The Cost for Biodiversity: Records of Ciliate–Nematode Epibiosis with the Description of Three New Suctorian Species
Abstract
:1. Introduction
2. Material and Methods
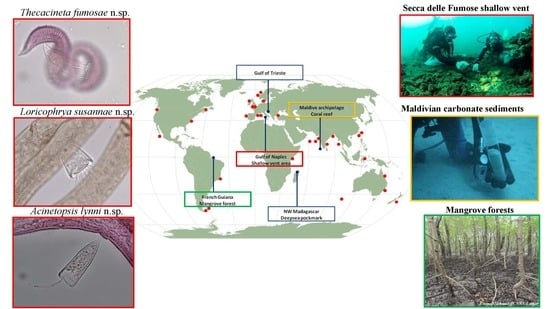
2.1. Research Areas and Sampling Strategy
2.1.1. Deep-Sea Pockmark: Madagascar Margin
2.1.2. Secca delle Fumose Shallow Vent: Gulf of Naples
2.1.3. Marine Protected Area: Gulf of Trieste
2.1.4. Mangrove Forests: French Guiana
2.1.5. Coral Reefs: Archipelago of Maldives
2.2. Samples Processing
3. Results
3.1. New Records of Associations between Nematodes and Ciliates from Deep-Sea and Shallow Water Systems
3.1.1. Deep-Sea Pockmark: Madagascar Margin
3.1.2. Secca delle Fumose Shallow Vent: Gulf of Naples
3.1.3. Marine Protected Area: Gulf of Trieste
3.1.4. Mangrove Forests: French Guiana
3.1.5. Coral Reefs: Archipelago of Maldives
3.2. Taxonomic Account of Ciliates: Systematic Position
3.3. Nematode-Ciliate Association: An Analysis
4. Discussion
4.1. Nematodes as ‘Promoters’ of Biodiversity
4.2. Advantages and Disadvantages of Nematode–Ciliate Association
4.3. The Cost for Biodiversity
4.4. Host–Epibiont Species–Specificity and the Environment
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ecological Studies. Marine Hard Bottom Communities; Wahl, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 206, p. 61. [Google Scholar] [CrossRef]
- Wahl, M.; Hay, M.E.; Enderlein, P. Effects of epibiosis on consumer-prey interactions. In Interactions and Adaptation Strategies of Marine Organisms. Proceedings of the 31st European Marine Biology Symposium, held in St. Petersburg, Russia, 9–13 September 1996; Kluwer Academic Publishers: New York, NY, USA, 1997; Volume 355, pp. 49–59. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Leborans, G. Epibiosis in Crustacea: An overview. Crustaceana 2010, 83, 549–640. [Google Scholar] [CrossRef]
- Fernandez-Leborans, G. Ciliate—Decapod epibiosis in two areas of the north-west Mediterranean coast. J. Nat. Hist. 2003, 37, 1655–1678. [Google Scholar] [CrossRef]
- Wahl, M. Ecological lever and interface ecology: Epibiosis modulates the interactions between host and environment. Biofouling 2008, 24, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.; Mark, O. The predominantly facultative nature of epibiosis: Experimental and observational evidence. Mar. Ecol. Prog. Ser. 1999, 187, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Mikac, B.; Semprucci, F.; Guidi, L.; Ponti, M.; Abbiati, M.; Balsamo, M.; Dovgal, I. Newly discovered associations between peritrich ciliates (Ciliophora: Peritrichia) and scale polychaetes (Annelida: Polynoidae and Sigalionidae) with a review of polychaete–peritrich epibiosis. Zoöl. J. Linn. Soc. 2019, 188, 939–953. [Google Scholar] [CrossRef]
- Fernandez-Leborans, G.; Chatterjee, T.; Grego, M. New records of epibiont ciliates (Ciliophora) on Harpacticoida (Copepoda, Crustacea) from the Bay of Piran (Gulf of Trieste, Northern Adriatic). Cah. Biol. Mar. 2012, 53, 53–63. [Google Scholar]
- Chiavelli, D.A.; Mills, E.L.; Threlkeld, S.T. Host preference, seasonality, and community interactions of zooplankton epibionts. Limnol. Oceanogr. 1993, 38, 574–583. [Google Scholar] [CrossRef]
- Davis, A.; White, G.A. Epibiosis in a guild of sessile subtidal invertebrates in south-eastern Australia: A quantitative survey. J. Exp. Mar. Biol. Ecol. 1994, 177, 1–14. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Clarke, A. Epibiotic communities on sublittoral macroinvertebrates at Signy Island, Antarctica. J. Mar. Biol. Assoc. UK 1995, 75, 689–703. [Google Scholar] [CrossRef]
