Effects of Environmental Traits and Landscape Management on the Biodiversity of Saproxylic Beetles in Mediterranean Oak Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
Description of Sampling Plots
2.2. Field and Laboratory Work
2.3. Data Analysis
3. Results
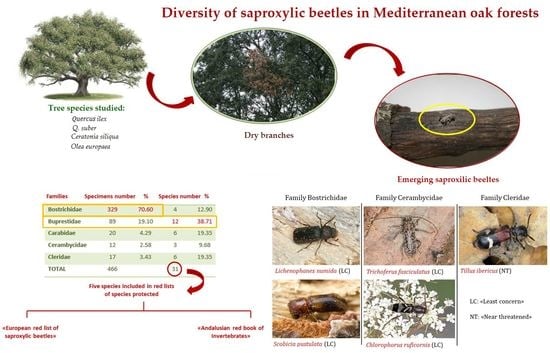
3.1. Faunistic Results
3.2. Saproxylic Fauna and Host Tree
3.3. Saproxylic Fauna and Environmental Features
3.4. Effect of the Environmental Temperature on the Chronology of Adult Emergencies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Campos, P.; Huntsinger, L.; Oviedo, J.L.; Starrs, P.F.; Diaz, M.; Standiford, R.B.; Montero, G. Mediterranean Oak Woodland Working Landscapes; Landscapes Series; Springer: Dordrecht, The Netherlands, 2013; pp. 61–89. [Google Scholar]
- Bugalho, M.N.; Caldeira, M.; Pereira, J.; Aronson, J.; Pausas, J. Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front. Ecol. Environ. 2011, 9, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Hernández, A.; Micó, E.; Galante, E. Temporal variation in saproxylic beetle assemblages in a Mediterranean ecosystem. J. Insect Conserv. 2014, 18, 993–1007. [Google Scholar] [CrossRef]
- Stokland, J.N.; Siitonen, J.; Jonsson, B.G. Biodiversity in Dead Wood. Ecology, Biodiversity, and Conservation; Cambridge University Press: Cambridge, UK, 2012; pp. 218–269. [Google Scholar]
- Davies, Z.G.; Tyler, C.; Stewart, G.B.; Pullin, A.S. Are current management recommendations for saproxylic invertebrates effective? A systematic review. Biodivers. Conserv. 2008, 17, 209–234. [Google Scholar] [CrossRef] [Green Version]
- Quinto, J.; Micó, E.; Martínez-Falcón, A.P.; Galante, E.; Marcos-García, M.A. Influence if tree hollow characteristics on the diversity of saproxylic insect guilds in Iberian Mediterranean woodlands. J. Insect Conserv. 2014, 18, 981–992. [Google Scholar] [CrossRef]
- Pérez-Moreno, I. Nuevas aportaciones al conocimiento de la fauna de Coleópteros saproxílicos (Coleoptera) del Sistema Ibérico Septentrional, I. Robledales del Valle Medio del Iregua (Sierra de Cameros, La Rioja, España). Bol. S.E.A. 2010, 46, 321–334. [Google Scholar]
- Horák, J.; Chobot, K.; Chumanová, E. Habitat preferences influencing populations, distribution, and conservation of the endangered saproxylic beetle Cucujus cinnaberinus (Coleoptera: Cucujidae) at the landscape level. Eur. J. Entomol. 2010, 107, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Horák, J.; Chumanová, E.; Hilszczaoski, J. Saproxylic beetle thrives on the openness in management: A case study on the ecological requirements of Cucujus cinnaberinus from Central Europe. Insect Conserv. Divers. 2011, 5, 403–413. [Google Scholar] [CrossRef]
- Nieto, A.; Alexander, K.N.A. European Red List of Saproxylic Beetles; Publications Office of the European Union: Luxembourg, 2010; p. 46. [Google Scholar]
- Radenkovid, S. The saproxylic hoverflies (Diptera: Syrphidae) of Serbia. J. Nat. Hist. 2013, 47, 87–127. [Google Scholar] [CrossRef]
- Cavalli, R.; Mason, F. Techniques for Reestablishment of Dead Wood for Saproxylic Fauna Conservation; LIFE nature project NAT/IT/99/6245 Bosco della Fontana (Mantova, Italy); Gianluigi Arcari Editore: Mantova, Italy, 2003; p. 109. [Google Scholar]
- Gallardo, P.; Cárdenas, A.M. Long term monitoring of saproxylic beetles from Mediterranean oak forests: An approach to the larval biology of the most representative species. J. Insect Conserv. 2016, 20, 999–1009. [Google Scholar] [CrossRef]
- DEFRA (Department for Environment, Food and Rural Affairs). Natural Environment, Adapting to Climate Change; Department for Environment, Food and Rural Affairs: London, UK, 2010; p. 51.
