Cover Crop Diversity as a Tool to Mitigate Vine Decline and Reduce Pathogens in Vineyard Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of Experiments
2.2. Effect of Cover Crop Diversity on Disease Symptoms
2.3. Location and Greenhouse Conditions
2.4. Soil
2.5. Pathogen Incubation and Inoculation
2.6. Germination and Growth of Plants
2.7. Incidence of Disease
2.8. Molecular Data
2.9. DNA Extraction
2.10. Droplet Digital Assay
2.11. Illumina Sequencing and Bioinformatics
2.12. Statistical Analyses
3. Results
3.1. Cover Crop Growth
3.2. Effect of Cover Crops on Incidence of Disease in Vines
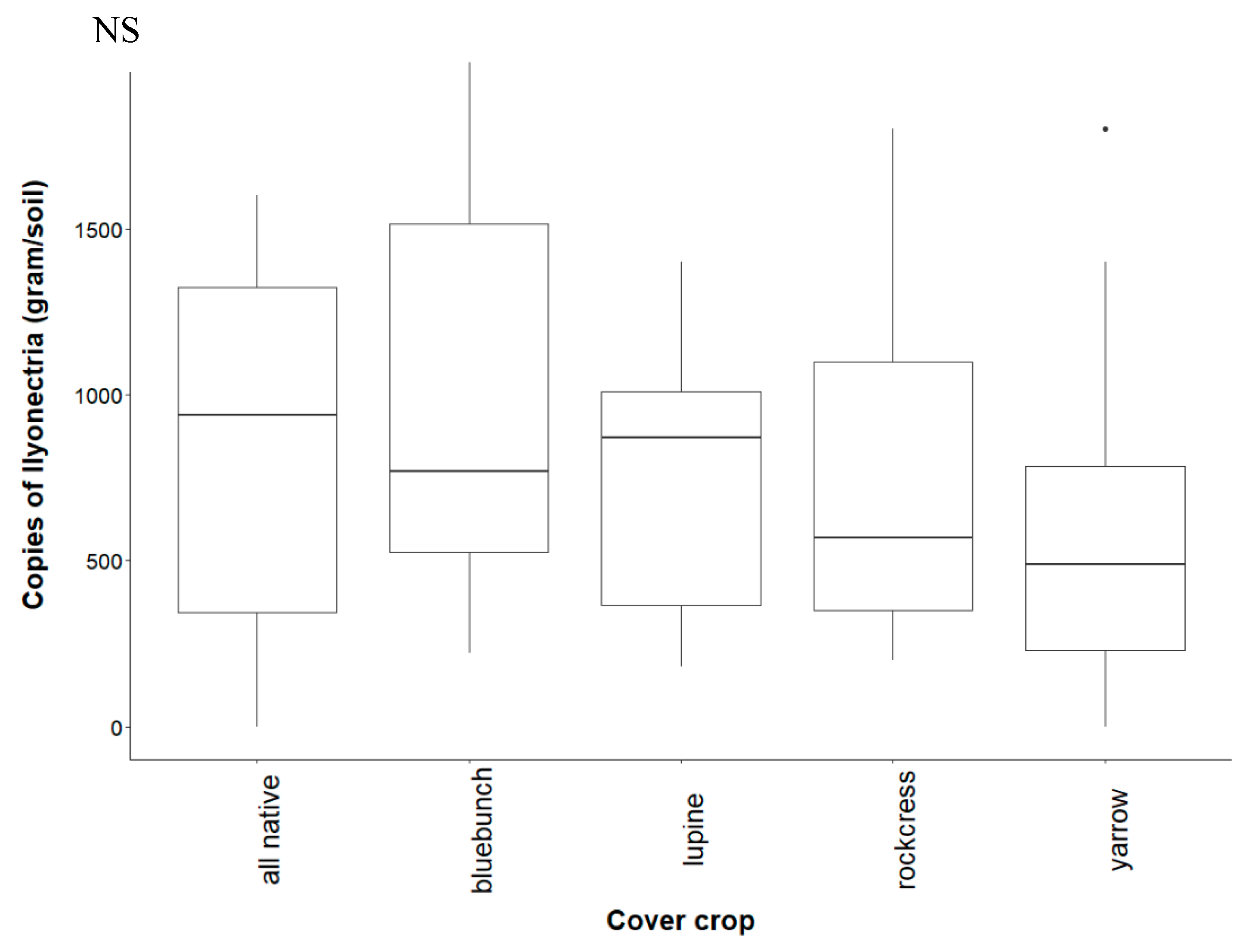
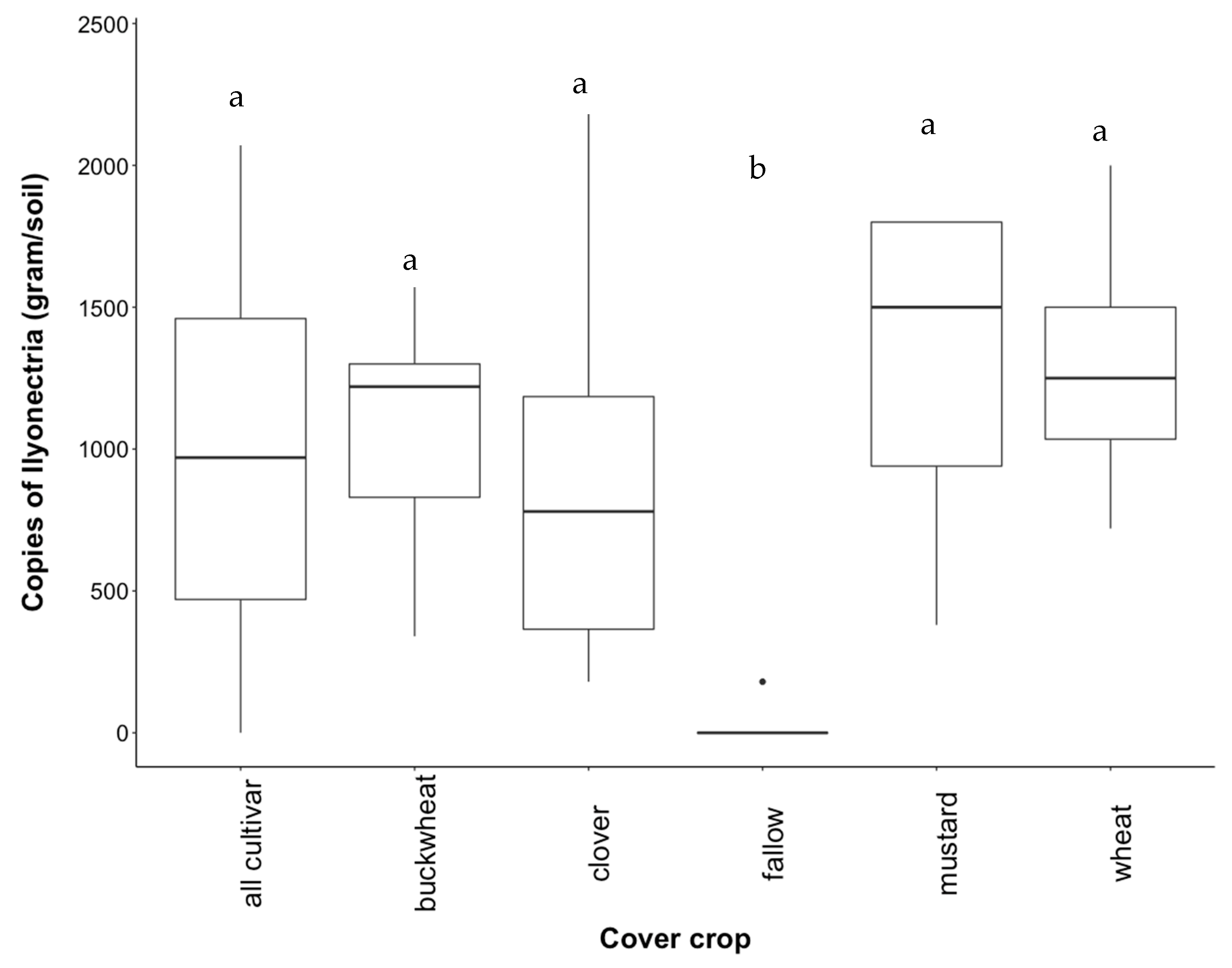
3.3. Recovery of Ilyonectria from Soil
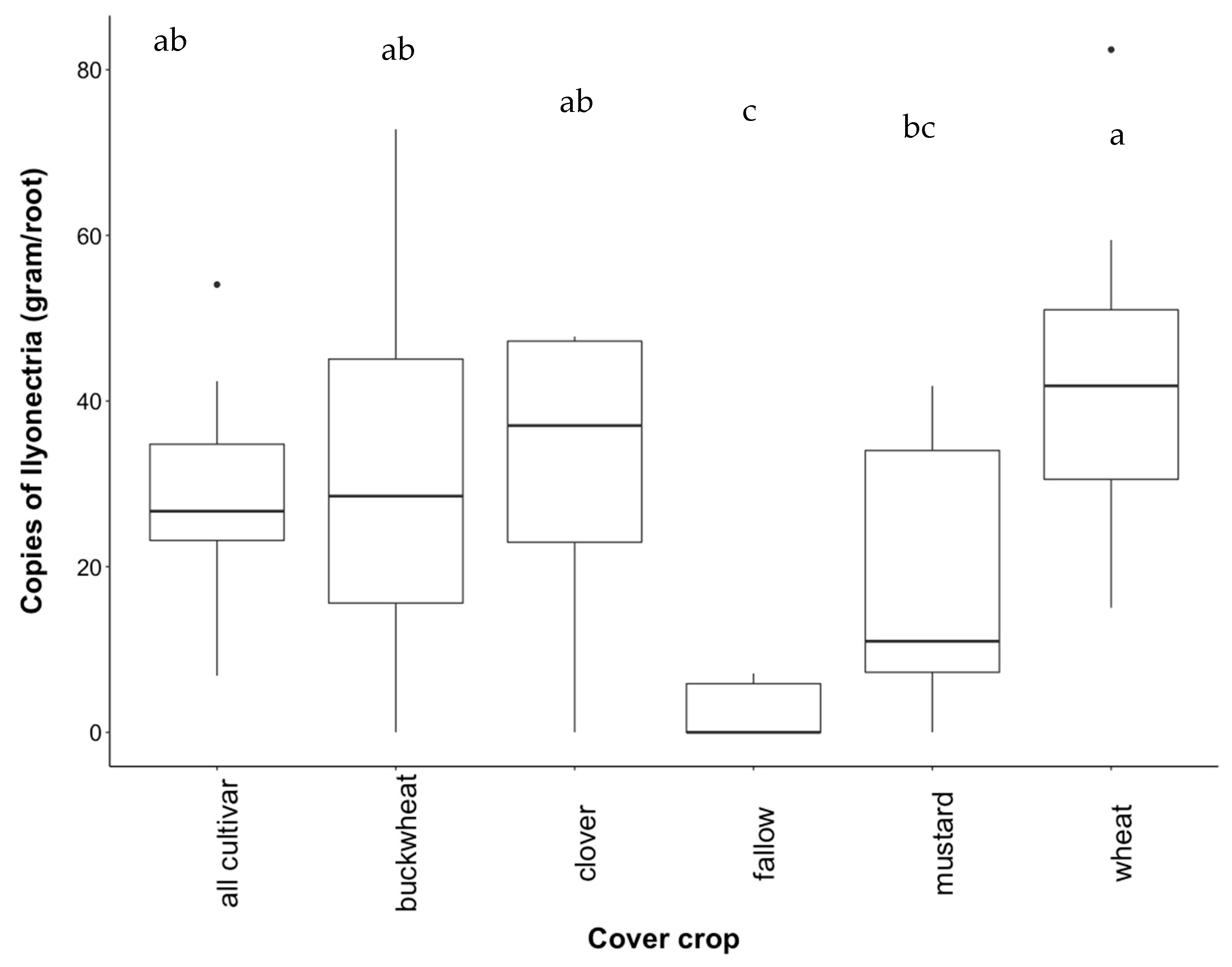
3.4. Recovery of Ilyonectria from Roots
3.5. Sequence Results
3.6. Effect of Cover Crops on Fungal Diversity
3.6.1. Alpha Diversity
3.6.2. Beta Diversity in Native and Cultivar Studies
4. Discussion
4.1. Effect of Cover Crop Diversity on Root Necrosis
4.2. Effect of Cover Crop Diversity on Abundance of Ilyonectria
4.3. Effect of Cover Crop Diversity on Fungal Diversity
4.4. Effect of Cover Crops on Community Composition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A






| Site Properties | Response |
|---|---|
| Fruit tree | Sweet cherry |
| Soil texture | Loamy sand |
| pH | 6.9 |
| Organic matter (%) | 2.3 |
| C/N ratio | 8.5 |
| Phosphorous (ppm) | 66 |
| Potassium (ppm) | 360 |
| Magnesium (ppm) | 170 |
| Calcium (ppm) | 1330 |
| Sodium (ppm) | 25 |
| Aluminum (ppm) | 13 |
| Sulfur (ppm) | 9 |
| Nitrate (ppm) | 23 |
| Date | Fertilizer Type | Amount Applied | Dilution |
|---|---|---|---|
| October 11 2018 | 15-15-18 | 150 mL | 50% |
| October 25 2018 | 15-15-18 | 150 mL | No |
| November 1 2018 | 15-15-18 | 150 mL | 50% |
| November 22 2018 | 15-15-18 | 150 mL | 33% |
| November 29 2018 | 15-15-18 | 150 mL | 40% |
| December 7 2018 | 15-15-18 | 150 mL | 40% |
| Sample | Input | filtered | DenoisedF | DenoisedR | Merged | Nonchim |
|---|---|---|---|---|---|---|
| B1-1 | 12,251 | 6665 | 6644 | 6644 | 6184 | 6176 |
| B1-10 | 14,589 | 8155 | 8133 | 8127 | 7909 | 7907 |
| B1-14 | 14,193 | 8257 | 8222 | 8183 | 7307 | 7290 |
| B1-15 | 16,920 | 9042 | 9020 | 9013 | 8424 | 8409 |
| B1-16 | 16,354 | 8844 | 8808 | 8789 | 8057 | 8053 |
| B1-20 | 21,936 | 9842 | 9807 | 9799 | 9040 | 9028 |
| B1-3 | 9439 | 4599 | 4589 | 4590 | 4276 | 4268 |
| B1-4 | 13,175 | 6559 | 6524 | 6520 | 6091 | 6086 |
| B1-6 | 10,873 | 6568 | 6514 | 6530 | 5964 | 5962 |
| B1-7 | 10,950 | 6140 | 6113 | 6094 | 5469 | 5451 |
| B10-14 | 6011 | 3446 | 3429 | 3431 | 3150 | 3146 |