- Cook, J.A.; Chubb, J.C. Veltkamp Epibionts of Asellus aquaticus (L.) (Crustacea, Isopoda): An SEM study. Freshw. Biol. 1998, 39, 423–438. [Google Scholar] [CrossRef]
- Magagnini, G.; Verni, F. Epibiosis of Scyphidia sp. (Ciliophora, Peritrichida) on Nerilla antennata (Archiannelida, Nerillidae). Boll. Zoöl. 1988, 55, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Ansari, K.G.M.T.; Guidi, L.; Dovgal, I.; Balsamo, M.; Semprucci, F. Some epibiont suctorian ciliates from meiofaunal organisms of Maldivian archipelago with description of a new ciliate species. Zootaxa 2017, 4258, 375. [Google Scholar] [CrossRef] [PubMed]
- Harder, T. Marine Epibiosis: Concepts, Ecological Consequences and Host Defence. In Marine and Industrial Biofouling; Springer: Berlin/Heidelberg, Germany, 2009; pp. 219–231. [Google Scholar]
- Chatterjee, T.; Fernandez-Leborans, G.; Ramteke, D.; Ingole, B.S. New records of epibiont Ciliates (Ciliophora) from Indian coast with descriptions of six new species. Cah. Biol. Mar. 2013, 54, 143–159. [Google Scholar]
- Dovgal, I.V. Evolution, phylogeny and classification of Suctorea (Ciliophora). Protistology 2002, 2, 194–270. [Google Scholar]
- Chatterjee, T.; Fernandez-Leborans, G.; Chan, B.K.K. New record of ciliate Thecacineta calix (Ciliophora: Suctorea) epibiont on Agauopsis halacarid mite (Acari, Halacaridae) from Taiwan. Scr. Sci. Nat. 2012, 2, 121–127. [Google Scholar]
- Dovgal, I.; Chatterjee, T.; Ingole, B. An overview of Suctorian ciliates (Ciliophora, Suctorea) as epibionts of halacarid mites (Acari, Halacaridae). Zootaxa 2008, 1810, 60–68. [Google Scholar] [CrossRef]
- Chatterjee, T.; Nanajkar, M.; Dovgal, I.; Sergeeva, N.; Bhave, S. New records of epibiont Thecacineta calix (Ciliophora, Suctorea) from the Caspian Sea and Angira Bank, Arabian Sea. Cah. Biol. Mar. 2019, 60, 445–451. [Google Scholar]
- Ansari, K.G.M.T.; Bhadury, P. Occurrence of epibionts associated with meiofaunal basibionts from the world’s largest mangrove ecosystem, the Sundarbans. Mar. Biodivers. 2016, 47, 539–548. [Google Scholar] [CrossRef]
- Bhattacharjee, D. Suctorian epibionts on Chromaspirina sp. (Nematoda: Desmodoridae) from the shallow continental shelf of the Bay of Bengal, northern Indian Ocean. Mar. Biodivers. Rec. 2014, 7, 1–3. [Google Scholar] [CrossRef]
- Zeppilli, D.; LeDuc, D.; Fontanier, C.; Fontaneto, D.; Fuchs, S.; Gooday, A.J.; Goineau, A.; Ingels, J.; Ivanenko, V.N.; Kristensen, R.M.; et al. Characteristics of meiofauna in extreme marine ecosystems: A review. Mar. Biodivers. 2017, 48, 35–71. [Google Scholar] [CrossRef] [Green Version]
- Schratzberger, M.; Ingels, J. Meiofauna matters: The roles of meiofauna in benthic ecosystems. J. Exp. Mar. Biol. Ecol. 2018, 502, 12–25. [Google Scholar] [CrossRef]
- Fernandez-Leborans, G.; Román, S.; Martin, D. A New Deep-Sea Suctorian-Nematode Epibiosis (Loricophrya-Tricoma) from the Blanes Submarine Canyon (NW Mediterranean). Microb. Ecol. 2017, 74, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.-X.; Dovgal, I. A new Thecacineta species (Ciliophora, Suctorea) on Desmodora pontica (Nematoda, Desmodorida) from a seagrass bed in Taiwan. Protistology 2015, 9, 75–78. [Google Scholar]
- Chatterjee, T.; Dovgal, I.; Fernandez-Leborans, G. A checklist of suctorian epibiont ciliates (Ciliophora) found on aquatic meiobenthic nematodes. J. Nat. Hist. 2019, 53, 2133–2143. [Google Scholar] [CrossRef]
