Accumulation and Effect of Heavy Metals on the Germination and Growth of Salsola vermiculata L. Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Germination Experiments
2.3. Morphological Analysis of the Seedlings
2.4. Metal Content Analysis
2.5. Statistical Analyzes
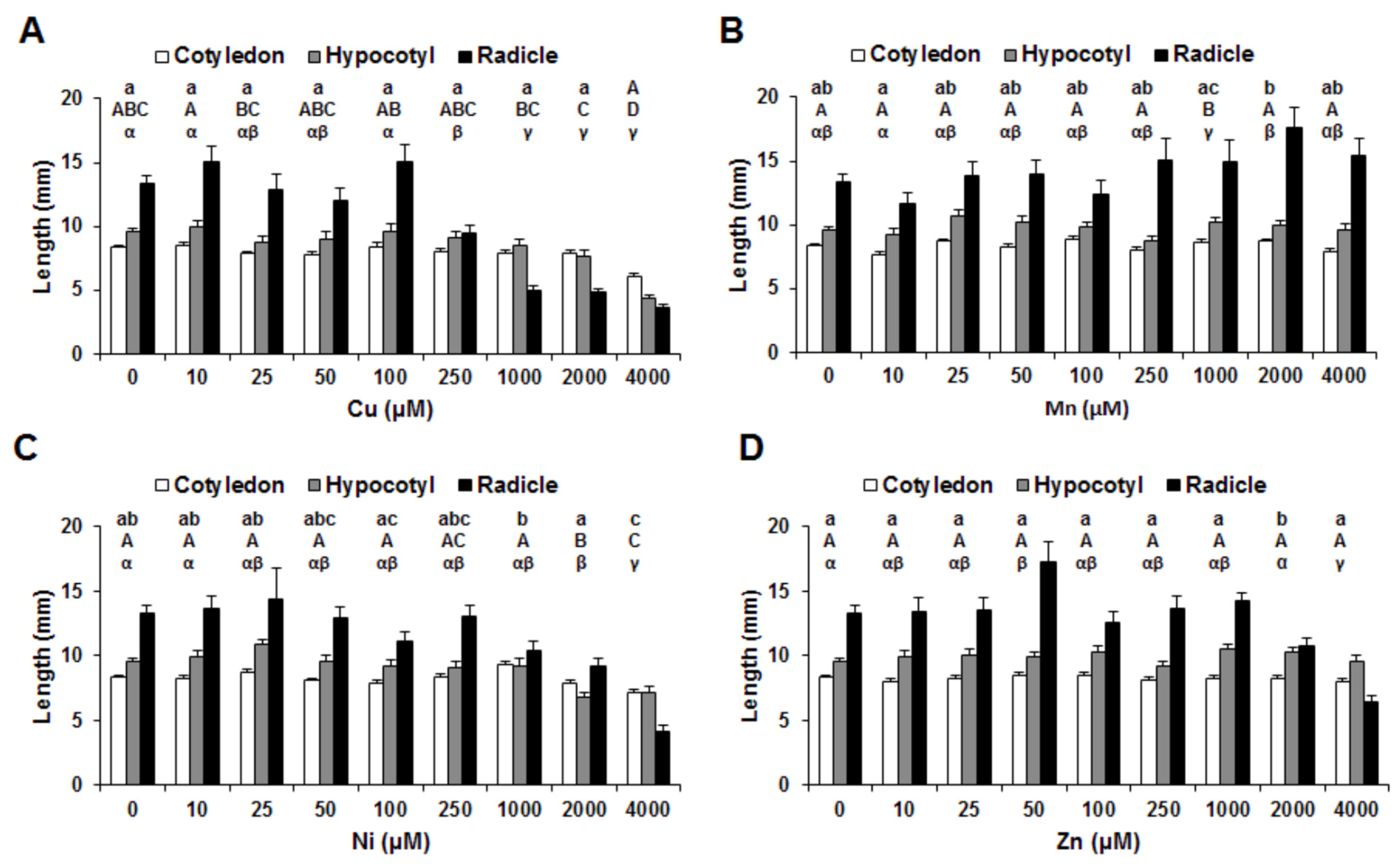
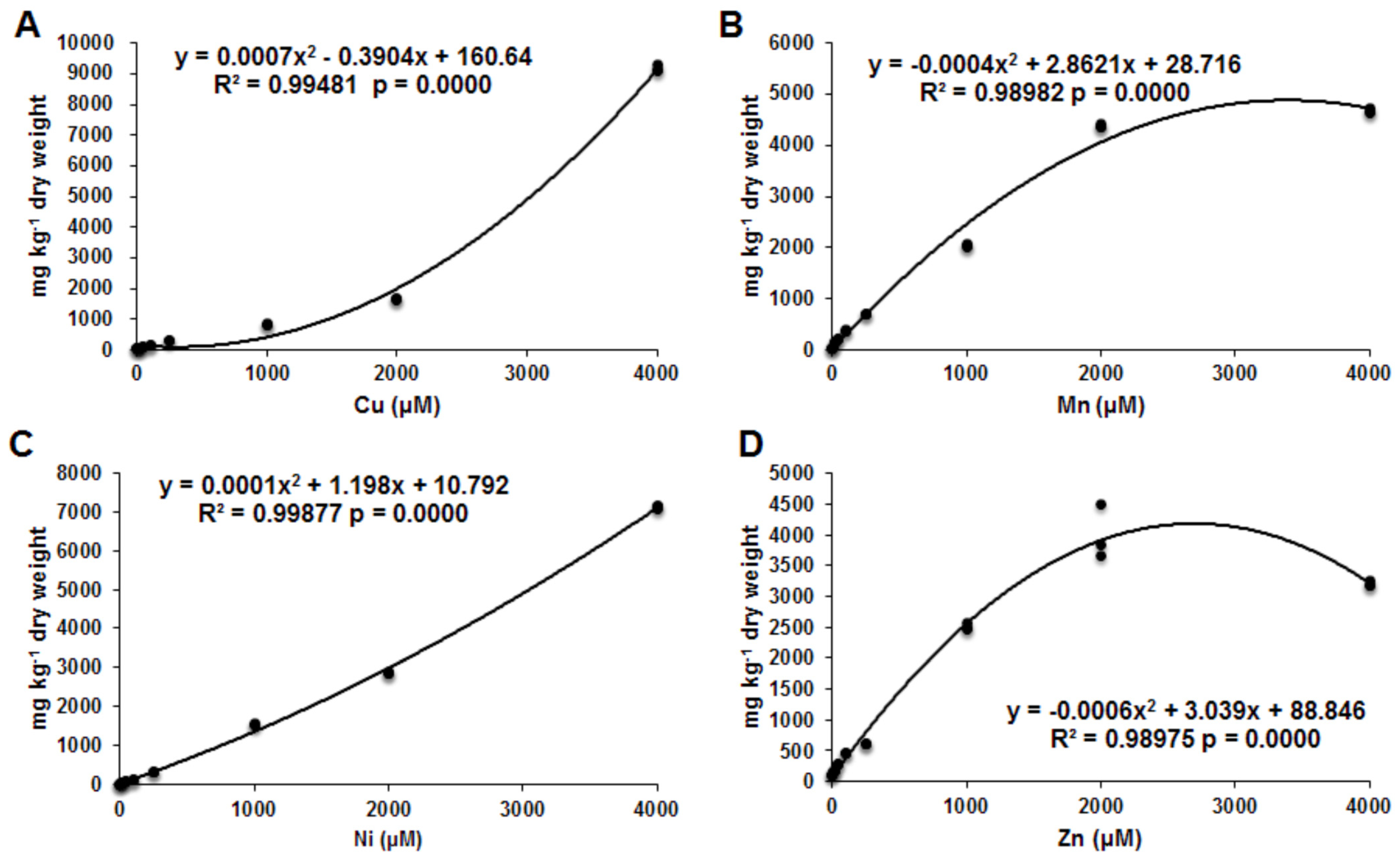
3. Results
4. Discussion
4.1. Copper
4.2. Manganese
4.3. Nickel
4.4. Zinc
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, T.P.; Bubb, J.M.; Lester, J.N. Metal accumulation within salt marsh environments: A review. Mar. Pollut. Bull. 1994, 28, 277–290. [Google Scholar] [CrossRef]
- Williams, T.P.; Bubb, J.M.; Lester, J.N. The occurrence and distribution of trace metals in halophytes. Chemosphere 1994, 28, 1189–1199. [Google Scholar] [CrossRef]
- Seneviratne, M.; Rajakaruna, N.; Rizwan, N.; Madawala, H.M.S.P.; Ok, Y.S.; Vithanage, M. Heavy metal-induced oxidative stress on seed germination and seedling development: A critical review. Environ. Geochem. Health 2019, 41, 1813–1831. [Google Scholar]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues progress eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef]
- Moreira, H.; Marques AP, G.C.; Rangel AO, S.S.; Castro, P.M.L. Heavy metal accumulation in plant species indigenous to a contaminated Portuguese site: Prospects for phytoremediation. Water Air Soil Pollut. 2011, 221, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Vithanage, M.; Dabrowska, B.B.; Mukherjee, B.; Sandhi, A.; Bhattacharya, P. Arsenic uptake by plants and possible phytoremediation applications: A brief overview. Environ. Chem. Lett. 2012, 10, 217–224. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; He, M.; Zeng, F.; Li, X. Accumulation of heavy metals in native plants growing on mininginfluenced sites in Jinchang: A typical industrial city (China). Environ. Earth Sci. 2017, 76, 446–460. [Google Scholar] [CrossRef]
- Nouri, H.; Borujeni, S.C.; Nirola, R.; Hassanli, A.; Beecham, S.; Alaghmand, S.; Saint, C.; Mulcahy, D. Application of green remediation on soil salinity treatment: A review on halophytoremediation. Process. Saf. Environ. Prot. 2017, 107, 94–107. [Google Scholar] [CrossRef]
- Oyuela-Leguizamo, M.A.; Fernández-Gómez, W.D.; Gutiérrez-Sarmiento, M.C. Native herbaceous plant species with potential use in phytoremediation of heavy metals spotlight on wetlands—A review. Chemosphere 2017, 168, 1230–1247. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.; Weis, P. Metal uptake transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Esposito, E. Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol. Adv. 2010, 28, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Bankaji, I.; Sleimi, N.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Effects of combined abiotic stresses on growth trace element accumulation and phytohormone regulation in two halophytic species. J. Plant Growth Regul. 2014, 33, 632–643. [Google Scholar] [CrossRef]
- Anjum, N.A.; Pereira, M.E.; Ahmad, I.; Duarte, A.C.; Umar, S.; Khan, N.A. Phytotechnologies: Remediation of Environmental Contaminants; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Anjum, N.A.; Ahmad, I.; Válega, M.; Mohmood, I.; Gill, S.S.; Tuteja, N.; Duarte, A.C.; Pereira, E. Salt marsh halophyte services to metal-metalloid remediation: Assessment of the processes and underlying mechanisms. Crit. Rev. Env. Sci. Technol. 2014, 44, 2038–2106. [Google Scholar] [CrossRef]
