian piedmont in Colombia. Pasturas Trop. 2000, 22, 19–25. [Google Scholar]
- Shearer, G.; Kohl, D.H. N2 fixation in field settings: Estimations based on natural abundance. Aust. J. Plant Physiol. 1986, 13, 699–744. [Google Scholar] [CrossRef]
- Wiggenhauser, M.; Bigalke, M.; Imseng, M.; Keller, A.; Archer, C.; Wilcke, W.; Frossard, E. Zinc isotope fractionation during grain filling of wheat and a comparison of zinc and cadmium isotope ratios in identical soil–plant systems. New Phytol. 2018, 219, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Zibilske, L.M. Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci. Soc. Am. J. 1991, 55, 892–895. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Pate, J.S.; Sanford, P.; Armstrong, E.L. Potential precision of the d15N natural abundance method in the field estimates of nitrogen fixation by crop and pasture legumes in south-west Australia. Aust. J. Agric. Res. 1994, 45, 119–132. [Google Scholar] [CrossRef]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, R.; Giller, K.; Alves, B.; Chalk, P.M. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; ACIAR: Canberra, Australia, 2008; Volume 136. [Google Scholar]
- Peoples, M.; Bell, M.; Bushby, H. Effect of rotation and inoculation with Bradyrhizobium on nitrogen fixation and yield of peanut (Arachis hypogaea L., cv. Virginia Bunch). Aust. J. Agric. Res. 1992, 43, 595–607. [Google Scholar] [CrossRef]
- Cadisch, G.; Hairiah, K.; Giller, K.E. Applicability of the natural N-15 abundance technique to measure N-2 fixation in Arachis hypogaea grown on an Ultisol. Neth. J. Agric. Sci. 2000, 48, 31–45. [Google Scholar]
- Okito, A.; Alves, B.; Urquiaga, S.; Boddey, R.M. Isotopic fractionation during N-2 fixation by four tropical legumes. Soil Biol. Biochem. 2004, 36, 1179–1190. [Google Scholar] [CrossRef]
- Rao, I.M. Advances in improving adaptation of common bean and Brachiaria forage grasses to abiotic stress in the tropics. In Handbook of Plant and Crop Physiology; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 847–889. [Google Scholar]
- Seiffert, N.; Zimmer, A. Contribución de Calopogonium mucunoides al contenido de nitrógeno en pasturas de Brachiaria decumbens. Pasturas Trop. 1988, 10, 8–13. [Google Scholar]
- Ribeiro, N.V.d.S.; Vidal, M.S.; Barrios, S.C.L.; Baldani, V.L.D.; Baldani, J.I. Genetic diversity and growth promoting characteristics of diazotrophic bacteria isolated from 20 genotypes of Brachiaria spp. Plant Soil 2020, 451, 187–205. [Google Scholar] [CrossRef]
- Thomas, R.J.; Lascano, C.E. The benefits of forage legumes for livestock production and nutrient cycling in pasture and agropastoral systems of acid soils savannas of Latin America. In Livestock and Sustainable Nutrient Cycling in Mixed Farming Systems of Sub-Sahara Africa; Powell, J.M., Fernandez-Rivera, S., Williams, T.O., Renard, C., Eds.; ILCA: Addis Ababa, Ethiopia, 1995; pp. 277–291. [Google Scholar]
- Rao, I.M.; Kerridge, P.C.; Macedo, M.C.M. Nutritional requirements of Brachiaria and adaptation to acid soils. In Brachiaria: Biology, Agronomy, and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; CIAT: Cali, Colombia, 1996; pp. 53–71. [Google Scholar]
- Rao, I.; Kerridge, P. Mineral nutrition of forage Arachis. In Bioogy and Agronomy of Forage Arachis; Kerridge, P., Hardy, B., CIAT: Cali, Colombia, 1994; pp. 71–83. [Google Scholar]