- Maresi, G.; Salvadori, C. Crown conditions and damages in two forest ecosystems in Trentino (Italy), Studi Trent. Sci. Nat. Acta Biol. 2005, 81, 253–260. [Google Scholar]
- Pardos, J.A. La contaminación atmosférica y los ecosistemas forestales. Forest Syst. 2006, 15, 55–70. [Google Scholar]
- Freer-Smith, P.H.; Broadmeadow, M.S.J.; Lynch, J.M. Forest, and climate change: The knowledgebase for action. In Forestry and Climate Change; Freer-Smith, P.H., Broadmeadow, M.S.J., Lynch, J.M., Eds.; CAB International: Wallingford, UK, 2007. [Google Scholar]
- Benito-Garzón, M.; Dios, R.S.; Sainz-Ollero, H. Effects of climate change on the distribution of Iberian tree species. Appl. Veg. Sci. 2008, 11, 1–10. [Google Scholar] [CrossRef]
- Myers, S.W.; Fraser, I.; Mastro, V.C. Evaluation of heat treatment schedules for emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2009, 102, 2048–2055. [Google Scholar] [CrossRef]
- Sobek, S.; Rajamohan, A.; Dillon, D.; Cumming, R.C.; Sinclair, B.J. High temperature tolerance and thermal plasticity in emerald ash borer Agrilus Planipennis Agr. Forest Entomol. 2011, 13, 333–340. [Google Scholar] [CrossRef]
- Cárdenas, A.; Gallardo, P. The effect of temperature on the preimaginal development of the Jewel beetle Coraebus florentinus (Coleoptera: Buprestidae). Eur. J. Entomol. 2012, 109, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, A.M.; Gallardo, P. The effects of oviposition on the development of the wood borer Coraebus florentinus (Coleoptera: Buprestidae). Eur. J. Entomol. 2013, 110, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Mushrow, L.; Morrison, A.; Sweeney, J.; Quiring, D. Heat as a phytosanitary treatment for the brown spruce longhorn beetle. Forest. Chron. 2004, 80, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Bains, S.S. Effect of temperature and moisture on the biology of Rhyzopertha dominica Fabricius (Bostrichidae: Coleoptera). Bull. Grain Technol. 1971, 9, 257–264. [Google Scholar]
- Ostaff, D.P.; Cech, M.Y. Heat Sterilization of Spruce-Pine-Fir Lumber Containing Pine Sawyer Beetle Larvae (Coleoptera: Cerambycid monochamus); Rep. OPX-200E; Canadian Forest Service: Ottawa, ON, Canada, 1978; p. 15.
