Impact of Climate Change on the Distribution of Four Closely Related Orchis (Orchidaceae) Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Occurrence Data and Predictor Variables
2.3. Maxent Modelling
2.4. Data Analysis
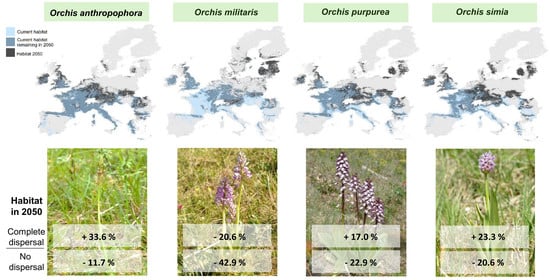
3. Results
4. Discussion
4.1. Changes in Distribution Area
4.2. Role of Climatic vs. Abiotic Variables
4.3. Colonization Potential
4.4. What about Crucial Interactions?
4.5. Implications for Conservation and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaston, K.J. The Structure and Dynamics of Geographic Ranges; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Sexton, J.P.; Montiel, J.; Shay, J.E.; Stephens, M.R.; Slatyer, R.A. Evolution of ecological niche breadth. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 183–206. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, B.R.; De Meester, L.; Bridge, T.C.L.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.M.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The broad footprint of climate change from genes to biomes to people. Science 2016, 354, 719. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef]
- Doak, D.F.; Morris, W.F. Demographic compensation and tipping points in climate-induced range shifts. Nature 2010, 467, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Lesica, P.; Crone, E.E. Arctic and boreal plant species decline at their southern range limits in the Rocky Mountains. Ecol. Lett. 2017, 20, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Vilà-Cabrera, A.; Premoli, A.C.; Jump, A.S. Refining predictions of population decline at species’ rear edges. Glob. Chang. Biol. 2019, 25, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.T.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Ehrlén, J.; Morris, W.F. Predicting changes in the distribution and abundance of species under environmental change. Ecol. Lett. 2015, 18, 303–314. [Google Scholar] [CrossRef]
- Merow, C.; Latimer, A.M.; Wilson, A.M.; McMahon, S.M.; Rebelo, A.G.; Silander, J.A. On using integral projection models to generate demographically driven predictions of species’ distributions: Development and validation using sparse data. Ecography 2014, 37, 1167–1183. [Google Scholar] [CrossRef]
- Franklin, J. Predictive vegetation mapping: Geographic modelling of biospatial patterns in relation to environmental gradients. Prog. Phys. Geogr. 1995, 19, 474–499. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Bush, A.; Mokany, K.; Catullo, R.; Hoffmann, A.; Kellermann, V.; Sgro, C.; McEvey, S.; Ferrier, S. Incorporating evolutionary adaptation in species distribution modelling reduces projected vulnerability to climate change. Ecol. Lett. 2016, 19, 1468–1478. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef]
- Gale, S.W.; Fischer, G.A.; Cribb, P.J.; Fay, M.F. Orchid conservation: Bridging the gap between science and practice. Bot. J. Linn. Soc. 2018, 186, 425–434. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Jacquemyn, H.; Kull, T.; Hutchings, M.J. The demography of terrestrial orchids: Life history, population dynamics and conservation. Bot. J. Linn. Soc. 2020, 192, 315–332. [Google Scholar] [CrossRef]
- Pfeifer, M.; Wiegand, K.; Heinrich, W.; Jetschke, G. Long-term demographic fluctuations in an orchid species driven by weather: Implications for conservation planning. J. Appl. Ecol. 2006, 43, 313–324. [Google Scholar] [CrossRef]
- van der Meer, S.; Jacquemyn, H.; Carey, P.D.; Jongejans, E. Recent range expansion of a terrestrial orchid corresponds with climate-driven variation in its population dynamics. Oecologia 2016, 181, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Jacquemyn, H.; Ochocki, B.M.; Brys, R.; Miller, T.E.X. Life history evolution under climate change and its influence on the population dynamics of a long-lived plant. J. Ecol. 2015, 103, 798–808. [Google Scholar] [CrossRef]
- Kretzschmar, H.; Eccarius, W.; Dietrich, H. The Orchid Genera Anacamptis, Orchis, Neotinea: Phylogeny, Taxonomy, Morphology, Biology, Distribution, Ecology and Hybridisation; EchinoMedia: Bürgel, Germany, 2007. [Google Scholar]
- Bateman, R.M.; Hollingsworth, P.M.; Preston, J.; Yi-Bo, L.; Pridgeon, A.M.; Chase, M.W. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae). Bot. J. Linn. Soc. 2003, 142, 1–40. [Google Scholar] [CrossRef]
- Claessens, J.; Kleynen, J. The Flower of the European Orchid: Form and Function; Jean Claessens & Jacques Kleynen: Voerendaal, The Netherlands, 2011; pp. 137–144. [Google Scholar]
