Biodiversity Management in a Mediterranean National Park: The Long, Winding Path from a Species-Centred to an Ecosystem-Centred Approach
Abstract
:1. Introduction
2. The Three Steps in the Approaches to Biodiversity Management
2.1. The Human-Centred Approach
2.2. The Species-Centred Approach
2.3. The Ecosystem-Centred Approach
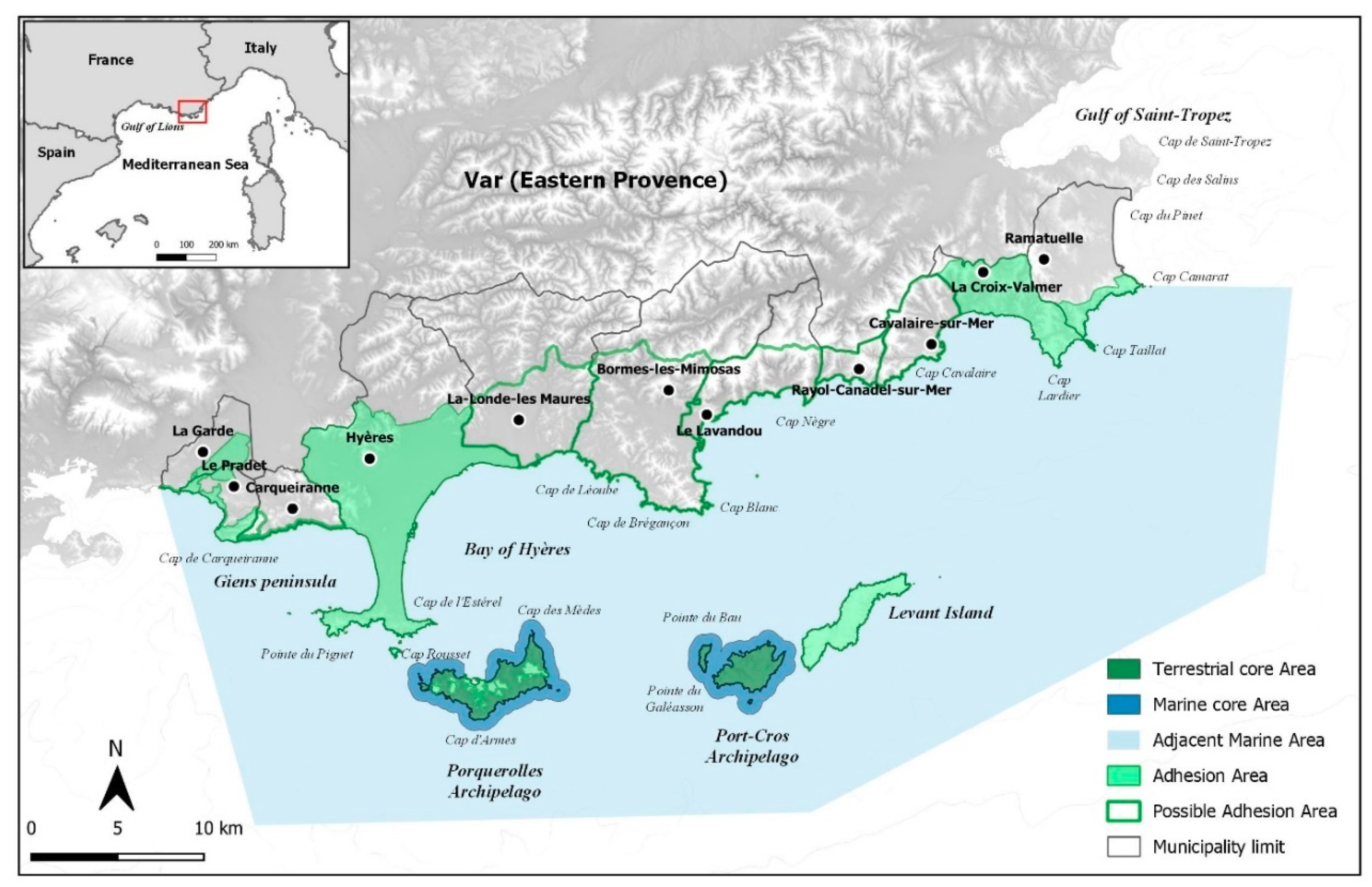
3. A Brief History of the Port-Cros National Park
4. Materials and Methods
5. Results and Discussion
5.1. Is the Marine Zoning Too Complex?
5.2. Should the Sea Urchin Paracentrotus Lividus and Other Macro-Herbivores Be Controlled?
5.3. Should Any Fishery Be Banned?
5.4. Should the Natural Closure of the Terrestrial Vegetation Be Controlled?
5.5. Should Natural Populations Be Enhanced?
5.6. The Natural Arrival of the Wild Boar Sus Scrofa
5.7. The Control of Marine Invasive Species
5.8. The Control of Terrestrial Invasive Species
5.9. Global Warming
5.10. Compensation of Ecological Damage and Conservation Easement
5.11. The Management of Biodiversity: Social and Public Acceptance
5.12. The Change over Time of the Port-Cros Management Strategy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedetti, G.; Franzosini, C.; Spoto, M. La riserva naturale marina di Miramare. Turismo ed educazione ambientale. Biennio 1989–1990. Parchi marini del Mediterraneo, problemi e prospettive. Atti del 2° convegno internazionale, San Teodoro, Italy, 1992; pp. 105–114.