- Faroni, L.R.A.; García-Mari, F. Influencia de la temperatura sobre los parámetros biológicos de Rhyzopertha dominica (F.). Bol. San. Veg. Plagas 1992, 18, 455–467. [Google Scholar]
- Cárdenas, A.M.; Gallardo, P. Assessment and monitoring damage by Coraebus florentinus (Coleoptera: Buprestidae) in Mediterranean oak forests. Open J. Ecol. 2018, 8, 324–338. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Mu, X.; Ruan, G.; Gao, Z.; Li, L.; Yan, G. Estimating fractional vegetation cover and the vegetation index of bare soil and highly dense vegetation with a physically based method. Int. J. Appl. Earth Obs. 2017, 58, 168–176. [Google Scholar] [CrossRef]
- Henderson, P.A. Practical Methods in Ecology; Blackwell Publishing: Cambridge, UK, 2003; p. 15. [Google Scholar]
- Bahillo de la Puebla, P.; López-Colón, J.I. Cléridos de Andalucía (Coleoptera, Cleridae); Delegación de Cultura del Excmo. Ayuntamiento de Utrera; Fundación El Monte y Sociedad Andaluza de Entomología: Utrera, Sevilla, 2001; p. 71. [Google Scholar]
- de la Puebla, P.B.; López-Colón, J.I.; García-París, M. Una especie nueva de Tillus Olivier, 1790 (Coleoptera, Cleridae) de la Península Ibérica. Graellsia 2003, 59, 57–62. [Google Scholar]
- Cobos, A. Fauna Ibérica de coleópteros Buprestidae; CSIC: Madrid, Spain, 1986; p. 364. [Google Scholar]
- Verdugo, A. Fauna de Buprestidae de la Península Ibérica y Baleares; Argania Editio: Barcelona, Spain, 2005; p. 350. [Google Scholar]
- Ortuño, V.M.; Toribio, M. Carabidae de la Península Ibérica y Baleares; Volume I. Trechinae, Bembidiini; Argania Editio: Barcelona, Spain, 2005; p. 455. [Google Scholar]
- Villiers, A. Faune des Coléoptères de France. I Cerambycidae; Editions Lechevalier: Paris, France, 1978; p. 611. [Google Scholar]
- Vives, E. Coleoptera Cerambycidae; Fauna Ibérica; Volume 12; CSIC: Madrid, Spain, 2000; p. 716. [Google Scholar]
- Bahillo de la Puebla, P.; López-Colón, J.I.; Baena, M. Los Bostrichidae Latreille 1802 de la fauna íbero-balear (Coleoptera). Heteropterus Rev. Entomol. 2007, 7, 147–227. [Google Scholar]
- Rochowicz, J.A. Bootstrapping analysis, inferential statistics, and EXCEL. Spreadsheets Education (eJSiE) 2011, 4, 1–23. [Google Scholar]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science Ltd.: Cambrige, UK, 2000; p. 592. [Google Scholar]
- Çokluk, Ö. Logistic regression: Concept and application. Educ. Sci. Theory Pract. 2010, 10, 1397–1407. [Google Scholar]
- McDonald, J.H. Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2014; p. 299. [Google Scholar]
- SPSS Inc. SPSS 14.0 for Windows Use Manual; SPSS Inc.: Chicago, IL, USA, 2006; p. 473. [Google Scholar]
- PAST Inc. PAST 3.25 for Windows Use Manual, (version 3.25); PAST Inc.: Oslo, Norway, 2017; p. 259. [Google Scholar]
- Grove, S. Saproxylic insect ecology and the sustainable management of forests. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 1–23. [Google Scholar] [CrossRef]
- Jonsson, B.G.; Kruys, N.; Raniuset, T. Ecology of species living on dead wood: Lessons for dead-wood management. Silva Fenn. 2005, 39, 289–389. [Google Scholar] [CrossRef] [Green Version]
- Peris-Felipo, F.J.; Jiménez-Peydró, R. Cerambycidae (Coleoptera) richness in Mediterranean landscapes of Spain: Diversity and community structure analysis. Biodivers. J. 2012, 3, 59–68. [Google Scholar]
- Ramilo, P. La Comunidad de Coleópteros Saproxílicos en Bosque Mediterráneo: Factores Ambientales que Condicionan sus Ensambles. Ph.D. Dissertation, University of Alicante, Alicante, Spain, 2018; p. 52. Available online: http://hdl.handle.net/10045/86894 (accessed on 14 July 2020).