| B10-16 | 13,831 | 7968 | 7941 | 7918 | 7248 | 7233 |
| B10-17 | 10,982 | 4927 | 4898 | 4896 | 4548 | 4506 |
| B10-18 | 10,059 | 5622 | 5605 | 5607 | 5053 | 5029 |
| B10-19 | 12,876 | 7437 | 7419 | 7397 | 6604 | 6579 |
| B10-22 | 21,342 | 11,584 | 11,551 | 11,564 | 10,118 | 10,041 |
| B10-6 | 29,911 | 15,434 | 15,381 | 15,404 | 14,299 | 14,200 |
| B10-7 | 8427 | 4811 | 4787 | 4784 | 4364 | 4352 |
| B10-9 | 13,366 | 6560 | 6530 | 6534 | 5964 | 5957 |
| B10-fal | 24,131 | 12,374 | 12,348 | 12,349 | 11,735 | 11,671 |
| B2-1 | 12,412 | 6563 | 6522 | 6512 | 6117 | 6079 |
| B2-10 | 19,272 | 11,002 | 10,973 | 10,965 | 10,166 | 10,161 |
| B2-11 | 8626 | 4766 | 4754 | 4751 | 4461 | 4400 |
| B2-12 | 17,980 | 9804 | 9768 | 9770 | 8801 | 8729 |
| B2-13 | 10,050 | 5568 | 5552 | 5539 | 5178 | 5158 |
| B2-17 | 18,591 | 10,111 | 10,080 | 10,067 | 8978 | 8935 |
| B2-18 | 6488 | 3754 | 3734 | 3735 | 3644 | 3640 |
| B2-21 | 20,710 | 10,897 | 10,864 | 10,863 | 10,170 | 10,086 |
| B2-22 | 20,429 | 10,227 | 10,190 | 10,178 | 9386 | 9305 |
| B2-5 | 12,175 | 7021 | 6998 | 6981 | 6355 | 6338 |
| B2-9 | 15,943 | 8738 | 8696 | 8686 | 8171 | 8105 |
| B3-1 | 14,605 | 8041 | 8015 | 8012 | 7281 | 7259 |
| B3-13 | 8386 | 4782 | 4765 | 4758 | 4303 | 4296 |
| B3-14 | 17,549 | 7704 | 7666 | 7648 | 7118 | 7107 |
| B3-15 | 13,922 | 5942 | 5919 | 5924 | 5398 | 5398 |
| B3-17 | 7866 | 4491 | 4473 | 4469 | 4090 | 4069 |
| B3-2 | 22,018 | 11,332 | 11,274 | 11,270 | 10,318 | 10,247 |
| B3-20 | 19,794 | 10,473 | 10,436 | 10,427 | 9681 | 9618 |
| B3-21 | 24,717 | 12,958 | 12,908 | 12,923 | 12,068 | 11,915 |
| B3-22 | 19,204 | 10,218 | 10,182 | 10,191 | 9640 | 9581 |
| B3-3 | 6693 | 3643 | 3631 | 3622 | 3322 | 3316 |
| B3-7 | 20,743 | 10,079 | 10,010 | 10,007 | 9315 | 9237 |
| B3-fal | 18,855 | 10,320 | 10,296 | 10,251 | 9587 | 9503 |
| B4-11 | 10,411 | 6031 | 6007 | 5994 | 5049 | 5049 |
| B4-12 | 14,153 | 7003 | 6958 | 6956 | 6417 | 6403 |
| B4-15 | 16,611 | 8708 | 8658 | 8662 | 7915 | 7840 |
| B4-16 | 19,340 | 10,285 | 10,240 | 10,241 | 9650 | 9558 |
| B4-18 | 8515 | 5131 | 5124 | 5122 | 4708 | 4697 |
| B4-2 | 25,973 | 13,688 | 13,617 | 13,625 | 12,315 | 12,203 |
| B4-21 | 23,882 | 12,563 | 12,498 | 12,473 | 11,638 | 11,530 |
| B4-5 | 23,443 | 12,027 | 11,955 | 11,961 | 11,003 | 10,927 |
| B4-7 | 20,209 | 10,317 | 10,270 | 10,265 | 9600 | 9531 |
| B5-10 | 12,457 | 6726 | 6689 | 6707 | 6415 | 6378 |
| B5-11 | 35,399 | 18,215 | 18,142 | 18,138 | 16,671 | 16,523 |
| B5-12 | 24,203 | 13,410 | 13,350 | 13,349 | 12,385 | 12,216 |
| B5-13 | 23,829 | 12,481 | 12,429 | 12,414 | 11,449 | 11,327 |
| B5-15 | 29,538 | 17,066 | 17,035 | 17,022 | 16,486 | 16,416 |
| B5-4 | 23,032 | 9630 | 9594 | 9580 | 8953 | 8943 |