- Dayton, P.K.; Sala, E.; Tegner, M.J.; Thrush, S. Marine reserves: Baselines and fishery enhancement. Bull. Mar. Sci. 2000, 66, 617–634. [Google Scholar]
- Boudouresque, C.F.; Cadiou, G.; Le Diréac’h, L. Marine protected areas: A tool for coastal areas management. In Strategic Management of Marine Ecosystems; Levner, E., Linkov, I., Proth, J.M., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 29–52. [Google Scholar]
- Farsac, L.; Boudouresque, C.F.; Barcelo, A.; Besnard, A. La recherche scientifique au sein des espaces protégés: Le cas du Parc national de Port-Cros (Provence, Méditerranée française). Sci. Rep. Port-Cros Natl. Park 2013, 27, 137–169. [Google Scholar]
- Sala, E.; Mayorga, J.; Bradley, D.; Cabral, R.B.; Atwood, T.B.; Auber, A.; Cheung, W.; Costello, C.; Ferretti, F.; Friedlander, A.M.; et al. Protecting the global ocean for biodiversity, food and climate. Nature 2021, 592, 397–402. [Google Scholar] [CrossRef]
- Di Franco, E.; Di Franco, A.; Calò, A.; Di Lorenzo, M.; Mangialajo, L.; Bussotti, S.; Bianchi, C.N.; Guidetti, P. Inconsistent relationships among protection, benthic assemblage, habitat complexity and fish biomass in Mediterranean temperate rocky reefs. Ecol. Indic. 2021, 128, 1–12. [Google Scholar] [CrossRef]
- Jones, K.R.; Klein, C.J.; Grantham, H.; Possingham, H.P.; Halpern, B.S.; Burgess, N.D.; Butchart, S.H.M.; Robinson, J.G.; Jingston, N.; Bhola, N.; et al. Area requirements to safeguard Earth’s marine species. One Earth 2020, 2, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Gill, D.A.; Mascia, M.B.; Ahmadia, G.N.; Glew, L.; Lester, S.E.; Barnes, M.; Craigie, I.; Darling, E.S.; Free, C.M.; Geldmann, J.; et al. Capacity shortfalls hinder the performance of marine protected areas globally. Nature 2017, 543, 665–669. [Google Scholar] [CrossRef]
- Rife, A.N.; Erisman, B.; Sanchez, A.; Aburto-Oropeza, O. When good intentions are not enough … Insights on networks of ‘paper parks’ marine protected areas. Conserv. Lett. 2013, 6, 200–212. [Google Scholar] [CrossRef] [Green Version]
- Sala, E.; Ballesteros, E.; Dendrinos, P.; Di Franco, A.; Ferretti, F.; Foley, D.; Fraschetti, S.; Friedlander, A.; Garrabou, J.; Güçlüsoy, H.; et al. The structure of Mediterranean reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 2012, 7, e32742. [Google Scholar] [CrossRef] [Green Version]
- Meinesz, A.; Blanfuné, A. 1983–2013: Development of marine protected areas along the French Mediterranean coasts and perspectives for achievement of the Aichi target. Mar. Pol. 2015, 54, 10–16. [Google Scholar] [CrossRef]
- Pieraccini, M.; Coppa, S.; De Lucia, G.A. Beyond marine paper parks? Regulation theory to assess and address environmental non-compliance. Aquat. Cons. Mar. Fresw. Ecosys. 2016, 27, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Claudet, J.; Loiseau, C.; Sostres, M.; Zupan, M. Underprotected marine protected areas in a global biodiversity hotspot. One Earth 2020, 2, 380–384. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Dominici, J.M.; Duriez, O.; Astruch, P.; Le Diréach, L.; Médail, F.; Sala, E.; Schohn, T.; Vicente, N. A terrestrial and marine nature reserve in the NW Mediterranean, Scàndula (Corsica): Biodiversity and lessons from 46 years of management. Sci. Rep. Port-Cros Natl. Park 2021, 35, 43–181. [Google Scholar]
- Leakey, R.; Lewin, R. The Sixth Extinction. Patterns of Life and the Future of Humankind; Doubleday: New York, NY, USA, 1995; pp. 1–271. [Google Scholar]
- Cronon, W. The trouble with wilderness, or, getting back to the wrong nature. Environ. Hist. 1996, 1, 7–28. [Google Scholar] [CrossRef] [Green Version]
- Simberloff, D. No reserve is an island: Marine reserves and nonindigenous species. Bull. Mar. Sci. 2000, 66, 567–580. [Google Scholar]
- Jackson, J.B.C.; Sala, E. Unnatural oceans. Sci. Mar. 2001, 65, 273–281. [Google Scholar] [CrossRef]
- De Planhol, X. Le Paysage Animal. l’Homme et la Grande Faune: Une Zoogéographie Historique; Fayard: Paris, France, 2004; pp. 1–1127. [Google Scholar]
- Thompson, J.D.; Mathevet, R.; Delanoë, O.; Gil-Fourrier, C.; Bonnin, M.; Cheylan, M. Ecological solidarity as a conceptual tool for rethinking ecological and social interdependence in conservation policy for protected areas and their surrounding landscape. C. R. Biol. 2011, 334, 412–419. [Google Scholar] [CrossRef]
- Harari, Y.N. Sapiens. A Brief History of Humankind; Harper Collins: New York, NY, USA, 2015; pp. viii + 1–445. [Google Scholar]
- Caro, T.; Darwin, J.; Forrestier, T.; Ledoux-Bloom, C.; Wells, C. Conservation in the Anthropocene. Conserv. Biol. 2011, 26, 185–188. [Google Scholar] [CrossRef]
- Lotze, H.K.; Coll, M.; Dunne, J.A. Historical changes in marine resources, food-web structure and ecosystem functioning in the Adriatic Sea, Mediterranean. Ecosystems 2011, 14, 198–222. [Google Scholar] [CrossRef]
- Lotze, H.K.; Worm, B. Historical baselines for large marine animals. Trends Ecol. Evol. 2008, 24, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.L.; Charpentier, A.; Bernal-Casasola, D.; Gardeisen, A.; Nores, C.; Pis Millán, J.A.; Mcgrath, K.; Speller, C.F. Forgotten Mediterranean calving grounds of grey and North Atlantic right whales: Evidence from Roman archaeological records. Proc. R. Soc. B 2018, 285, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D.; Maclean, J. In a Perfect Ocean—The State of Fisheries and Ecosystems in the North Atlantic Ocean; Island Press: Washington, DC, USA, 2003; p. 175. [Google Scholar]
- Willis, K.J.; Birks, H.J.B. What is natural? The need for a long-term perspective in biodiversity conservation. Science 2006, 314, 1261–1265. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef]
- Boero, F. Episodic events: Their relevance to ecology and evolution. Mar. Ecol. 1996, 17, 237–250. [Google Scholar] [CrossRef]
- Bakun, A. Linking climate to population variability in marine ecosystems characterized by non-simple dynamics: Conceptual templates and schematic constructs. J. Mar. Syst. 2010, 79, 361–373. [Google Scholar] [CrossRef]
- Overland, J.E.; Alheit, J.; Bakun, A.; Hurrell, J.W.; Mackas, D.L.; Miller, A.J. Climate controls on marine ecosystems and fish populations. J. Mar. Syst. 2010, 79, 305–315. [Google Scholar] [CrossRef]
- Faget, D. Éloge Vagabond de la Méditerranée; Philippe Rey: Paris, France, 2020; pp. 1–351. [Google Scholar]
- Gravina, M.F.; Bonifazi, A.; Del Pasqua, M.; Giampaoletti, J.; Lezzi, M.; Ventura, D.; Giangrande, A. Perception of changes in marine benthic habitats: The relevance of taxonomic and ecological memory. Diversity 2020, 12, 480. [Google Scholar] [CrossRef]