- Jayasri, M.A.; Suthindhiran, K. Effect of zinc and lead on the physiological and biochemical properties of aquatic plant Lemna minor: Its potential role in phytoremediation. Appl. Water Sci. 2017, 7, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Lefèvre, I.; Marchal, G.; Corrèal, E.; Zanuzzi, A.; Lutts, S. Variation in response to heavy metals during vegetative growth in Dorycnium pentaphyllum Scop. Plant Growth Regul. 2009, 59, 1–11. [Google Scholar] [CrossRef]
- Bae, J.; Benoit, D.L.; Watson, A.K. Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ. Pollut. 2016, 213, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, S.E.; Gharineh, M.H.; Afshari, R.T.; Ebrahimi, A. Effects of some heavy metals on seed germination characteristics of canola (Brassica napus) wheat (Triticum aestivum) and safflower (Carthamus tinctorious) to evaluate phytoremediation potential of these crops. J. Agric. Sci. 2012, 4, 11. [Google Scholar]
- Márquez-García, B.; Márquez, C.; Sanjosé, I.; Nieva FJ, J.; Rodríguez-Rubio, P.; Muñoz-Rodríguez, A.F. The effects of heavy metals on germination and seedling characteristics in two halophyte species in Mediterranean marshes. Mar. Pollut. Bull. 2013, 70, 119–124. [Google Scholar] [CrossRef]
- Peralta, J.R.; Gardea-Torresdey, J.L.; Tiemann, K.J.; Gomez, E.; Arteaga, S.; Rascon, E.; Parsons, J.G. Uptake and effects of five heavy metals on seed germination and plant growth in alfalfa (Medicago sativa L.). Bull. Environ. Contam. Toxicol. 2001, 66, 727–734. [Google Scholar] [CrossRef]
- Singh, D.; Nath, K.; Sharma, Y.K. Response of wheat seed germination and seedling growth under copper stress. J. Environ. Biol. 2007, 28, 409–414. [Google Scholar] [PubMed]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Kranner, I.; Colville, L. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011, 72, 93–105. [Google Scholar] [CrossRef]
- Ko, K.S.; Lee, P.K.; Kong, I.C. Evaluation of the toxic effects of arsenite chromate cadmium and copper using a battery of four bioassays. Appl. Microbiol. Biotechnol. 2012, 95, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, W.; Sun, Y.; Huo, X.; Li, S.; Zhou, Q. Phytoremediation of heavy metal contaminated saline soils using halophytes: Current progress and future perspectives. Environ. Rev. 2017, 25, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Fourati, E.; Wali, M.; Vogel-Mikuš, K.; Abdelly, C.; Ghnaya, T. Nickel tolerance accumulation and subcellular distribution in the halophytes Sesuvium portulacastrum and Cakile maritima. Plant Physiol. Biochem. 2016, 108, 295–303. [Google Scholar] [CrossRef]
- Milić, D.; Luković, J.; Ninkov, J.; Zeremski-Škorić, T.; Zorić, L.; Vasin, J.; Milić, S. Heavy metal content in halophytic plants from inland and maritime saline areas. Cent. Eur. J. Biol. 2012, 7, 307–317. [Google Scholar] [CrossRef]
- Wali, M.; Fourati, E.; Hmaeid, N.; Ghabriche, R.; Poschenrieder, C.; Abdelly, C.; Ghnaya, T. NaCl alleviates Cd toxicity by changing its chemical forms of accumulation in the halophyte Sesuvium portulacastrum. Environ. Sci. Pollut. Res. 2015, 22, 10769–10777. [Google Scholar] [CrossRef]
