Characterization of the Multidimensional Functional Space of the Aquatic Macroinvertebrate Assemblages in a Biosphere Reserve (Central México)
Abstract
:1. Introduction
2. Materials and Methods
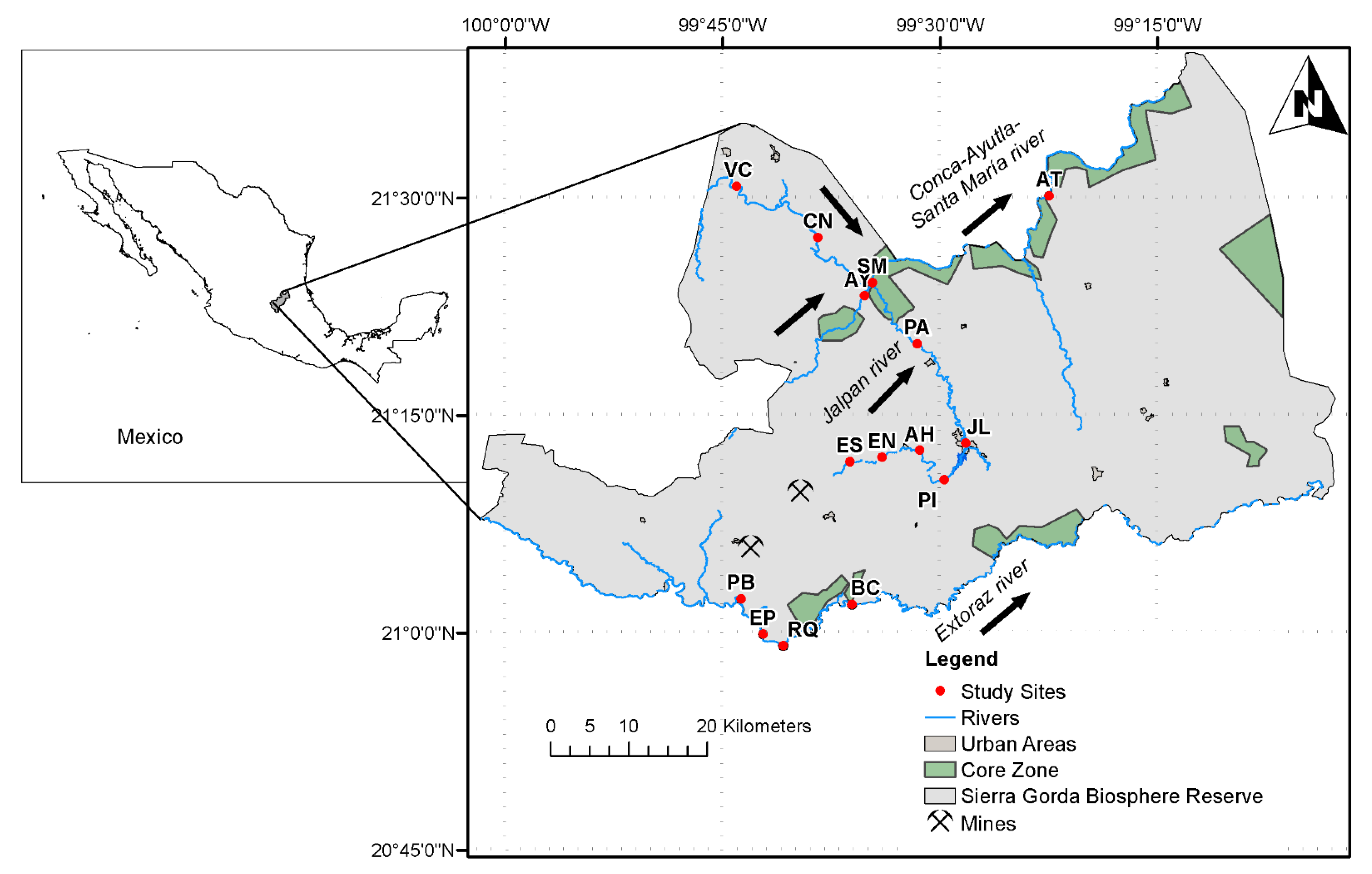
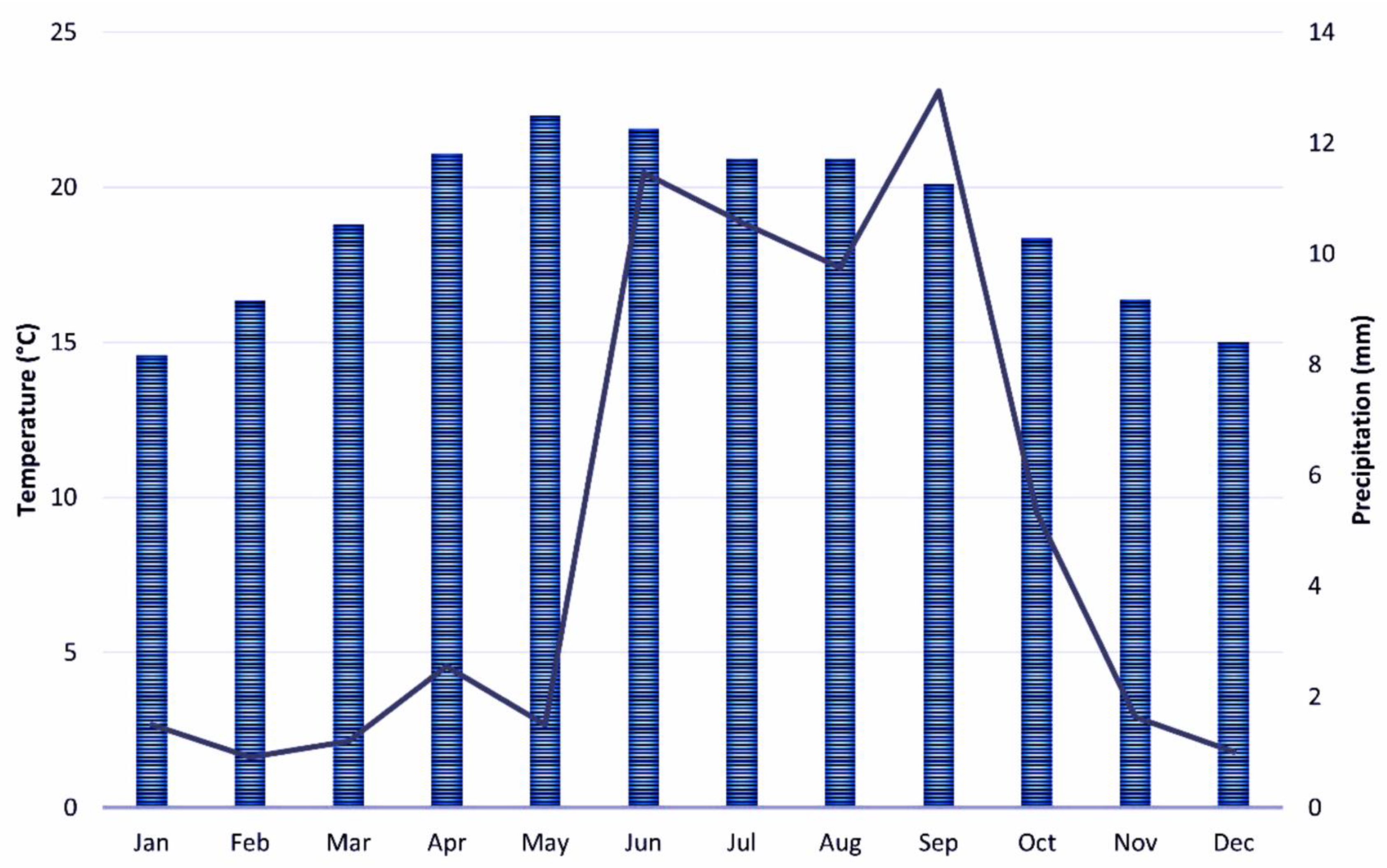
2.1. Study Area
2.2. Field Sampling and Environmental Variables
2.3. Characterization of Sites with Environmental Indices
2.4. Macroinvertebrate Monitoring
2.5. Characterization of the Multifunctional Space
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Strayer, D.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. North Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, D.; Graham, N.; Villéger, S.; Mason, N.W.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Rico-Sánchez, A.E.; Sundermann, A.; López-López, E.; Torres-Olvera, M.J.; Mueller, S.A.; Haubrock, P.J. Biological diversity in protected areas: Not yet known but already threatened. Glob. Ecol. Conserv. 2020, 22, e01006. [Google Scholar] [CrossRef]
- Almeida, B.D.A.; Green, A.J.; Sebastián-González, E.; Dos Anjos, L. Comparing species richness, functional diversity and functional composition of waterbird communities along environmental gradients in the neotropics. PLoS ONE 2018, 13, e0200959. [Google Scholar] [CrossRef]
- Schmera, D.; Heino, J.; Podani, J.; Erős, T.; Dolédec, S. Functional diversity: A review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiol. 2017, 787, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Allan, J.D.; Castillo, M.M.; Capps, K.A. Stream Ecology: Structure and Function of Running Waters; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Luiza-Andrade, A.; Montag, L.; Juen, L. Functional diversity in studies of aquatic macroinvertebrates community. Science 2017, 111, 1643–1656. [Google Scholar] [CrossRef]
- Rezende, R.S.; Santos, A.M.; Henke-Oliveira, C.; Gonçalves, J.F., Jr. Effects of spatial and environmental factors on benthic a macroinvertebrate community. Zoologia 2014, 31, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Melo, A.S.; Froehlich, C.G. Macroinvertebrates in neotropical streams: Richness patterns along a catchment and assemblage structure between 2 seasons. J. N. Am. Benthol. Soc. 2001, 20, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Resh, V.H. Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environ. Monit. Assess. 2008, 138, 131–138. [Google Scholar] [CrossRef]
- Oliveira, A.; Callisto, M. Benthic macroinvertebrates as bioindicators of water quality in an Atlantic forest fragment. Iheringia Série Zool. 2010, 100, 291–300. [Google Scholar] [CrossRef]
- Ruiz-Picos, R.A.; Kohlmann, B.; Sedeño-Díaz, J.E.; López-López, E. Assessing ecological impairments in Neotropical rivers of Mexico: Calibration and validation of the Biomonitoring Working Party Index. Int. J. Environ. Sci. Technol. 2017, 14, 1835–1852. [Google Scholar] [CrossRef]
- Serna, D.J.; Tamaris-Turizo, C.E.; Gutiérrez Moreno, L.C. Spatial and temporal distribution of Trichoptera (Insecta) larvae in the Manzanares river Sierra Nevada of Santa Marta (Colombia). Rev. Biol. Trop. 2015, 63, 465–477. [Google Scholar] [CrossRef]