- Weigel, J.Y.; Féral, F.; Cazalet, B. Introduction. In Governance of Marine Protected Areas in the Least-Developed Countries. Case Studies from West Africa; FAO Fisheries and Aquaculture Technical Paper; Weigel, J.Y., Féral, F., Cazalet, B., Eds.; FAO: Rome, Italy, 2011; Volume 548, pp. 1–9. [Google Scholar]
- Di Franco, A.; Thiriet, P.; Di Carlo, G.; Dimitriadis, C.; Francour, P.; Gutiérrez, N.L.; Jeudy de Grissac, A.; Koutsoubas, D.; Milazzo, M.; Otero, M.D.M.; et al. Five key attributes can increase marine protected areas performance for small-scale fisheries management. Sci. Rep. 2016, 6, 38135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Yan, S.; Feng, R.; Song, D.; Chen, X. Development of an environmental performance indicator framework to evaluate management effectiveness for Jiaozhou Bay Coastal Wetland Special Marine Protected Area, Qingdao, China. Ocean Coast. Manag. 2017, 142, 71–89. [Google Scholar] [CrossRef]
- Bennett, N.J.; Di Franco, A.; Calò, A.; Nethery, E.; Niccolini, F.; Milazzo, M.; Guidetti, P. Local support for conservation is associated with perceptions of good governance, social impacts, and ecological effectiveness. Cons. Lett. 2019, 12, e12640. [Google Scholar] [CrossRef]
- Sala, E.; Knowlton, N. Global marine biodiversity trends. Annu. Rev. Environ. Resour. 2006, 31, 93–122. [Google Scholar] [CrossRef] [Green Version]
- Boudouresque, C.F. Insights into the diversity of the biodiversity concept. Sci. Rep. Port-Cros Natl. Park 2014, 28, 65–86. [Google Scholar]
- Boudouresque, C.F.; Astruch, P.; Bănaru, D.; Blanfuné, A.; Carlotti, F.; Faget, D.; Goujard, A.; Harmelin-Vivien, M.; Le Diréach, L.; Pagano, M.; et al. Global change and the management of Mediterranean coastal habitats: A plea for a socio-ecosystem-based approach. In Evolution of Marine Coastal Ecosystems under the Pressure of Global Change. Proceedings of Coast Bordeaux Symposium and of the 17th French-Japanese Oceanography Symposium; Ceccaldi, J.H., Hénocque, Y., Komatsu, T., Prouzet, P., Sautour, B., Yoshida, J., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 297–320. [Google Scholar]
- Buffon, G.L. (as: Leclerc, comte de Buffon); Histoire Naturelle, Générale et Particulière, avec la Description du Cabinet du Roi. Tome Douzième; Imprimerie Royale: Paris, France, 1764. [Google Scholar]
- Buffon, G.L. (as: Leclerc, comte de Buffon); Histoire Naturelle, Générale et Particulière, avec la Description du Cabinet du Roi. Tome Quinzième; Imprimerie Royale: Paris, France, 1767. [Google Scholar]
- Gourret, P. Provence des Pêcheurs. Réimpression 1981; Serre: Paris, France, 1894; pp. 1–360. [Google Scholar]
- De La Blanchère, H. Les Oiseaux Utiles et les Oiseaux Nuisibles aux Champs—Jardins—Forêts—Plantations—Vignes, etc.; J. Rothschild: Paris, France, 1878; p. 387. [Google Scholar]
- Faget, D. Dauphins. In Dictionnaire de la Méditerranée; Actes Sud: Arles, France, 2016; pp. 330–334. [Google Scholar]
- Balmford, A.; Bond, W. Trends in the state of nature and their implications for human well-being. Ecol. Lett. 2005, 8, 1218–1234. [Google Scholar] [CrossRef] [PubMed]
- Geijzendorffer, I.R.; Roche, P.K. Can biodiversity monitoring schemes provide indicators for ecosystem services? Ecol. Indic. 2013, 33, 148–157. [Google Scholar] [CrossRef]
- Pesche, D.; Méral, P.; Hrabanski, M.; Bonnin, M. Ecosystem services and payments for environmental services: Two sides of the same coin? In Governing the Provision of Ecosystem Services; Muradian, R., Rival, L., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 69–87. [Google Scholar]
- European Union. Regulation (EU) N° 1143/2014 of the European Parliament and the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. Off. J. Eur. Union 2014, L 317/36. [Google Scholar]
- Mongruel, R.; Méral, P.; Doussan, I.; Levrel, H. l’Institutionnalisation de l’Approche par les Services Ecosystémiques: Dimensions Scientifiques, Politiques et Juridiques. In Valeurs de la Biodiversité et Services Ecosystémiques, Perspectives Interdisciplinaires; Update Sciences et, Technologies; Roche, P., Geijzendorffer, I., Levrel, H., Maris, V., Eds.; Quae: Versailles, France, 2016; pp. 191–216. [Google Scholar]
- Paoli, C.; Montefalcone, M.; Morri, C.; Vassallo, P.; Bianchi, C.N. Ecosystem functions and services of the marine animal forests. In Marine animal forests; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1271–1312. [Google Scholar]
- Rigo, I.; Paoli, C.; Dapueto, G.; Pergent-Martini, C.; Pergent, G.; Oprandi, A.; Montefalcone, M.; Bianchi, C.N.; Morri, C.; Vassallo, P. The natural capital value of the seagrass Posidonia oceanica in the North-Western Mediterranean. Diversity 2021, 13, 499. [Google Scholar] [CrossRef]
- Simberloff, D. Flagships, umbrellas and keystones: Is single-species management passé in the landscape era? Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Jordán, F. Keystone species and food webs. Phil. Trans. R. Soc. B 2009, 364, 1733–1741. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, P.; Debussche, M.; Thompson, J.D. Regional priority setting for rare species based on a method combining three criteria. Biol. Conserv. 2010, 143, 1501–1509. [Google Scholar] [CrossRef]
- Astruch, P.; Boudouresque, C.F.; Bonhomme, D.; Goujard, A.; Antonioli, P.A.; Bonhomme, P.; Perez, T.; Ruitton, S.; De Saint-Martin, T.; Verlaque, M. Mapping and state of conservation of benthic marine habitats and assemblages of Port-Cros National Park (Provence, France, northwestern Mediterranean Sea). Sci. Rep. Port-Cros Natl. Park 2012, 26, 45–90. [Google Scholar]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Verlaque, M. Decline and local extinction of Fucales in the French Riviera: The harbinger of future extinctions? Mediterr. Mar. Sci. 2015, 16, 206–224. [Google Scholar] [CrossRef] [Green Version]
- Thibaut, T.; Blanfuné, A.; Verlaque, M.; Boudouresque, C.F.; Ruitton, S. The Sargassum conundrum: Highly rare, threatened or locally extinct in the NW Mediterranean and still lacking protection. Hydrobiologia 2016, 781, 25–42. [Google Scholar] [CrossRef]
- Verlaque, M.; Boudouresque, C.F.; Perret-Boudouresque, M. Mediterranean seaweeds listed as threatened under the Barcelona Convention: A critical analysis. Sci. Rep. Port-Cros Natl. Park 2019, 33, 179–214. [Google Scholar]