- Ouni, Y.; Mateos-Naranjo, E.; Abdelly, C.; Lakhdar, A. Interactive effect of salinity and zinc stress on growth and photosynthetic responses of the perennial grass Polypogon monspeliensis. Ecol. Eng. 2016, 95, 171–179. [Google Scholar] [CrossRef]
- Sousa, A.I.; Caçador, I.; Lillebø, A.I.; Pardal, M.A. Heavy metal accumulation in Halimione portulacoides: Intra- and extra-cellular metal binding sites. Chemosphere 2008, 70, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 509–528. [Google Scholar] [CrossRef]
- Aronson, J. Salt Tolerant Plant of the World; University of Arizona Press: Tucson, AZ, USA, 1989. [Google Scholar]
- Fernández-Illescas, F.; Nieva FJ, J.; Silva, I.; Tormo, R.; Muñoz-Rodríguez, A.F. Pollen production of Chenopodiaceae species at habitat and landscape scale in Mediterranean salt marshes: An ecological and phenological study. Rev. Palaeobot. Palynol. 2010, 161, 127–136. [Google Scholar] [CrossRef]
- Contreras-Cruzado, I.; Infante-Izquierdo, M.D.; Márquez-García, B.; Hermoso-López, V.; Polo, A.; Nieva, F.J.; Cartes-Barroso, J.B.; Castillo, J.M.; Muñoz-Rodríguez, A.F. Relationships between spatio-temporal changes in the sedimentary environment and halophytes zonation in salt marshes. Geoderma 2017, 305, 173–187. [Google Scholar] [CrossRef]
- Castro, R.; Pereira, S.; Lima, A.; Corticeiro, S.; Valega, M.; Pereira, E.; Duarte, A.; Figueira, E. Accumulation distribution and cellular partitioning of mercury in several halophytes of a contaminated salt marsh. Chemosphere 2006, 76, 1348–1355. [Google Scholar] [CrossRef]
- Reboreda, R.; Caçador, I. Halophyte vegetation influences in salt marsh retention capacity for heavy metals. Environ. Pollut. 2007, 146, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Válega, M.; Lillebø, A.I.; Pereira, M.E.; Caçador, I.; Duarte, A.C.; Pardal, M.A. Mercury in salt marshes ecosystems: Halimione portulacoides as biomonitor. Chemosphere 2008, 73, 1224–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, B.; Caetano, M.; Almeida, P.; Vale, C.; Caçador, I. Accumulation and biological cycling of heavy metal in four salt marsh species from Tagus estuary (Portugal). Environ. Pollut. 2010, 158, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Ahmad, I.; Válega, M.; Pacheco, M.; Figueira, E.; Duarte, A.C.; Pereira, E. Impact of seasonal fluctuations on the sediment-mercury its accumulation and partitioning in Halimione portulacoides and Juncus maritimus collected from Ria de Aveiro Coastal Lagoon (Portugal). Water Air Soil Pollut. 2011, 22, 1–15. [Google Scholar] [CrossRef]
- Sleimi, N.; Bankaji, I.; Dallai, M.; Kefi, O. Accumulation des éléments traces et tolérance au stress métallique chez les halophytes colonisant les bordures de la lagune de Bizerte (Tunisie). Rev. D’ecologie 2014, 69, 49–59. [Google Scholar]
- Almeida, C.M.R.; Diasa, A.C.; Mucha, A.P.; Bordaloa, A.A.; Vasconcelos, M.T.S.D. Study of the influence of different organic pollutants on Cu accumulation by Halimione portulacoides. Estuar. Coast. Shelf Sci. 2009, 85, 627–632. [Google Scholar] [CrossRef]