- Moretti, M.S.; Loyola, R.D.; Becker, B.; Callisto, M. Leaf abundance and phenolic concentrations codetermine the selection of case-building materials by Phylloicus sp. (Trichoptera, Calamoceratidae). Hydrobiologia 2009, 630, 199–206. [Google Scholar] [CrossRef]
- Kohlmann, B.; Vásquez, D.; Arroyo, A.; Springer, M. Taxonomic and Functional Diversity of Aquatic Macroinvertebrate Assemblages and Water Quality in Rivers of the Dry Tropics of Costa Rica. Front. Environ. Sci. 2021, 309. [Google Scholar] [CrossRef]
- Colzani, E.; Siqueira, T.; Suriano, M.T.; Roque, F.O. Responses of aquatic insect functional diversity to landscape changes in Atlantic forest. Biotropica 2013, 45, 343–350. [Google Scholar] [CrossRef]
- Motta Díaz, A.J.; Longo, M.; Aranguren-Riaño, N. Temporal variation of taxonomic diversity and functional traits of aquatic macroinvertebrates in temporary rivers on Old Providence Island, Colombia. Actual. Biológicas 2017, 39, 82–100. [Google Scholar] [CrossRef] [Green Version]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Mason, N.W.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Morrone, J.J. Biogeographic regionalization of the mexican transition zone. In The Mexican Transition Zone; Springer: Cham, Switzerland, 2020; pp. 103–155. [Google Scholar] [CrossRef]
- Servicio Geológico Mexicano Conoce GeoInfoMex en 3D. Available online: https://www.gob.mx/sgm/articulos/conoce-el-sistema-de-consulta-de-informacion-geocientifica-geoinfomex?idiom=es (accessed on 18 February 2021).