- Luna-Murillo, A.; Obregón, R. Nuevas aportaciones a la fauna de Bostrichidae (Coleoptera) de la provincial de Córdoba (Andalucía, España). Bol. Soc. Andaluza Ent. 2013, 21, 46–57. [Google Scholar]
- Nardi, G.; Mifsud, D.A. The Bostrichidae of the Maltese Islands (Coleoptera). ZooKeys 2015, 481, 69–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sverdrup-Thygeson, A.; Birkemoe, T. What window traps can tell us: Effect of placement, forest openness and beetle reproduction in retention trees. J. Insect Conserv. 2009, 13, 183–191. [Google Scholar] [CrossRef]
- Horák, J.; Chobot, K.; Horáková, J. Hanging on by the tips of the tarsi: A review of the plight of the critically endangered saproxylic beetle in European forests. J. Nat. Conserv. 2012, 20, 101–108. [Google Scholar] [CrossRef]
- Widerberg, M.K.; Ranius, T.; Drobyshev, I.; Nilsson, U.; Lindbladh, M. Increased openness around retained oaks increases richness of saproxylic beetles. Biodivers. Conserv. 2012, 21, 3035–3059. [Google Scholar] [CrossRef]
- Micó, E.; García-López, A.; Sánchez, A.; Juárez, M.; Galante, E. What can physical, biotic and chemical features of a three hollow tell us about their associated diversity? J. Insect Conserv. 2015, 19, 141–153. [Google Scholar] [CrossRef]
- Recalde, J.I.; San Martín, A.F. Coleópteros xilófagos asociados a ramas de Quercus muertas por la acción del bupréstido Coraebus florentinus (Herbst, 1801) en la Navarra media. Heteropterus Rev. Ent. 2003, 3, 43–50. [Google Scholar]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptative capacity and vulnerability of European forest ecosystems. Forest Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Netherer, S.; Schopf, A. Potential effects of climate change on insect herbivores in European forests-general aspects and the pine processionary moth as specific example. Forest Ecol. Manag. 2010, 259, 831–838. [Google Scholar] [CrossRef]
- Alexander, K.N.A. Changing distribution of Cantharidae and Buprestidae within Great Britain (Coleoptera). In Proceedings of the 13th International Colloquium of the European Invertebrate Survey, Leiden, The Netherlands, 2–5 September 2003; Reemer, M., van Helsdingen, P.S., Kleukes, R.M., Eds.; European Invertebrate Survey: Leiden, The Netherlands, 2001; pp. 87–91. [Google Scholar]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; Oxford University Press: New York, NY, USA, 1986; p. 411. [Google Scholar]
- Gaylord, M.L.; Kolb, T.E.; Wallin, F.K.; Wagner, M.R. Seasonality, and lure preference of bark beetles (Curculionidae: Scotylinae) and associates in a Northern Arizona ponderosa pine forest. Environ. Entomol. 2006, 35, 3747. [Google Scholar] [CrossRef]
- Stork, N.E.; Hammond, P.M. Species richness and temporal partitioning in the beetle fauna of oak trees (Quercus robur L.) in Richmond Park, UK. Insect Conserv. Diver. 2013, 6, 67–81. [Google Scholar] [CrossRef]