- Caetano, M.; Vale, C.; Cesario, R.; Fonseca, N. Evidence for preferential depths of metal retention in roots of salt marsh plants. Sci. Total Environ. 2008, 390, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Santos-Echeandía, J.; Vale, C.; Caetano, M.; Pereira, P.; Prego, R. Effect of tidal flooding on metal distribution in pore waters of marsh sediments and its transport to water column (Tagus estuary Portugal). Mar. Environ. Res. 2010, 70, 358–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Alcaraz, M.N.; Conesa, H.M.; Del Carmen Tercero, M.; Schulin, R.; Álvarez-Rogel, J.; Egea, C. The combined use of liming and Sarcocornia fruticosa development for phytomanagement of salt marsh soils polluted by mine wastes. J. Hazard. Mater. 2011, 186, 805–813. [Google Scholar] [CrossRef]
- Lutts, S.; Lefèvre, I.; Delpérée, C.; Kivits, S.; Dechamps, C.; Robledo, A.; Correal, E. Heavy metal accumulation by the halophyte species Mediterranean saltbush. J. Environ. Qual. 2004, 33, 1271–1279. [Google Scholar] [CrossRef]
- Abbad, A.; El Hadrami, A.; Benchaabane, A. Germination responses of the Mediterranean saltbush (Atriplex halimus L.) to NaCl treatment. J. Agron. Crop. Sci. 2004, 3, 111–114. [Google Scholar]
- Manousaki, E.; Kalogerakis, N. Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): Metal uptake in relation to salinity. Environ. Sci. Pollut. Res. 2009, 16, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Naranjo, E.; Andrades-Moreno, L.; Cambrollé, J.; Perez-Martin, A. Assessing the effect of copper on growth copper accumulation and physiological responses of grazing species Atriplex halimus: Ecotoxicological implications. Ecotoxicol. Environ. Saf. 2013, 90, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Nedjimi, B.; Daoud, Y. Cadmium accumulation in Atriplex halimus subsp. schweinfurthii and its influence on growth proline root hydraulic conductivity and nutrient uptake. Flora 2009, 204, 316–324. [Google Scholar]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Andrades-Moreno, L. Accumulation and tolerance characteristics of cadmium in a halophytic Cd-hyperaccumulator Arthrocnemum macrostachyum. J. Hazard. Mater. 2010, 184, 299–307. [Google Scholar] [CrossRef]
- Kachout, S.S.; Ben Mansoura, A.; Mechergui, R.; Leclerc, J.C.; Rejeb, M.N.; Ouerghi, Z. Accumulation of Cu Pb Ni and Zn in the halophyte plant Atriplex grown on polluted soil. J. Sci. Food Agric. 2012, 92, 336–342. [Google Scholar] [CrossRef]
- Eissa, M.A. Impact of compost on metals phytostabilization potential of two halophytes species. Int. J. Phytoremediation 2015, 17, 662–668. [Google Scholar] [CrossRef]
- Vromman, D.; Flores-Bavestrello, A.; Šlejkovec, Z.; Lapaille, S.; Teixeira-Cardoso, C.; Briceño, M.; Kumar, M.; Martínez, J.-P.; Lutts, S. Arsenic accumulation and distribution in relation to young seedling growth in Atriplex atacamensis. Sci. Total Environ. 2011, 412, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Midhat, L.; Ouazzani, N.; Esshaimi, M.; Ouhammou, A.; Mandi, L. Assessment of heavy metals accumulation by spontaneous vegetation: Screening for new accumulator plant species grown in Kettara mine-Marrakech Southern Morocco. Int. J. Phytoremediation 2017, 19, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Pedro, C.A.; Santos, M.S.; Ferreira, S.M.; Gonçalves, S.C. The influence of cadmium contamination and salinity on the survival growth and phytoremediation capacity of the saltmarsh plant Salicornia ramosissima. Mar. Environ. Res. 2013, 92, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Bankaji, I.; Caçador, I.; Sleimi, N. Physiological and biochemical responses of Suaeda fruticosa to cadmium and copper stresses: Growth nutrient uptake antioxidant enzymes phytochelatin and glutathione levels. Environ. Sci. Pollut. Res. 2015, 22, 13058–13069. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, J.; Liu, L.; Qin, S.; Yu, J.; Liu, J. Petroleum pollution and its ecological impact on Salsola glauca Bunge in the Yellow River Delta Nature Reserve China. Fresenius Environ. Bull. 2011, 20, 904–1909. [Google Scholar]
