Plant-Pollinator Networks in Savannas of Burkina Faso, West Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas and Study Design
2.2. Sampling Flowering Plants, Bees and Their Interactions
2.3. Flower Visitation Networks
2.4. Statistical Analyses
3. Results
3.1. Plant and Bee Richness in Relation to Land-Use Intensity and Climatic Seasonality
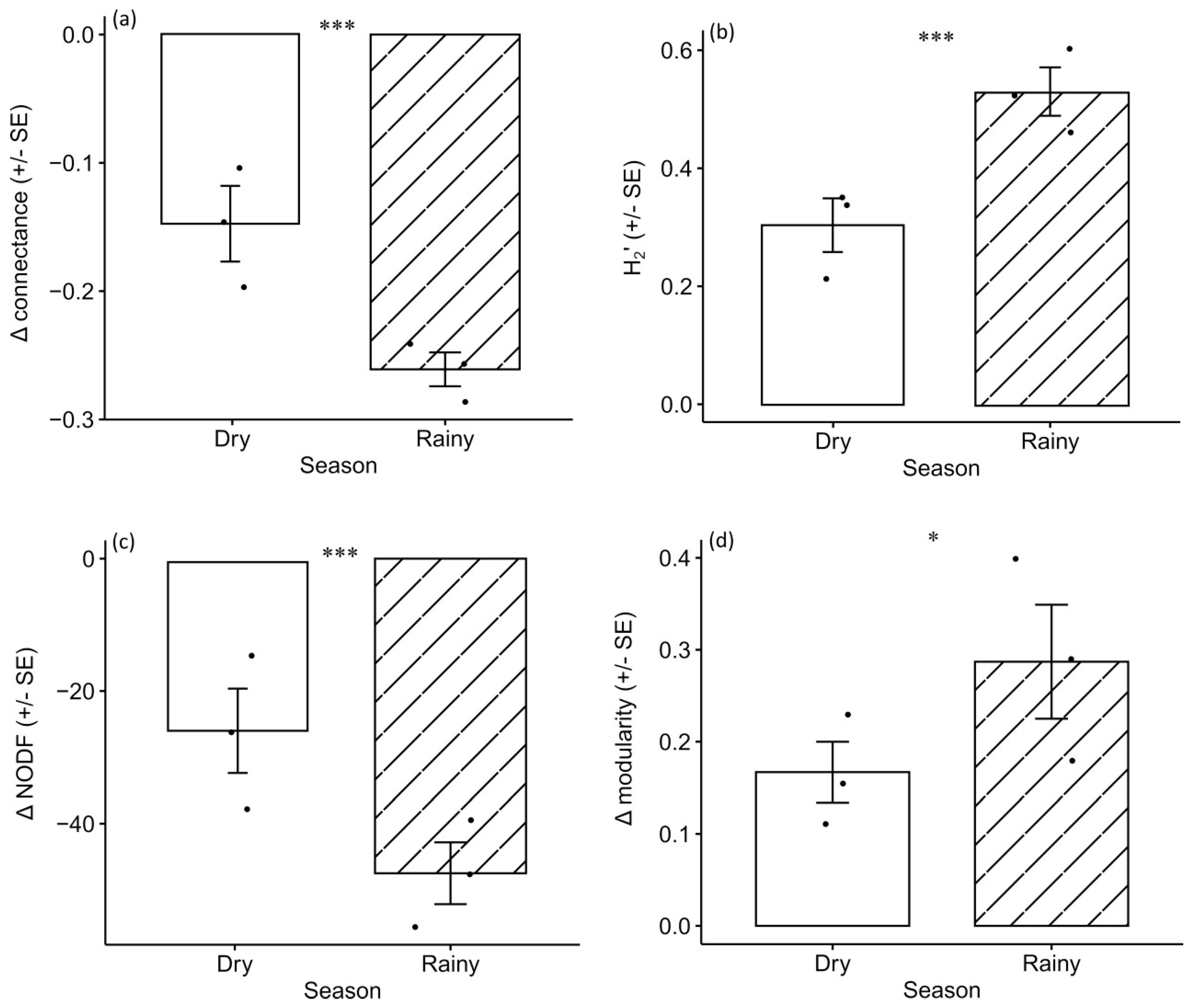
3.2. Plant-Bee Network Architecture in Relation to Land-Use Intensity and Climatic Seasonality
4. Discussion
4.1. Bee and Flowering Plant Richness, Land-Use Intensity and Climatic Seasonality
4.2. Plant-Bee Network Architecture, Land-Use Intensity and Climatic Seasonality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Potts, S.G. The Assessment Report on Pollinators, Pollination and food Production: Summary for Policymakers; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; ISBN 978-92-807-3568-0. [Google Scholar]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; LeBuhn, G.; Aizen, M.A. A meta-analysis of bees′ responses to anthropogenic disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, A.D.; Kühn, I.; Frenzel, M.; Kuhlmann, M.; Poschlod, P.; Potts, S.G.; Roberts, S.P.M.; Schweiger, O. Wild bee and floral diversity co-vary in response to the direct and indirect impacts of land use. Ecosphere 2017, 8, 02008. [Google Scholar] [CrossRef]
- Theodorou, P.; Albig, K.; Radzevičiūtė, R.; Settele, J.; Schweiger, O.; Murray, T.E.; Paxton, R.J. The structure of flower visitor networks in relation to pollination across an agricultural to urban gradient. Funct. Ecol. 2017, 31, 838–847. [Google Scholar] [CrossRef]
- Spiesman, B.J.; Inouye, B.D. Habitat loss alters the architecture of plant—Pollinator interaction networks. Ecology 2013, 94, 2688–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, C.S.; Maruyama, P.K.; Aoki, C.; Sigrist, M.R.; Raizer, J.; Gross, C.L.; De Araujo, A.C. Temporal variation in plant-pollinator networks from seasonal tropical environments: Higher specialization when resources are scarce. J. Ecol. 2018, 106, 2409–2420. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Mutualistic Networks; Princeton University Press: Princeton, NJ, USA, 2014; ISBN 9780691131269. [Google Scholar]
- Tylianakis, J.M.; Laliberté, E.; Nielsen, A.; Bascompte, J. Conservation of species interaction networks. Biol. Conserv. 2010, 143, 2270–2279. [Google Scholar] [CrossRef]
- Falcão, J.C.; Dáttilo, W.; Izzo, T.J. Efficiency of different planted forests in recovering biodiversity and ecological interactions in Brazilian Amazon. For. Ecol. Manag. 2015, 339, 105–111. [Google Scholar] [CrossRef]
- Burkle, L.A.; Alarcón, R. The future of plant-pollinator diversity: Understanding interaction networks across time, space, and global change. Am. J. Bot. 2011, 98, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, P.K.; Vizentin-Bugoni, J.; Sonne, J.; Martín González, A.M.; Schleuning, M.; Araujo, A.C.; Baquero, A.C.; Cardona, J.; Cardona, P.; Cotton, P.A.; et al. The integration of alien plants in mutualistic plant-hummingbird networks across the Americas: The importance of species traits and insularity. Divers. Distrib. 2016, 22, 672–681. [Google Scholar] [CrossRef] [Green Version]