- Grace, M.K.; Akçakaya, H.R.; Bennett, E.L.; Brooks, T.M.; Heath, A.; Hedges, S.; Hilton-Taylor, C.; Hoffmann, M.; Hochkirch, A.; Jenkins, R.; et al. Testing a global standard for quantifying species recovery and assessing conservation impact. Conserv. Biol. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A. Population biology, conservation threats and status of Mediterranean striped dolphins (Stenella coeruleoalba). J. Cetacean Res. Manag. 2000, 2, 17–26. [Google Scholar]
- Baş, A.A.; Affinito, F.; Martin, S.; Vollmer, A.; Gansen, C.; Morris, N.; Frontier, N.; Nikpaljevic, N.; Vujović, A. Bottlenose Dolphins and Striped Dolphins: Species Distribution, Behavioural Patterns, Encounter Rates, Residency Patterns and Hotspots in Montenegro, South Adriatic; Montenegro Dolphin Project Annual Report; Marine Mammal Research Association: Turkey, 2017; pp. 1–64. [Google Scholar]
- Bearzi, G.; Reeves, R.R. Shifting baselines of cetacean conservation in Europe. ICES J. Mar. Sci. 2021, 78, 2337–2341. [Google Scholar] [CrossRef]
- Brambilla, M.; Gustin, M.; Celada, C. Species appeal predicts conservation status. Biol. Conserv. 2013, 160, 209–213. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Lawton, J.H. What do species do in ecosystems? Oikos 1994, 71, 367–374. [Google Scholar] [CrossRef]
- Power, M.E.; Mills, L.S. The keystone cops meet in Hilo. Trends Ecol. Evol. 1995, 10, 182–184. [Google Scholar] [CrossRef]
- Le Berre, M.; Diadema, K.; Pires, M.; Noble, V.; De Barros, G.; Gavotto, O. Stratégie de conservation de la flore vasculaire en région Sud Provence-Alpes-Côte d’Azur. 1.—Hiérarchisation des enjeux. Sci. Rep. Port-Cros Natl. Park 2020, 34, 101–135. [Google Scholar]
- Goldstein, B. The struggle over ecosystem management at Yellowstone. BioScience 1992, 42, 183–187. [Google Scholar] [CrossRef]
- Borja, A.; Menchaca, I.; Garmendia, J.M.; Franco, J.; Larreta, J.; Sagarminaga, Y.; Schembri, Y.; González, R.; Antón, R.; Micalef, T.; et al. Big insights from a small country: The added value of integrated assessment in the marine environmental status evaluation of Malta. Front. Mar. Sci. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Personnic, S.; Boudouresque, C.F.; Astruch, P.; Ballesteros, E.; Blouet, S.; Bellan-Santini, D.; Bonhomme, P.; Thibault-Botha, D.; Feunteun, E.; Harmelin-Vivien, M.; et al. An ecosystem-based approach to assess the status of a Mediterranean ecosystem, the Posidonia oceanica seagrass meadow. PLoS ONE 2014, 9, e98994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruitton, S.; Personnic, S.; Ballesteros, E.; Bellan-Santini, D.; Boudouresque, C.F.; Chevaldonné, P.; Bianchi, C.N.; David, R.; Féral, J.P.; Guidetti, P.; et al. An ecosystem-based approach to assess the status of the Mediterranean coralligenous habitat. In Proceedings of the 2nd Mediterranean Symposium on the Conservation of Coralligenous and Other Calcareous Bio-Concretions, Portorož, Slovenia, 29–30 October 2014; Bouafif, C., Langar, H., Ouerghi, A., Eds.; RAC/SPA: Tunis, Tunisia, 2014; pp. 153–158. [Google Scholar]
- Boudouresque, C.F.; Personnic, S.; Astruch, P.; Ballesteros, E.; Bellan-Santini, D.; Bonhomme, P.; Botha, D.; Feunteun, E.; Harmelin-Vivien, M.; Pergent, G.; et al. Ecosystem-based versus species-based approach for assessment of the human impact on the Mediterranean seagrass Posidonia oceanica. In Marine productivity: Perturbations and Resilience of Socio-Ecosystems; Ceccaldi, H., Hénocque, Y., Koike, Y., Komatsu, T., Stora, G., Tusseau-Vuillemin, M.H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 235–241. [Google Scholar]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Personnic, S.; Ruitton, R.; Ballesteros, E.; Bellan-Santini, D.; Bianchi, C.N.; Bussotti, S.; Cebrian, E.; et al. An ecosystem-based approach to assess the status of Mediterranean algae-dominated shallow rocky reefs. Mar. Pollut. Bull. 2017, 117, 311–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astruch, P.; Boudouresque, C.F.; Changeux, T.; Faget, D.; Lascève, M.; Le Diréach, L.; Massinelli, L.; Moussy, F. From a species-centred to an ecosystem-based management approach, a case study of the saltmarshes of Hyères (Provence, France). In Planning, Nature and Ecosystem Services; Gargiulo, C., Zoppi, C., Eds.; FedOAPress: Naples, Italy, 2019; pp. 29–38. [Google Scholar]
- Astruch, P.; Goujard, A.; Rouanet, É.; Boudouresque, C.F.; Verlaque, M.; Berthier, L.; Daniel, B.; Harmelin, J.G.; Peirache, M.; Peterka, A.; et al. Assessment of the conservation status of coastal detrital sandy bottoms in the Mediterranean Sea: An ecosystem-based approach in the framework of the ACDSEA project. In Proceedings of the 3rd Symposium on the Conservation of Coralligenous and Other Calcareous Bio-Constructions, Antalya, Turkey, 15–16 January 2019; Langar, H., Ouerghi, A., Eds.; RAC/SPA: Tunis, Tunisia, 2019; pp. 23–29. [Google Scholar]
- Galil, B.S. Alien species in the Mediterranean Sea—Which, when, where, why? Hydrobiologia 2008, 606, 105–116. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Gatto, F.; Zenetos, A.; Cardoso, A.C. How many marine aliens in Europe? Manag. Biol. Invasions 2013, 4, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E.M. The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F.; Blanfuné, A.; Fernandez, C.; Lejeusne, C.; Pérez, T.; Ruitton, S.; Thibault, D.; Thibaut, T.; Verlaque, M. Marine Biodiversity—Warming vs. biological invasions and overfishing in the Mediterranean Sea: Take care, ‘One train can hide another’. MOJ Ecol. Env. Sci. 2017, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Boudouresque, C.F.; Ruitton, S.; Verlaque, M. Large-scale disturbances, regime shift and recovery in littoral systems subject to biological invasions. In Large-Scale Disturbances (Regime Shifts) and Recovery in Aquatic Ecosystems: Challenges for Management towards Sustainability; Velikova, V., Chipev, N., Eds.; Unesco: Paris, France, 2005; pp. 85–101. [Google Scholar]
- Boudouresque, C.F.; Klein, J.; Ruitton, S.; Verlaque, M. Biological Invasion: The Thau Lagoon, a Japanese biological island in the Mediterranean Sea. In Global Change: Mankind-Marine Environment Interactions; Ceccaldi, H.J., Dekeyser, I., Girault, M., Stora, G., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 151–156. [Google Scholar]
- Halpern, B.S.; Lester, S.E.; Mcleod, K.L. Placing marine protected areas onto the ecosystem-based management seascape. Proc. Natl. Acad. Sci. USA 2010, 107, 18312–18317. [Google Scholar] [CrossRef] [Green Version]
