Abstract
Acanthurids (surgeonfishes) are an abundant and diverse group of herbivorous fishes on coral reefs. While their contribution to trophic linkages and dynamics in coral reef systems has received considerable attention, the role of linkages involving their parasites has not. As both consumers of fish tissue and prey to microcarnivores, external parasites may play a significant role in trophic transfer between primary consumers (and hence their predominantly algae-based diet) and the broader coral reef community. Stable isotope analysis is a common tool for studying trophic linkages which can be used for studies involving parasites. We examined the stable isotope ecology (13C and 15N) of copepod (Caligus atromaculatus) and monogenean (Neobenedenia sp.) ectoparasites collected from two species of Caribbean acanthurids (Acanthurus coeruleus and Acanthurus bahianus). There were significant intraspecific differences in isotope discrimination factors between parasites collected from the two different host species as well as interspecific differences between parasites collected from the same host species. Discrimination factors for 15N were consistently positive but varied in magnitude depending on host and parasite species and were slightly lower than what would be expected for consumers. The 13C discrimination factors for both monogeneans and copepods collected from A. coeruleus were consistently positive but were negative for copepods collected from A. bahianus. These findings emphasize the complexity of the stable isotope trophic interactions occurring between parasites and their hosts, highlighting the value of these types of host-parasite isotopic studies.
Keywords:
Surgeonfish; Acanthuridae; coral reefs; ectoparasites; Caligus; Neobenedenia; stable isotope ecology; food webs; nanoEA 1. Introduction
Near-shore scleractinian coral reefs harbor the greatest biodiversity in the world’s oceans [1,2,3]. This high biodiversity contained within a relatively small area facilitates an unparalleled network of complex interactions involving the biotic and abiotic community [4]. As with any ecosystem, a functioning coral reef system depends on and is structured by activities of its component trophic groups and its integrity is maintained via the flow of energy amongst these groups [5,6]. Thus, understanding the trophic interconnectivity of key trophic groups is essential for unravelling complex coral reef ecosystems.
An emerging modern view of ecology centers around the idea that the role of both parasites and micropredators in ecological communities has been greatly underestimated [7,8,9,10] and recent outbreaks of disease and parasite infestations in coral reef systems have further stimulated research into their role in coral reef energy dynamics. Parasites comprise the majority of biodiversity on coral reefs [3,11,12,13,14] and results to date indicate that these organisms are capable of modifying and directly or indirectly controlling the flow of energy in food webs and affecting host populations [10,15,16,17]. This, paired with their high abundance, biomass, and diversity, leads to the logical conclusion that they are ecologically significant organisms whose impacts extend to the community and ecosystem levels [15,18,19,20]. In some estuarine systems the overall biomass of parasites surpasses that of apex predators [21,22]. It is therefore surprising that in most food-web studies, parasites are often overlooked as key components of food-web energy dynamics [15,16,23,24]. This omission is largely due to their relatively small size and cryptic nature. The addition of parasites to food web models can increase connectance [25], food chain length [8,26], and overall species diversity [27]. Parasites can also indirectly alter energy flow by increasing the susceptibility of infected hosts to predation (e.g., [28]). Due to their intimate connection to hosts, parasites are often not considered to be readily available prey items. However, Johnson et al. [10] suggest that they are not only critical to energy transfer as consumers, but also as prey items acquired through concomitant predation, grooming/cleaning, or during free-living stages.
Herbivorous reef fishes are among the best studied trophic groups in coral reef systems [29,30,31]. They have major direct and indirect impacts on reef trophic dynamics via their role as grazers and primary consumers, serving as prey for other species, and in some cases through territorial behavior [32,33,34,35,36,37]. It is likely that given the diversity and abundance of parasites that they host, herbivorous fishes may also be influencing the trophic dynamics of reef ecosystems in other, more discrete ways. While their contribution to reef trophic dynamics through herbivory and as a prey source for piscivorous fishes has been well-studied, the role of parasites in trophodynamics of these fishes has been ignored.
Surgeonfishes (Acanthuridae) are among the most diverse and abundant herbivorous fishes in coral reef systems [38]. Although they are known to be primarily “herbivores,” they exhibit significant interspecific variability within their diet. This includes selectively feeding on different types of algae and incidents of omnivory through ingestion of invertebrates while grazing on algae [39,40,41]. They are also known to harbor multiple internal and external parasites [42,43,44,45] which, given the high biomass of surgeonfishes, could contribute significantly to the energy transfer, to parasite consumers and coral reef communities at large. For example, surgeonfishes are frequent visitors to cleaning stations, where external gnathiid isopods, copepods, and monogeneans are eaten by cleaners [46,47,48]. These trophic interactions may represent a significant transfer of algal-derived biomass (in terms of carbon) between primary consumers and reef cleaning organisms.
Stable isotope analysis (SIA) has been used to help assess the links and magnitude of energy flow between organisms, which are the foundational components of food-web modeling [49,50]. In typical predator/prey interactions, carbon stable isotopes reflect primary carbon sources [51,52] and nitrogen stable isotopes are used to infer relative trophic levels among consumer groups [52,53,54]. Parasites and micropredators, however, employ a wide variety of unique feeding and life history strategies, and as a result have evolved complex metabolic processes for the digestion and assimilation of their diets [55]. It is not surprising that the limited (yet growing) number of studies focused on the stable isotope ecology of these organisms are finding that the classic isotopic patterns in carbon and nitrogen cannot be as easily applied to these cryptic yet ecologically significant organisms [56,57,58,59,60,61,62,63,64].
A significant source of variation in the isotopic values between parasites and “traditional” predators, as well as among different parasite species, is the site of attachment and the specific host tissue and/or fluids consumed by the parasites. Broadly, parasites fall into two groups, based on site of attachment: endoparasites and ectoparasites. Endo, or internal, parasites, including certain nematodes and helminths, live inside the host and typically enter the host via consumption, whereas ectoparasites (e.g., ticks, fleas, and leeches) attach to the outside of the host. Two of the most common ectoparasites in marine systems are monogeneans and parasitic copepods.
Monogeneans are parasitic flatworms (Platyhelminthes: Monogenea) that are found attached to the fins, skin, and gills of a wide variety of fish, and feed on blood or mucus, depending on attachment site [65,66]. These parasites can have detrimental impacts on the health of both wild and captive fish populations [67,68] and may be an important dietary source for reef associated cleaning organisms [47,69,70,71]. Despite their ecological and economic significance, the only information available on the stable isotope ecology of parasitic monogeneans is for a single freshwater species [72] which is found attached to host fish gill tissues, feeding on blood [73]. The monogeneans were enriched relative to their host fishes: 13C increased by up to 0.22‰ whereas 15N increased by ~2‰, consistent with expectations for typical consumers relative to their diet.
In contrast to monogeneans, copepods are a more diverse group, with only about 1/3 of the species functioning as parasites/micropredators [74]. However, they infect a wide range of fish hosts, with significant impacts on individuals and populations [75] and are also likely to be significant dietary components for reef cleaner organisms [71]. Available data on the stable isotope ecology of parasitic copepods is also limited, with only three studies of note [56,57,76] where they quantified isotope discrimination, which is the difference in the isotopic ratio (13C, 15N) between the parasite and its host. Results from these preliminary studies indicate a surprising pattern of the copepods, exhibiting near consistent depletion in both 13C and 15N relative to host tissues (gill, muscle, skin, and eye) with a wide range of reported values for both. Interspecific differences in feeding strategies and attachment site of the parasites as well as interspecific difference in host physiology are speculated to be the cause of these somewhat unusual patterns of isotope fractionation [56,57,76] but there remains a significant amount of ambiguity surrounding the driving forces behind these patterns.
This study was inspired by the absence of data on the role of parasites in the trophic dynamics of surgeonfishes. Given the unusual patterns of stable isotope discrimination that have been documented for parasites and micropredators at large, the focus of this study was to determine if the carbon and nitrogen stable isotope discrimination patterns of a common eastern Caribbean monogenean, Neobenedenia sp. and parasitic copepod Caligus atromaculatus infecting two common, congeneric, Caribbean surgeonfishes (Acanthuridae) follow the patterns expected based on previous studies for these types of parasites.