- Carabias Lillo, J.; Provencio, E.; de la Maza Elvira, J.; Ruiz Corzo, M. Programa de Manejo Reserva de la Biosfera Sierra Gorda. 1999. Available online: http://www.paot.org.mx/centro/ine-semarnat/anp/AN15.pdf (accessed on 18 September 2021).
- Ejecutivo, P. Decreto de la Reserva de la Biosfera Sierra Gorda. 1997, pp. 1–11. Available online: http://dof.gob.mx/nota_detalle.php?codigo=4879875&fecha=19/05/1997 (accessed on 18 February 2021).
- Sanginés, A.E. Pobreza y Medio Ambiente en México: Teoría y Evaluación de una Política Pública; Instituto Nacional de Ecología/Universidad Iberoamericana/Instituto Nacional de Administración: Ciudad de México, Mexico, 2003; ISBN 9688594520. [Google Scholar]
- Instituto Nacional de Estadística, G.e I. México en Cifras. Available online: https://www.inegi.org.mx/app/areasgeograficas/?ag=22 (accessed on 25 August 2021).
- Baird, R.B. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- Barbour, M.T.; Stribling, J.B.; Verdonschot, P.F.M. The multihabitat approach of USEPA’s rapid bioassessment protocols: Benthic macroinvertebrates. Limnetica 2006. [Google Scholar] [CrossRef]
- Cornejo, A.; Lopez, E.; Bernal, J. Protocolo de Biomonitoreo para la Vigilancia de la Calidad del Agua en Afluentes Superficiales de Panama; Instituto Conmemorativo Gorgas de Estudios de la Salud: Panamá, Panama, 2019; 81p, ISBN 978-9962-13-053-6. [Google Scholar]
- Rodríguez-Romero, A.J.; Rico-Sánchez, A.E.; Mendoza-Martínez, E.; Gómez-Ruiz, A.; Sedeño-Díaz, J.E.; López-López, E. Impact of changes of land use on water quality, from tropical forest to anthropogenic occupation: A multivariate approach. Water 2018, 10, 1518. [Google Scholar] [CrossRef] [Green Version]
- INEGI Instituto Nacional de Estadística, Geografía e Informática. 2020. Available online: https://www.inegi.org.mx/temas/mapadigital/ (accessed on 14 September 2021).
- Glovis, Servicio Geológico de EE. UU. Servicio Geológico. 2021. Available online: https://glovis.usgs.gov/app (accessed on 15 July 2021).