| B5-6 | 7570 | 3983 | 3967 | 3965 | 3565 | 3562 |
| B5-9 | 11,398 | 4582 | 4558 | 4557 | 4269 | 4268 |
| B6-11 | 11,204 | 6595 | 6572 | 6555 | 6071 | 6052 |
| B6-12 | 8155 | 4512 | 4484 | 4491 | 4028 | 4021 |
| B6-13 | 10,779 | 5783 | 5755 | 5744 | 5194 | 5178 |
| B6-19 | 29,866 | 16,427 | 16,367 | 16,360 | 15,012 | 14,867 |
| B6-2 | 17,688 | 9582 | 9411 | 9366 | 8683 | 8677 |
| B6-20 | 8519 | 4795 | 4783 | 4782 | 4331 | 4317 |
| B6-21 | 22,819 | 12,354 | 12,305 | 12,295 | 11,632 | 11,567 |
| B6-3 | 5341 | 2866 | 2855 | 2851 | 2574 | 2573 |
| B6-4 | 6254 | 847 | 832 | 835 | 771 | 769 |
| B6-5 | 5377 | 2209 | 2198 | 2187 | 2000 | 1995 |
| B7-1 | 24,124 | 12,565 | 12,508 | 12,504 | 11,635 | 11,547 |
| B7-10 | 25,220 | 13,851 | 13,789 | 13,798 | 13,062 | 12,847 |
| B7-11 | 9586 | 5557 | 5530 | 5516 | 5131 | 5111 |
| B7-15 | 26,469 | 14,189 | 14,156 | 14,150 | 13,580 | 13,489 |
| B7-17 | 16,917 | 8897 | 8856 | 8842 | 8204 | 8161 |
| B7-19 | 13,208 | 8050 | 7998 | 7990 | 7187 | 7167 |
| B7-20 | 10,609 | 6169 | 6151 | 6140 | 5595 | 5589 |
| B7-22 | 29,654 | 16,540 | 16,492 | 16,503 | 15,391 | 15,259 |
| B7-3 | 19,382 | 10,255 | 10,220 | 10,216 | 9450 | 9450 |
| B7-5 | 11,105 | 5371 | 5353 | 5342 | 4865 | 4863 |
| B7-7 | 12,032 | 6861 | 6824 | 6819 | 6298 | 6291 |
| B7-fal | 24,746 | 13,168 | 13,147 | 13116 | 12,135 | 12,111 |
| B8-12 | 20,947 | 10,878 | 10,833 | 10,825 | 9907 | 9825 |
| B8-13 | 14,151 | 7813 | 7771 | 7781 | 7010 | 7009 |
| B8-18 | 13,899 | 6928 | 6888 | 6886 | 6373 | 6342 |
| B8-2 | 26,482 | 14,400 | 14,337 | 14,317 | 13,350 | 13,228 |
| B8-20 | 18,913 | 9475 | 9447 | 9451 | 8770 | 8695 |
| B8-21 | 14,129 | 7922 | 7901 | 7893 | 7391 | 7332 |
| B8-22 | 18,843 | 9856 | 9801 | 9807 | 9007 | 8966 |
| B8-5 | 20,503 | 10,688 | 10,648 | 10,648 | 10,081 | 10,023 |
| B8-6 | 13,991 | 7014 | 6976 | 6985 | 6394 | 6378 |
| B8-fal | 4603 | 2518 | 2478 | 2466 | 2362 | 2358 |
| B9-10 | 11,953 | 5229 | 5209 | 5202 | 4679 | 4670 |
| B9-14 | 6549 | 3955 | 3938 | 3941 | 3602 | 3599 |
| B9-16 | 11,558 | 6775 | 6752 | 6755 | 6119 | 6111 |
| B9-19 | 11,146 | 5144 | 5116 | 5115 | 4804 | 4744 |
| B9-3 | 10,354 | 4664 | 4648 | 4644 | 4081 | 4076 |
| B9-4 | 13,701 | 6974 | 6945 | 6933 | 6440 | 6422 |
| B9-6 | 9487 | 4444 | 4414 | 4411 | 4093 | 4089 |
| B9-9 | 19,158 | 10,136 | 10,094 | 10,088 | 9287 | 9246 |
| B9-fal | 7037 | 4131 | 4117 | 4116 | 3850 | 3836 |
| PRE-1 | 9227 | 5446 | 5423 | 5416 | 4963 | 4954 |
| PRE-10 | 41,280 | 20,182 | 20,115 | 20,095 | 18,033 | 17,904 |
| PRE-2 | 10,046 | 5575 | 5566 | 5559 | 5085 | 5084 |
| PRE-3 | 16,164 | 7724 | 7665 | 7679 | 7081 | 7074 |
| PRE-4 | 25,025 | 11,935 | 11,915 | 11,903 | 11,141 | 11,055 |