2. Materials and Methods
The capsalid monogeneans of Neobenedenia sp. are some of the more notorious monogeneans due to their wide geographic and host range, infecting multiple species of hosts when most monogeneans tend to be host species specific [77]. Typical of monogeneans, oncomiracidia larvae hatch from eggs deposited on the substrate and attach to the host, where they remain for life [78,79]. While some monogeneans attach to the gills, Neobenedenia and other capsalids attach to the body and fins of the host. In the eastern Caribbean, Neobenedenia have been found in low numbers on a wide range of fish species [80,81] but are particularly common on surgeonfishes (Acanthuridae), especially the blue tang (Acanthurus bahianus) [43,82]. They are also eaten by cleaner shrimps [47].
Copepods of the genus Caligus exhibit little host specificity parasitizing a wide range of host fish families [83]. As adults these copepods are exclusively parasitic, generally utilizing a single host during its life cycle [84] but there is increasing evidence that some species may parasitize multiple hosts [85]. Little information is available for C. atromaculatus but they are primarily found on the skin of host fish and are believed to consume host skin tissue, mucus, and/or blood [86,87].
Two species of Acanthurids were selected as hosts for this study. The blue tang, Acanthurus coeruleus, and the ocean surgeonfish, Acanthurus bahianus. These herbivorous fishes are common in coral reefs and adjacent habitats throughout the Caribbean region [88]. Both are hosts to C. atromaculatus and N. melleni, and are common at our study sites [43]. Although closely related species, A. coeruleus and A. bahianus appear to have significant differences in terms of both feeding ecologies and digestive physiology [39]. This provided an opportunity to examine any differences in patterns of stable isotope discrimination between two different species of parasite collected from the same species of host, and to compare differences between hosts. Blood was selected for analysis of host stable isotopes as it could be collected without sacrificing fish and is one of the presumed dietary sources for both species of parasites studied.
Sampling was conducted from June-August 2012, 2013, and 2018 at Greater Lameshur Bay (GLB), St. John, U.S. Virgin Islands (USVI, 18°19′ N, 65°44′ W), and Brewers Bay (BRB), St. Thomas, U.S. Virgin Islands (Figure 1). Fish hosts were collected at night using hand nets and flashlights with SCUBA or by snorkeling. Once netted, fish were carefully transferred to holding tanks prior to processing and were assigned a unique fish host ID which was used to pair tissue and parasite samples collected from each individual host fish. Parasites were collected by administering freshwater baths to host [43] and then sieving the contents of the bath to collect the parasites. Adult monogeneans and adult copepods were sorted from the contents of the sieve under a dissecting scope and labeled with their corresponding fish ID and preserved in 80% ethanol. Ethanol has been determined to be an adequate preservation method for parasites with no significant preservative associated artifacts in stable isotope analysis for a variety of invertebrate organisms [60,89,90,91]. While we did not specifically test for ethanol preservation effects on isotope values for these specific parasites, based on studies of similar organisms that found no significant change in isotope values with ethanol preservation, ethanol artifacts were considered negligible in the current study. Host fish which harbored a sufficient number of parasites for isotope analysis were sampled for blood (non-lethal) using an insulin needle and syringe via the caudal or dorsal aortas. Blood samples were immediately dried at 50–60 °C for 24–36 h. Once completely dried, samples were stored in airtight cryovials and packed with desiccant for transport. Monogeneans were only analyzed for A. coeruleus due to a lack of sufficient numbers of monogeneans collected from A. bahianus. This work was completed under Arkansas State University IACUC number 778227-1.
Figure 1.
Location of study sites Brewers Bay (BRB) and Greater Lameshur Bay (GLB) where host fish and parasites were collected.
Parasites and host blood were processed for stable isotope analysis according to methods described in Demopoulos and Sikkel [60] and Jenkins et al. [61]. In order to meet the minimum and maximum mass requirements for accurate stable isotope analysis, individual parasites from the same host were either pooled (ranging from 2–11 individuals) or subsampled. Subsampling of individual parasites was done under the microscope using a scalpel and care was taken to split individuals along their axis of symmetry to ensure an appropriate subsample. Parasite mass was recorded after drying samples. For pooled parasite samples with more than one individual, the average mass of the pooled individuals was used as a proxy. Prior to analysis, copepod samples were acidified using 10% PtCl to remove inorganic carbon associated with their chitinous exoskeleton [92].
Two elemental analyzers were used to collect stable isotope data for the samples used in this study. Host blood and a subset of the parasite samples (large individual copepods and pooled monogeneans when sample sizes permitted) were analyzed at Washington State University, WA, using a Costech (Valencia, CA, USA) elemental analyzer interfaced to a GV Instruments Ltd (Manchester, UK) IsoprimeTM isotope ratio mass spectrometer. The remaining samples were analyzed at the University of California, Santa Cruz using an automated “nanoEA” trapping system [93], which combines a modified Carlo Erba CE1108 Elemental Analyzer and Thermo Electron Gas Bench II to cryofocus small samples (1–8 mg C) in order to enhance signal to noise. Reproducibility of the analysis and consistency of data between the two mass spectrometers was confirmed using a bovine liver standard [49] within ± 0.2‰ for both δ13C and δ15N on both elemental analyzers used. Dual 13C and 15N analysis for samples was not always possible due to limitations in machine sensitivity and/or machine error so sample sizes vary between carbon and nitrogen data (see Supplemental Table S1).
Stable isotope ratios are expressed as δ values, in units of per mil (‰), using the following equation:
where R is the ratio of 13C/12C or 15N/14N and standards are PeeDee Belemnite and atmospheric nitrogen gas for carbon and nitrogen, respectively. Data were examined for potential lipid contribution based on the C:N data and we found no indication of a lipid effect across samples [94], so we did not lipid-correct the isotope data for any of the taxa.
δ13C or δ15N = ([Rsample/Rstandard] – 1) × 103
Isotopic discrimination factors between paired samples (i.e., between copepods and blood from their respective host) are expressed as ∆ values, in units of per mil (‰), using the following equation:
For parasite – host blood discrimination:
∆13C or ∆15N = (δparasite – δhost blood)
Linear mixed effect (LME) modeling was used to test for isotopic differences between host tissues and associated paired parasites. By including the unique host fish IDs as a random effect, LME modeling accounts for instances of pseudoreplication, which arose from having replicates of parasites collected from the same fish host. LME models were fit using the restricted likelihood ratio estimation method as part of the lmer function of the R package lme4 [95]. All data analyses were conducted using the R statistical program [96].
Model selection was determined using a type-II (hierarchical) analysis of variance (Wald X2) on the initial model to test for significance of the candidate fixed effects and interactions using the car package in R [97]. Significant predictors (Table 1 for a list of factors tested), identified through the Wald X2 analysis of variance, were then refit using the maximum likelihood estimation method (ML), and likelihood ratio test was used to confirm significance of model relative to a null model (also using ML) fitted with only individual host fish ID as a random intercept to account for instances of pseudo-replication. Preliminary comparisons (Students t-test) of data from the two sites indicated no significant variations in stable isotopes of host fish or parasites between the two sites (i.e., p > 0.05), therefore results from both sites were pooled for all comparisons. The proportion of variance in the observed data that was explained by the fixed and random effects was calculated based on marginal and conditional R2 (R2GLMM(m) and R2GLMM(c) [98] using the r.squaredGLMM function from the MuMIn package in R [99]. The validity of the assumptions of linearity, homoscedasticity, and normality were assessed using fitted vs. residual, scale-location, and Q-Q diagnostic plots. Significance of isotope discrimination factors and pairwise means comparisons were assessed with model estimated marginal means and associated 95% confidence intervals generated using the package emmmeans [100] (Tables S2 and S3). Additionally, we conducted a one-way ANOVA to identify if time was a factor explaining the isotopic variance for a subset of the samples (A. coeruleus blood, copepods, and monogeneans). These were selected because they had sufficient sample sizes at all collection times. We compared C:N ratios of host blood with parasite-host discrimination factors using Pearson correlation. Differences in C:N ratios of host blood across sampling years were assessed with a Welch one-way test for A. coeruleus and a Wilcoxon rank-sum test for A. bahianus. Lastly, a one-way analysis of variance of A. coeruleus blood (insufficient sample size for A. bahianus) vs. sample month was conducted.

Table 1.
Results from type-II (hierarchical) analysis-of variance (Wald X2) on full models which included all factors of interest. Factors which were deemed significant (p-values in bold) were fitted to the final model.