- Powell, J.A.; Logan, J.A. Insect seasonality: Circle map analysis of temperature driven life cycles. Theor. Popul. Biol. 2005, 67, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Haack, R.A.; Slansky, F.J. Nutritional ecology of Wood-feeding Coleoptera, Lepidoptera and Hymenoptera. In Nutrition Ecology of Insects, Mites, Spiders, and Related Invertebrates; Slansky, F.J., Rodríguez, J.E., Eds.; Wiley-Interscience: New York, NY, USA, 1985; pp. 449–486. [Google Scholar]
- Walczyńska, A.; Danko, M.; Kozłowski, J. The considerable adult size variability in wood feeders is optimal. Ecol. Entomol. 2010, 35, 16–24. [Google Scholar] [CrossRef]
- Scriven, J.J.; Whitehorn, P.R.; Goulson, D.; Tinsley, M.C. Bergmann’s body size rule operates in facultatively endothermic insects: Evidence from a complex of cryptic bumblebee species. PLoS ONE 2016, 11, e0163307. [Google Scholar] [CrossRef] [PubMed]
- Jonsell, M.; Weslien, J.; Ehnström, B. Substrate requirements of red-listed saproxylic invertebrates in Sweden. Biodivers. Conserv. 1998, 7, 749–764. [Google Scholar] [CrossRef]
- Ranius, T.; Jansson, N. The influence of forest regrowth, original canopy cover and tree size on saproxylic beetles associated with old oaks. Biol. Conserv. 2000, 95, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Økland, B.A. Comparison of three methods of trapping saproxylic beetles. Eur. J. Entomol. 1996, 93, 195–209. [Google Scholar]
- Janssen, P.; Fortin, D.; Hébert, C. Beetle diversity in a matrix of old-growth boreal forest: Influence of habitat heterogeneity at multiple scales. Ecography 2009, 32, 423–432. [Google Scholar] [CrossRef]
- Lachat, T. Deadwood: Quantitative and qualitative requirements for the conservation of saproxylic biodiversity. In Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity; Kraus, D., Krumm, F., Eds.; European Forest Institute: Freiburg, Germany, 2013; p. 284. [Google Scholar]
- Brin, A.; Brustel, H.; Jactel, H. Species variables or environmental variables as indicators of forest biodiversity: A case study using saproxylic beetles in Maritime pine plantations. Ann. For. Sci. 2009, 66, 306. [Google Scholar] [CrossRef] [Green Version]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a surrogate for forest biodiversity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organism. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Martínez-Pastur, G.; Messier, C. Retention forestry to maintain multifunctional forests: A world perspective. Bioscience 2012, 62, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Iglesias, M. ¿Cómo gestionar la madera muerta y conservar a los organismos saproxílicos? Quercus 2014, 336, 32–38. [Google Scholar]




| Plot | Q. ilex | Q. suber | O. europaea | C. siliqua |
|---|---|---|---|---|
| P1 | 16 | 12 | 8 | 0 |
| P2 | 12 | 0 | 8 | 8 |
| P3 | 9 | 8 | 8 | 0 |
| P4 | 12 | 0 | 8 | 8 |
| P5 | 20 | 0 | 0 | 0 |
| Trial | Average Temperature (°C) | Number of Branches | |||
|---|---|---|---|---|---|
| Q. ilex | Q. suber | O. europaea | C. siliqua | ||
| T 1 | 20.72 ± 1.57 | 18 | 5 | 8 | 4 |
| T 2 | 22.85 ± 0.42 | 17 | 5 | 8 | 4 |
| T 3 | 25.40 ± 0.32 | 17 | 5 | 8 | 4 |