| PRE-5 | 21,858 | 11,157 | 11,125 | 11,117 | 10,273 | 10,187 |
| PRE-6 | 31,867 | 17,222 | 17,163 | 17,145 | 15,889 | 15,758 |
| PRE-7 | 29,499 | 14,842 | 14,816 | 14,809 | 13,730 | 13,649 |
| PRE-8 | 17,772 | 9465 | 9443 | 9436 | 8714 | 8689 |
| PRE-9 | 22,917 | 12,095 | 12,053 | 12,062 | 11,220 | 11,125 |
| Experiment | Cover Crop Treatment | Below-Ground Biomass | Above-Ground Biomass |
|---|---|---|---|
| Native study | All native | 0.885 a | 2.715 a |
| Bluebunch | 0.795 a | 3.041 a | |
| Lupine | 0.319 b | 0.730 b | |
| Rockcress | 0.138 b | 1.380 b | |
| Yarrow | 1.056 a | 2.822 a | |
| Cultivar study | All cultivar | 1.238 a | 6.917 a |
| Buckwheat | 0.694 b | 4.771 b | |
| Clover | 0.527 b | 7.483 a | |
| Mustard | 0.121 c | 1.834 c | |
| Wheat | 2.272 d | 3.663 d |
References
- Statistics Canada. 2016 Census of Agriculture. 2019. Available online: https://www.statcan.gc.ca/eng/survey/agriculture/3438 (accessed on 29 February 2020).
- Klodd, A.E.; Eissenstat, D.M.; Wolf, T.K.; Centinari, M. “Coping with cover crop competition in mature grapevines. Plant Soil 2016, 400, 391–402. [Google Scholar] [CrossRef]
- Messiga, A.J.; Gallant, K.S.; Sharifi, M.; Hammermeister, A.; Fuller, K.; Tango, M.; Fillmore, S. Grape yield and quality response to cover crops and amendments in a vineyard in Nova Scotia, Canada. Am. J. Enol. Vitic. 2016, 67, 77–85. [Google Scholar] [CrossRef]
- Rahman, L.; Somers, T. Suppression of root knot nematode (Meloidogyne javanica) after incorporation of Indian mustard cv. Nemfix as green manure and seed meal in vineyards. Australas. Plant Pathol. 2005, 34, 77–83. [Google Scholar] [CrossRef]
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing Grapevine Trunk Diseases With Respect to Etiology and Epidemiology: Current Strategies and Future Prospects. Plant Dis. 2017, 102, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Úrbez-Torres, J.R.; Haag, P.; Bowen, P.; O’Gorman, D.T. Grapevine Trunk Diseases in British Columbia: Incidence and Characterization of the Fungal Pathogens Associated with Black Foot Disease of Grapevine. Plant Dis. 2013, 98, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Maluta, D.R.; Larignon, P. Pied-noir: Mieux vaut prévenir. Viticulture 1991, 11, 71–72. [Google Scholar]
- Anderson, K. Lessons for Other Industries from Australia’s Booming Wine Industry. SSRN Electron. J. 2005. [Google Scholar] [CrossRef] [Green Version]
- Sumner, D.; Bombrun, H. An Economic Survey of the Wine and Winegrape Industry in the United States and Canada. 2001. Available online: http://www.academia.edu/download/44327963/Winegrape.pdf (accessed on 29 July 2019).