- Zhang, X.; Lia, M.; Yang, H.; Lia, X.; Cui, Z. Physiological responses of Suaeda glauca and Arabidopsis thaliana inphytoremediation of heavy metals. J. Environ. Manag. 2018, 223, 132–139. [Google Scholar] [CrossRef]
- Panda, A.; Rangani, J.; Kumari, A.; Parida, A.K. Efficient regulation of Arsenic translocation to shoot tissue and modulation of phytochelatin levels and antioxidative defense system confers salinity and arsenic tolerance in the halophyte Suaeda maritima. Environ. Exp. Bot. 2017, 143, 149–171. [Google Scholar] [CrossRef]
- Wu, H.; Liu, X.; Zhao, J.; Yu, J. Regulation of metabolites gene expression and antioxidant enzymes to environmentally relevant lead and zinc in the halophyte Suaeda salsa. J. Plant Growth Regul. 2013, 32, 353–361. [Google Scholar] [CrossRef]
- Shrestha, B.; Lipe, S.; Johnson, K.A.; Zhang, T.Q.; Retzlaff, W.; Lin, Z.Q. Soil hydraulic manipulation and organic amendment for the enhancement of selenium volatilization in a soil-pickleweed system. Plant Soil 2006, 288, 189–196. [Google Scholar] [CrossRef]
- Creager, R.A. The biology of mediterranean saltwort Salsola vermiculata. Weed Technol. 1988, 2, 369–374. [Google Scholar] [CrossRef]
- Muñoz-Rodríguez, A.F.; Sanjosé, I.; Márquez-García, B.; Infante-Izquierdo, M.D.; Polo-Ávila, A.; Nieva, F.J.; Castillo, J.M. Germination syndromes in response to salinity of Chenopodiaceae halophytes along the intertidal gradient. Aquat. Bot. 2017, 139, 48–56. [Google Scholar] [CrossRef]
- Fernández-Illescas, F.; Cabrera, J.; Nieva FJ, J.; Márquez-García, B.; Sánchez-Gullón, E.; Muñoz-Rodríguez, A.F. Production of aborted pollen in marsh species of Chenopodiaceae: Evidence of partial male sterility in Suadeaea and Salsoleae species. Plant Syst. Evol. 2010, 288, 167–176. [Google Scholar] [CrossRef]
- Nelson, C.H.; Lamothe, P.J. Heavy metal anomalies in the Tinto and Odiel River and estuary system Spain. Estuaries 1993, 16, 496–511. [Google Scholar] [CrossRef]
- Sainz, A.; Grande, J.A.; de la Torre, M.L. Characterization of heavy metal discharge into the Ria of Huelva. Environ. Int. 2004, 30, 557–566. [Google Scholar] [CrossRef]
- Pérez-López, R.; Nieto, J.M.; López-Cascajosa, M.J.; Díaz-Blanco, M.J.; Sarmiento, A.M.; Oliveira, V.; Sánchez-Rodas, D. Evaluation of heavy metals and arsenic speciation discharged by the industrial activity on the Tinto–Odiel estuary SW Spain. Mar. Pollut. Bull. 2011, 62, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Boularbah, A.; Schwartz, C.; Bitton, G.; Morel, J.L. Heavy metals contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 2006, 63, 811–817. [Google Scholar] [CrossRef]
- Muñoz-Rodríguez, A.F.; Rodríguez-Rubio, P.; Nieva FJ, J.; Fernández-Illescas, F.; Sánchez-Gullón, E.; Soto, J.M.; Hermoso-López, V.; Márquez-García, B. The importance of bracteoles in ensuring Atriplex halimus germination under optimal conditions. Fresenius Environ. Bull. 2012, 21, 3521–3526. [Google Scholar]