3. Results
3.1. Carbon Stable Isotopes
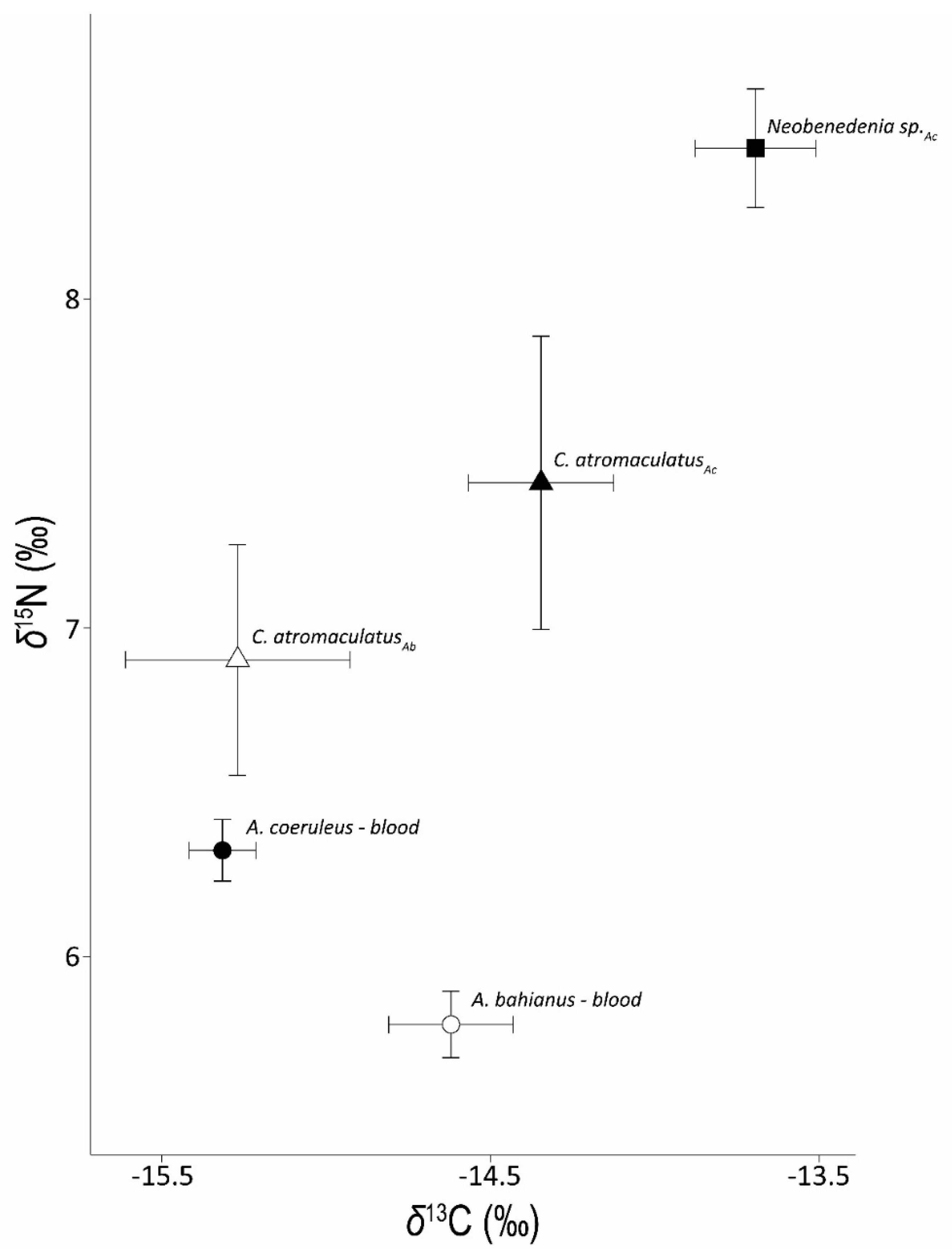
Acanthurus coeruleus blood (δ13C = −15.3 ± 0.1‰) was slightly depleted relative to A. bahianus (δ13C = −14.6 ± 0.2‰, Figure 2). Monogeneans collected from A. coeruleus were enriched relative to A. coeruleus blood (δ13C = −13.7 ± 0.2‰) and had an average Δ13C of 1.9 ± 0.1‰ (Table 2) for parasite-host pairs. We examined the variation in δ13C data for the monogeneans relative to A. coeruleus blood (Supplemental Figure S1a) and found low variation among parasite δ13C values across fish hosts. Copepods collected from A. coeruleus were also enriched in 13C relative to host blood (δ13C = −14.4 ± 0.2‰, Figure 2) and had an average Δ13C discrimination factor of 1.0 ± 0.2‰. In contrast to the positive Δ13C discrimination factor observed for copepods feeding on A. coeruleus, copepods collected from A. bahianus were consistently depleted in 13C relative to host blood (δ13C = −15.3 ± 0.3‰, Figure 2) with an average parasite-host Δ13C of −0.6 ± 0.1‰.
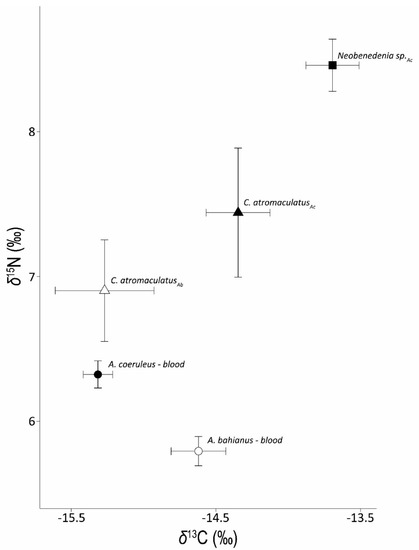
Figure 2.
Mean ± SE δ13C and δ15N data for host fish blood (circles) and associated Caligus atromaculatus (triangles) and Neobenedenia sp. (square) collected from A. coeruleus (Ac) and A. bahianus (Ab) hosts.

Table 2.
Summary of stable isotope data (‰, mean ± SE, range in parentheses), discrimination factors (∆13C, ∆15N), carbon to nitrogen ratios (C:N), and sample sizes (n) for host fish blood and parasites.
Host species and an interaction between host species and sample type (host blood, monogenean, copepod) accounted for a significant amount of the observed variance in δ13C (R2m/R2c = 0.24/0.66) when fitted to a model with individual host “Host ID” as a random effect (Table 3). Both parasite type and host species appeared to be significant predictors of parasite-host Δ13C and there was a significant negative relationship between the parasite-host Δ13C and parasite mass for monogeneans (Table 3).

Table 3.
Model estimated coefficients.
Model estimates indicated no significant difference in blood δ13C between the two species of hosts (p = 0.97), however estimates for copepods indicated a significant difference depending on host species (p = 0.04). Estimated 95% confidence intervals indicated that the Δ13C discrimination factors for copepods and monogeneans collected from A. coeruleus and copepods collected from A. bahianus were all significant (i.e., do not span zero, Table S3). In addition, the estimated Δ13C means for the different parasites indicate significantly different Δ13C discrimination factors associated with each of the different groups of parasites (monogeneans from A. coeruleus, copepods from A. coeruleus, and copepods from A. bahianus, Table S2).
3.2. Nitrogen Stable Isotopes
Acanthurus coeruleus blood (δ15N = 6.3 ± 0.1‰) was enriched in 15N relative to A. bahianus blood (δ15N = 5.8 ± 0.1‰, Figure 2). Both monogeneans (δ15N = 8.5 ± 0.2‰) and copepod parasites (δ15N = 7.4 ± 0.4‰) collected from A. coeruleus were enriched in 15N relative to host blood (Figure 2) with average parasite-host Δ15N discrimination factors of 2.0 ± 0.1‰ and 0.9 ± 0.4‰, respectively. We examined the variation in δ15N data for the monogeneans relative to A. coeruleus blood (Supplemental Figure S1b) and found high variation among parasites across individual fish hosts. Copepods collected from A. bahianus hosts (δ15N = 6.9 ± 0.3‰) were enriched in 15N relative to A. bahianus blood (δ15N = 5.8 ± 0.1‰, Figure 2) with an observed mean parasite-host Δ15N discrimination factor of 1.4 ± 0.3‰.