- Biella, P.; Ollerton, J.; Barcella, M.; Assini, S. Network analysis of phenological units to detect important species in plant-pollinator assemblages: Can it inform conservation strategies? Commun. Ecol. 2017, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dalsgaard, B. Land-use and climate impacts on plant–pollinator interactions and pollination services. Diversity 2020, 12, 168. [Google Scholar] [CrossRef]
- Vizentin-Bugoni, J.; Maruyama, P.K.; de Souza, C.S.; Ollerton, J.; Rech, A.R.; Sazima, M. Plant-pollinator networks in the tropics: A review. In Ecological Networks in the Tropics; Dáttilo, W., Rico-Gray, V., Eds.; Springer: Cham, Switzerland, 2018; pp. 73–91. ISBN 978-3-319-68227-3. [Google Scholar]
- Trøjelsgaard, K.; Báez, M.; Espadaler, X.; Nogales, M.; Oromí, P.; La Roche, F.; Olesen, J.M. Island biogeography of mutualistic interaction networks. J. Biogeogr. 2013, 40, 2020–2031. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B. The Miombo in Transition. Woodlands and Welfare in Africa; CIFOR: Bogor, Java, 1996; ISBN 9798764072. [Google Scholar]
- Grace, J.; Jose, J.S.; Meir, P.; Miranda, H.S.; Montes, R.A. Productivity and carbon fluxes of tropical savannas. J. Biogeogr. 2006, 33, 387–400. [Google Scholar] [CrossRef]
- Furley, P.A.; Proctor, J.; Ratter, J.A. Nature and Dynamics of Forest-Savanna Boundaries, 1st ed.; Chapman & Hall: London, UK, 1992; ISBN 0412443708. [Google Scholar]
- Poilecot, P.; Bonfou, K.; Dosso, H.; Lauginie, F.; N′Dri, K.; Nicole, M.; Sangare, Y. Un Écosystème de Savane Soudanienne: Le Parc National de la Comoé; Technical Report No. IVC/87/007; United Nations Educational, Scientific and Cultural Organization: Paris, France, 1991. [Google Scholar]
- Abbadie, L.; Gignoux, J.; Lepage, M.; Roux, X. Lamto. Structure, Functioning, and Dynamics of a Savanna Ecosystem; Springer: New York, NY, USA, 2006; ISBN 9780387338576. [Google Scholar]
- Koulibaly, A.; Goetze, D.; Traoré, D.; Porembski, S. Protected versus exploited savanna: Characteristics of the Sudanian vegetation in Ivory Coast. Candollea 2006, 61, 425–452. [Google Scholar]
- Dimobe, K.; Ouédraogo, A.; Soma, S.; Goetze, D.; Porembski, S.; Thiombiano, A. Identification of driving factors of land degradation and deforestation in the Wildlife Reserve of Bontioli (Burkina Faso, West Africa). Glob. Ecol. Conserv. 2015, 4, 559–571. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Gomez, S.O.; Torres-Vitolas, C.A.; Schreckenberg, K.; Honzák, M.; Cruz-Garcia, G.S.; Willcock, S.; Palacios, E.; Pérez-Miñana, E.; Verweij, P.A.; Poppy, G.M.; et al. Analysis of ecosystem services provision in the Colombian Amazon using participatory research and mapping techniques. Ecosyst. Serv. 2015, 13, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Vrebos, D.; Staes, J.; Vandenbroucke, T.; D′Haeyer, T.; Johnston, R.; Muhumuza, M.; Kasabeke, C.; Meire, P. Mapping ecosystem service flows with land cover scoring maps for data-scarce regions. Ecosyst. Serv. 2015, 13, 28–40. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Livestock in the Balance; Food and Agriculture Organization: Rome, Italy, 2009; ISBN 978-92-5-106215-9. [Google Scholar]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Stein, K.; Coulibaly, D.; Stenchly, K.; Goetze, D.; Porembski, S.; Lindner, A.; Konaté, S.; Linsenmair, E.K. Bee pollination increases yield quantity and quality of cash crops in Burkina Faso, West Africa. Sci. Rep. 2017, 7, 17691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, K.; Stenchly, K.; Coulibaly, D.; Pauly, A.; Dimobe, K.; Steffan-Dewenter, I.; Konaté, S.; Goetze, D.; Porembski, S.; Linsenmair, K.E.; et al. Impact of human disturbance on bee pollinator communities in savanna and agricultural sites in Burkina Faso, West Africa. Ecol. Evol. 2018, 8, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Eardley, C.D.; Gikungu, M.; Schwarz, M.P. Bee conservation in Sub-Saharan Africa and Madagascar: Diversity, status and threats. Apidologie 2009, 40, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [Green Version]
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and temporal trends of global pollination benefit. PLoS ONE 2012, 7, e35954. [Google Scholar] [CrossRef] [Green Version]
- Guuroh, R.T.; Ruppert, J.C.; Ferner, J.; Čanak, K.; Schmidtlein, S.; Linstädter, A. Drivers of forage provision and erosion control in West African savannas—A macroecological perspective. Agric. Ecosyst. Environ. 2018, 251, 257–267. [Google Scholar] [CrossRef]
- Grote, R.; Lehmann, E.; Brümmer, C.; Brüggemann, N.; Szarzynski, J.; Kunstmann, H. Modelling and observation of biosphere–atmosphere interactions in natural savannah in Burkina Faso, West Africa. Phys. Chem. Earth Parts A B C 2009, 34, 251–260. [Google Scholar] [CrossRef]
- Hema, E.M.; Barnes, R.F.W.; Guenda, W. Distribution of savannah elephants (Loxodonta africana Blumenbach 1797) within Nazinga game ranch, Southern Burkina Faso. Afr. J. Ecol. 2011, 49, 141–149. [Google Scholar] [CrossRef]
- White, F. Vegetation of Africa—A Descriptive Memoir to Accompany the UNESCO/AETFAT/UNSO Vegetation Map of Africa; Natural Resources Research Report No. 20; United Nations Educational, Scientific and Cultural Organization: Paris, France, 1983; 356p. [Google Scholar]
- Dimobe, K.; Goetze, D.; Ouédraogo, A.; Forkuor, G.; Wala, K.; Porembski, S.; Thiombiano, A. Spatio-temporal dynamics in land use and habitat fragmentation within a protected area dedicated to tourism in a Sudanian savanna of West Africa. J. Landsc. Ecol. 2017, 10, 75–95. [Google Scholar] [CrossRef] [Green Version]
- Drissa, C.; Tuo, Y.; Koné, M.; Balima, L.H.; Konaté, S.; Linsenmair, K.E.; Porembski, S.; Goetze, D.; Stein, K. Savanna woody plants and their provision of food resources to bees in southern Burkina Faso, West Africa. J. For. Landsc. Res. 2020, 5, 14–23. [Google Scholar] [CrossRef]
- Hagen, M.; Kissling, W.D.; Rasmussen, C.; De Am Aguiar, M.; Brown, L.E.; Carstensen, D.W.; Alves-Dos-Santos, I.; Dupont, Y.L.; Edwards, F.K.; Genini, J.; et al. Biodiversity, species interactions and ecological networks in a fragmented world. Adv. Ecol. Res. 2012, 46, 89–210. [Google Scholar]
- Thébault, E.; Fontaine, C. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science 2010, 329, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Gaiarsa, M.P.; Guimarães, P.R. Interaction strength promotes robustness against cascading effects in mutualistic networks. Sci. Rep. 2019, 9, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, E.A.; Feit, B.; Requier, F.; Friberg, H.; Jonsson, M. Assessing the resilience of biodiversity-driven functions in agroecosystems under environmental change. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–123. [Google Scholar]