- Townsend, R.F.; Kirsten, J.; Vink, N. Farm size, productivity and returns to scale in agriculture revisited: A case study of wine producers in South Africa. Agric. Econ. 1998, 19, 175–180. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Haag, P.; Bowen, P.; Lowery, T.; O’Gorman, D.T. Development of a DNA Macroarray for the Detection and Identification of Fungal Pathogens Causing Decline of Young Grapevines. Phytopathology 2015, 105, 1373–1388. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; di Marco, S. Identifying practices likely to have impacts on grapevine trunk disease infections: A European nursery survey. Phytopathol. Mediterr. 2015, 54, 313–324. [Google Scholar] [CrossRef]
- Hofstetter, V.; Buyck, B.; Croll, D.; Viret, O.; Couloux, A.; Gindro, K. What if esca disease of grapevine were not a fungal disease? Fungal Divers. 2012, 54, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Morales-Cruz, A.; Figueroa-Balderas, R.; García, J.F.; Tran, E.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Profiling grapevine trunk pathogens in planta: A case for community-targeted DNA metabarcoding. BMC Microbiol. 2018, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, T.T.; Forge, T.A.; Nelson, L.M. Pseudomonads contribute to regulation of Pratylenchus penetrans (Nematoda) populations on apple. Can. J. Bot. 2018, 64, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, J.M.; González-García, S.; Cobos, R.; Olego, M.Á; Ibañez, A.; Díez-Galán, A.; Garzón-Jimeno, E.; Coque, J.J.R. Use of endophytic and rhizosphere actinobacteria from grapevine plants to reduce nursery fungal graft infections that lead to young grapevine decline. Appl. Environ. Microbiol. 2017, 83, e01564–17. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, M.; Diniz, I.; Cabral, A.; Rego, C.; Oliveira, H. Unveiling inoculum sources of black foot pathogens in a commercial grapevine nursery. Phytopathol. Mediterr. 2013, 52, 298–312. [Google Scholar]
- Canada, H. Pesticide Product Information Database. 2017. Available online: https://pesticide-registry.canada.ca/en/product-search.html (accessed on 30 January 2020).
- Schneider, S.M.; Rosskopf, E.N.; Leesch, J.G.; Chellemi, D.O.; Bull, C.T.; Mazzola, M. United States Department of Agriculture - Agricultural Research Service research on alternatives to methyl bromide: Pre-plant and post-harvest. Pest Manag. Sci. 2003, 59, 814–826. [Google Scholar] [CrossRef]
- Bendavid-Val, R.; Rabinowitch, H.D.; Katan, J.; Kapulnik, Y. Viability of VA-mycorrhizal fungi following soil solarization and fumigation. Plant Soil 1997, 195, 185–193. [Google Scholar] [CrossRef]
- Cabrera, J.A.; Hanson, B.D.; Gerik, J.S.; Gao, S.; Qin, R.; Wang, D. Pre-plant soil fumigation with reduced rates under low permeability films for nursery production, orchard and vineyard replanting. Crop Prot. 2015, 75, 34–39. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The biochemical activity of soil contaminated with fungicides. J. Environ. Sci. Heal.—Part B Pestic. Food Contam. Agric. Wastes 2019, 54, 252–262. [Google Scholar] [CrossRef]
- Fourie, P.H.; Halleen, F. Proactive Control of Petri Disease of Grapevine Through Treatment of Propagation Material. Plant Dis. 2004, 88, 241–1245. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; Armengol, J.; Salazar, D.; López-Cortés, I.; García-Jiménez, J. Effect of hot-water treatments above 50°C on grapevine viability and survival of Petri disease pathogens. Crop Prot. 2009, 28, 280–285. [Google Scholar] [CrossRef]
- Waite, H.; Morton, L. Hot water treatment, trunk diseases and other critical factors in the production of high-quality grapevine planting material. Phytopathol. Mediterr. 2007, 46, 5–17. [Google Scholar]
- Corti, G.; Cavallo, E.; Cocco, S.; Biddoccu, M.; Brecciaroli, G.; Agnelli, A. Evaluation of Erosion Intensity and Some of Its Consequences in Vineyards from Two Hilly Environments Under a Mediterranean Type of Climate, Italy. In Soil Erosion Issues in Agriculture; Godone, D., Stanchi, S., Eds.; IntechOpen: London, UK, 2011. [Google Scholar]
- Bair, K.E.; Davenport, J.R.; Stevens, R.G. Release of Available Nitrogen after Incorporation of a Legume Cover Crop in Concord Grape. HortScience 2008, 43, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Irvin, N.A.; Hagler, J.R.; Hoddle, M.S. Measuring natural enemy dispersal from cover crops in a California vineyard. Biol. Control 2018, 126, 15–25. [Google Scholar] [CrossRef]
- Khanh, T.D.; Chung, M.I.; Xuan, T.D.; Tawata, S. The exploitation of crop allelopathy in sustainable agricultural production. J. Agron. Crop Sci. 2005, 191, 172–184. [Google Scholar] [CrossRef]
- Monteiro, A.; Lopes, C.M. Influence of cover crop on water use and performance of vineyard in Mediterranean Portugal. Agric. Ecosyst. Environ. 2007, 121, 336–342. [Google Scholar] [CrossRef]
- Bleach, C.M.; Jones, E.E.; Jaspers, M.V. Biofumigation using brassicaceous plant products to control Cylindrocarpon black foot disease in New Zealand soils. Phytopathol. Mediterr. 2010, 49, 10–1007. [Google Scholar]
- Whitelaw-Weckert, M.; Rahman, L.; Cappello, J.; Bartrop, K. Preliminary findings on the grapevine yield response to Brassica biofumigation soil treatment. Phytopathol. Mediterr. 2014, 53, 587. [Google Scholar]
- Mazzola, M.; Granatstein, D.M.; Elfving, D.C.; Mullinix, K.; Gu, Y.H. Cultural management of microbial community structure to enhance growth of apple in replant soils. Phytopathology 2002, 92, 1363–1366. [Google Scholar] [CrossRef] [Green Version]
- Wiggins, B.E.; Kinkel, L.L. Green Manures and Crop Sequences Influence Potato Diseases and Pathogen Inhibitory Activity of Indigenous Streptomycetes. Phytopathology 2005, 95, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostfeld, R.S.; Keesing, F. Effects of Host Diversity on Infectious Disease. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 157–182. [Google Scholar] [CrossRef]
- Steinauer, K.; Chatzinotas, A.; Eisenhauer, N. Root exudate cocktails: The link between plant diversity and soil microorganisms? Ecol. Evol. 2016, 6, 7387–7396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, N.; Beßler, H.; Engels, C.; Gleixner, G.; Habekost, M.; Milcu, A.; Partsch, S.; Sabais, A.C.W.; Scherber, C.; Steinbeiss, S. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 2010, 91, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Mariotte, P.; Mehrabi, Z.; Bezemer, T.M.; de Deyn, G.B.; Kulmatiski, A.; Drigo, B.; Veen, G.F.; van der Heijden, M.G.A.; Kardol, P. Plant–soil feedback: Bridging natural and agricultural sciences. Trends Ecol. Evol. 2018, 33, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant-microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef] [Green Version]