| T 4 | 18.54 ± 1.31 | 17 | 5 | 8 | 4 |
| Family | P1 | P2 | P3 | P4 | P5 | Total | Family | P1 | P2 | P3 | P4 | P5 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bostrichidae | Carabidae | ||||||||||||
| L. numida | 1 | 0 | 1 | 0 | 0 | 2 | C. bifasciatus | 0 | 0 | 0 | 1 | 0 | 1 |
| S. pustulata | 0 | 0 | 1 | 2 | 0 | 3 | Dromius sp. | 0 | 0 | 0 | 3 | 0 | 3 |
| S. sexdentatum | 0 | 0 | 0 | 92 | 0 | 92 | M. luctuosus | 10 | 0 | 0 | 0 | 1 | 11 |
| T. impressum | 0 | 0 | 0 | 231 | 1 | 232 | P. linearis | 0 | 0 | 0 | 0 | 1 | 1 |
| ------------ | - | - | - | -- | - | - | P. obtusus | 0 | 0 | 0 | 0 | 1 | 1 |
| Buprestidae | T. obtusus | 2 | 0 | 1 | 0 | 0 | 3 | ||||||
| A. adspersula | 12 | 2 | 2 | 0 | 3 | 19 | ---------- | - | - | - | - | - | - |
| A. degener | 1 | 0 | 0 | 1 | 12 | 14 | Cerambycidae | ||||||
| A. angustulus | 1 | 0 | 0 | 0 | 0 | 1 | C. ruficornis | 0 | 0 | 0 | 1 | 0 | 1 |
| A. graminis | 4 | 0 | 1 | 0 | 0 | 5 | P. timida | 0 | 0 | 1 | 5 | 0 | 6 |
| A. hastulifer | 1 | 0 | 0 | 0 | 0 | 1 | T. fasciculatus | 0 | 1 | 0 | 4 | 0 | 5 |
| A. laticornis | 1 | 0 | 1 | 0 | 0 | 2 | ----------- | - | - | - | - | - | - |
| Agrilus sp. | 1 | 0 | 0 | 0 | 0 | 1 | Cleridae | ||||||
| A. dimidiata | 0 | 1 | 0 | 0 | 0 | 1 | D. albofasciatus | 0 | 0 | 0 | 1 | 0 | 1 |
| A. hungarica | 0 | 0 | 1 | 0 | 1 | 2 | O. domesticus | 1 | 0 | 0 | 1 | 1 | 3 |
| A. millefolii | 32 | 1 | 7 | 0 | 0 | 40 | T. univittatus | 0 | 0 | 0 | 7 | 0 | 7 |
| A. thalassophila | 0 | 0 | 0 | 1 | 0 | 1 | T. ibericus | 0 | 1 | 0 | 0 | 2 | 3 |
| A. umbellatarum | 0 | 0 | 0 | 2 | 0 | 2 | T. leucopsideus | 0 | 0 | 1 | 0 | 0 | 1 |
| ------------- | - | - | - | - | - | - | T. octopunctatus | 0 | 0 | 0 | 0 | 1 | 1 |
| Family | Qi | Qs | Oe | Cs | Family | Qi | Qs | Oe | Cs |
|---|---|---|---|---|---|---|---|---|---|
| Bostrichidae | Carabidae | ||||||||
| L. númida | 1 | 1 | 0 | 0 | C. bifasciatus | 1 | 0 | 0 | 0 |
| S. pustulata | 0 | 1 | 0 | 2 | Dromius sp. | 0 | 0 | 2 | 1 |
| S. sexdentatum | 0 | 0 | 0 | 92 | M. luctuosus | 1 | 10 | 0 | 0 |
| T. impressum | 1 | 0 | 0 | 231 | P. linearis | 1 | 0 | 0 | 0 |
| Buprestidae | P. obtusus | 1 | 0 | 0 | 0 | ||||
| A. adspersula | 19 | 0 | 0 | 0 | T. obtusus | 0 | 3 | 0 | 0 |
| A. degener | 14 | 0 | 0 | 0 | Cerambycidae | ||||
| A. angustulus | 0 | 1 | 0 | 0 | C. ruficornis | 1 | 0 | 0 | 0 |
| A. graminis | 0 | 4 | 1 | 0 | P. timida | 1 | 0 | 0 | 5 |
| A. hastulifer | 0 | 1 | 0 | 0 | T. fasciculatus | 2 | 0 | 0 | 3 |
| A. laticornis | 1 | 1 | 0 | 0 | Cleridae | ||||
| Agrilus sp. | 1 | 0 | 0 | 0 | D. albofasciatus | 0 | 0 | 0 | 1 |
| A. dimidiata | 0 | 0 | 1 | 0 | O. domesticus | 3 | 0 | 0 | 0 |
| A. hungarica | 1 | 0 | 1 | 0 | T. univittatus | 0 | 0 | 0 | 7 |
| A. millefolii | 40 | 0 | 0 | 0 | T. ibericus | 3 | 0 | 0 | 0 |
| A. thalassophila | 0 | 0 | 1 | 0 | T. leucopsideus | 1 | 0 | 0 | 0 |
| A. umbellatarum | 0 | 0 | 0 | 2 | T. octopunctatus | 1 | 0 | 0 | 0 |
| Oe | Cs | P | Oe | Qi | P | O | Qs | P | |
| Richness | 5 | 9 | 0.99 | 5 | 19 | 0.40 | 5 | 8 | 0.84 |