- Cresson, P.; Fabri, M.C.; Bouchoucha, M.; Brach Papa, C.; Chavanon, F.; Jadaud, A.; Knoery, J.; Miralles, F.; Cossa, D. Mercury in organisms from the Northwestern Mediterranean slope: Importance of food sources. Sci. Total Environ. 2014, 497–498, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giakoumi, S.; Halpern, B.S.; Michel, L.N.; Gobert, S.; Sini, M.; Boudouresque, C.F.; Gambi, M.C.; Katsanevakis, S.; Lejeune, P.; Montefalcone, M.; et al. Towards a framework for assessment and management of cumulative human impacts on marine food webs. Conserv. Biol. 2015, 29, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Ourgaud, M.; Ruitton, S.; Bell, J.; Letourneur, Y.; Harmelin, J.G.; Harmelin-Vivien, M. Response of a seagrass fish assemblage to improved wastewater treatment. Mar. Pollut. Bull. 2015, 90, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, K.; Rose, G.; Devillers, R. How fisher-influenced marine closed areas contribute to ecosystem-based management: A review and performance indicator scorecard. Fish Fish. 2017, 18, 860–876. [Google Scholar] [CrossRef]
- Shin, Y.J.; Houle, J.E.; Akoglu, E.; Blanchard, J.L.; Bundy, A.; Coll, M.; Demarcq, H.; Fu, C.; Fulton, E.A.; Heymans, J.J.; et al. The specificity of marine ecological indicators to fishing in the face of environmental change: A multi-model evaluation. Ecol. Indic. 2018, 89, 317–326. [Google Scholar] [CrossRef]
- Bougeant, P. Un Parc en forme d’île: Bilan des vingt-cinq premières années de fonctionnement du Parc National de Port-Cros. In Proceedings of the Atti del 1° Convegno Internazionale ‘Parchi Marini del Mediterraneo, Aspetti Naturalistici E Gestionali’, San Teodoro, Italy, 28–30 April 1989; pp. 75–83. [Google Scholar]
- Boudouresque, C.F.; Barcelo, A.; Harmelin, J.G.; Martin, G.; Maurer, C.; Médail, F.; Sellier, G.; Viviani, R.A. The Scientific Council of a national park, the Port-Cros National Park: 50 years of conservation culture. Sci. Rep. Port-Cros Natl. Park 2013, 27, 297–317. [Google Scholar]
- Boudouresque, C.F.; Médail, F.; Ponel, P.; Astruch, P.; Barcelo, A.; Blanfuné, A.; Changeux, T.; Chevaldonné, P.; Cheylan, G.; Le Diréach, L.; et al. Species-based or ecosystem-based approaches to conservation practices: Lessons from the Port-Cros National Park (South-East France, Mediterranean Sea). Life Environ. 2020, 70, 89–112. [Google Scholar]
- Barcelo, A.; Boudouresque, C.F. Rôle de la recherche dans un parc national: 50 ans de recherche dans le Parc national de Port-Cros. Bull. Soc. Zool. Fr. 2012, 137, 11–24. [Google Scholar]
- Astruch, P.; Boudouresque, C.F.; Rouanet, É.; Le Direach, L.; Bonhomme, P.; Bonhomme, D.; Goujard, A.; Ruitton, S.; Harmelin, J.G. A quantitative and functional assessment of fish assemblages of the Port-Cros Archipelago (Port-Cros National Park, north-western Mediterranean Sea). Sci. Rep. Port-Cros Natl. Park 2018, 32, 17–82. [Google Scholar]
- Barcelo, A.; Bernardi, P.; Buzaud, C.; Creusefond, M.; Despinoy, P.; Gabriel, J.; Hily, G.; Mazzella, C.; Millier, L.; Cresp, G.; et al. Mode de gouvernance pour la gestion concertée du coeur marin de l’île de Porquerolles, au sein du Parc national de Port-Cros (Provence, France): Retour des acteurs sur 10 années de pratique. Sci. Rep. Port-Cros Natl. Park 2018, 32, 83–111. [Google Scholar]
- Les Amoureux de Porquerolles. Perspectives pour l’Avenir de l’île de Porquerolles; Les Amoureux de Porquerolles: Hyères, France, 2021; pp. 1–15. [Google Scholar]
- Sellier, G. Processus de mise en place de la charte au nouveau périmètre du Parc national de Port-Cros. In Entretiens de Port-Cros; 8, 7–9 Septembre 2015, Porquerolles, France; Fondation Total: Paris, France, 2015; pp. 86–90. [Google Scholar]
- Hogg, K.; Markantonatou, V.; Noguera-Mendez, P.; Semitiel-Garcia, M. Incentives for good governance: Getting the balance right for Port-Cros National Park (Mediterranean Sea, France). Sci. Rep. Port-Cros Natl. Park 2016, 30, 165–178. [Google Scholar]
- Barcelo, A.; Aboucaya, A.; Boudouresque, C.F.; Gillet, P.; Harmelin, J.G.; Jarin, M.; Martin, G.; Maurer, C.; Médail, F.; Peirache, M.; et al. The scientific strategy of the Port-Cros National Park for the 2013–2022 period. Sci. Rep. Port-Cros Natl. Park 2013, 27, 485–492. [Google Scholar]
- Martin, G.J. Les conseils scientifiques des parcs nationaux. Réflexions à partir de l’expérience du Parc national de Port-Cros. Rev. Jur. Environ. 2020, 4, 659–665. [Google Scholar]
- Simon, G.; Moutou, F. Le projet phoque moine français (1984–1995). La Gaz. Des Grands Prédateurs 2010, 37, 28–29. [Google Scholar]
- Boudouresque, C.F. The driving force behind the doctrine and scientific strategy of the Port-Cros National Park (Provence, France): Jannick Olivier (1948–2019). Sci. Rep. Port-Cros Natl. Park 2020, 34, 23–43. [Google Scholar]
- Andral, B.; Stanisiere, J.Y.; Sauzade, D.; Damier, E.; Thebault, H.; Galgani, F.; Boissery, P. Monitoring chemical contamination levels in the Mediterranean based on the use of mussel caging. Mar. Pollut. Bull. 2004, 49, 704–712. [Google Scholar] [CrossRef]
- Amalric, L.; Leclerc, G. Grandes nacres: Opération transplantation dans le Var. Var-Matin, 9 December 2018; 48. [Google Scholar]
- Azzola, A.; Bavestrello, G.; Bertolino, M.; Bianchi, C.N.; Bo, M.; Enrichetti, F.; Morri, C.; Oprandi, A.; Toma, M.; Montefalcone, M. You cannot conserve a species that has not been found: The case of the marine sponge Axinella polypoides in Liguria, Italy. Aquat. Conserv. Mar. Freshw. Ecos. 2021, 31, 737–747. [Google Scholar] [CrossRef]
- Cadiou, G.; Boudouresque, C.F.; Bonhomme, P.; Le Diréach, L. The management of artisanal fishing within the Marine Protected Area of the Port-Cros National Park (northwest Mediterranean Sea): A success story? ICES J. Mar. Sci. 2009, 66, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Boudouresque, C.F.; Astruch, P.; Changeux, T.; Ruitton, S.; Thibaut, T. Marine protected areas: Multi-Use Management (MUM) vs. No-Take Zones (NTZ) and the efficiency of locally managed artisanal fishery. Rapp. Comm. Intl. Mer Mediterr. 2019, 42, 266. [Google Scholar]
- Martin, G.J. La politique juridique du Parc national de Port-Cros entre 2011 et 2017. Bilan, perspectives etpréconisations. Sci. Rep. Port-Cros Natl. Park 2020, 34, 167–261. [Google Scholar]
- Parc National De Port-Cros. Yachting, Fishing, Diving around Port-Cros Island and Access to the Harbour; Parc National de Port-Cros: Hyères, France, 2021; pp. 1–6. Available online: www.portcros-parcnational.fr/fr/download/file/fid/10678 (accessed on 15 September 2021).