Sample type and host species were both significant predictors of δ15N and the magnitude of these effects was consistent across levels (i.e., no interactions, Table 1). For the final model, fixed effects alone accounted for 24% of the variance observed and this only increased to 25% when factoring in Host ID as a random effect (R2m/R2c = 0.24/0.25). Parasite-host Δ15N appeared to be significantly influenced by host species but not the type of parasite. Both host blood δ15N and parasite mass were significant predictors of Parasite-host Δ15N but varied in effect depending on parasite type. Parasite mass had a positive effect on parasite-host Δ15N for copepods but the opposite effect for monogeneans (Table 3). Parasite-host Δ15N decreased with increasing host blood δ15N for both copepods and monogeneans. However, the opposite relationship was indicated for monogeneans with parasite-host Δ15N decreasing with increasing host blood δ15N (Table 3). With the final model, fixed effects alone accounted for 18% of the observed variance and 46% when host ID was included as a random effect (R2m/R2c = 0.18/0.46).
Model estimates for δ15N indicated the observed differences in host blood and copepod δ15N between the two host species was not significant (p = 0.15 and 0.15, Table S2). Model generated CIs indicated significant positive Δ15N discrimination factors for both parasite types regardless of host species (Table S3). Additionally, model estimates indicated a significant difference in parasite – host Δ15N between the two species of parasites collected from A. coeruleus (p = 0.01) and no significant difference Δ15N between copepods from the two species of hosts (p = 0.81, Table S2).
3.3. Stable Isotope Patterns over Time and with C:N
For A. coeruleus blood, there was no difference among years in δ13C (p = 0.196, df = 2) or δ15N (p = 0.235, df = 2). For copepods collected on A. coeruleus, there was also no difference among years for δ13C (p = 0.31, df = 2), but there was a significant effect for δ15N (p = 0.0316, df = 2), with 2018 having higher δ15N than either 2013 or 2012. For collection month, A. coeruleus blood was not significantly different in δ13C (p = 0.101, df = 2), but there were differences in δ15N (p < 0.001, df = 2), with June having the highest (6.8 ± 0.1‰) and July the lowest (5.8 ± 0.2‰) values. Likewise, for monogeneans collected from A. coeruleus, δ13C did not differ among months (p = 0.192, df = 2); however, for δ15N significant differences among months were evident (p < 0.001, df = 2), with June having the highest (9.1 ± 0.3‰) and July the lowest (7.4 ± 0.2‰) values, mirroring the results from the host blood. For A. coeruleus-associated copepods, there were no significant differences among months for δ13C (p = 0.839, df = 2) or δ15N (June and July only, because there were insufficient sample sizes from August, t = −0.063, df = 19, p-value = 0.723). Due to sampling limitations, temporal analysis of A. bahianus blood was not possible because a majority of those samples were collected in July.
For A. coeruleus, we compared the discrimination factors for monogeneans relative to C:N data using Pearson’s correlation tests, and found no significant difference in Δ13C and host blood C:N (p = 0.936, df = 42, t-statistic = 0.080). However, there was a significant correlation between Δ15N and host blood C:N (p = 0.019, df = 68, t-statistic = −2.41), with a weak correlation coefficient of −0.28. For copepods associated with A. coeruleus, there was no significant correlation for either Δ13C (p = 0.626) or for Δ15N (p = 0.768, df = 21, t-statistic = −0.299). Likewise, for A. bahianus, there was no significant correlation between C:N of host blood and copepod discrimination factors for Δ13C (p = 0.870, df = 13, t-statistic = −0.166) or for Δ15N (p = 0.948, df = 15, t-statistic = −0.066).
For both A. coeruleus and A. bahianus we compared host blood C:N values across the different sampling years. For A. coeruleus, a Welch one-way test indicated no significant difference between the three sampling years (F = 1.85, df = 2, p = 0.190). A Wilcoxon rank-sum test of A. bahianus host blood C:N indicated a significant difference (W = 54.5, p = 0.016) between 2012 (C:Nmean = 3.8 ± 0.1, n = 4) and 2018 (C:Nmean = 3.7 ± 0.0, n = 4). Due sample size limitations, we were only able to assess variation of C:N values across the sampling months for A. coeruleus. A one-way analysis of variance indicated significant differences between the sampling months (C:Nmean = 3.6 ± 0.0 for June , July, and August; p = 0.001, df = 2). For both the year and month assessments the magnitude of all significant differences fell within the margin of error for our C:N (determined based on standard error for C:N of bovine liver standards run with parasite and fish tissue samples) data and were considered not significant.
4. Discussion
This study constitutes one of the most robust analyses of the stable isotope ecology of parasitic monogeneans and copepods from any fish species to date. While there is a significant lack of data regarding the stable isotope ecology of parasites in general, it is especially sparse for copepods and monogeneans. With one exception [63] previous studies on the stable isotope ecology of these parasites are limited to sample sizes ranging from 1–7 (see Table 4). This is not surprising given the exceptionally cryptic nature of these parasites compared to larger ectoparasites such as gnathiid and cymothoid isopods which have received more attention in recent studies [60,61,101,102].

Table 4.
Summary of data on parasite-host discrimination factors (‰) and sample sizes (n) for monogeneans and parasitic copepods. Data from this study are in bold. Samples sizes for present study are reported for both ∆13C and ∆15N.
The differences in direction of the parasite-host Δ13C discrimination factor for the copepods is particularly interesting given the phylogenetic and ecological similarities between the two species of hosts [39]. Caligus atromaculatus copepods collected from A. bahianus were consistently depleted in 13C relative to host blood, consistent with other studies of parasitic copepods [56,57,63] (see Table 4) as well as other terrestrial and marine micropredators and parasites [56,57,58,60,61,103]. On the other hand, the same species of copepod were significantly enriched in 13C relative to A. coeruleus blood (Figure 2, Table 2) exhibiting typical patterns of 13C fractionation associated with consumers relative to their prey [54,104]. Variations in Δ13C discrimination factors between the same species of parasite feeding on different species of hosts is consistent with published results from parasitic copepods [57] and other species of fish parasites [61,72]. However, the shift in direction of parasite-host Δ13C, from positive for A. coeruleus to negative for A. bahianus for these copepods is unusual and may be the due, in part, to the physiological and dietary differences between two species of hosts [39].
The variation in stable isotope turnover rates among organisms is another factor to consider when resolving between-species differences in discrimination factors [105]. If the isotopic turnover rates for either of the parasites examined in this study are significantly slower than those for either species of host fish, their respective stable isotope signals may be representative of different time scales and not always at equilibrium. This would be especially relevant if there is temporal variation in the diet of the host as the parasite would retain a residual isotopic signal of previous diets longer than the hosts’ blood. In the context of this study, such conditions might provide a reasonable explanation for the differences 13C discrimination between C. atromaculatus from the two host species if there is temporal variability in the diet of A. coeruleus or A. bahianus. For A. coeruleus blood, 13C values were consistent over time, indicating that these fish are either feeding on consistent food sources and/or their sources of carbon have consistent isotope values over time. It is possible that variations in food selection for A. bahianus may exist outside of this timescale, but this would require future studies to resolve. Thus, it is difficult to speculate on the possibility of shift in diet for the host fish occurring outside of the timescales examined in this study without knowing more about the isotopic turnover rates for A. coeruleus and A. bahianus and their associated parasites. There is a wide range of reported 13C and 15N turnover rates for fish blood [106,107,108,109], some exceeding the timescale of our study (June–August, 3 months total). To the best of our knowledge, turnover rates for either of our host species or acanthurids in general are unpublished. Furthermore, there do not appear to be any published data on turnover rates for copepods or monogeneans in general. In lieu of information on the species-specific discrimination rates we can speculate that given the short lifespan of Neobenedenia sp. [110] and the tight coupling between the A. coeruleus blood and monogenean isotope data that these parasites are recording the fish isotope composition over similar time scales. Controlled feeding experiments would be needed to resolve whether blood and monogeneans have similar turnover timescales. For C. atromaculatus, the life cycle of its congener, C. rogercresseyi, indicates that they may stay attached to the fish host up to 45 days [111], which may exceed typical turnover times for blood [106,107,108,109].