- Donnarumma, L.; Appolloni, L.; Chianese, E.; Bruno, R.; Baldrighi, E.; Guglielmo, R.; Russo, G.F.; Zeppilli, D.; Sandulli, R. Environmental and Benthic Community Patterns of the Shallow Hydrothermal Area of Secca Delle Fumose (Baia, Naples, Italy). Front. Mar. Sci. 2019, 6, 685. [Google Scholar] [CrossRef] [Green Version]
- Baldrighi, E.; Zeppilli, D.; Appolloni, L.; Donnarumma, L.; Chianese, E.; Russo, G.F.; Sandulli, R. Meiofaunal communities and nematode diversity characterizing the Secca delle Fumose shallow vent area (Gulf of Naples, Italy). PeerJ 2020, 8, e9058. [Google Scholar] [CrossRef]
- Franzo, A.; Guilini, K.; Cibic, T.; Del Negro, P. Interactions between free-living nematodes and benthic diatoms: Insights from the Gulf of Trieste (northern Adriatic Sea). Mediterr. Mar. Sci. 2018, 19, 538–554. [Google Scholar] [CrossRef] [Green Version]
- Semprucci, F.; Balsamo, M. New records and distribution of marine free-living nematodes in the Maldivian Archipelago. Proc. Biol. Soc. Wash. 2014, 127, 35–46. [Google Scholar] [CrossRef]
- Olu, K. PAMELA-MOZ01 Cruise. 2014. Available online: https://campagnes.flotteoceanographique.fr/campagnes/14001000/ (accessed on 1 March 2020). [CrossRef]
- Fontanier, C.; Garnier, E.; Brandily, C.; Dennielou, B.; Bichon, S.; Gayet, N.; Eugene, T.; Rovere, M.; Grémare, A.; Deflandre, B. Living (stained) benthic foraminifera from the Mozambique Channel (eastern Africa): Exploring ecology of deep-sea unicellular meiofauna. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 115, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Fontanier, C.; Mamo, B.; Toucanne, S.; Bayon, G.; Schmidt, S.; Deflandre, B.; Dennielou, B.; Jouet, G.; Garnier, E.; Sakai, S.; et al. Are deep-sea ecosystems surrounding Madagascar threatened by land-use or climate change? Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 131, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Kench, P.S.; Brander, R.W. Wave Processes on Coral Reef Flats: Implications for Reef Geomorphology Using Australian Case Studies. J. Coast. Res. 2006, 221, 209–223. [Google Scholar] [CrossRef]
- Semprucci, F.; Colantoni, P.; Baldelli, G.; Rocchi, M.B.; Balsamo, M. The distribution of meiofauna on back-reef sandy platforms in the Maldives (Indian Ocean). Mar. Ecol. 2010, 31, 592–607. [Google Scholar] [CrossRef]
- Semprucci, F.; Frontalini, F.; Losi, V.; Du Châtelet, E.A.; Cesaroni, L.; Sandulli, R.; Coccioni, R.; Balsamo, M. Biodiversity and distribution of the meiofaunal community in the reef slopes of the Maldivian archipelago (Indian Ocean). Mar. Environ. Res. 2018, 139, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Heip, C.; Vincx, M.; Vranken, G. The ecology of marine nematodes. Oceanogr. Mar. Biol. Annu. Rev. 1985, 23, 399–489. [Google Scholar]
- Seinhorst, J.W. A rapid method for the transfer of nematodes from fixative toanhydrous glicerine. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef] [Green Version]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes. Part. I. British Enoplids; Synopses of the British Fauna, Volume 28; Cambridge University Press: Cambridge, UK, 1983; p. 307. [Google Scholar]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes. Part II. British Chromadorids; Synopses of the British Fauna, Volume 38; E.J Brill: Leiden, The Netherlands, 1988; p. 502. [Google Scholar]
- Warwick, R.M.; Platt, H.M.; Somerfield, P.J. Free-Living Marine Nematodes. Part III. Monhysterids; Synopses of the British Fauna, Volume 53; Field Studies Council: Shrewsbury, UK, 1988; p. 296. [Google Scholar]