- Vergés, A.; Alcoverro, T.; Ballesteros, E. Role of fish herbivory in structuring the vertical distribution of canopy algae Cystoseira spp. in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2009, 375, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L., Jr.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalization of temperate marine ecosystems: Climate mediated changes in herbivory and community phase shifts. Proc. Roy. Soc. B 2014, 281, 20140846. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Paracentrotus lividus. In Sea Urchins: Biology and Ecology, 4th ed.; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Waltham, MA, USA, 2020; pp. 447–485. [Google Scholar]
- Buñuel, X.; Alcoverro, T.; Pagès, J.F.; Romero, J.; Ruiz, J.M.; Arthur, R. The dominant seagrass herbivore Sarpa salpa shifts its shoaling and feeding strategies as they grow. Sci. Rep. 2020, 10, 110622. [Google Scholar] [CrossRef]
- Papadakis, O.; Tsirintanis, K.; Lioupa, V.; Katsanevakis, S. The neglected role of omnivorous fish in the overgrazing of Mediterranean rocky reefs. Mar. Ecol. Prog. Ser. 2021, 673, 107–116. [Google Scholar] [CrossRef]
- Verlaque, M.; Nedelec, H. Biologie de Paracentrotus lividus (Lamarck) sur substrat rocheux en Corse (Méditerranée, France): Alimentation des adultes. Vie Milieu 1983, 33, 191–201. [Google Scholar]
- Verlaque, M.; Nedelec, H. Note préliminaire sur les relations biotiques Paracentrotus lividus (Lmk.) et herbier de posidonies. Rapp. Comm. Intl. Mer Méditerr. 1983, 28, 157–158. [Google Scholar]
- Sala, E.; Zabala, M. Fish predation and the structure of the sea urchin Paracentrotus lividus populations in the NW Mediterranean. Mar. Ecol. Prog. Ser. 1996, 140, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Sala, E.; Boudouresque, C.F. The role of fishes in the organization of a Mediterranean sublittoral community. I: Algal communities. J. Exp. Mar. Biol. Ecol. 1997, 212, 25–44. [Google Scholar] [CrossRef]
- Sala, E.; Boudouresque, C.F.; Harmelin-Vivien, M. Fishing, trophic cascades and the structure of algal assemblages: Evaluation of an old but untested paradigm. Oikos 1998, 82, 425–439. [Google Scholar] [CrossRef]
- Guidetti, P. Consumers of sea urchins, Paracentrotus lividus and Arbacia lixula, in shallow Mediterranean rocky reefs. Helg. Mar. Res. 2004, 58, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.D.; Scheibling, R.E.; Rassweiler, A.; Johnson, C.R.; Shears, N.; Connell, S.D.; Salomon, A.K.; Norderhaug, K.M.; Péres-Matus, A.; Hernández, J.C.; et al. Global regime shift of catastrophic sea urchin overgrazing. Phil. Trans. R. Soc. B 2015, 370, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Ballesteros, E.; Francour, P.; Guidetti, P.; Meinesz, A.; Thibaut, T.; Mangialajo, L. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of Marine Protected Areas. Adv. Oceanogr. Limnol. 2013, 4, 83–101. [Google Scholar] [CrossRef]
- Bernal-Ibàñez, A.; Cacabelos, E.; Melo, R.; Gestoso, I. The role of sea urchins in marine forests from Azores, Webbnesia, and Cabo Verde: Human pressures, climate-change effects and restoration opportunities. Front. Mar. Sci. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Hereu, B.; Casals, D.; Ortega, J.; Rovira, G. Suivi des Populations D’échinodermes du Parc National de Port-Cros. Juillet 2019; Parc national de Port-Cros and Universitat Barcelona: Barcelona, Spain, 2019. [Google Scholar]
- Couvray, S.; Miard, T.; Bunet, R.; Martin, Y.; Grillasca, J.P.; Bonnefont, J.L.; Coupé, S. Experimental release of Paracentrotus lividus sea urchin juveniles in exploited sites along the French Mediterranean coast. J. Shellfish Res. 2015, 34, 1–9. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Blanfuné, A.; Pergent, G.; Pergent-Martini, C.; Perret-Boudouresque, M.; Thibaut, T. Impacts of marine and lagoon aquaculture on macrophytes in Mediterranean benthic ecosystems. Front. Mar. Sci. 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Caltagirone, A.; Lefevre, J.R.; Rico, V.; Semroud, R. Macrozoobenthos de la réserve naturelle de Scandola (Corse, Méditerranée nord-occidentale). Analyse pluriannuelle de l’‘effet réserve’. MEDPAN News 1992, 3, 15–20. [Google Scholar]
- Boudouresque, C.F.; Nedelec, H.; Shepherd, S.A. The decline of a population of the sea-urchin Paracentrotus lividus in the bay of Port-Cros (Var). Rapp. P.V. Réun. Commiss. Internation. Explor. Sci. Médit. 1981, 27, 223–224. [Google Scholar]
- Azzolina, J.F.; Boudouresque, C.F.; Nedelec, H. Seasonal and year-to-year changes of the edible sea-urchin Paracentrotus lividus populations in the bay of Port-Cros (Var, France). Rapp. Comm. Intl. Mer Méditerr 1983, 28, 265–266. [Google Scholar]
- Azzolina, J.F.; Boudouresque, C.F.; Nedelec, H. Dynamique des populations de Paracentrotus lividus dans la baie de Port-Cros (Var): Données préliminaires. Sci. Rep. Port-Cros Natl. Park 1985, 11, 61–81. [Google Scholar]
- Boudouresque, C.F.; Augier, H.; Belsher, T.; Coppejans, E.; Perret, M. Végétation marine de l’île de Port-Cros (Parc National). X. La régression du récif-barrière de posidonies. Trav. Sci. Parc Natl. Port-Cros 1975, 1, 41–46. [Google Scholar]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Cottalorda, J.M.; Hereu, B.; Susini, M.L.; Verlaque, M. Unexpected temporal stability of Cystoseira and Sargassum forests in Port-Cros, one of the oldest Mediterranean marine National Parks. Cryptogam. Algol. 2016, 37, 61–90. [Google Scholar] [CrossRef]
- Ferrari, B. Etude Synécologique de Posidonia oceanica et de Sarpa salpa le Long de la côte Rocheuse des Albères (Pyrénées-Orientales, France); Influence d’une Aire Marine Protégée. Ph.D. Thesis, Université de Perpignan, Perpignan, France, 2006; pp. 1–289. [Google Scholar]
- Ferrari, B.; Raventos, N.; Planes, S. Assessing effects of fishing prohibition on Posidonia oceanica seagrass meadows in the Marine Natural Reserve of Cerbère-Banyuls. Aquat. Bot. 2008, 88, 295–302. [Google Scholar] [CrossRef]