Trophic discrimination factors, Δ15N, associated with the monogeneans from A. coeruleus hosts were consistent with previous published values from monogenean and fish host- stable isotopes (Table 4). Monogeneans in both the present study and that of Sures et al. [72] were enriched in 15N (~2‰) relative to host tissue, consistent with 15N trophic discrimination between a consumer and its diet [53] (e.g., a single trophic step). The copepods collected from both A. coeruleus and A. bahianus were similarly enriched (albeit to a lesser extent) in 15N relative to blood from their respective host species, but their associated Δ15N discrimination factors fall below the threshold of what is typically expected for consumers. The overall trend for other species of parasitic copepods (including another species of Caligidae) [57] appears to be negative 15N discrimination with only few instances of positive discrimination relative to host blood (Table 4). This low discrimination of 15N is consistent with observations for gnathiid isopods [60,61]. More efficient digestion and assimilation of blood is associated with less fractionation, and thus, lower discrimination, of 15N compared to other host tissues [105].
Significant differences in the carbon stable isotope data between the copepods collected from A. coeruleus versus A. bahianus indicate that differences in host physiology may be playing a significant role in the turnover rates of 13C between hosts and parasites. Isotopically speaking, the blood of A. coeruleus and A. bahianus do not appear significantly different (Figure 2, Table S2), however, they are known to have markedly different feeding habits and digestive physiology [39]. It is well documented that differences in diet, condition, and metabolic physiology can have significant impacts on the isotopic turnover rates between an organism and its diet [105,112,113] which could in turn impact the isotopic turnover rates of associated parasites. However, much of the work on the topic has focused on consumers and not parasites. Given their unique physiology and feeding ecology, it is unclear whether parasites would behave similarly without additional studies. The handful of studies which have examined stable isotope turnover rates for a single species of parasite infecting multiple host species indicate that there are likely host specific traits influencing the rate of isotope discrimination between a parasite and its host [57,61]. As such, it is possible that physiological differences between the two host species may be contributing to the differences in parasite – host Δ13C between C. atromaculatus feeding on A. coeruleus versus A. bahianus and resolving physiological differences would require future studies.
One of the major challenges faced when trying to draw comparisons between parasite stable isotope studies is differences in tissues selected for studies (Table 4) due to the possibility that selected host tissues may not be an accurate representation of the parasites diet [56,57,60]. Blood was selected for this study because it is easy to collect from hosts with minimal loss of life and it is likely the primary food source for both species of parasites examined in this study [65,66,86,87]. This is not, however, definitive proof that these parasites were feeding on host blood which leaves open the possibility that the observed discrimination may be the result of a mismatch in actual isotopic values of the assumed dietary source (blood) and the true diet of the parasite (other host tissue). It should also be noted that comparisons between this study and others can be impacted by the difference in host tissues being analyzed and highlights the importance of careful consideration of which host tissues to analyze in parasite isotope studies [61]. We also note that while ethanol preservation effects on isotope values on crustaceans, including copepods, as well as soft bodied invertebrates, have been identified as negligible in other studies [60,114,115], future analysis examining the effects on monogeneans specifically should be conducted. Regardless, the emerging theme is that the driving forces behind the more complex patterns in stable isotope ecology associated with micropredators and parasites has been largely attributed to their complex life histories and feeding strategies [56,61] and the overall complexity of the biological and physiological factors which are known to influence the stable isotope ecology of organisms in general [112,113,116,117,118].
5. Conclusions
Parasite-host trophic fractionation patterns for both 13C and 15N were significantly different when comparing the two different types of parasites (i.e., copepods vs. monogeneans) collected from the same host species as well as when comparing the same species of parasites (C. atromaculatus) collected from the two different species of hosts (A. coeruleus vs. A. bahianus). Identifying the driving forces behind the wide array of isotope discrimination factors for parasites is a logical next step but requires significantly more scientific attention on the differences in the physiology and feeding ecology of these parasites and their hosts. Furthermore, identifying the specific tissues that are being selected by the parasites and examining how their unique metabolic processes influence isotopic patterns would advance our understanding of their stable isotope ecology. It is increasingly apparent that the stable isotope ecology of host-parasite systems does not always conform to conventional notions regarding the behavior of stable isotopes in ecological systems and these findings contribute to a growing realization that parasite stable isotope ecology requires significantly more scientific attention. This is particularly important given the increasing pressure from parasite minded ecologists to restructure the existing food web models in order to accurately account for parasites [15,16,23,24].
Given the high biomass and diversity of herbivorous reef fishes that includes wide variation in diet, habitat association, behavior, and parasite communities, understanding the role of parasites in their trophic ecology will require studies involving multiple species and localities, as well as analysis of multiple host tissues for stable isotope analysis. In addition, while there was no apparent temporal shift in host blood stable isotopes for either of our host fish, future studies would benefit from a more thorough understanding of the temporal feeding habits of hosts as well as species specific discrimination rates for both hosts and parasites. Future isotope studies of parasites would also benefit from species-specific comparisons of the effects of preservation methods. The demonstrated utility of isotope analysis of preserved and archived specimens may also allow for expanding the inclusion of multiple parasite species and hosts. We hope our findings help stimulate such studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/12/11/429/s1, Table S1: Summary of sampling design, Table S2: Pairwise comparisons of model estimated means, Table S3: Model estimated 95% confidence intervals, Figure S1: Distribution of parasite isotope values relative to host blood for Neobenedenia sp. collected from the same host fish. All data presented herein are available at https://doi.org/10.5066/P9QE4FW6.
Author Contributions
Conceptualization, P.C.S., A.W.J.D., and W.G.J.; methodology, P.C.S., A.W.J.D., and W.G.J.; software, W.G.J.; validation, W.G.J. and A.W.J.D.; formal analysis, W.G.J.; investigation, P.C.S., W.G.J., and M.D.N.; resources, P.C.S., A.W.J.D., W.G.J., and M.D.N.; data curation, W.G.J., A.W.J.D.; writing—original draft preparation, W.G.J. and P.C.S.; writing—review and editing, A.W.J.D., P.C.S., and M.D.N.; visualization, W.G.J. and M.D.N.; supervision, A.W.J.D. and P.C.S.; project administration, A.W.J.D. and P.C.S.; funding acquisition, P.C.S. and A.W.J.D.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the US National Science Foundation, grant number OCE-1536794 (P.C.S., PI) and by the USGS Environments Program.