- Bae, J.; Mercier, G.; Watson, A.K.; Benoit, D.L. Seed germination test for heavy metal phytotoxicity assessment. Can. J. Plant Sci. 2014, 94, 1519–1521. [Google Scholar] [CrossRef] [Green Version]
- Barba-Brioso, C.; Fernández-Caliani, J.C.; Miras, A.; Cornejo, J.; Galán, E. Multisource water pollution in a highly anthropized wetland system associated with the estuary of Huelva (SW Spain). Mar. Pollut. Bull. 2010, 60, 1259–1269. [Google Scholar] [CrossRef]
- Infante-Izquierdo, M.D.; Hernandez, P.; Polo, A.; Marquez-Garcia, B.; Nieva FJ, J.; Davila, C.; Molina, C.; Muñoz-Rodriguez, A.F. Effects of light salt and burial depth on the germination and initial seedling development of Oenothera drummondii. Fresenius Environ. Bull. 2017, 26, 5502–5510. [Google Scholar]
- Wilkins, D.A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Lei, Y.; Korpelainen, H.; Li, C. Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere 2007, 68, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Al Harbawee, W.E.Q.; Kluchagina, A.N.; Anjum, N.A.; Bashmakov, D.I.; Lukatkin, A.S.; Pereira, E. Evaluation of cotton burdock (Arctium tomentosum Mill.) responses to multi-metal exposure. Environ. Sci Pollut. Res. 2017, 24, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Luque, C.J.; Castellanos, E.M.; Castillo, J.M.; González, M.; González-Vilches, M.C.; Figueroa, M.E. Metals in halophytes of a contaminated Estuary (Odiel saltmarshes SW Spain). Mar. Pollut. Bull. 1999, 38, 49–51. [Google Scholar] [CrossRef]
- Stenner, R.D.; Nikless, G. Heavy metals in organisms of the Atlantic coast of Southwestern Spain and Portugal. Mar. Pollut. Bull. 1975, 6, 89–92. [Google Scholar] [CrossRef]
- Feng, J.; Lin, Y.; Yang, Y.; Shen, Q.; Huang, J.; Wang, S.; Zhu, X.; Li, Z. Tolerance and bioaccumulation of Cd and Cu in Sesuvium portulacastrum. Ecotoxicol. Environ. Saf. 2018, 147, 306–312. [Google Scholar] [CrossRef]
- Oh, S.-J.; Koh, S.-C. Copper and zinc uptake capacity of a Sorghum-sudangrass hybrid selected for in situ phytoremediation of soils polluted by heavy metals. J. Environ. Sci. Int. 2015, 24, 1501–1511. [Google Scholar] [CrossRef]
- Clarkson, D.T. The uptake and translocation of manganese by plant roots. In Manganese in Soil and Plants; Graham, R.D., Hannam, R.J., Uren, N.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 101–111. [Google Scholar]
- Foy, C.D.; Chaney, R.L.; White, M.C. The physiology of metal toxicity in plants. Annu. Rev. Physiol. 1978, 29, 511. [Google Scholar]
- Horst, W.J.; Marschner, H. Effect of silicon on manganese tolerance of bean plants (Phaseolus vulgaris L.). Plant Soil 1978, 50, 287. [Google Scholar] [CrossRef]
- Lidon, F.C.; Teixeira, M.G. Rice tolerance to excess Mn: Implications in the chloroplast lamellae and synthesis of a novel Mn protein. Plant Physiol. Biochem. 2000, 38, 969–978. [Google Scholar] [CrossRef]
- Ahmad, M.S.A.; Ashraf, M. Essential roles and hazardous effects of nickel in plants. Rev. Environ. Contam. Toxicol. 2011, 214, 125–167. [Google Scholar] [PubMed]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of nickel in plants: A role in plant stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef] [Green Version]
- Ain, Q.; Akhtar, J.; Amjad, M.; Haq, M.A.; Saqib, Z.A. Effect of Enhanced Nickel Levels on Wheat Plant Growth and Physiology under Salt Stress. Commun. Soil Sci. Plant Anal. 2016, 47, 2538–2546. [Google Scholar] [CrossRef]