| Abundance | 6 | 344 | 0 | 6 | 95 | 0 | 6 | 22 | 0 |
| Dominance | 0.22 | 0.52 | 0.09 | 0.22 | 0.24 | 0.85 | 0.22 | 0.27 | 0.71 |
| Shannon | 1.56 | 0.90 | 0.09 | 1.56 | 1.92 | 0.87 | 1.56 | 1.64 | 0.93 |
| Evenness | 0.95 | 0.27 | 0.42 | 0.95 | 0.36 | 0.03 | 0.95 | 0.64 | 0.06 |
| Qi | Cs | P | Qi | Qs | P | Cs | Qs | P | |
| Richness | 19 | 9 | 0.18 | 19 | 8 | 0.22 | 9 | 8 | 1 |
| Abundance | 95 | 344 | 0 | 95 | 22 | 0 | 344 | 22 | 0 |
| Dominance | 0.24 | 0.52 | 0.001 | 0.24 | 0.27 | 0.68 | 0.52 | 0.27 | 0.018 |
| Shannon | 1.92 | 0.90 | 0.001 | 1.92 | 1.64 | 0.53 | 0.90 | 1.64 | 0.008 |
| Evenness | 0.36 | 0.27 | 0.82 | 0.36 | 0.64 | 0.27 | 0.27 | 0.64 | 0.59 |
| P1 | P2 | P | P1 | P3 | P | P1 | P4 | p | P1 | P5 | P | |
| Richness | 12 | 5 | 0.67 | 12 | 10 | 0.96 | 12 | 14 | 0.99 | 12 | 10 | 0.89 |
| Abundance | 67 | 6 | 0 | 67 | 17 | 0 | 67 | 352 | 0 | 67 | 25 | 0 |
| Dominance | 0.29 | 0.22 | 0.64 | 0.29 | 0.21 | 0.42 | 0.29 | 0.50 | 0.001 | 0.29 | 0.27 | 0.71 |
| Shannon | 1.66 | 1.56 | 0.93 | 1.66 | 1.95 | 0.47 | 1.66 | 1.01 | 0.003 | 1.66 | 1.78 | 0.66 |
| Evenness | 0.44 | 0.95 | 0.05 | 0.44 | 0.70 | 0.34 | 0.44 | 0.19 | 0.13 | 0.44 | 0.60 | 0.50 |
| P2 | P3 | p | P2 | P4 | P | P2 | P5 | P | P3 | P4 | P | |
| Richness | 5 | 10 | 0.31 | 5 | 14 | 0.97 | 5 | 10 | 0.57 | 10 | 14 | 1 |
| Abundance | 6 | 17 | 0 | 6 | 352 | 0 | 6 | 25 | 0 | 17 | 352 | 0 |
| Dominance | 0.22 | 0.21 | 0.94 | 0.22 | 0.50 | 0.09 | 0.22 | 0.27 | 0.75 | 0.21 | 0.50 | 0.029 |
| Shannon | 1.56 | 1.95 | 0.59 | 1.56 | 1.01 | 0.29 | 1.56 | 1.78 | 0.82 | 1.95 | 1.01 | 0.003 |
| Evenness | 0.95 | 0.70 | 0.13 | 0.95 | 0.19 | 0.18 | 0.95 | 0.60 | 0.06 | 0.70 | 0.19 | 0.42 |
| P3 | P5 | P | P4 | P5 | P | |||||||
| Richness | 10 | 10 | 1 | 14 | 10 | 1 | ||||||
| Abundance | 17 | 25 | 0 | 352 | 25 | 0 | ||||||
| Dominance | 0.21 | 0.27 | 0.38 | 0.50 | 0.27 | 0.027 | ||||||
| Shannon | 1.95 | 1.78 | 0.61 | 1.01 | 1.78 | 0.013 | ||||||
| Evenness | 0.70 | 0.60 | 0.34 | 0.19 | 0.60 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas, A.M.; Gallardo, P.; Salido, Á.; Márquez, J. Effects of Environmental Traits and Landscape Management on the Biodiversity of Saproxylic Beetles in Mediterranean Oak Forests. Diversity 2020, 12, 451. https://doi.org/10.3390/d12120451
Cárdenas AM, Gallardo P, Salido Á, Márquez J. Effects of Environmental Traits and Landscape Management on the Biodiversity of Saproxylic Beetles in Mediterranean Oak Forests. Diversity. 2020; 12(12):451. https://doi.org/10.3390/d12120451
Chicago/Turabian StyleCárdenas, Ana M., Patricia Gallardo, Ángela Salido, and José Márquez. 2020. "Effects of Environmental Traits and Landscape Management on the Biodiversity of Saproxylic Beetles in Mediterranean Oak Forests" Diversity 12, no. 12: 451. https://doi.org/10.3390/d12120451
APA StyleCárdenas, A. M., Gallardo, P., Salido, Á., & Márquez, J. (2020). Effects of Environmental Traits and Landscape Management on the Biodiversity of Saproxylic Beetles in Mediterranean Oak Forests. Diversity, 12(12), 451. https://doi.org/10.3390/d12120451