Acknowledgments
We would like to thank members of the Sikkel lab at Arkansas State University, the Benthic Ecology lab of the USGS Wetland and Aquatic Research Center, the University of the Virgin Islands Center for Marine and Environmental Studies, and the staff of the Virgin Islands Environmental Resource Station (VIERS) for logistical support and use of their facilities. We would also like to thank Dyke Andreasen and the UC Santa Cruz Stable Isotope Laboratory for their assistance with applying novel NanoEA technology to this study. This is contribution number 224 from the University of the Virgin Islands Center for Marine and Environmental Studies. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reaka-Kudla, M.L. The global biodiversity of coral reefs: a comparison with rain forests. In Biodiversity II: Understanding and Protecting Our Biological Resources; Reaka-Kudla, M.L., Wilson, D.E., Wilson, E.O., Eds.; Joseph Henry Press: Washington, DC, USA, 1997; pp. 83–108. ISBN 0309052270. [Google Scholar]
- Roberts, C.; McClean, C.; Vernon, J.; Hawkins, J.; Allen, G.; McAllister, D.; Mittermeier, C.; Schueler, F.; Spalding, M.; Wells, F.; et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science (80-) 2002, 295, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, N.; Brainard, R.E.; Fisher, R.; Moews, M.; Plaisance, L.; Caley, M.J. Coral reef biodiversity. In Life in the World’s Oceans; McIntyre, A.D., Ed.; John Wiley & Sons: West Sussex, UK, 2010. [Google Scholar]
- Dornelas, M.; Connolly, S.R.; Hughes, T.P. Coral reef diversity refutes the neutral theory of biodiversity. Nature 2006, 440, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Bellwood, D.R.; Streit, R.P.; Brandl, S.J.; Tebbett, S.B. The meaning of the term ‘function’ in ecology: a coral reef perspective. Funct. Ecol. 2019, 33, 948–961. [Google Scholar] [CrossRef]
- Brandl, S.J.; Rasher, D.B.; Côté, I.M.; Casey, J.M.; Darling, E.S.; Lefcheck, J.S.; Duffy, J.E. Coral reef ecosystem functioning: eight core processes and the role of biodiversity. Front. Ecol. Environ. 2019, 17, 445–454. [Google Scholar] [CrossRef]
- Hudson, P.J.; Dobson, A.P.; Lafferty, K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006, 21, 381–385. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Dobson, A.P.; Kuris, A.M. Parasites dominate food web links. Proc. Natl. Acad. Sci. USA 2006, 103, 11211–11216. [Google Scholar] [CrossRef]
- Wood, C.L.; Byers, J.E.; Cottingham, K.L.; Altman, I.; Donahue, M.J.; Blakeslee, A.M.H. Parasites alter community structure. Proc. Natl. Acad. Sci. USA 2007, 104, 9335–9339. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Dobson, A.; Lafferty, K.D.; Marcogliese, D.J.; Memmott, J.; Orlofske, S.A.; Poulin, R.; Thieltges, D.W. When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol. Evol. 2010, 25, 362–371. [Google Scholar] [CrossRef]
- Rohde, K. Latitudinal gradients in species diversity: the search for the primary cause. Oikos 1992, 65, 514–527. [Google Scholar] [CrossRef]
- Rohde, K. Latitudinal gradients in species diversity and rapoport’s rule revisited: a review of recent work and what can parasites teach us about the causes of the gradients? Ecography (Cop.) 1999, 22, 593–613. [Google Scholar] [CrossRef]
- Poulin, R.; Morand, S. The diversity of parasites. Q. Rev. Biol. 2000, 75, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Grutter, A.S.; Cribb, T.H. Structure of the parasite communities of a coral reef fish assemblage (Labridae): testing ecological and phylogenetic factors. J. Parasitol. 2007, 93, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K.D.; Allesina, S.; Arim, M.; Briggs, C.J.; De Leo, G.; Dobson, A.P.; Dunne, J.A.; Johnson, P.T.J.; Kuris, A.M.; Marcogliese, D.J.; et al. Parasites in food webs: the ultimate missing links. Ecol. Lett. 2008, 11, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Marcogliese, D.J.; Cone, D.K. Food webs: A plea for parasites. Trends Ecol. Evol. 1997, 12, 320–325. [Google Scholar] [CrossRef]
- Jephcott, T.G.; Sime-Ngando, T.; Gleason, F.H.; Macarthur, D.J. Host-parasite interactions in food webs: diversity, stability, and coevolution. Food Webs 2016, 6, 1–8. [Google Scholar] [CrossRef]
- De Meeûs, T.; Renaud, F. Parasites within the new phylogeny of eukaryotes. Trends Parasitol. 2002, 18, 247–250. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Kuris, A.M. Trophic strategies, animal diversity and body size. Trends Ecol. Evol. 2002, 17, 507–513. [Google Scholar] [CrossRef]
- Poulin, R. Evolutionary Ecology of Parasites; Princeton University Press: Princeton, NJ, USA, 2011; ISBN 9781400840809. [Google Scholar]
- Kuris, A.M.; Hechinger, R.F.; Shaw, J.C.; Whitney, K.L.; Aguirre-Macedo, L.; Boch, C.A.; Dobson, A.P.; Dunham, E.J.; Fredensborg, B.L.; Huspeni, T.C.; et al. Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 2008, 454, 515–518. [Google Scholar] [CrossRef]
- Preston, D.L.; Orlofske, S.A.; Lambden, J.P.; Johnson, P.T.J. Biomass and productivity of trematode parasites in pond ecosystems. J. Anim. Ecol. 2013, 82, 509–517. [Google Scholar] [CrossRef]
- Byers, J.E. Including parasites in food webs. Trends Parasitol. 2009, 25, 55–57. [Google Scholar] [CrossRef]
- Sukhdeo, M.V.K. Food Webs for parasitologists: A Review. J. Parasitol. 2010, 96, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, P.A.; Lafferty, K.D.; Knudsen, R.; Primicerio, R.; Klemetsen, A.; Kuris, A.M. Food web topology and parasites in the pelagic zone of a subarctic lake. J. Anim. Ecol. 2009, 78, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.D.; Sukhdeo, M.V.K. Parasites alter the topology of a stream food web across seasons. Oecologia 2008, 156, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Dunne, J.A.; Lafferty, K.D.; Dobson, A.P.; Hechinger, R.F.; Kuris, A.M.; Martinez, N.D.; McLaughlin, J.P.; Mouritsen, K.N.; Poulin, R.; Reise, K.; et al. Parasites affect food web structure primarily through increased diversity and complexity. PLoS Biol. 2013, 11, e1001579. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Kimo Morris, A. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 1996, 77, 1390–1397. [Google Scholar] [CrossRef]
- Hay, M.E. Fish-seaweed interaction on coral reefs: effects of herbivorous fishes and adaptation of their prey. In The Ecology of Fishes on Coral Reef; Academic Press: San Diego, CA, USA, 1991; pp. 96–119. [Google Scholar]
- Choat, J.H.; Clements, K.D.; Robbins, W.D. The trophic status of herbivorous fishes on coral reefs. Mar. Biol. 2002, 140, 613–623. [Google Scholar] [CrossRef]
- Hixon, M.A. 60 Years of coral reef fish ecology: Past, present, future. Bull. Mar. Sci. 2011, 87, 727–765. [Google Scholar] [CrossRef]
- Lobel, P.S. Herbivory by damselfishes and their role in coral reef community ecology. Bull. Mar. Sci. 1980, 30, 273–289. [Google Scholar]
- Polunin, N.V.C.; Klumpp, D.W. Algal food supply and grazer demand in a very productive coral-reef zone. J. Exp. Mar. Bio. Ecol. 1992, 164, 1–15. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Bellwood, D.R. Biologically mediated sediment fluxes on coral reefs: sediment removal and off-reef transportation by the surgeonfish Ctenochaetus striatus. Mar. Ecol. Prog. Ser. 2010, 415, 237–245. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Goatley, C.H.R.; Brandl, S.J.; Bellwood, O. Fifty million years of herbivory on coral reefs: fossils, fish and functional innovations. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133046. [Google Scholar] [CrossRef] [PubMed]
- Marshell, A.; Mumby, P.J. The role of surgeonfish (Acanthuridae) in maintaining algal turf biomass on coral reefs. J. Exp. Mar. Bio. Ecol. 2015, 473, 152–160. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Goatley, C.H.R.; Bellwood, D.R. Clarifying functional roles: algal removal by the surgeonfishes Ctenochaetus striatus and Acanthurus nigrofuscus. Coral Reefs 2017, 36, 803–813. [Google Scholar] [CrossRef]