- Chen, C.; Huang, D.; Liu, J. Functions and Toxicity of Nickel in Plants: Recent Advances and Future Prospects. J. Clean. Prod. 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Parlak, K.U. Effect of nickel on growth and biochemical characteristics of wheat (Triticum aestivum L.) seedlings. NJAS—Wagening. J. Life Sci. 2016, 76, 1–5. [Google Scholar] [CrossRef]
- Gupta, V.; Jatav, P.K.; Verma, R.; Kothari, S.L.; Kachhwaha, S. Nickel accumulation and its effect on growth physiological and biochemical parameters in millets and oats. Environ. Sci. Pollut. Res. 2017, 24, 23915–23925. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.; Kozhevnikova, A. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Nedhi, A.; Singh, L.J.; Singh, S.I. Effect of cadmium and nickel on germination early seedling growth and photosynthesis of wheat and pigeon pea. Int. J. Trop. Agric. 1990, 8, 141–147. [Google Scholar]
- Thakur, S.; Sharma, S.S. Characterization of seed germination seedling growth and associated metabolic responses of Brassica juncea L. cultivars to elevated nickel concentrations. Protoplasma 2016, 253, 571–580. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.; Fariduddin, Q.; Hayat, S.; Ahmad, A. Nickel: An Overview of Uptake Essentiality and Toxicity in Plants. Bull. Environ. Contam. Toxicol. 2011, 86, 1–17. [Google Scholar] [CrossRef]
- Kozhevnikova, A.D.; Erlikh, N.T.; Zhukovskaya, N.V.; Obroucheva, N.V.; Ivanov, V.B.; Belinskaya, A.A.; Khutoryanskaya, M.Y.; Seregin, I.V. Nickel and zinc effects accumulation and distribution in ruderal plants Lepidium ruderale and Capsella bursa-pastoris. Acta Physiol. Plant 2014, 36, 3291–3305. [Google Scholar] [CrossRef]
- Mohseni, R.; Ghaderian, S.M.; Ghasemi, R.; Schat, H. Differential effects of iron starvation and iron excess on nickel uptake kinetics in two Iranian nickel hyperaccumulators Odontarrhena bracteata and Odontarrhena inflata. Plant Soil 2018, 428, 153–162. [Google Scholar] [CrossRef]
- Pandey, S.N. Accumulation of heavy metals (Cd Cr Cu Ni and Zn) in Raphanus sativus L. and Spinacia oleracea L. plants irrigated with industrial effluent. J. Environ. Biol. 2006, 27, 381–384. [Google Scholar] [PubMed]
- Ivanov, Y.V.; Kartashov, A.V.; Ivanova, A.I.; Savochkin, Y.V.; Kuznetsov, V.V. Effects of zinc on Scots pine (Pinus sylvestris L.) seedlings grown in hydroculture. Plant Physiol. Biochem. 2016, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Homer, F.A.; Morrison, R.S.; Brooks, R.R.; Clemens, J.; Reeves, R.D. Comparative studies of nickel cobalt and copper uptake by some nickel hyperaccumulators of the genus Alyssum. Plant Soil 1991, 138, 195–2115. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and excluders: Strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
| Concentration (μM) | Germination (%) | t50 (Days) | IT Cotyledon | IT Hypocotyl | IT Roots |
|---|---|---|---|---|---|
| Cu (μM) | |||||
| 0 | 94.65 ± 0.04 a | 1.43 ± 0.12 a | 100 | 100 | 100 |
| 10 | 94.66 ± 0.04 ab | 1.35 ± 0.14 a | 102 | 104 | 113 |
| 25 | 92.00 ± 0.08 ab | 1.53 ± 0.14 a | 94 | 91 | 97 |
| 50 | 96.00 ± 0.04 ab | 1.47 ± 0.1 a | 93 | 94 | 91 |
| 100 | 92.00 ± 0.06 ab | 1.28 ± 0.24 a | 101 | 100 | 113 |
| 250 | 93.33 ± 0.04 ab | 1.30 ± 0.19 a | 97 | 96 | 71 * |