- Prado, P.; Farina, S.; Tomas, F.; Romero, J.; Alcoverro, T. Marine protection and meadow size alter fish herbivory in seagrasses ecosystems. Mar. Ecol. Prog. Ser. 2008, 371, 11–21. [Google Scholar] [CrossRef]
- Goujard, A.; Astruch, P.; Bonhomme, P.; Boudouresque, C.F. Cartographie du Récif Barrière de Posidonie et des Peuplements Associés, Importance des Herbivores, de la Baie de Port-Cros (Parc National, Var, France); GIS Posidonie: Marseille, France, 2010; pp. 1–51. [Google Scholar]
- Boussard, A.; Barralon, E.; Boudouresque, C.F.; Boursault, M.; Goujard, A.; Pergent, G.; Pergent-Martini, C.; Rouanet, É.; Schohn, T. Almost a century of monitoring of the Posidonia barrier reef at Port-Cros (Provence) and the platform reef at Saint-Florent (Corsica). In Proceedings of the 6th Mediterranean Symposium on Marine Vegetation, Antalya, Turkey, 14–15 January 2019; Langar, H., Ouerghi, A., Eds.; RAC/SPA: Tunis, Tunisia, 2019; pp. 41–46. [Google Scholar]
- Combelles, S. Pêche Amateur dans les Eaux du Parc National de Port-Cros; Parc National de Port-Cros and Laboratoire de Zoologie et d’Écologie de l’Université d’Orsay: Paris, France, 1991; pp. 1–63. [Google Scholar]
- Boudouresque, C.F.; Cadiou, G.; Guerin, B.; Le Direach, L.; Robert, P. Is there a negative interaction between biodiversity conservation and artisanal fishing in a Marine Protected Area, the Port-Cros National Park (France, Mediterranean Sea). Sci. Rep. Port-Cros Natl. Park 2004, 20, 147–160. [Google Scholar]
- Robert, P. Évolution de la gouvernance: L’exemple de la pêche dans les eaux du Parc national de Port-Cros. Sci. Rep. Port-Cros Natl. Park 2013, 27, 319–324. [Google Scholar]
- Robert, P. La recherche au service de la gestion: Les moyens d’inventer. In GIS Posidonie: Plus de 30 ans au Service de la Protection et de la Gestion du Milieu Marin; Le Diréach, L., Boudouresque, C.F., Eds.; GIS Posidonie: Marseille, France, 2013; pp. 69–73. [Google Scholar]
- Le Diréach, L.; Boudouresque, C.F.; Bonhomme, P.; Cadiou, G.; Ourgaud, M.; Rouanet, É. Exploitation des ressources halieutiques par la pêche artisanale dans et autour des aires marines protégées: Socio-écosystème, conservation et gouvernance. In Moissonner la mer. Économies, Sociétés et Pratiques Halieutiques Méditerranéennes (Xve-Xxie Siècle); Butin, G., Faget, D., Raveux, O., Rivoal, S., Eds.; Karthala, Maison Méditerranéenne des Sciences de l’homme: Aix-en-Provence, France, 2018; pp. 351–380. [Google Scholar]
- Marchessaux, D. Distribution et statut des populations du phoque moine Monachus monachus (Hermann, 1779). Mammalia 1989, 53, 621–642. [Google Scholar] [CrossRef]
- Ferretti, F.; Myers, R.A.; Serena, F.; Lotze, H.K. Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 2008, 22, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.P. Les îles d’Hyères. Fragments d’Histoire; Actes Sud: Arles, France, 1997; pp. 1–176. [Google Scholar]
- Lavagne, A.; Bigeard, N.; Delaye, F.; Masotti, V. Étude de la dynamique forestière de l’île de Port-Cros de 1968 à 2004. Sci. Rep. Port-Cros Natl. Park 2007, 22, 195–232. [Google Scholar]
- Médail, F.; Cheylan, G.; Ponel, P. Dynamique des paysages et de la biodiversité terrestres du Parc national de Port-Cros (Var, France): Enseignements de cinquante années de gestion conservatoire. Sci. Rep. Port-Cros Natl. Park 2013, 27, 171–262. [Google Scholar]
- Jahandiez, E. Les Iles d’Hyères. Monographie des Iles D’or, 3rd ed.; (Laffite Reprints, 1977); Rebufa et Rouard: Toulon, France, 1929; p. 447. [Google Scholar]
- Médail, F.; Loisel, R.; Rolando, C. Éléments pour une gestion dynamique des populations de quatre végétaux protégés des îles d’Hyères (Var, France). Sci. Rep. Port-Cros Natl. Park 1995, 16, 19–54. [Google Scholar]
- Loisel, R.; Rolando, C.; Trocello, M. Les Pelouses de la Classe des Tuberarietea guttatae sur les îles de Port-Cros et Porquerolles. Caractéristiques Floristiques, Edaphiques et Syntaxonomiques, Facteurs de Régression; Rapport Laboratoire de Botanique et Ecologie Méditerranéenne; Université Aix-Marseille III et Parc national de Port-Cros: Marseille et Hyères, France, 1996; pp. 37 + annexes. [Google Scholar]
- Landrieu, G.; Gilg, O. Les réservoirs de la nature. In Biodiversité: Paroles d’Acteurs; Lemoine-Danese, M.L., Ed.; FRB (Fondation pour la Recherche sur la Biodiversité): Paris, France, 2010; pp. 69–76. [Google Scholar]
- Quezel, P.; Médail, F. Écologie et Biogéographie des Forêts du Bassin Méditerranéen; Elsevier (Collection Environnement): Paris, France, 2003; pp. 1–573. [Google Scholar]
- Pickett, S.T.A.; White, P.S. (Eds.) The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: New York, NY, USA, 1985; p. 472. [Google Scholar]
- Métropole Toulon Provence Méditerranée. À la Découverte des Salins d’Hyères; Métropole Toulon Provence Méditerranée: Toulon, France, 2019; pp. 1–44. [Google Scholar]
- De Wit, R. Can abandoned salinas be managed as coastal lagoons? Life Environ. 2020, 70, 225–233. [Google Scholar]
- Barraud, J. Un nouvel espace pour faire son nid aux Vieux Salins. Var-Matin, 26 September 2021. [Google Scholar]
- Duguet, R.; Priol, P.; Deso, G.; Geoffroy, D. Mise à jour des connaissances sur le discoglosse sarde Discoglossus sardus Tschudi in Otth, 1837 dans l’île de Port-Cros en 2018: Habitats potentiels, état de la population et mesures de gestion. Sci. Rep. Port-Cros Natl. Park 2019, 33, 101–126. [Google Scholar]
- Fritz, U.; Auer, M.; Bertolero, A.; Cheylan, M.; Fattizzo, T.; Hundsdörfer, A.K.; Martín Sampayo, M.; Pretus, J.L.; Šlroký, P.; Wink, M. A rangewide phylogeography of Hermann’s tortoise, Testudo hermanni (Reptilia: Testudines: Testudinidae): Implications for taxonomy. Zool. Scr. 2006, 35, 531–543. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Bianchi, C.N. Une idée neuve: La protection des espèces marines. In GIS Posidonie: Plus de 30 ans au Service de la Protection et de la Gestion du Milieu Marin; Le Diréach, L., Boudouresque, C.F., Eds.; GIS Posidonie: Marseille, France, 2013; pp. 85–91. [Google Scholar]