- Tarpley, J.D.; Schneider, S.R.; Money, R.L. Global Vegetation Indices from the NOAA-7 Meteorological Satellite. J. Clim. Appl. Meteorol. 1984, 23, 491–494. [Google Scholar] [CrossRef]
- Dinius, S.H. Design of an Index of Water Quality. JAWRA J. Am. Water Resour. Assoc. 1987, 23, 833–843. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. Taxonomic sufficiency in freshwater ecosystems: Effects of taxonomic resolution, functional traits, and data transformation. Freshw. Sci. 2013, 32, 762–778. [Google Scholar] [CrossRef]
- Thorp and Covich’s Freshwater Invertebrates. In Thorp and Covich’s Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2018. [CrossRef]
- Thorp, J.H.; Rogers, D.C. Thorp, J.H.; Rogers, D.C. Thorp and Covich’s Freshwater Invertebrates. In Ecology and Classification of North American Freshwater Invertebrates; Academic press: Cambridge, MA, USA, 2014; ISBN 9780080889818. [Google Scholar]
- Merritt, R.W.; Cummins, K.W. (Eds.) An Introduction to the Aquatic Insects of North America; Kendall Hunt: Dubuque, IA, USA, 2008. [Google Scholar]
- Poff, N.L.; Olden, J.D.; Vieira, N.K.; Finn, D.S.; Simmons, M.P.; Kondratieff, B.C. Functional trait niches of North American lotic insects: Traits-based ecological applications in light of phylogenetic relationships. J. N. Am. Benthol. Soc. 2006, 25, 730–755. [Google Scholar] [CrossRef] [Green Version]
- Vieira, N.K.; Poff, N.L.; Carlisle, D.M.; Moulton, S.R.; Koski, M.L.; Kondratieff, B.C. A database of lotic invertebrate traits for North America. US Geol. Surv. Data Ser. 2006, 187, 1–15. [Google Scholar] [CrossRef]
- Jesus, T.M.G.M.D. Ecological, anatomical and physiological traits of benthic macroinvertebrates: Their use on the health characterization of freshwater ecosystems. Limnetica 2008, 27, 079–092. [Google Scholar]
- Ramírez, A.; Gutiérrez-Fonseca, P.E. Functional feeding groups of aquatic insect families in Latin America: A critical analysis and review of existing literature. Rev. Biol. Trop. 2014, 62, 155–167. [Google Scholar] [CrossRef]
- Mondy, C.P.; Usseglio-Polatera, P. Using fuzzy-coded traits to elucidate the non-random role of anthropogenic stress in the functional homogenisation of invertebrate assemblages. Freshw. Biol. 2014, 59, 584–600. [Google Scholar] [CrossRef]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in E uropean fish assemblages. Glob. Ecol. Biogeogr. 2013, 22, 671–681. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundar, S.; Heino, J.; Roque, F.D.O.; Simaika, J.P.; Melo, A.S.; Tonkin, J.D.; Silva, D.P. Conservation of freshwater macroinvertebrate biodiversity in tropical regions. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1238–1250. [Google Scholar] [CrossRef]
- Torres-Olvera, M.J.; Durán-Rodríguez, O.Y.; Torres-García, U.; Pineda-López, R.; Ramírez-Herrejón, J.P. Validation of an index of biological integrity based on aquatic macroinvertebrates assemblages in two subtropical basins of central Mexico. Lat. Am. J. Aquat. Res. 2018, 46, 945–960. [Google Scholar] [CrossRef]
- Voß, K.; Schäfer, R.B. Taxonomic and functional diversity of stream invertebrates along an environmental stress gradient. Ecol. Indic. 2017, 81, 235–242. [Google Scholar] [CrossRef]