- Tylianakis, J.M.; Morris, R.J. Ecological networks across environmental gradients. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 25–48. [Google Scholar] [CrossRef]
- Dormann, C.F.; Frund, J.; Bluthgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. TOECOL J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Cuartas-Hernández, S.; Medel, R. Topology of plant—Flower-visitor networks in a tropical mountain forest: Insights on the role of altitudinal and temporal variation. PLoS ONE 2015, 10, e0141804. [Google Scholar] [CrossRef] [Green Version]
- Escobedo-Kenefic, N.; Landaverde-González, P.; Theodorou, P.; Cardona, E.; Dardón, M.J.; Domínguez, C.A. Disentangling the effects of local resources, landscape heterogeneity and climatic seasonality on bee diversity and plant-pollinator networks in tropical highlands. Oecologia 2020, 194, 333–344. [Google Scholar] [CrossRef]
- Kaiser-Bunbury, C.N.; Blüthgen, N. Integrating network ecology with applied conservation: A synthesis and guide to implementation. AoB Plants 2015, 7. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Dormann, C.F.; Strauss, R. A method for detecting modules in quantitative bipartite networks. Methods Ecol. Evol. 2014, 5, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Clauset, A.; Moore, C.; Newman, M.E.J. Hierarchical structure and the prediction of missing links in networks. Nature 2008, 453, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Patefield, W.M. Algorithm AS 159: An efficient method of generating random R × C tables with given row and column totals. Appl. Stat. 1981, 30, 91. [Google Scholar] [CrossRef]
- Schleuning, M.; Ingmann, L.; Strauss, R.; Fritz, S.A.; Dalsgaard, B.; Dehling, D.M.; Plein, M.; Saavedra, F.; Sandel, B.; Svenning, J.C.; et al. Ecological, historical and evolutionary determinants of modularity in weighted seed-dispersal networks. Ecol. Lett. 2014, 17, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, B.; Schleuning, M.; Maruyama, P.K.; Dehling, D.M.; Sonne, J.; Vizentin-Bugoni, J.; Zanata, T.B.; Fjeldså, J.; Böhning-Gaese, K.; Rahbek, C.; et al. Opposed latitudinal patterns of network-derived and dietary specialization in avian plant-frugivore interaction systems. Ecography 2017, 40, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 2015, 67. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- McKinney, M.L. Extinction vulnerability and selectivity: Combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 1997, 28, 495–516. [Google Scholar] [CrossRef]
- Kassen, R. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 2002, 15, 173–190. [Google Scholar] [CrossRef] [Green Version]
- Bommarco, R.; Biesmeijer, J.C.; Meyer, B.; Potts, S.G.; Pöyry, J.; Roberts, S.P.M.; Steffan-Dewenter, I.; Ockinger, E. Dispersal capacity and diet breadth modify the response of wild bees to habitat loss. Proc. Biol. Sci. 2010, 277, 2075–2082. [Google Scholar] [CrossRef] [Green Version]