- Bezerra, T.N.; Decraemer, W.; Eisendle-Flöckner, U.; Hodda, M.; Holovachov, O.; Leduc, D.; Miljutin, D.; Mokievsky, V.; Peña Santiago, R.; Sharma, J.; et al. Nemys: World Database of Nematodes. 2019. Available online: http://nemys.ugent.be (accessed on 7 October 2019). [CrossRef]
- Dovgal, I.V. Fauna of Ukraine: In 40 Vol. Volume 36: Ciliates—Ciliophora. Issue 1: Class Suctorea; Naukova Dumka: Kyiv, Ukraine, 2013; p. 267. [Google Scholar]
- Ghosh, M.; Mandal, S. Living with Nematode: An Epibiont Trematosoma rotunda Associated with Basibiont Desmodora scaldensis from Matla Estuary, Sundarbans, India. Thalassas 2019, 35, 619–624. [Google Scholar] [CrossRef]
- Chen, C.; Golovatch, S.I.; Chang, H.-W. Identity of the east Asian millipede Habrodesmus inexpectatus Attems, 1944 (Diplopoda: Polydesmida: Paradoxosomatidae). J. Nat. Hist. 2008, 42, 2547–2556. [Google Scholar] [CrossRef]
- Chatterjee, T.; Nanajkar, M.; Dovgal, I. New record of Loricophrya stresemanni (Ciliophora, Suctorea) as epibiont on nematodes from the India Ocean and notes on the genus Loricophrya. Cah. Biol. Mar. 2019, 60, 283–288. [Google Scholar]
- Collin, B. Etudes monographiques sur les Acinetiens. II. Morphologie, physiologie, systematique. Arch. Zool. Exp. Gen. 1912, 51, 1–457. [Google Scholar]
- Dovgal, I.; Chatterjee, T.; Ingole, B. New records of Thecacineta cothurnioides and Trematosoma rotunda (Ciliophora, Suctorea) as epibionts on nematodes from Indian Ocean. Protistology 2009, 6, 19–23. [Google Scholar]
- Lynn, D.H. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2008; p. 605. [Google Scholar]
- Robin, M.C. Mémoire sur la structure et la reproduction de quelques infusoires tentaculés suceurs et flagellés. Journal l’Anatomie Physiologie Normale Pathologiques l’homme Animaux 1979, 529–583. [Google Scholar]
- Root, F.M. A New Suctorian from Woods Hole. Trans. Am. Microsc. Soc. 1922, 41, 77. [Google Scholar] [CrossRef]
- Swarczewsky, B. Sur Kenntnis der Baikalprotistenfauna. Die an der Baikalgammariden lebeden Infusorien. 1–6. 4. Acinetidae. Archive Protistenkunde 1929, 63, 362–449. [Google Scholar]
- Jankowski, A.V. Review of Taxa Phylum Ciliophora Doflein, 1901; Protista: Handbook on Zoology. Volume Part. 2; Alimov, A.F., Ed.; Nauka: St. Petersburg, Russia, 2007; pp. 415–993. [Google Scholar]
- Grell, K.G.; Meister, A. Die Ultrastruktur von Acinetopsis rara Robin (Suctoria). I. Tentakeln und Nahrungsaufnahnme. Protistologica 1982, 18, 67–84. [Google Scholar]
- Batisse, A. Sous-Classe des Suctoria Claparede et Lachmann, 1858; Traite de Zoologie. Anatomie, Systematique, Biologie. Tome II. Infusoires Cilies. Fascicule 2. Systematique; De Puytorac, P., Ed.; Masson: Paris, France; Milan, Italy; Barcelone, Spain, 1994; pp. 493–563. [Google Scholar]
- Sergeeva, N.; Dovgal, I. First finding of epibiont peritrich and suctorian ciliates (Ciliophora) on oligochaetes and harpacticoid copepods from the deep-water hypoxic/anoxic conditions of the Black Sea. Ecol. Montenegr. 2014, 1, 49–54. [Google Scholar]
- Lynn, D.H.; Gómez-Gutiérrez, J.; Strüder-Kypke, M.; Shaw, C.T. Ciliate species diversity and host-parasitoid codiversification in the apostome genus Pseudocollinia (Ciliophora, Apostomatia, Pseudocollinidae) that infect krill, with description of Pseudocollinia similis n. sp., a parasitoid of the krill Thysanoessa spinifera. Dis. Aquat. Org. 2014, 112, 89–102. [Google Scholar]
- Sergeeva, N.; Dovgal, I. Loricophrya bosporica n. sp. (Ciliophora, Suctorea) epibiont of Desmoscolex minutus (Nematoda, Desmoscolecida) from oxic/anoxic boundary of the Black Sea Istanbul Strait’s outlet area. Zootaxa 2016, 4061, 596. [Google Scholar] [CrossRef]