- Garbeva, P.; Postma, J.; van Veen, J.A.; van Elsas, J.D. Effect of above-ground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environ. Microbiol. 2006, 8, 233–246. [Google Scholar] [CrossRef]
- van Elsas, J.D.; Garbeva, P.; Salles, J. Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens. Biodegradation 2002, 13, 29–40. [Google Scholar] [CrossRef]
- Latz, E.; Eisenhauer, N.; Rall, B.C.; Allan, E.; Roscher, C.; Scheu, S.; Jousset, A. Plant diversity improves protection against soil-borne pathogens by fostering antagonistic bacterial communities. J. Ecol. 2012, 100, 597–604. [Google Scholar] [CrossRef]
- Latz, E.; Eisenhauer, N.; Scheu, S.; Jousset, A. Plant identity drives the expression of biocontrol factors in a rhizosphere bacterium across a plant diversity gradient. Funct. Ecol. 2015, 29, 1225–1234. [Google Scholar] [CrossRef]
- Lee, B.D.; Dutta, S.; Ryu, H.; Yoo, S.J.; Suh, D.S.; Park, K. Induction of systemic resistance in panax ginseng against phytophthora cactorum by native bacillus amyloliquefaciens HK34. J. Ginseng Res. 2015, 39, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.; Ruan, Y.; Xue, C.; Zhong, S.; Li, R.; Shen, Q. Soils naturally suppressive to banana Fusarium wilt disease harbor unique bacterial communities. Plant Soil 2015, 393, 21–33. [Google Scholar] [CrossRef]
- Irvin, N.A.; Tracy, R.P.; Thomas, M.P.; Hoddle, M. Evaluating the potential of buckwheat and cahaba vetch as nectar producing cover crops for enhancing biological control of Homalodisca vitripennis in California vineyards. Biol. Control 2014, 76, 10–18. [Google Scholar] [CrossRef]
- Langenhoven, S.D.; Halleen, F.; Spies, C.F.J.; Stempien, E.; Mostert, L. Detection and quantification of black foot and crown and root rot pathogens in grapevine nursery soils in the Western Cape of South Africa. Phytopathol. Mediterr. 2018, 57, 519–537. [Google Scholar] [CrossRef]
- Shields, M.W.; Tompkins, J.M.; Saville, D.J.; Meurk, C.D.; Wratten, S. Potential ecosystem service delivery by endemic plants in New Zealand vineyards: Successes and prospects. PeerJ 2016, 4, e2042. [Google Scholar] [CrossRef] [Green Version]
- Tompkins, J.-M. Ecosystem Services Provided by Native New Zealand Plants in Vineyards. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2010. [Google Scholar]
- Vukicevich, E.; Lowery, D.T.; Bennett, J.A.; Hart, M. Influence of Groundcover Vegetation, Soil Physicochemical Properties, and Irrigation Practices on Soil Fungi in Semi-arid Vineyards. Front. Ecol. Evol. 2019, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Holland, T.; Vukicevich, E.; Thomsen, C.; Pogiatzis, A.; Hart, M.; Bowen, P. Arbuscular Mycorrhizal Fungi in Viticulture: Should We Use Biofertilizers? Catal. Discov. Pract. 2018, 2, 59–63. [Google Scholar] [CrossRef]
- Revillini, D.; Gehring, C.A.; Johnson, N.C. The role of locally adapted mycorrhizas and rhizobacteria in plant–soil feedback systems. Funct. Ecol. 2016, 30, 1086–1098. [Google Scholar] [CrossRef] [Green Version]
- Lupwayi, N.Z.; Kennedy, A.C. Grain legumes in Northern Great plains: Impacts on selected biological soil processes. Agron. J. 2007, 99, 1700–1709. [Google Scholar] [CrossRef]
- Ratcliff, W.C.; Kadam, S.V.; Denison, R.F. Poly-3-hydroxybutyrate (PHB) supports survival and reproduction in starving rhizobia. FEMS Microbiol. Ecol. 2008, 65, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Mehrabi, Z.; Bell, T.; Lewis, O.T. Plant-soil feedbacks from 30-year family-specific soil cultures: Phylogeny, soil chemistry and plant life stage. Ecol. Evol. 2015, 5, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Rúa, M.A.; Antoninka, A.; Antunes, P.M.; Chaudhary, V.B.; Gehring, C.; Lamit, L.J.; Piculell, B.J.; Bever, J.D.; Zabinski, C.; Meadow, J.F.; et al. Home-field advantage? Evidence of local adaptation among plants, soil, and arbuscular mycorrhizal fungi through meta-analysis. BMC Evol. Biol. 2016, 16, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.S.; Schweitzer, J.A.; Turk, P.; Bailey, J.K.; Hart, S.C.; Shuster, S.M.; Whitham, T.G. Soil-mediated local adaptation alters seedling survival and performance. Plant Soil 2012, 352, 243–251. [Google Scholar] [CrossRef]
- Tian, K.; Kong, X.; Gao, J.; Jia, Y.; Lin, H.; He, Z.; Ji, Y.; Bei, Z.; Tian, X. Local root status: A neglected bio-factor that regulates the home-field advantage of leaf litter decomposition. Plant Soil 2018, 431, 175–189. [Google Scholar] [CrossRef]
- Veen, G.F.; Snoek, B.L.; Bakx-Schotman, T.; Wardle, D.A.; van der Putten, W.H. Relationships between fungal community composition in decomposing leaf litter and home-field advantage effects. Funct. Ecol. 2019, 33, 1524–1535. [Google Scholar] [CrossRef] [Green Version]
- Madritch, M.D.; Greene, S.L.; Lindroth, R.L. Genetic mosaics of ecosystem functioning across aspen-dominated landscapes. Oecologia 2009, 160, 119–127. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Q.; Bai, E.; Lü, X.; Li, Q.; Xia, J.; Kardol, P.; Liang, W.; Wang, Z.; Han, X.; et al. Nitrogen deposition weakens plant-microbe interactions in grassland ecosystems. Glob. Chang. Biol. 2013, 19, 3688–3697. [Google Scholar] [CrossRef]
- Lambers, H.; Mougel, C.; Jaillard, B.; Hinsinger, P. Plant-microbe-soil interactions in the rhizosphere: An evolutionary perspective. Plant Soil 2009, 321, 83–115. [Google Scholar] [CrossRef]
- Watson, T.; Nelson, L.; Jones, M.; Urbez-Torres, J.R. Non-Fumigant Alternative Soil Management Practices for Mitigating Replant Disease of Fruit Trees: Mechanisms Contributing to Pratylenchus Penetrans Suppression. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, April 2018. [Google Scholar]
- Watson, T.T.; Nelson, L.M.; Forge, T.A. Preplant Soil Incorporation of Compost to Mitigate Replant Disease: Soil Biological Factors Associated with Plant Growth Promotion in Orchard Soil. Compost Sci. Util. 2018, 26, 286–296. [Google Scholar] [CrossRef]
- Overmann, J. Mahoney lake: A case study of the ecological significance of phototrophic sulfur bacteria. In Advances in Microbial Ecology; Jones, J.G., Ed.; Springer Science & Business Media: Berlin, Germany, 1997. [Google Scholar]