- Winfree, R.; Griswold, T.; Kremen, C. Effect of human disturbance on bee communities in a forested ecosystem. Conserv. Biol. 2007, 21, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Steffan-Dewenter, I.; Kuhn, A. Honeybee foraging in differentially structured landscapes. Proc. Biol. Sci. 2003, 270, 569–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffan-Dewenter, I.; Munzenberg, U.; Burger, C.; Thies, C.; Tscharntke, T. Scale-dependent effects of landscape context on three pollinator guilds. Ecology 2002, 83, 1421. [Google Scholar] [CrossRef]
- Arbonnier, M. Arbres, Arbustes et Lianes des Zones Sèches D′Afrique de L′ouest; Centre for International Development: Montpellier, France, 2000; ISBN 287614431X. [Google Scholar]
- Pauly, A. Classification des Nomiinae africains (Hymenoptera apoidea Halictidae): Musée royal de L´Afrique centrale Tervuren, Belgique. Ann. Sci. Zoologiques 1990, 261, 1–206. [Google Scholar]
- Eardley, C.D.; Daly, H.V. Bees of the Genus Ceratina Latreille in Southern Africa (Hymenoptera: Apoidea). Entomofauna; Biologiezentrum: Linz, Austria, 2007; pp. 1–96. [Google Scholar]
- Lever, J.J.; Van Nes, E.H.; Scheffer, M.; Bascompte, J. The sudden collapse of pollinator communities. Ecol. Lett. 2014, 17, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Bastolla, U.; Fortuna, M.A.; Pascual-Garcia, A.; Ferrera, A.; Luque, B.; Bascompte, J. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 2009, 458, 1018–1020. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [Green Version]
- Naranjo, C.; Iriondo, J.M.; Riofrio, M.L.; Lara-Romero, C. Evaluating the structure of commensalistic epiphyte-phorophyte networks: A comparative perspective of biotic interactions. AoB Plants 2019, 11, 11. [Google Scholar] [CrossRef]
- Olesen, J.M.; Jordano, P. Geographic patterns in plant-pollinator mutualistic networks. Ecology 2002, 83, 2416–2424. [Google Scholar]
- Stouffer, D.B.; Bascompte, J. Compartmentalization increases food-web persistence. Proc. Natl. Acad. Sci. USA 2011, 108, 3648–3652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, A.; Totland, Ø. Structural properties of mutualistic networks withstand habitat degradation while species functional roles might change. Oikos 2014, 123, 323–333. [Google Scholar] [CrossRef]






Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, K.; Coulibaly, D.; Balima, L.H.; Goetze, D.; Linsenmair, K.E.; Porembski, S.; Stenchly, K.; Theodorou, P. Plant-Pollinator Networks in Savannas of Burkina Faso, West Africa. Diversity 2021, 13, 1. https://doi.org/10.3390/d13010001
Stein K, Coulibaly D, Balima LH, Goetze D, Linsenmair KE, Porembski S, Stenchly K, Theodorou P. Plant-Pollinator Networks in Savannas of Burkina Faso, West Africa. Diversity. 2021; 13(1):1. https://doi.org/10.3390/d13010001
Chicago/Turabian StyleStein, Katharina, Drissa Coulibaly, Larba Hubert Balima, Dethardt Goetze, Karl Eduard Linsenmair, Stefan Porembski, Kathrin Stenchly, and Panagiotis Theodorou. 2021. "Plant-Pollinator Networks in Savannas of Burkina Faso, West Africa" Diversity 13, no. 1: 1. https://doi.org/10.3390/d13010001
APA StyleStein, K., Coulibaly, D., Balima, L. H., Goetze, D., Linsenmair, K. E., Porembski, S., Stenchly, K., & Theodorou, P. (2021). Plant-Pollinator Networks in Savannas of Burkina Faso, West Africa. Diversity, 13(1), 1. https://doi.org/10.3390/d13010001