- White, J.C.; Hill, M.J.; Bickerton, M.A.; Wood, P.J. Macroinvertebrate taxonomic and functional trait compositions within lotic habitats affected by river restoration practices. Environ. Manag. 2017, 60, 513–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maasri, A. A global and unified trait database for aquatic macroinvertebrates: The missing piece in a global approach. Front. Environ. Sci. 2019, 7, 65. [Google Scholar] [CrossRef]
- Usseglio-Polatera, P.; Bournaud, M.; Richoux, P.; Tachet, H. Biomonitoring through biological traits of benthic macroinvertebrates: How to use species trait databases? In Assessing the Ecological Integrity of Running Waters; Springer: Dordrecht, The Netherlands, 2000; pp. 153–162. [Google Scholar] [CrossRef]
- Tomanova, S.; Moya, N.; Oberdorff, T. Using macroinvertebrate biological traits for assessing biotic integrity of neotropical streams. River Res. Appl. 2008, 24, 1230–1239. [Google Scholar] [CrossRef]
- Martini, S.; Larras, F.; Boyé, A.; Faure, E.; Aberle, N.; Archambault, P.; Ayata, S.D. Functional trait-based approaches as a common framework for aquatic ecologists. Limnol. Oceanogr. 2021, 66, 965–994. [Google Scholar] [CrossRef]
- Díaz-Rojas, C.A.; Motta-Díaz, Á.J.; Aranguren-Riaño, N. Study of the taxonomic and functional diversity of the macroinvertebrate assemblages in an Andean mountain river. Rev. Biol. Trop. 2020, 68, 132–149. [Google Scholar] [CrossRef]
- Washko, S.; Bogan, M.T. Global patterns of aquatic macroinvertebrate dispersal and functional feeding traits in aridland rock pools. Front. Environ. Sci. 2019, 7, 106. [Google Scholar] [CrossRef]
- Theodoropoulos, C.; Vourka, A.; Stamou, A.; Rutschmann, P.; Skoulikidis, N. Response of freshwater macroinvertebrates to rainfall-induced high flows: A hydroecological approach. Ecol. Indic. 2017, 73, 432–442. [Google Scholar] [CrossRef]
- Martínez-Trinidad, S.; Hernández Silva, G.; Ramírez Islas, M.E.; Martínez Reyes, J.; Solorio Munguía, G.; Solís Valdez, S.; García Martínez, R. Total mercury in terrestrial systems (air-soil-plant-water) at the mining region of San Joaquín, Queretaro, Mexico. Geofísica Int. 2013, 52, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Rico-Sánchez, A.E.; Rodríguez-Romero, A.J.; Sedeño-Díaz, J.E.; López-López, E.; Sundermann, A. Aquatic Macroinvertebrate Assemblages in Rivers Under the Influence of Mining Activities. Sci. Rep. 2021, in press. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, Z.; Hou, X.; Ma, Z.; Chen, B. River-groundwater interaction affected species composition and diversity perpendicular to a regulated river in an arid riparian zone. Glob. Ecol. Conserv. 2021, 27, e01595. [Google Scholar] [CrossRef]
- Sowa, A.; Krodkiewska, M.; Halabowski, D. How does mining salinisation gradient affect the structure and functioning of macroinvertebrate communities? Water Air Soil Pollut. 2020, 231, 1–19. [Google Scholar] [CrossRef]
- La Jornada. 2019. Available online: https://www.jornada.com.mx/2019/05/24/estados/028n1est (accessed on 22 August 2021).