- Ivanova, E.; Dovgal, I.V.; Newton, A. First records of epibiont ciliates (Ciliophora) in methane enriched sediments with species redescriptions. Ecol. Montenegr. 2017, 10, 51–57. [Google Scholar]
- Sartini, B.; Marchesini, R.; D’ávila, S.; D’Agosto, M.; Dias, R.J.P. Diversity and Distribution of Peritrich Ciliates on the Snail Physa acuta Draparnaud, 1805 (Gastropoda: Physidae) in a Eutrophic Lotic System. Zool. Stud. 2018, 57, 2018–2057. [Google Scholar]
- Fernandez-Leborans, G.; Gabilondo, R. Inter-annual variability of the epibiotic community on Pagurus bernhardus from Scotland. Estuar. Coast. Shelf Sci. 2006, 66, 35–54. [Google Scholar] [CrossRef]
- Key, M.M.; Winston, J.E.; Volpe, J.W.; Jeffries, W.B.; Voris, H.K. Bryozoan fouling of the blue crab Callinectes sapidus at Beaufort, North Carolina. Bull. Mar. Sci. 1999, 64, 513–533. [Google Scholar]
- Fisher, R. Ciliate Hitch-hikers—Nematode ecto-commensals from tropical Australian sea grass meadows. J. Mar. Biol. Assoc. UK 2003, 83, 445–446. [Google Scholar] [CrossRef]
- Panigrahi, S.; Bindu, V.K.; Bramha, S.N.; Samantara, M.K.; Mohanty, A.K.; Satpathy, K.K.; Dovgal, I. Report of Thecacineta calix (Ciliophora: Suctoria) on nematode Desmodora from the intertidal sediments of Southwest Bay of Bengal. Indian J. Geo-Mar. Sci. 2015, 44, 1840–1843. [Google Scholar]
- Decraemer, W.; Coomans, A.; Baldwin, J. Morphology of Nematoda. Nematoda 2013, 2, 1–60. [Google Scholar]
- Riemann, F.; Riemann, O. The enigmatic mineral particle accumulations on the cuticular rings of marine desmoscolecoid nematodes—Structure and significance explained with clues from live observations. Meiofauna Mar. 2010, 18, 1–10. [Google Scholar]
- Ingole, B.; Singh, R.; Sautya, S.; Dovgal, I.; Chatterjee, T. Report of epibiont Thecacineta calix (Ciliophora: Suctorea) on deep-sea Desmodora (Nematoda) from the Andaman Sea, Indian Ocean. Mar. Biodivers. Rec. 2010, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Pujol, N.; Davis, P.; Ewbank, J.J. The Origin and Function of Anti-Fungal Peptides in C. elegans: Open Questions. Front. Immunol. 2012, 3, 237. [Google Scholar] [CrossRef] [Green Version]
- Tarr, D.E.K. Nematode antimicrobial peptides. Invert. Surv. J. 2012, 9, 122–133. [Google Scholar]







| System Investigated | Deep-Sea Pockmark | Shallow Water HV | Nearby MPA | Mangrove Forest | Coral Reef |
|---|---|---|---|---|---|
| Study site | NW Madagascar margin | Gulf of Naples | Gulf of Trieste | French Guiana | Archipelago of Maldives |
| Geographical area | Indian Ocean | Tyrrhenian Sea | Adriatic Sea | Atlantic Ocean | Indian Ocean |
| Sampling depth | 528–775 m | 9–14 m | 18 m | 0–50 cm | 19–63 m |
| Sediment tipology | Muddy | Sandy | Sandy-mud | Muddy | Sandy (in reef); silty (out reef) |
| Sampling period | Sept.–Oct. 2014 | November 2017 | September 2011 | November 2017 | May 2013 |
| N. of stations sampled | 2 (Site 1; Site 2) | 4 (H; G; CN and CS) | 1 (St. C1) | 3 (St. 1; St.2 and St.3) | 4 (M1, M4—in; M8, M9—out reef) |
| N. of stations with infested nematodes | 1 (Site 1—inside pockmark) | 3 (G; CN and CS) | 1 (St. C1) | 2 (St. 1 and St.2) | 4 (M1; M4; M8 and M9) |
| Sampling technique | MUC | Manual corer | Manual corer | Manual corer | Manual corer |
| Sediment layer(s) considered | 0–1 cm | 0–1; 1–3; 3–5; 5–10 cm | 0–10 cm | 0–2; 2–10; 10–16 cm | 0–5 cm |
| Sive sized used | 1 mm–32µm | 1 mm–32 µm | 1 mm–38 µm | 1 mm–32 µm | 0.5 mm–42 µm |