- Jacquemyn, H.; Brys, R. Lack of strong selection pressures maintains wide variation in floral traits in a food-deceptive orchid. Ann. Bot. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Honnay, O.; Cammue, B.P.A.; Brys, R.; Lievens, B. Low specificity and nested subset structure characterize mycorrhizal associations in five closely-related species of the genus Orchis. Mol. Ecol. 2010, 19, 4086–4095. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Brys, R.; Hutchings, M.J. Biological flora of the British Isles: Orchis anthropophora (L.) All. (Aceras anthropophorum (L.) W.T. Aiton). J. Ecol. 2011, 99, 1551–1565. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Merckx, V.; Brys, R.; Tyteca, D.; Cammue, B.P.A.; Honnay, O.; Lievens, B. Analysis of network architecture reveals phylogenetic constraints on mycorrhizal specificity in the genus Orchis (Orchidaceae). New Phytol. 2011, 192, 518–528. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Cammue, B.P.A.; Honnay, O.; Lievens, B. Mycorrhizal associations and reproductive isolation in three closely-related Orchis species. Ann. Bot. 2011, 107, 347–356. [Google Scholar] [CrossRef]
- Oja, J.; Kohout, P.; Tedersoo, L.; Kull, T.; Kõljalg, U. Temporal patterns of orchid mycorrhizal fungi in meadows and forests as revealed by 454 pyrosequencing. New Phytol. 2015, 205, 1608–1618. [Google Scholar] [CrossRef]
- Schatz, B.; Geoffroy, A.; Dainat, B.; Bessiere, J.M.; Buatois, B.; Hossaert-McKey, M.; Selosse, M.A. A case study of modified interactions with symbionts in a hybrid Mediterranean orchid. Am. J. Bot. 2010, 97, 1278–1288. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Honnay, O.; Roldán-Ruiz, I.; Lievens, B.; Wiegand, T. Non-random spatial structuring of orchids in a hybrid zone of three Orchis species. New Phytol. 2012, 193, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Brys, R.; Honnay, O.; Roldán-Ruiz, I. Asymmetric gene introgression in two closely related Orchis species: Evidence from morphometric and genetic analyses. BMC Evol. Biol. 2012, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation Project: Chicago, IL, USA, 2019. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A.; Richardson, K. WorldClim Version 1.3; University of California: Berkeley, CA, USA, 2005. [Google Scholar]
- Van Liedekerke, M.; Jones, A.; Panagos, P. ESDBv2 Raster Library—A Set of Rasters Derived from the European Soil Database Distribution v2. 0; CD-ROM, EUR 19945 EN; European Commission and the European Soil Bureau Network: Ispra, Italy, 2006. [Google Scholar]
- Ballabio, C.; Lugato, E.; Fernandez-Ugalde, O.; Orgiazzi, A.; Jones, A.; Borrelli, P.; Montanarella, L.; Panagos, P. Mapping LUCAS topsoil chemical properties at European scale using Gaussian process regression. Geoderma 2019, 355, 113912. [Google Scholar] [CrossRef] [PubMed]
- Hiederer, R. Mapping Soil Properties for Europe—Spatial Representation of Soil Database Attributes; EUR26082EN Scientific and Technical Research series; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Amatulli, G.; Domisch, S.; Tuanmu, M.N.; Parmentier, B.; Ranipeta, A.; Malczyk, J.; Jetz, W. A suite of global, cross-scale topographic variables for environmental and biodiversity modeling. Sci. Data 2018, 5, 180040. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing (Version 3.6.2); R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org (accessed on 15 January 2020).
- Leutner, B.; Horning, N.; Schwalb-Willmann, J.; Hijmans, R. RStoolbox: Tools for Remote Sensing Data Analysis, R package version 0.1 7; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species ’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps, R package version 1.0.8; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Stevanović, V. Factors affecting the distribution and abundance of orchids in grasslands and herbaceous wetlands. Syst. Biodivers. 2016, 14, 355–370. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; O’Neill, J.P.; Becker, J.J.; Werner, S.; Rasmussen, H.N.; Bruns, T.D.; Taylor, D.L. Abundance and distribution of Corallorhiza odontorhiza reflect variations in climate and ectomycorrhizae. Ecol. Monogr. 2009, 79, 619–635. [Google Scholar] [CrossRef]
- Acharya, K.P.; Vetaas, O.R.; Birks, H.J.B. Orchid species richness along Himalayan elevational gradients. J. Biogeogr. 2011, 38, 1821–1833. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I.; Karagiannakidou, V.; Alifragis, D. Niche analysis and conservation of the orchids of east Macedonia (NE Greece). Acta Oecol. 2008, 33, 27–35. [Google Scholar] [CrossRef]