- Williams-Subiza, E.A.; Brand, C. Functional response of benthic macroinvertebrates to fire disturbance in patagonian streams. Hydrobiologia 2021, 848, 1575–1591. [Google Scholar] [CrossRef]
- Juvigny-Khenafou, N.P.; Piggott, J.J.; Atkinson, D.; Zhang, Y.; Macaulay, S.J.; Wu, N.; Matthaei, C.D. Impacts of multiple anthropogenic stressors on stream macroinvertebrate community composition and functional diversity. Ecol. Evol. 2021, 11, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Barnum, T.R.; Weller, D.E.; Williams, M. Urbanization reduces and homogenizes trait diversity in stream macroinvertebrate communities. Ecol. Appl. 2017, 27, 2428–2442. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. Consecuencias del dominio: Una revisión de los efectos de la uniformidad en los procesos de los ecosistemas locales y regionales. Ecología 2008, 89, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Mykrä, H.; Heino, J. Decreased habitat specialization in macroinvertebrate assemblages in anthropogenically disturbed streams. Ecol. Complex. 2017, 31, 181–188. [Google Scholar] [CrossRef]
- Croijmans, L.; De Jong, J.F.; Prins, H.H.T. Oxygen is a better predictor of macroinvertebrate richness than temperature—a systematic review. Environ. Res. Lett. 2021, 16, 023002. [Google Scholar] [CrossRef]
- Carrera-Villacrés, D.V.; Crisanto-Perrazo, T.; Ortega-Escobar, H.; Ramírez-García, J.; Espinosa-Victoria, D.; Ramírez-Ayala, C.; Sánchez-Bernal, E. Salinidad cuantitativa y cualitativa del sistema hidrográfico Santa María-Río Verde, México. Tecnol. Cienc. Del Agua 2015, 6, 69–83. [Google Scholar]
- Gál, B.; Szivák, I.; Heino, J.; Schmera, D. The effect of urbanization on freshwater macroinvertebrates–knowledge gaps and future research directions. Ecol. Indic. 2019, 104, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Verkaik, I.; Vila-Escale, M.; Rieradevall, M.; Baxter, C.V.; Lake, P.S.; Minshall, G.W.; Prat, N. Stream macroinvertebrate community responses to fire: Are they the same in different fire-prone biogeographic regions? Freshw. Sci. 2015, 34, 1527–1541. [Google Scholar] [CrossRef]



| Environmental Variables/Mainstream | Extoraz_Feb_17 | Extoraz_Jul_17 | Jalpan_Feb_17 | Jalpan_Jul_17 | Jalpan_Jun_19 | Concá_Ayutla_StMaría_Feb_17 | Concá_Ayutla_StMaría_Jul_17 |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 22.65 ± 1.01 | 25.31 ± 1.03 | 17.57 ± 0.67 | 22.00 ± 1.35 | 24.52 ± 1.85 | 23.22 ± 0.81 | 24.94 ± 0.47 |
| Conductivity (ms/cm) | 0.70 ± 0.22 | 0.89 ± 0.17 | 0.33 ± 0.01 | 0.37 ± 0.03 | 0.38 ± 0.02 | 0.53 ± 0.03 | 0.28 ± 0.02 |
| Disolved oxygen (mg/L) | 9.60 ± 0.55 | 7.30 ± 0.33 | 9.41 ± 0.30 | 7.63 ± 0.32 | 7.67 ± 1.04 | 9.68 ± 0.32 | 8.10 ± 0.20 |
| Oxygen saturation (%) | 110.00 ± 6.66 | 92.21 ± 1.65 | 103.00 ± 3.05 | 89.94 ± 3.72 | 94.62 ± 11.52 | 106.46 ± 3.98 | 93.74 ± 1.84 |
| pH | 8.06 ± 0.07 | 7.77 ± 0.12 | 8.07 ± 0.06 | 8.01 ± 0.15 | 8.48 ± 0.08 | 7.84 ± 0.06 | 7.83 ± 0.06 |
| Turbidity (NTU) | 17.07 ± 10.91 | 251.57 ± 130.43 | 15.87 ± 6.65 | 41.16 ± 21.40 | 7.69 ± 2.75 | 14.78 ± 6.30 | 1002.42 ± 403.33 |