| Extraction method | Ludox colloidal silica | Ludox colloidal silica | Ludox colloidal silica | Ludox colloidal silica | Ludox colloidal silica |
| Reference | Sanchez et al. (under review) | [29] | [30] | Michelet et al. (under review) | [31] |
| Study Site | Station | No. IN | B Gender | BG | ES | No. E/B | Position on the Basibiont Body |
|---|---|---|---|---|---|---|---|
| NW Madagascar margin-deep sea pockmark | St.1 | 15 | female (13)-male (3) | Desmodora | Paracineta homari | 1–4 | Middle body and tail region |
| St.1 | 17 | female (12)-male (4) | Desmodora | Trematostoma rotunda | 1–4 | Middle body and head region | |
| St.1 | 17 | female (10)-male (6) | Desmodora | Paracineta homari | 1–4 | Cloaca region | |
| 15% | |||||||
| Gulf of Naples-shallow vent area | Site G | 25 | female (15)-male (6)-juv. (4) | Desmodora | Trematostoma rotunda | 1–8 | Along the entire body length |
| Gayser | Site G | 14 | female (6)-male (5)-juv. (3) | Paradesmodora | Paracineta homari | 1–6 | Along the entire body length |
| Site G | 2 | male (2) | Polysigma | Thecacineta calix | 2–5 | Along the entire body length | |
| Site G | 2 | male (2) | Pseudochromadora | Paracineta homari | 1 | Tail region, before anus | |
| Site G | 8 | female (4)-male (4) | Pseudodesmodora | Thecacineta calix | 2–12 | Along the entire body length | |
| Site G | 2 | male (2) | Desmodoridae | Loricophrya bosporica | 1–2 | Tail region | |
| Site G | 1 | female | Desmodora | Acinetopsis lynni sp. n. | 4 | Middle body and tail region | |
| 5% | |||||||
| Gulf of Naples-shallow vent area | Site CN | 9 | female (2)-male (3)-juv. (4) | Desmodora | Trematostoma rotunda | 1–5 | Along the entire body length |
| Inactive site | Site CN | 7 | female (1)-male (4)-juv. (2) | Pseudodesmodora | Thecacineta calix | 1–5 | Along the entire body length |
| Site CN | 1 | male | Sygmophoranema | Thecacineta calix | 1 | Tail region | |
| 2% | |||||||
| Gulf of Naples-shallow vent area | Site CS | 6 | female (1)-male (4)-juv. (1) | Desmodora | Paracineta homari | 1–5 | Along the entire body length |
| Inactive site | Site CS | 3 | Juvenile | Paradesmodora | Paracineta homari | 1 | Middle body |
| Site CS | 1 | Juvenile | Perspiria | Loricophrya susannae n. sp. | 1 | Middle body | |
| Site CS | 2 | male (1)-juv. (1) | Pseudodesmodora | Thecacineta calix | 1–6 | Along the entire body length | |
| Site CS | 3 | female (2)-juv. (1) | Chromaspirina | Loricophrya susannae n. sp. | 1 | Middle body | |
| Site CS | 2 | male | Polysigma | Thecacineta calix | 1–2 | Along the entire body length | |
| Site CS | 1 | female | Prochaetosoma | Thecacineta calix | 1 | Middle body | |
| Site CS | 1 | female | Perepsilonema | Thecacineta fumosae n. sp. | 1 | Middle body | |
| 2% | |||||||
| Gulf of Trieste | St. C1 | 1 | juvenile | Pseudochromadora | phoronts of Apostomatia | 19 | Along the entire body length |
| 0.2% | |||||||
| French Guiana-Mangrove forest | St. 1 | 4 | male (1)-female (3) | Desmodora | Paracineta homari | 5–6 | Along the entire body length |
| Polluted area | St. 1 | 2 | female | Desmoscolex | Loricophrya bosporica | 1 | Tail region |
| 0.5% | |||||||
| French Guiana-Mangrove forest | St. 2 | 15 | male | Desmodora | Loricophrya bosporica | 6 | Along the entire body length |
| Medium polluted area | St. 2 | 1 | female | Spirinia | Cothurnia sp. | 2 | Tail region |
| 2.7% | |||||||
| Archipelago of Maldives - coral reef | M1 | 1 | male | Paradesmodora | Loricophrya sivertseni and L. stresemanni | 2 | After cloaca opening |