| 1000 | 98.66 ± 0.02 a | 1.30 ± 0.05 a | 95 | 88 | 38 * |
| 2000 | 93.33 ± 0.08 a | 1.43 ± 0.16 a | 95 | 80 | 36 * |
| 4000 | 80.00 ± 0.06 b | 1.35 ± 0.10 a | 73 | 46 * | 28 * |
| Mn (μM) | |||||
| 0 | 94.65 ± 0.04 a | 1.43 ± 0.12 a | 100 | 100 | 100 |
| 10 | 93.33 ± 0.02 a | 1.33 ± 0.37 a | 92 | 96 | 88 |
| 25 | 91.83 ± 0.04 a | 1.44 ± 0.10 a | 104 | 112 | 104 |
| 50 | 94.66 ± 0.02 a | 1.35 ± 0.12 a | 98 | 107 | 105 |
| 100 | 89.33 ± 0.06 a | 1.36 ± 0.23 a | 106 | 102 | 93 |
| 250 | 96.00 ± 0.04 a | 1.55 ± 0.03 a | 96 | 92 | 113 |
| 1000 | 89.11 ± 0.09 a | 1.40 ± 0.09 a | 103 | 106 | 113 |
| 2000 | 98.66 ± 0.02 a | 1.40 ± 0.24 a | 104 | 104 | 133 |
| 4000 | 90.50 ± 0.04 a | 1.33 ± 0.18 a | 95 | 100 | 116 |
| Ni (μM) | |||||
| 0 | 94.65 ± 0.04 a | 1.43 ± 0.12 a | 100 | 100 | 100 |
| 10 | 97.33 ± 0.02 a | 1.38 ± 0.08 a | 99 | 103 | 103 |
| 25 | 98.66 ± 0.02 a | 1.41 ± 0.10 a | 104 | 113 | 108 |
| 50 | 94.66 ± 0.06 a | 1.30 ± 0.22 a | 96 | 100 | 97 |
| 100 | 100.00 ± 0.00 a | 1.31 ± 0.17 a | 94 | 96 | 83 |
| 250 | 89.22 ± 0.04 a | 1.36 ± 0.06 a | 100 | 94 | 98 |
| 1000 | 94.55 ± 0.06 a | 1.34 ± 0.08 a | 111 | 96 | 78 |
| 2000 | 94.55 ± 0.02 a | 1.53 ± 0.09 a | 95 | 70 * | 69 * |
| 4000 | 90.66 ± 0.02 a | 1.31 ± 0.23 a | 85 * | 74 * | 31 * |
| Zn (μM) | |||||
| 0 | 94.65 ± 0.04 a | 1.43 ± 0.12 a | 100 | 100 | 100 |
| 10 | 93.33 ± 0.02 ab | 1.31 ± 0.16 a | 96 | 103 | 101 |
| 25 | 97.33 ± 0.02 a | 1.66 ± 0.32 ab | 99 | 104 | 102 |
| 50 | 98.61 ± 0.02 a | 1.48 ± 0.14 ab | 101 | 103 | 130 * |
| 100 | 92.00 ± 0 ab | 1.67 ± 0.16 ab | 102 | 107 | 95 |
| 250 | 96.00 ± 0.04 a | 1.61 ± 0.12 ab | 96 | 95 | 102 |
| 1000 | 96.00 ± 0.04 a | 1.56 ± 0.06 ab | 99 | 110 | 107 |
| 2000 | 94.66 ± 0.06 a | 1.59 ± 0.04 ab | 99 | 106 | 81 |
| 4000 | 79.83 ± 0.1 b | 1.77 ± 0.08 b | 96 | 100 | 49 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanjosé, I.; Navarro-Roldán, F.; Infante-Izquierdo, M.D.; Martínez-Sagarra, G.; Devesa, J.A.; Polo, A.; Ramírez-Acosta, S.; Sánchez-Gullón, E.; Jiménez-Nieva, F.J.; Muñoz-Rodríguez, A.F. Accumulation and Effect of Heavy Metals on the Germination and Growth of Salsola vermiculata L. Seedlings. Diversity 2021, 13, 539. https://doi.org/10.3390/d13110539
Sanjosé I, Navarro-Roldán F, Infante-Izquierdo MD, Martínez-Sagarra G, Devesa JA, Polo A, Ramírez-Acosta S, Sánchez-Gullón E, Jiménez-Nieva FJ, Muñoz-Rodríguez AF. Accumulation and Effect of Heavy Metals on the Germination and Growth of Salsola vermiculata L. Seedlings. Diversity. 2021; 13(11):539. https://doi.org/10.3390/d13110539
Chicago/Turabian StyleSanjosé, Israel, Francisco Navarro-Roldán, María Dolores Infante-Izquierdo, Gloria Martínez-Sagarra, Juan Antonio Devesa, Alejandro Polo, Sara Ramírez-Acosta, Enrique Sánchez-Gullón, Francisco Javier Jiménez-Nieva, and Adolfo Francisco Muñoz-Rodríguez. 2021. "Accumulation and Effect of Heavy Metals on the Germination and Growth of Salsola vermiculata L. Seedlings" Diversity 13, no. 11: 539. https://doi.org/10.3390/d13110539
APA StyleSanjosé, I., Navarro-Roldán, F., Infante-Izquierdo, M. D., Martínez-Sagarra, G., Devesa, J. A., Polo, A., Ramírez-Acosta, S., Sánchez-Gullón, E., Jiménez-Nieva, F. J., & Muñoz-Rodríguez, A. F. (2021). Accumulation and Effect of Heavy Metals on the Germination and Growth of Salsola vermiculata L. Seedlings. Diversity, 13(11), 539. https://doi.org/10.3390/d13110539