| Salinity (UPS) | 0.32 ± 0.08 | 0.43 ± 0.08 | 0.16 ± 0.00 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.25 ± 0.01 | 0.15 ± 0.01 |
| NO2 (mg/L) | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.12 ± 0.09 | 0.06 ± 0.06 | 0.07 ± 0.07 | 0.01 ± 0.00 | 0.03 ± 0.01 |
| NO3 (mg/L) | 1.28 ± 0.29 | 3.55 ± 2.64 | 1.63 ± 0.17 | 0.43 ± 0.15 | 0.97 ± 0.20 | 1.57 ± 0.11 | 1.16 ± 0.32 |
| NH3 (mg/L) | 0.20 ± 0.04 | 1.32 ± 0.35 | 0.56 ± 0.27 | 0.42 ± 0.11 | 0.22 ± 0.17 | 0.79 ± 0.65 | 3.89 ± 2.73 |
| Total Nitrogen (mg/L) | 3.03 ± 0.97 | 8.07 ± 1.63 | 2.65 ± 0.34 | 6.63 ± 0.56 | 2.99 ± 0.24 | 1.95 ± 0.38 | 13.45 ± 3.27 |
| PO4 (mg/L) | 0.16 ± 0.04 | 0.47 ± 0.28 | 0.26 ± 0.05 | 0.35 ± 0.09 | 0.52 ± 0.27 | 0.18 ± 0.08 | 0.70 ± 0.25 |
| Total Phosphorous (mg/L) | 1.51 ± 0.98 | 1.35 ± 0.41 | 0.34 ± 0.04 | 1.40 ± 0.82 | 1.13 ± 0.35 | 0.37 ± 0.08 | 1.34 ± 0.52 |
| SO4 (mg/L) | 81.12 ± 14.06 | 92.37 ± 20.83 | 12.70 ± 0.51 | 15.50 ± 0.85 | 16.00 ± 0.85 | 72.30 ± 18.84 | 21.80 ± 7.70 |
| Chlorides (mg/L) | 20.36 ± 7.24 | 21.24 ± 6.08 | 8.99 ± 0.40 | 7.28 ± 0.76 | 0.99 ± 0.38 | 10.29 ± 1.31 | 8.69 ± 1.77 |
| Alkalinity (mg/L) | 193.12 ± 8.40 | 233.00 ± 13.77 | 195.80 ± 11.49 | 224.50 ± 18.62 | 183.63 ± 6.66 | 192.40 ± 6.76 | 109.40 ± 30.28 |
| Hardness (mg/L) | 126.75 ± 43.33 | 244.50 ± 81.43 | 59.40 ± 8.66 | 179.66 ± 13.18 | 159.40 ± 3.18 | 99.60 ± 33.17 | 99.80 ± 17.61 |
| Suspended solids (mg/L) | 14.25 ± 11.60 | 281.25 ± 123.76 | 1.24 ± 0.51 | 30.66 ± 17.09 | 13.40 ± 5.29 | 4.62 ± 3.36 | 733.00 ± 289.88 |
| Color (Pt/Co U.) | 2.75 ± 1.18 | 20.75 ± 4.17 | 1.00 ± 0.01 | 7.83 ± 3.45 | 9.20 ± 3.15 | 2.00 ± 0.63 | 40.60 ± 16.15 |
| Fecal coliforms (MPN/100 mL) | 24.00 ± 6.64 | 645.75 ± 265.32 | 301.00 ± 186.00 | 658.83 ± 205.06 | 243.42 ± 214.80 | 111.40 ± 88.13 | 133.60 ± 82.32 |
| BOD5 (mg/L) | 3.15 ± 0.91 | 3.03 ± 0.32 | 5.14 ± 0.78 | 3.18 ± 0.47 | 0.30 ± 0.13 | 2.57 ± 0.46 | 2.62 ± 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Romero, A.J.; Rico-Sánchez, A.E.; Sedeño-Díaz, J.E.; López-López, E. Characterization of the Multidimensional Functional Space of the Aquatic Macroinvertebrate Assemblages in a Biosphere Reserve (Central México). Diversity 2021, 13, 546. https://doi.org/10.3390/d13110546
Rodríguez-Romero AJ, Rico-Sánchez AE, Sedeño-Díaz JE, López-López E. Characterization of the Multidimensional Functional Space of the Aquatic Macroinvertebrate Assemblages in a Biosphere Reserve (Central México). Diversity. 2021; 13(11):546. https://doi.org/10.3390/d13110546
Chicago/Turabian StyleRodríguez-Romero, Alexis Joseph, Axel Eduardo Rico-Sánchez, Jacinto Elías Sedeño-Díaz, and Eugenia López-López. 2021. "Characterization of the Multidimensional Functional Space of the Aquatic Macroinvertebrate Assemblages in a Biosphere Reserve (Central México)" Diversity 13, no. 11: 546. https://doi.org/10.3390/d13110546
APA StyleRodríguez-Romero, A. J., Rico-Sánchez, A. E., Sedeño-Díaz, J. E., & López-López, E. (2021). Characterization of the Multidimensional Functional Space of the Aquatic Macroinvertebrate Assemblages in a Biosphere Reserve (Central México). Diversity, 13(11), 546. https://doi.org/10.3390/d13110546