- Randall, J.E. Surgeonfishes of the World; Bishop Museum Press: Honolulu, HI, USA, 2001; ISBN 1566475619. [Google Scholar]
- Tilghman, G.C.; Klinger-Bowen, R.E.; Francis-Floyd, R. Feeding electivity indices in surgeonfish (Acanthuridae) of the Florida keys. Aquarium Sci. Conserv. 2001, 3, 215–223. [Google Scholar] [CrossRef]
- Miyake, S.; Ngugi, D.K.; Stingl, U. Diet strongly influences the gut microbiota of surgeonfishes. Mol. Ecol. 2015, 24, 656–672. [Google Scholar] [CrossRef]
- Duran, A.; Adam, T.C.; Palma, L.; Moreno, S.; Collado-Vides, L.; Burkepile, D.E. Feeding behavior in Caribbean surgeonfishes varies across fish size, algal abundance, and habitat characteristics. Mar. Ecol. 2019, 40. [Google Scholar] [CrossRef]
- Lo, C.M.; Morgan, J.A.T.; Galzin, R.; Cribb, T.H. Identical digeneans in coral reef fishes from French Polynesia and the Great Barrier Reef (Australia) demonstrated by morphology and molecules. Int. J. Parasitol. 2001, 31, 1573–1578. [Google Scholar] [CrossRef]
- Sikkel, P.C.; Nemeth, D.; McCammon, A.; Williams, E.H., Jr. Habitat and species differences in prevalence and intensity of Neobenedenia melleni (Monogenea: Capsalidae) on sympatric Caribbean surgeonfishes (Acanthuridae). J. Parasitol. 2009, 95, 63–68. [Google Scholar] [CrossRef]
- Bernal, M.A.; Floeter, S.R.; Gaither, M.R.; Longo, G.O.; Morais, R.; Ferreira, C.E.L.; Vermeij, M.J.A.; Rocha, L.A. High prevalence of dermal parasites among coral reef fishes of Curaçao. Mar. Biodivers. 2016, 46, 67–74. [Google Scholar] [CrossRef]
- Santos, T.R.N.; Sikkel, P.C. Habitat associations of fish-parasitic gnathiid isopods in a shallow reef system in the central Philippines. Mar. Biodivers. 2017, 49, 83–96. [Google Scholar] [CrossRef]
- Bshary, R.; Grutter, A.S. Parasite distribution on client reef fish determines cleaner fish foraging patterns. Mar. Ecol. Prog. Ser. 2002, 235, 217–222. [Google Scholar] [CrossRef]
- McCammon, A.; Sikkel, P.C.; Nemeth, D. Effects of three Caribbean cleaner shrimps on ectoparasitic monogeneans in a semi-natural environment. Coral Reefs 2010, 29, 419–426. [Google Scholar] [CrossRef]
- Huebner, L.K.; Chadwick, N.E. Patterns of cleaning behaviour on coral reef fish by the anemoneshrimp Ancylomenes pedersoni. J. Mar. Biol. Assoc. UK 2012, 92, 1557–1562. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Boecklen, W.J.; Yarnes, C.T.; Cook, B.A.; James, A.C. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 411–440. [Google Scholar] [CrossRef]
- Deniro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Minagawa, M.; Wada, E. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 1984, 48, 1135–1140. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: model, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Hatcher, M.J.; Dunn, A.M. Parasites in Ecological Communities: From Interactions to Ecosystems; Cambridge University Press: Cambridge, UK, 2011; ISBN 9780511987359. [Google Scholar]
- Pinnegar, J.K.; Campbell, N.; Polunin, N.V.C. Unusual stable isotope fractionation patterns observed for fish host-parasite trophic relationships. J. Fish Biol. 2001, 59, 494–503. [Google Scholar] [CrossRef]
- Deudero, S.; Pinnegar, J.K.; Polunin, N.V.C. Insights into fish host-parasite trophic relationships revealed by stable isotope analysis. Dis. Aquat. Organ. 2002, 52, 77–86. [Google Scholar] [CrossRef]
- Voigt, C.C.; Kelm, D.H. Host preferences of bat flies: following the bloody path of stable isotopes in a host–parasite food chain. Can. J. Zool. 2006, 84, 397–403. [Google Scholar] [CrossRef]
- Schmidt, O.; Dautel, H.; Newton, J.; Gray, J.S. Natural isotope signatures of host blood are replicated in moulted ticks. Ticks Tick. Borne. Dis. 2011, 2, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Demopoulos, A.W.J.; Sikkel, P.C. Enhanced understanding of ectoparasite-host trophic linkages on coral reefs through stable isotope analysis. Int. J. Parasitol. 2015, 4, 125–134. [Google Scholar] [CrossRef]
- Jenkins, W.G.; Demopoulos, A.W.J.; Sikkel, P.C. Host feeding ecology and trophic position significantly influence isotopic discrimination between a generalist ectoparasite and its hosts: implications for parasite-host trophic studies. Food Webs 2018, 16. [Google Scholar] [CrossRef]
- Thieltges, D.W.; Goedknegt, M.A.; O’Dwyer, K.; Senior, A.M.; Kamiya, T. Parasites and stable isotopes: a comparative analysis of isotopic discrimination in parasitic trophic interactions. Oikos 2019, 128, 1329–1339. [Google Scholar] [CrossRef]
- Gilbert, B.M.; Nachev, M.; Jochmann, M.A.; Schmidt, T.C.; Köster, D.; Sures, B.; Avenant-Oldewage, A. You are how you eat: differences in trophic position of two parasite species infecting a single host according to stable isotopes. Parasitol. Res. 2020, 119, 1393–1400. [Google Scholar] [CrossRef]
- Weston, M.J. Investigating trophic interactions between parasites and their marine fish hosts using stable isotope analysis. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2017. [Google Scholar]
- Whittington, I.D. Diversity “down under”: monogeneans in the Antipodes (Australia) with a prediction of monogenean biodiversity worldwide. Int. J. Parasitol. 1998, 28, 1481–1493. [Google Scholar] [CrossRef]
- Whittington, I.D. The capsalidae (Monogenea: Monopisthocotylea): A review of diversity, classification and phylogeny with a note about species complexes. Folia Parasitologica 2004, 51, 109–122. [Google Scholar] [CrossRef]
- Montero, F.E.; Crespo, S.; Padrós, F.; De La Gándara, F.; García, A.; Raga, J.A. Effects of the gill parasite Zeuxapta seriolae (Monogenea: Heteraxinidae) on the amberjack Seriola dumerili Risso (Teleostei: Carangidae). Aquaculture 2004, 232, 153–163. [Google Scholar] [CrossRef]
- Thoney, D.A.; Hargis, W.J. Monogenea (platyhelminthes) as hazards for fish in confinement. Annu. Rev. Fish Dis. 1991, 1, 133–153. [Google Scholar] [CrossRef]
- Cowell, L.E.; Watanabe, W.O.; Head, W.D.; Grover, J.J.; Shenker, J.M. Use of tropical cleaner fish to control the ectoparasite Neobenedenia melleni (Monogenea: Capsalidae) on seawater-cultured Florida red tilapia. Aquaculture 1993, 113, 189–200. [Google Scholar] [CrossRef]
- Grutter, A.S.; Deveney, M.R.; Whittington, I.D.; Lester, R.J.G. The effect of the cleaner fish Labroides dimidiatus on the capsalid monogenean Benedenia lolo parasite of the labrid fish Hemigymnus melapterus. J. Fish Biol. 2002, 61, 1098–1108. [Google Scholar] [CrossRef]
- Becker, J.H.; Grutter, A.S. Cleaner shrimp do clean. Coral Reefs 2004, 23, 515–520. [Google Scholar] [CrossRef]
- Sures, B.; Nachev, M.; Gilbert, B.M.; Dos Santos, Q.M.; Jochmann, M.A.; Köster, D.; Schmidt, T.C.; Avenant-Oldewage, A. The monogenean Paradiplozoon ichthyoxanthon behaves like a micropredator on two of its hosts, as indicated by stable isotopes. J. Helminthol. 2019, 93, 71–75. [Google Scholar] [CrossRef]
- Avenant-Oldewage, A.; Le Roux, L.E.; Mashego, S.N.; Van Vuuren, B.J. Paradiplozoon ichthyoxanthon n. sp. (Monogenea: Diplozoidae) from Labeobarbus aeneus (Cyprinidae) in the Vaal River, South Africa. J. Helminthol. 2014, 88, 166–172. [Google Scholar] [CrossRef]
- Humes, A.G. How many copepods? In Ecology and Morphology of Copepods; Ferrari, F.D., Bradley, B.P., Eds.; Springer: Dordrecht, Netherlands, 1994; pp. 1–7. [Google Scholar]
- Sikkel, P.C.; Welicky, R.L. The Ecological Significance of Parasitic Crustaceans. In Parasitic Crustacea. Zoological Monographs, vol 3; Smit, N., Bruce, N., Hadfield, K., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 421–477. [Google Scholar]
- Gilbert, B.M.; Nachev, M.; Jochmann, M.A.; Schmidt, T.C.; Köster, D.; Sures, B.; Avenant-Oldewage, A. Stable isotope analysis spills the beans about spatial variance in trophic structure in a fish host – parasite system from the Vaal River System, South Africa. Int. J. Parasitol. Parasites Wildl. 2020, 12, 134–141. [Google Scholar] [CrossRef]
- Whittington, I.D.; Horton, M.A. A revision of neobenedenia yamaguti, 1963 (Monogenea: Capsalidae) including a redescription of N. melleni (maccallum, 1927) yamaguti, 1963. J. Nat. Hist. 1996, 30, 1113–1156. [Google Scholar] [CrossRef]