| Inner reef | M4 | 1 | female | Croconema | Thecacineta calix | 5 | Intestine region and caudal region before anus |
| M4 | 1 | male | Paradesmodora | Thecacineta cothurnioides | 1 | Second half of the body, before cloaca | |
| 1% | |||||||
| Outer reef | M8 | 1 | juvenile | Desmodorella | Paracineta sp. | 1 | First half of pharingeal region |
| M9 | 1 | female | Echinodesmodora | Thecacineta cothurnioides | 1 | Second half of intestine region | |
| 1% |
| (A) Epibiont Effects on Basibiont | |
| Cost | Benefit |
| Change of properties of basibiont surface | Epibiont mechanisms against predators |
| into basibiont–epibiont–water interface | that can serve concurrently for the basibiont |
| Increase friction with the water = decrease | Associational resistance |
| basibiont velocity | |
| Increase predation risk by modifying chemical signals | Camouflage |
| and by increasing the basibiont weight = motility | Nutrient flow from epibiont and vitamins |
| reduction | |
| Decrease the visus of the basibiont and cause | Ciliate epibionts adhere to external cuticular |
| wounds on the cuticle | layer of the basibiont with no effect and without |
| causing any arm = neutral effect | |
| A high infestation makes difficult for the host | |
| to maintain the position into the water and sediment | |
| Harmful energetic cost especially when energy | |
| conditions are limited | |
| Decreased reproduction, growth and survivorship: | |
| epibiont can be a hindrance to mating | |
| Starvation for food competition with epibiont | |
| in the case of sharing of feeding source(s) | |
| Increase basibiont vulnerability to infections | |
| and energy cost for locomotion | |
| Diminish gas exchanges if they colonize | |
| gas exchange surface | |
| Epibionts as cause for stress on basibiont by | |
| compromising the capacity of defense and competition | |
| (B) Basibiont Effects on Epibiont | |
| Cost | Benefit |
| Exposure to inadequate environmental conditions | Available surface to colonize on which to live |
| Removing of epibiont by abrasion or by moult | Shock-absorbing substratum |
| Capture of the basibiont by predator | Dispersion of epibiont mobile life stage and gene |
| flow | |
| Competition with other ciliates attached to the same | Free transport for the epibiont |
| basibiont or with conspecific when abundant | |
| Shared doom with basibiont | Protection against predators |
| Exposure to detrimental host defense | Favorable hydrodynamic conditions |
| Increasing in availability of nutrients for the epibiont | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldrighi, E.; Dovgal, I.; Zeppilli, D.; Abibulaeva, A.; Michelet, C.; Michaud, E.; Franzo, A.; Grassi, E.; Cesaroni, L.; Guidi, L.; et al. The Cost for Biodiversity: Records of Ciliate–Nematode Epibiosis with the Description of Three New Suctorian Species. Diversity 2020, 12, 224. https://doi.org/10.3390/d12060224
Baldrighi E, Dovgal I, Zeppilli D, Abibulaeva A, Michelet C, Michaud E, Franzo A, Grassi E, Cesaroni L, Guidi L, et al. The Cost for Biodiversity: Records of Ciliate–Nematode Epibiosis with the Description of Three New Suctorian Species. Diversity. 2020; 12(6):224. https://doi.org/10.3390/d12060224
Chicago/Turabian StyleBaldrighi, Elisa, Igor Dovgal, Daniela Zeppilli, Alie Abibulaeva, Claire Michelet, Emma Michaud, Annalisa Franzo, Eleonora Grassi, Lucia Cesaroni, Loretta Guidi, and et al. 2020. "The Cost for Biodiversity: Records of Ciliate–Nematode Epibiosis with the Description of Three New Suctorian Species" Diversity 12, no. 6: 224. https://doi.org/10.3390/d12060224