- Lindahl, B.D.; Nilsson, R.H.; Tedersoo, L.; Abarenkov, K.; Carlsen, T.; Kjøller, R.; Kõljalg, U.; Pennanen, T.; Rosendahl, S.; Stenlid, J.; et al. Fungal community analysis by high-throughput sequencing of amplified markers—a user’s guide. New Phytol. 2013, 199, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Holland, T.; Bowen, P.; Kokkoris, V.; Urbez-Torres, J.R.; Hart, M. Does inoculation with arbuscular mycorrhizal fungi reduce trunk disease in grapevine rootstocks? Horticulturae 2019, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Kokkoris, V.; Li, Y.; Hamel, C.; Hanson, K.; Hart, M. Site specificity in establishment of a commercial arbuscular mycorrhizal fungal inoculant. Sci. Total Environ. 2019, 660, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, 259–264. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [Green Version]
- Martino, C.; Morton, J.T.; Marotz, C.A.; Thompson, L.R.; Tripathi, A.; Knight, R.; Zengler, K. A novel sparse compositional technique reveals microbial perturbations. Am. Soc. Microbiol. 2019, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Neilsen, D.; Smith, C.A.S.; Frank, G.; Koch, W.; Alila, Y.; Merritt, W.S.; Taylor, W.G.; Barton, M.; Hall, J.W.; Cohen, S.J.; et al. Potential impacts of climate change on water availability for crops in the Okanagan Basin, British Columbia. Can. J. Soil. Sci 2006, 86, 921–936. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Kivlin, S.N.; Du, J.; Eviner, V.T. The temporal development and additivity of plant-soil feedback in perennial grasses. Plant Soil 2013, 369, 141–150. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Reich, P.B.; Scheu, S. Increasing plant diversity effects on productivity with time due to delayed soil biota effects on plants. Basic Appl. Ecol. 2012, 13, 571–578. [Google Scholar] [CrossRef]
- Vogel, A.; Ebeling, A.; Gleixner, G.; Roscher, C.; Scheu, S.; Ciobanu, M.; Koller-France, E.; Lange, M.; Lochner, A.; Meyer, S.T.; et al. A new experimental approach to test why biodiversity effects strengthen as ecosystems age. Adv. Ecol. Res. 2019, 61, 221–264. [Google Scholar]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Ball, B.A.; Bradford, M.A.; Coleman, D.C.; Hunter, M.D. Linkages between below and aboveground communities: Decomposer responses to simulated tree species loss are largely additive. Soil Biol. Biochem. 2009, 41, 1155–1163. [Google Scholar] [CrossRef]
- Cumagun, C.J.R. Managing plant diseases and promoting sustainability and productivity with Trichoderma: The Philippine experience. J. Agric. Sci. Technol. 2012, 14, 699–714. [Google Scholar]
- Naher, L.; Yusuf, U.K.; Ismail, A.; Hossain, K. Trichoderma spp.: A biocontrol agent for sustainable management of plant diseases. Pak. J. Bot. 2014, 46, 1489–1493. [Google Scholar]
- Fourie, P.H.; Halleen, F. Occurrence of grapevine trunk disease pathogens in rootstock mother plants in South Africa. Australas. Plant Pathol. 2004, 33, 313–315. [Google Scholar] [CrossRef]
- Cobos, R.; Mateos, R.M.; Álvarez-Pérez, J.M.; Olego, M.A.; Sevillano, S.; González-García, S.; Garzón-Jimeno, E.; Coque, J.J.R. Effectiveness of natural antifungal compounds in controlling infection by grapevine trunk disease pathogens through pruning wounds. Appl. Environ. Microbiol. 2015, 81, 6474–6483. [Google Scholar] [CrossRef] [Green Version]
- Clark, A. Managing Cover Crops Profitably; Sustainable Agriculture Research and Education: College Park, MD, USA, 2012. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Berlanas, C.; Andrés-Sodupe, M.; López-Manzanares, B.; Maldonado-González, M.M.; Gramaje, D. Effect of white mustard cover crop residue, soil chemical fumigation and Trichoderma spp. root treatment on black-foot disease control in grapevine. Pest Manag. Sci. 2018, 74, 2864–2873. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, S.; Hewavitharana, M.; Strauss, S.S. Brassica seed meal soil amendments transform the rhizosphere microbiome and improve apple production through resistance to pathogen re-infestation. Phytopathology 2014, 105, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Mazzola, M. Interaction of Brassicaceae seed meal soil amendment and apple rootstock genotype on microbiome structure and replant disease suppression. Phytopathology 2019, 109, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drakopoulos, D.; Luz, C.; Torrijos, R.; Meca, G.; Weber, P.; Banziger, I.; Voegele, R.T.; Six, J.; Vogelgsang, S. Use of Botanicals to Suppress Different Stages of the Life Cycle of Fusarium graminearum. Phytopathology 2019, 109, 2116–2123. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.; Kumar, J. Management of fusarium wilt of chickpea using brassicas as biofumigants. Legum. Res. 2017, 40, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Mazzola, M.; Granatstein, D.M.; Elfving, D.C.; Mullinix, K. Suppression of specific apple root pathogens by Brassica napus seed meal amendment regardless of glucosinolate content. Phytopathology 2001, 91, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Mowlick, S.; Yasukawa, H.; Inoue, T.; Takehara, T.; Kaku, N.; Ueki, K.; Ueki, A. Suppression of spinach wilt disease by biological soil disinfestation incorporated with Brassica juncea plants in association with changes in soil bacterial communities. Crop Prot. 2013, 54, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.A. Patterns of Rust Infection as a Function of Host Genetic Diversity and Host Density in Natural Populations of the Apomictic Crucifer, Arabis holboellii. Evolution 1993, 47, 111. [Google Scholar] [CrossRef]
- Ma, Y.; Gentry, T.; Hu, P.; Pierson, E.; Gu, M.; Yin, S. Impact of brassicaceous seed meals on the composition of the soil fungal community and the incidence of Fusarium wilt on chili pepper. Appl. Soil Ecol. 2015, 90, 41–48. [Google Scholar] [CrossRef]
- Wen, L.; Lee-Marzano, S.; Ortiz-Ribbing, L.M.; Gruver, J.; Hartman, G.L.; Eastburn, D.M. Suppression of soilborne diseases of soybean with cover crops. Plant Dis. 2017, 101, 1918–1928. [Google Scholar] [CrossRef] [Green Version]
- BC Wine Grape Council. 2010 Best Practices Guide for Grapes; British Columbia Wine Grape Council: Peachland, BC, Canada, 2010. [Google Scholar]