- Bowles, M.; Zettler, L.; Bell, T.; Kelsey, P. Relationships between soil characteristics, distribution and restoration potential of the federal threatened eastern prairie fringed orchid, Platanthera leucophaea (Nutt.) Lindl. Am. Midl. Nat. 2005, 154, 273–286. [Google Scholar] [CrossRef]
- Štípková, Z.; Romportl, D.; Černocká, V.; Kindlmann, P. Factors associated with the distributions of orchids in the Jeseníky Mountains, Czech Republic. Eur. J. Environ. Sci. 2017, 7, 135–145. [Google Scholar] [CrossRef]
- Honnay, O.; Verheyen, K.; Butaye, J.; Jacquemyn, H.; Bossuyt, B.; Hermy, M. Possible effects of habitat fragmentation and climate change on the range of forest plant species. Ecol. Lett. 2002, 5, 525–530. [Google Scholar] [CrossRef]
- Arditti, J.; Ghani, A.K.A. Tansley Review No. 110. Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef]
- Willems, J.H. Establishment and development of a population of Orchis simia Lamk. in The Netherlands, 1972 to 1981. New Phytol. 1982, 91, 757–765. [Google Scholar] [CrossRef]
- Crackles, E. The Monkey Orchid in Yorkshire. Naturalist 1975, 932, 25–26. [Google Scholar]
- Tsiftsis, S.; Djordjević, D. Modelling sexually deceptive orchid species distributions under future climates: The importance of plant-pollinator interactions. Sci. Rep. 2020, 10, 10623. [Google Scholar] [CrossRef]
- Henneresse, T.; Tyteca, D. Insect visitors and potential pollinators of Orchis militaris (Orchidaceae) in Southern Belgium. J. Insect Sci. 2016, 16, 104. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Terrestrial Orchids: From Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- McCormick, M.K.; Jacquemyn, H. What constrains the distribution of orchid populations? New Phytol. 2014, 202, 392–400. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Kull, T.; Tali, K. Mycorrhizal interactions of orchids colonizing Estonian mine tailing hills. Am. J. Bot. 2008, 95, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Těšitelová, T.; Těšitel, J.; Jersáková, J.; Říhová, G.; Selosse, M.A. Symbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am. J. Bot. 2012, 99, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Greiser, C.; Hylander, K.; Meineri, E.; Luoto, M.; Ehrlén, J. Climate limitation at the cold edge: Contrasting perspectives from species distribution modelling and a transplant experiment. Ecography 2019, 43, 637–647. [Google Scholar] [CrossRef]
- Hausfather, Z.; Peters, G.P. Emissions—The ‘business as usual’ story is misleading. Nature 2020, 577, 618–620. [Google Scholar] [CrossRef]
- Duffy, K.J.; Waud, M.; Schatz, B.; Petanidou, T.; Jacquemyn, H. Latitudinal variation in mycorrhizal diversity associated with a European orchid. J. Biogeogr. 2019, 46, 968–980. [Google Scholar] [CrossRef]




| Species | Levins’ B | Potential Habitat Loss (%) | ||
|---|---|---|---|---|
| RCP 2.6 | RCP 4.5 | RCP 8.5 | ||
| Orchis anthropophora | 0.3748 ± 0.0011 | 8.6087 | 12.2136 | 22.5989 |
| Orchis militaris | 0.4687 ± 0.0012 | 29.5588 | 36.8489 | 45.2645 |
| Orchis purpurea | 0.3912 ± 0.0008 | 22.3596 | 27.5207 | 38.8076 |
| Orchis simia | 0.2797 ± 0.0011 | 24.4939 | 37.1175 | 51.4098 |
| Species | Orchis anthropophora | Orchis militaris | Orchis purpurea | Orchis simia |
|---|---|---|---|---|
| Orchis anthropophora | 1 | 0.7073 | 0.8531 | 0.8032 |
| Orchis militaris | 1 | 0.7374 | 0.6423 | |
| Orchis purpurea | 1 | 0.8331 | ||
| Orchis simia | 1 |
| Species | Mean Annual Temperature (°C) | Annual Precipitation (mm3) |
|---|---|---|
| Orchis anthropophora | 10.89 ± 0.06 | 795.84 ± 3.41 |
| Orchis militaris | 9.42 ± 0.04 | 800.67 ± 3.88 |
| Orchis purpurea | 10.63 ± 0.03 | 761.29 ± 2.04 |
| Orchis simia | 10.79 ± 0.06 | 785.20 ± 5.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, A.; Janssens, S.; Jacquemyn, H. Impact of Climate Change on the Distribution of Four Closely Related Orchis (Orchidaceae) Species. Diversity 2020, 12, 312. https://doi.org/10.3390/d12080312
Evans A, Janssens S, Jacquemyn H. Impact of Climate Change on the Distribution of Four Closely Related Orchis (Orchidaceae) Species. Diversity. 2020; 12(8):312. https://doi.org/10.3390/d12080312
Chicago/Turabian StyleEvans, Alexandra, Sam Janssens, and Hans Jacquemyn. 2020. "Impact of Climate Change on the Distribution of Four Closely Related Orchis (Orchidaceae) Species" Diversity 12, no. 8: 312. https://doi.org/10.3390/d12080312
APA StyleEvans, A., Janssens, S., & Jacquemyn, H. (2020). Impact of Climate Change on the Distribution of Four Closely Related Orchis (Orchidaceae) Species. Diversity, 12(8), 312. https://doi.org/10.3390/d12080312