- Hirazawa, N.; Mitsuboshi, T.; Hirata, T.; Shirasu, K. Susceptibility of spotted halibut Verasper variegatus (Pleuronectidae) to infection by the monogenean Neobenedenia girellae (Capsalidae) and oral therapy trials using praziquantel. Aquaculture 2004, 238, 83–95. [Google Scholar] [CrossRef]
- Hirazawa, N.; Takano, R.; Hagiwara, H.; Noguchi, M.; Narita, M. The influence of different water temperatures on Neobenedenia girellae (Monogenea) infection, parasite growth, egg production and emerging second generation on amberjack Seriola dumerili (Carangidae) and the histopathological effect of this parasite on fish skin. Aquaculture 2010, 299, 2–7. [Google Scholar] [CrossRef]
- Dyer, W.G.; Williams, E.H.; Bunkley-Williams, L. Neobenedenia pargueraensis n. sp. (Monogenea: Capsalidae) from the Red Hind, Epinephelus guttatus, and comments about Neobenedenia melleni. J. Parasitol. 1992, 78, 399. [Google Scholar] [CrossRef]
- Bullard, S.; Benz, G.; Overstreet, R.; Williams, E.; Hemdal, J. Six new host records and an updated list of wild hosts for Neobenedenia melleni (MacCallum) (Monogenea: Capsalidae). Comp. Parasitol. 2000, 67, 190–196. [Google Scholar]
- Loerch, S.M.; McCammon, A.M.; Sikkel, P.C. Low susceptibility of invasive Indo-Pacific lionfish Pterois volitans to ectoparasitic Neobenedenia in the eastern Caribbean. Environ. Biol. Fishes 2015, 98, 1979–1985. [Google Scholar] [CrossRef]
- Cressey, R.F. Parasitic copepods from the Gulf of Mexico and Caribbean Sea, III: Caligus. Smithson. Contrib. Zool. 1991, 1–53. [Google Scholar] [CrossRef]
- Dojiri, M.; Ho, J.S. Systematics of the Caligidae, Copepods Parasitic on Marine Fishes; Brill Academic Publishers: Leiden, Netherlands, 2013; Volume 18, ISBN 9789004204249. [Google Scholar]
- Morales-Serna, F.N.; Medina-Guerrero, R.M.; Fajer-Avila, E.J. Sea lice (Copepoda: Caligidae) parasitic on fishes reported from the Neotropical region. Neotrop. Biodivers. 2016, 2, 141–150. [Google Scholar] [CrossRef]
- Johnson, S.C.; Treasurer, J.W.; Bravo, S.; Nagasawa, K.; Kabata, Z. A Review of the impact of parasitic copepods on marine aquaculture. Zool. Stud. 2004, 43, 229–243. [Google Scholar]
- Gonçalves, A.T.; Farlora, R.; Gallardo-Escárate, C. Transcriptome survey of the lipid metabolic pathways involved in energy production and ecdysteroid synthesis in the salmon louse Caligus rogercresseyi (Crustacea: Copepoda). Comp. Biochem. Physiol. Part B 2014, 176, 9–17. [Google Scholar] [CrossRef]
- Rocha, L.A.; Bass, A.L.; Robertson, D.R.; Bowen, B.W. Adult habitat preferences, larval dispersal, and the comparative phylogeography of three Atlantic surgeonfishes (Teleostei: Acanthuridae). Mol. Ecol. 2002, 11, 243–251. [Google Scholar] [CrossRef]
- Hobson, K.A.; Gloutney, M.L.; Gibbs, H.L. Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis. Can. J. Zool. 1997, 75, 1720–1723. [Google Scholar] [CrossRef]
- Syväranta, J.; Vesala, S.; Rask, M.; Ruuhijärvi, J.; Jones, R.I. Evaluating the utility of stable isotope analyses of archived freshwater sample materials. Hydrobiologia 2008, 600, 121–130. [Google Scholar] [CrossRef]
- Gloutney, M.L.; Hobson, K.A. Field preservation techniques for the analysis of stable-carbon and nitrogen isotope ratios in eggs. J. Field Ornithol. 1998, 69, 223–227. [Google Scholar]
- Demopoulos, A.W.J.; Gualtieri, D.; Kovacs, K. Food-web structure of seep sediment macrobenthos from the Gulf of Mexico. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 1972–1981. [Google Scholar] [CrossRef]
- Polissar, P.J.; Fulton, J.M.; Junium, C.K.; Turich, C.C.; Freeman, K.H. Measurement of 13 C and 15 N isotopic composition on nanomolar quantities of C and N. Anal. Chem. 2009, 81, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using {lme4}. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 August 2020).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd edition; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 15 August 2020).
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 15 August 2020).
- Fanelli, E.; Cartes, J.E.; Rumolo, P.; Sprovieri, M. Food-web structure and trophodynamics of mesopelagic-suprabenthic bathyal macrofauna of the Algerian Basin based on stable isotopes of carbon and nitrogen. Deep. Res. Part I Oceanogr. Res. Pap. 2009, 56, 1504–1520. [Google Scholar] [CrossRef]
- Welicky, R.L.; Demopoulos, A.W.J.; Sikkel, P.C. Host-dependent differences in resource use associated with Anilocra spp. parasitism in two coral reef fishes, as revealed by stable carbon and nitrogen isotope analyses. Mar. Ecol. 2017, 38. [Google Scholar] [CrossRef]
- Stapp, P.; Salkeld, D.J. Inferring host-parasite relationships using stable isotopes: implications for disease transmission and host specificity. Ecology 2009, 90, 3268–3273. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Carter, W.A.; Bauchinger, U.; McWilliams, S.R. The importance of isotopic turnover for understanding key aspects of animal ecology and nutrition. Diversity 2019, 11, 84. [Google Scholar] [CrossRef]
- Buchheister, A.; Latour, R.J. Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Can. J. Fish. Aquat. Sci. 2010, 67, 445–461. [Google Scholar] [CrossRef]
- Yohannes, E.; Grimm, C.; Rothhaupt, K.-O.; Behrmann-Godel, J. The effect of parasite infection on stable isotope turnover rates of δ15n, δ13c and δ34s in multiple tissues of eurasian perch perca fluviatilis. PLoS ONE 2017, 12, e0169058. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, P.B.; Flecker, A.S. Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Oecologia 2006, 148, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Matley, J.K.; Fisk, A.T.; Tobin, A.J.; Heupel, M.R.; Simpfendorfer, C.A. Diet-tissue discrimination factors and turnover of carbon and nitrogen stable isotopes in tissues of an adult predatory coral reef fish, Plectropomus leopardus. Rapid Commun. Mass Spectrom. 2016, 30, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Brazenor, A.K.; Hutson, K.S. Effects of temperature and salinity on the life cycle of Neobenedenia sp. (Monogenea: Capsalidae) infecting farmed barramundi (Lates calcarifer). Parasitol. Res. 2015, 114, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Carvajal, J. Life cycle of Caligus rogercresseyi, (Copepoda: Caligidae) parasite of Chilean reared salmonids. Aquaculture 2003, 220, 101–117. [Google Scholar] [CrossRef]
- Hobson, K.A.; Alisauskas, R.T.; Clark, R.G. Stable-nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress: implications for isotopic analyses of diet. Condor 1993, 95, 394. [Google Scholar] [CrossRef]
- Hobson, K.A.; Clark, R.G. Assessing avian diets using sable isotopes II: factors influencing diet-tissue fractionation. Condor 1992, 94, 189–197. [Google Scholar] [CrossRef]
- Sarakinos, H.C.; Johnson, M.L.; Vander Zanden, M.J. A synthesis of tissue-preservation effects on carbon and nitrogen stable isotope signatures. Can. J. Zool. 2002, 80, 381–387. [Google Scholar] [CrossRef]
- Chouvelon, T.; Chappuis, A.; Bustamante, P.; Lefebvre, S.; Mornet, F.; Guillou, G.; Violamer, L.; Dupuy, C. Trophic ecology of European sardine Sardina pilchardus and European anchovy Engraulis encrasicolus in the Bay of Biscay (north-east Atlantic) inferred from δ13C and δ15N values of fish and identified mesozooplanktonic organisms. J. Sea Res. 2014, 85, 277–291. [Google Scholar] [CrossRef]
- Krueger, H.W.; Sullivan, C.H. Models for carbon isotope fractionation between diet and bone. In Stable Isotopes in Nutrition; Turnlund, J.R., Johnson, P.E., Eds.; American Chemical Society: Washington, D.C., USA, 1984; pp. 205–220. [Google Scholar]
- Vanderklift, M.A.; Ponsard, S. Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 2003, 136, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.T.; Felicetti, L.A.; Sponheimer, M. The effect of dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecologia 2005, 144, 534–540. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).