- Agustí-Brisach, C.; Gramaje, D.; León, M.; García-Jiménez, J.; Armengol, J. Evaluation of vineyard weeds as potential hosts of black-foot and petri disease pathogens. Plant Dis. 2011, 95, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbez-Torres, J.R.; O’Gorman, D.T.; Boule, J.; Walker, M.; Pollard-Flamand, J. Pruning time can reduce grapevine trunk diseases infection under British Columbia environmental con-ditions. Phytopathol. Mediterr. 2019, 58, 426. [Google Scholar]
- Boruah, H.P.D.; Kumar, B.S.D. Plant Disease Suppression and Growth Promotion by a Fluorescent Pseudomonas Strain. Folia Microbiol. 2002, 47, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ridout, M.; Newcombe, G. Disease suppression in winter wheat from novel symbiosis with forest fungi. Fungal Ecol. 2016, 20, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Inderjit; van der Putten, W. H. Impacts of soil microbial communities on exotic plant invasions. Trends Ecol. Evol. 2010, 25, 512–519. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, W.H.; Klironomos, J.N.; Wardle, D.A. Microbial ecology of biological invasions. ISME J. 2007, 1, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Broz, A.K.; Manter, D.K.; Vivanco, J.M. Soil fungal abundance and diversity: Another victim of the invasive plant Centaurea maculosa. ISME J. 2007, 1, 763–765. [Google Scholar] [CrossRef] [Green Version]
- Klironomos, J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef]
- Friesen, M.L.; Porter, S.S.; Stark, S.C.; von Wettberg, E.J.; Sachs, J.L.; Martinez-Romero, E. Microbially Mediated Plant Functional Traits. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenstock, N.; Ellström, M.; Oddsdottir, E.; Sigurdsson, B.D.; Wallander, H. Carbon sequestration and community composition of ectomycorrhizal fungi across a geothermal warming gradient in an Icelandic spruce forest. Fungal Ecol. 2019, 40, 32–42. [Google Scholar] [CrossRef]
- Garbeva, P.; van Veen, J.A.; van Elsas, J.D. Microbial Diversity In Soil: Selection of Microbial Populations by Plant and Soil Type and Implications for Disease Suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef]
- Veen, G.F.; Fry, E.L.; Hooven, F.C.t.; Kardol, P.; Morriën, E.; de Long, J.R. The Role of Plant Litter in Driving Plant-Soil Feedbacks. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Yang, H.; Chang, Z. Effect of biofumigation and chemical fumigation on soil microbial community structure and control of pepper Phytophthora blight. World J. Microbiol. Biotechnol. 2014, 30, 507–518. [Google Scholar] [CrossRef]
- Glenn, M.G.; Chew, F.S.; Williams, P.H. Influence of glucosinolate content of Brassica (Cruciferae) roots on growth of vesicular–arbuscular mycorrhizal fungi. New Phytol. 1988, 10, 217–225. [Google Scholar] [CrossRef]
- Koron, D.; Sonjak, S.; Regvar, M. Effects of non-chemical soil fumigant treatments on root colonisation with arbuscular mycorrhizal fungi and strawberry fruit production. Crop Prot. 2014, 55, 35–41. [Google Scholar] [CrossRef]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [Google Scholar] [CrossRef]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H.A. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [Google Scholar] [CrossRef]
- Clair, J.B.S.; Kilkenny, F.F.; Johnson, R.C.; Shaw, N.L.; Weaver, G. Genetic variation in adaptive traits and seed transfer zones for Pseudoroegneria spicata (bluebunch wheatgrass) in the northwestern United States. Evol. Appl. 2013, 6, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Thelen, G.C.; Barth, S.; Ramsey, P.W.; Gannon, J.E. Soil fungi alter interactions between the invader Centaurea maculosa and North American natives. Ecology 2004, 85, 062–1071. [Google Scholar] [CrossRef] [Green Version]
- Hayman, D.S. Mycorrhizae of nitrogen-fixing legumes. MIRCEN J. Appl. Microbiol. Biotechnol. 1986, 2, 121–145. [Google Scholar] [CrossRef]
- Nieva, A.S.; Bailleres, M.A.; Llames, M.E.; Taboada, M.A.; Ruiz, O.A.; Menéndez, A. Promotion of Lotus tenuis in the Flooding Pampa (Argentina) increases the soil fungal diversity. Fungal Ecol. 2018, 33, 80–91. [Google Scholar] [CrossRef]
- Benitez, M.S.; Taheri, W.I.; Lehman, R.M. Selection of fungi by candidate cover crops. Appl. Soil Ecol. 2016, 103, 72–82. [Google Scholar] [CrossRef]
- Sanchez, I.I.; Fultz, L.M.; Lofton, J.; Haggard, B. Soil Biological Response to Integration of Cover Crops and Nitrogen Rates in a Conservation Tillage Corn Production System. Soil Sci. Soc. Am. J. 2019, 83, 1356–1367. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Ruddell, D.; Mazzola, M. Carbon source-dependent antifungal and nematicidal volatiles derived during anaerobic soil disinfestation. Eur. J. Plant Pathol. 2014, 140, 39–52. [Google Scholar] [CrossRef]




| Treatment | Latin Binomial | Study | Diversity | Group | Life Cycle |
|---|---|---|---|---|---|
| Crimson clover | Trifolium incarnatum | Cultivar | 1 | Legume | Annual |
| Wheat | Triticum aestivum | Cultivar | 1 | Grass | Annual |
| Buckwheat | Fagopyrum esculentum | Cultivar | 1 | Forb | Annual |
| White mustard | Sinapis alba | Cultivar | 1 | Brassica | Annual |
| All cultivar | Cultivar | 4 | Annual | ||
| Fallow | N/A | Cultivar | 0 | N/A | N/A |
| Silky Lupine | Lupinus sericeus | Native | 1 | Legume | Perennial |
| Bluebunch wheatgrass | Pseudoroegneria spicata | Native | 1 | Grass | Perennial |
| White yarrow | Achillea millefolium | Native | 1 | Forb | Perennial |
| Holboell’s rockcress | Bochera hoellbelii | Native | 1 | Brassica | Perennial |
| All native | Native | 4 | Perennial |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richards, A.; Estaki, M.; Úrbez-Torres, J.R.; Bowen, P.; Lowery, T.; Hart, M. Cover Crop Diversity as a Tool to Mitigate Vine Decline and Reduce Pathogens in Vineyard Soils. Diversity 2020, 12, 128. https://doi.org/10.3390/d12040128
Richards A, Estaki M, Úrbez-Torres JR, Bowen P, Lowery T, Hart M. Cover Crop Diversity as a Tool to Mitigate Vine Decline and Reduce Pathogens in Vineyard Soils. Diversity. 2020; 12(4):128. https://doi.org/10.3390/d12040128
Chicago/Turabian StyleRichards, Andrew, Mehrbod Estaki, José Ramón Úrbez-Torres, Pat Bowen, Tom Lowery, and Miranda Hart. 2020. "Cover Crop Diversity as a Tool to Mitigate Vine Decline and Reduce Pathogens in Vineyard Soils" Diversity 12, no. 4: 128. https://doi.org/10.3390/d12040128
APA StyleRichards, A., Estaki, M., Úrbez-Torres, J. R., Bowen, P., Lowery, T., & Hart, M. (2020). Cover Crop Diversity as a Tool to Mitigate Vine Decline and Reduce Pathogens in Vineyard Soils. Diversity, 12(4), 128. https://doi.org/10.3390/d12040128