- Cantarutti, R.B.; Tarre, R.; Macedo, R.; Cadisch, G.; Rezende, C.D.; Pereira, J.M.; Braga, J.M.; Gomide, J.A.; Ferreira, E.; Alves, B.J.R.; et al. The effect of grazing intensity and the presence of a forage legume on nitrogen dynamics in Brachiaria pastures in the Atlantic forest region of the south of Bahia, Brazil. Nutr. Cycl. Agroecosyst. 2002, 64, 257–271. [Google Scholar] [CrossRef]
- Rao, I.M.; Borrero, V.; Ricaurte, J.; Garcia, R. Adaptive attributes of tropical forage species to acid soils. V. Differences in phosphorus acquisition from less available inorganic and organic sources of phosphate. J. Plant Nutr. 1999, 22, 1175–1196. [Google Scholar] [CrossRef]
- Oberson, A.; Bünemann, E.K.; Friesen, D.K.; Rao, I.M.; Smithson, P.C.; Turner, B.L.; Frossard, E. Improving phosphorus fertility in tropical soils through biological interventions. In Biological Approaches to Sustainable Soil Systems; Uphoff, N., Ball, A.S., Fernandes, E., et al., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 531–546. [Google Scholar]
- Oberson, A.; Friesen, D.K.; Tiessen, H.; Morel, C.; Stahel, W. Phosphorus status and cycling in native savanna and improved pastures on an acid low-P Colombian Oxisol. Nutr. Cycl. Agroecosyst. 1999, 55, 77–88. [Google Scholar] [CrossRef]
- Bünemann, E.; Smithson, P.C.; Jama, B.; Frossard, E.; Oberson, A. Maize productivity and nutrient dynamics in maize-fallow rotations in western Kenya. Plant Soil 2004, 264, 195–208. [Google Scholar] [CrossRef]
- Rao, I.M.; Barrios, E.; Amezquita, E.; Friesen, D.K.; Thomas, R.; Oberson, A.; Singh, B.R. Soil phosphorus dynamics, acquisition and cycling in crop-pasture-fallow systems in low fertility tropical soils of Latin America. In Modelling Nutrient Management in Tropical Cropping Systems; Delve, R.J., Probert, M.E., Eds.; Australian Center for International Agricultural Research (ACIAR): Canberra, Australia, 2004; Volume 114, pp. 126–134. [Google Scholar]
- Nakamura, T.; Miranda, C.H.B.; Ohwaki, Y.; Valéio, J.R.; Kim, Y.; Macedo, M.C.M. Characterization of nitrogen utilization by Brachiaria grasses in Brazilian savannas (Cerrados). Soil Sci. Plant Nutr. 2005, 51, 973–979. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon Isotopes in Photosynthesis: Fractionation techniques may reveal new aspects of carbon dynamics in plants. BioScience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- De Moraes, J.F.L.; Volkoff, B.; Cerri, C.C.; Bernoux, M. Soil properties under Amazon forest and changes due to pasture installation in Rondônia, Brazil. Geoderma 1996, 70, 63–81. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef] [Green Version]


| Pasture Type | pH a | Total N (mg g Dry Soil−1) b | NH4+ (mg kg Dry Soil−1) c | NO3− (mg kg Dry Soil−1) c | Total C (mg g Dry Soil−1) b | Bray-II P (mg kg Dry Soil−1) d | C:N | δ15N (‰) b | δ13C (‰) b |
|---|---|---|---|---|---|---|---|---|---|
| Grass-alone | 4.8 ± 0.3 | 2.9 ± 0.5 | 4.1 ± 1.0 | 3.1 ± 2.1 | 32.4 ± 5.6 | 1.27 ± 0.5 | 11.2 ± 0.7 | 5.9 ± 0.7 | −20.6 ± 1.6 |
| Grass-legume | 4.8 ± 0.1 | 2.6 ± 0.7 | 3.9 ± 2.3 | 3.4 ± 4.5 | 28.2 ± 7.4 | 1.09 ± 0.6 | 10.6 ± 0.8 | 6.1 ± 1.2 | −21.0 ± 1.6 |
| Pasture Type | Botanical Fraction | N Concentration (g N kg DM−1) | P Concentration (g P kg DM−1) | C Concentration (g C kg DM−1) | C:N | δ15N (‰) | δ13C (‰) |
|---|---|---|---|---|---|---|---|
| Grass alone | Forbs | 18.8 ± 4.1 c | 1.3 ± 0.4 b | 411.1 ± 5.4 a | 22.7 ± 4.7 a | 5.3 ± 1.1 d | −25.0 ± 5.1 b |
| Principal grass | 14.9 ± 3.5 b | 1.1 ± 0.3 b | 424.0 ± 5.4 b | 30.0 ± 7.6 a | 4.5 ± 3.1 c | −13.3 ± 0.5 d | |
| Secondary grass | 15.3 ± 5.3 bc | 1.4 ± 0.3 b | 420.5 ± 8.1 ab | 30.5 ± 11.4 a | 2.5 ± 3.0 c | −20.9 ± 6.2 c | |
| Legumes | 22.1 ± 4.8 d | 1.2 ± 0.1 b | 447.9 ± 9.0 c | 20.7 ± 4.9 a | −0.6 ± 0.6 a | −31.0 ± 0.0 a | |
| Plant litter | 6.8 ± 1.8 a | 0.4 ± 0.1 a | 424.8 ± 11.7 b | 67.1 ± 20.1 b | 1.9 ± 2.3 b | −16.9 ± 3.8 c | |
| Total * | 17.4 ± 3.9 A | 1.2 ± 0.3 A | 426.2 ± 11.5 A | 25.8 ± 7.1 A | 4.6 ± 2.9 B | −17.1 ± 3.8 A | |
| Grass legume | Forbs | 18.4 ± 3.1 c | 1.4 ± 0.4 b | 417.4 ± 7.5 ab | 23.1 ± 3.7 a | 5.7 ± 2.5 d | −25.4 ± 5.3 b |
| Principal grass | 14.8 ± 3.5 b | 1.3 ± 0.5 b | 426.9 ± 5.0 b | 30.1 ± 6.8 a | 3.8 ± 2.9 c | −12.7 ± 0.4 d | |
| Secondary grass | 15.4 ± 2.1 bc | 1.4 ± 0.3 b | 419.8 ± 5.0 ab | 27.6 ± 3.7 a | 3.6 ± 3.2 c | −18.0 ± 3.8 c | |
| Legumes | 27.8 ± 3.3 d | 1.3 ± 0.3 b | 422.1 ± 9.2 ab | 15.3 ± 1.8 a | 0.4 ± 1.0 a | −29.6 ± 0.3 a | |
| Plant litter | 7.9 ± 1.3 a | 0.5 ± 0.2 a | 417.1 ± 8.6 ab | 53.6 ± 7.3 b | 1.1 ± 2.1 b | −16.0 ± 0.9 c | |
| Total * | 20.5 ± 3.2 B | 1.2 ± 0.2 A | 404.9 ± 46.2 A | 20.0 ± 3.3 A | 2.1 ± 1.7 A | −19.9 ± 3.5 A | |
| Source of variation ** | Pasture type | ns | ns | p < 0.05 | ns | ns | ns |
| Botanical fraction | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Pasture type x botanical fraction | ns | ns | p < 0.001 | ns | ns | ns | |
| Farm (random) | p < 0.001 | p < 0.001 | p < 0.05 | ns | p < 0.001 | p < 0.001 |
| Pasture Type | Species | δ15N (‰) | Ndfa (%) |
|---|---|---|---|
| Grass alone | U. brizantha | 4.7 ± 4.2 ab | - |
| U. decumbens | 5.8 ± 1.1 b | - | |
| U. humidicola | 3.2 ± 4.2 ab | - | |
| Grass legume | U. brizantha | 4.9 ± 2.7 ab | - |
| U. decumbens | 5.0 ± 0.0 ab | - | |
| U. humidicola | 2.0 ± 3.3 ab | - | |
| A. pintoi | 0.4 ± 0.9 a | 83.2 ± 14.0 | |
| P. phaseoloides | 1.3 ± 0.6 ab | 67.5 ± 9.2 | |
| Source of variation | Pasture type | ns | - |
| Species | p < 0.05 | - | |
| Pasture type x species | ns | - | |
| Farm (random) | ns | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas, D.M.; Velasquez, J.; Arango, J.; Obregon, K.; Rao, I.M.; Rosas, G.; Oberson, A. Urochloa Grasses Swap Nitrogen Source When Grown in Association with Legumes in Tropical Pastures. Diversity 2020, 12, 419. https://doi.org/10.3390/d12110419
Villegas DM, Velasquez J, Arango J, Obregon K, Rao IM, Rosas G, Oberson A. Urochloa Grasses Swap Nitrogen Source When Grown in Association with Legumes in Tropical Pastures. Diversity. 2020; 12(11):419. https://doi.org/10.3390/d12110419
Chicago/Turabian StyleVillegas, Daniel M., Jaime Velasquez, Jacobo Arango, Karen Obregon, Idupulapati M. Rao, Gelber Rosas, and Astrid Oberson. 2020. "Urochloa Grasses Swap Nitrogen Source When Grown in Association with Legumes in Tropical Pastures" Diversity 12, no. 11: 419. https://doi.org/10.3390/d12110419
APA StyleVillegas, D. M., Velasquez, J., Arango, J., Obregon, K., Rao, I. M., Rosas, G., & Oberson, A. (2020). Urochloa Grasses Swap Nitrogen Source When Grown in Association with Legumes in Tropical Pastures. Diversity, 12(11), 419. https://doi.org/10.3390/d12110419