- Laborel-Deguen, F. Essai de réintroduction de Patella ferruginea Gmelin (Gasteropoda) dans le Parc national de Port-Cros (Var, France). Sci Rep Port-Cros Natl Park 1988, 14, 141–146. [Google Scholar]
- Cheylan, G.; Geoffroy, D. Colonisation des îles d’Hyères (Var, sud de la France) par le sanglier Sus scrofa. Sci. Rep. Port-Cros Natl. Park 2020, 34, 45–56. [Google Scholar]
- Sáez-Royuela, C.; Tellería, J.L. The increased population of the wild boar (Sus scrofa L.) in Europe. Mammal Rev. 1986, 16, 97–101. [Google Scholar] [CrossRef]
- Ballouard, J.M.; Kauffman, C.; Besnard, A.; Ausanneau, M.; Amiguet, M.; Billy, G.; Caron, S.; Fosseries, G.; Ferrari, T.; Mariani, V.; et al. Recent invaders in small Mediterranean islands: Wild boars impact snakes in Port-Cros National Park. Diversity 2021, 13, 498. [Google Scholar] [CrossRef]
- Dubois, S.; Fenwick, N.; Ryan, E.A.; Baker, L.; Baker, S.E.; Beausoleil, N.J.; Carter, S.; Cartwright, B.; Costa, F.; Draper, C.; et al. International consensus for ethical wildlife control. Conserv. Biol. 2017, 30, 753–760. [Google Scholar] [CrossRef] [Green Version]
- McNeely, J.A. Protected areas for the twenty-first century: Working to provide benefits for Society. Unasylva 1994, 45, 3–7. [Google Scholar]
- Boudouresque, C.F.; Verlaque, M. Nature conservation, Marine Protected Areas, sustainable development and the flow of invasive species to the Mediterranean Sea. Sci. Rep. Port-Cros Natl Park 2005, 21, 29–54. [Google Scholar]
- Katsanevakis, S.; Issaris, Y.; Poursanidis, D.; Thessalou-Legaki, M. Vulnerability of marine habitats to the invasive green alga Caulerpa racemosa var. cylindracea within a marine protected area. Mar. Environ. Res. 2010, 70, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudouresque, C.F.; Verlaque, M. An overview of species introduction and invasion processes in marine and coastal lagoon habitats. Cah. Biol. Mar. 2012, 53, 309–317. [Google Scholar]
- Mannino, A.M.; Balistreri, P. Invasive alien species in Mediterranean Marine Protected Areas: The Egadi Islands (Italy) case study. Biodiversity 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; University of Chicago: Chicago, IL, USA, 1958; pp. i–xiv + 1–181. [Google Scholar]
- Connell, J.H.C. Diversity in tropical rain forests and coral reefs. High diversity of trees and corals is maintained only in a nonequilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Dunstan, P.K.; Johnson, C.R. Invasion rates increase with species richness in a marine epibenthic community by two mechanisms. Oecologia 2004, 138, 285–292. [Google Scholar] [CrossRef]
- Corriero, G.; Pierri, C.; Accoroni, S.; Alabiso, G.; Bavestrello, G.; Barbone, E.; Bastianini, M.; Bazzoni, A.M.; Bernardi Aubry, F.; Boero, F.; et al. Ecosystem vulnerability to alien and invasive species: A case study on marine habitats along the Italian coast. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 392–409. [Google Scholar] [CrossRef]
- Meinesz, A.; Belsher, T.; Thibaut, T.; Antolic, B.; Ben Mustapha, K.; Boudouresque, C.F.; Chiaverini, D.; Cinelli, F.; Cottalorda, J.M.; Djellouli, A.; et al. The introduced alga Caulerpa taxifolia continues to spread in the Mediterranean. Biol. Invasions 2001, 3, 201–210. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: Invasive versus introduced macrophytes. Mar. Pollut. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef]
- Verlaque, M.; Afonso-Carrillo, J.; Gil-Rodriguez, M.C.; Durand, C.; Boudouresque, C.F.; Le Parco, Y. Blitzkrieg in a marine invasion: Caulerpa racemosa var. cylindracea (Bryopsidales, Chlorophyta) reaches the Canary Islands (NE Atlantic). Biol. Invasions 2004, 6, 269–281. [Google Scholar] [CrossRef]
- Bax, N.; Hayes, K.; Marshall, A.; Parry, D.; Thresher, R. Man-Made Marinas as Sheltered Islands for Alien Organisms: Establishment and Eradication of an Alien Invasive Marine Species. Turning the Tide: The Eradication of Invasive Species; Veitch, C.R., Clout, M.N., Eds.; IUCN SSC Invasive Specialist Group, IUCN: Gland, Switzerland, 2002; pp. 26–39. [Google Scholar]
- Locke, A.; Hanson, J.M. Rapid response to non-indigenous species. 1. Goals and history of rapid response in the marine environment. Aquat. Inv. 2009, 4, 237–247. [Google Scholar] [CrossRef]
- Boudouresque, C.F. Protected marine species, prevention of species introduction and the national environmental agencies of Mediterranean countries: Professionalism or amateurishness? In Actes du Congrès International ‘Environnement et Identité en Méditerranée’, Corte, France, 3–5 July 2002; Université de Corse Pascal Paoli: Corte, France, 2002; Volume 4, pp. 75–85. [Google Scholar]
- Cottalorda, J.M.; Robert, P.; Charbonnel, E.; Dimeet, J.; Menager, V.; Tillman, M.; De Vaugelas, J.; Volto, E. Eradication de la colonie de Caulerpa taxifolia découverte en 1994 dans les eaux du Parc National de Port-Cros (Var, France). In Second International Workshop on Caulerpa taxifolia; Ribera, M.A., Ballesteros, E., Boudouresque, C.F., Gomez, A., Gravez, V., Eds.; Publications Universitat Barcelona: Barcelona, Spain, 1996; pp. 149–155. [Google Scholar]
- Cottalorda, J.M.; Barcelo, A.; Bergère, H.; Houard, T.; Lefebvre, C.; Robert, P. Le Parc national de Port-Cros: Une structure de référence dans la mise en œuvre de stratégies de contrôle de la chlorobionte envahissante Caulerpa taxifolia (Vahl) C. Agardh. Sci. Rep. Port-Cros Natl. Park 2010, 24, 105–126. [Google Scholar]
- Cottalorda, J.M.; Barcelo, A.; Barral, M.; Bergère, H.; Formentin, J.Y.; Pironneau, E.; Houard, T. Résultats de la campagne d’octobre 2010 de recherche et d’éradication de la Chlorobionte envahissante Caulerpa taxifolia (Vahl) C. Agardh dans les eaux du Parc national de Port-Cros. Sci. Rep. Port-Cros Natl. Park 2011, 25, 199–202. [Google Scholar]
- Cottalorda, J.M.; Houard, T.; Barcelo, A.; Barral, M.; Bergere, H.; Formentin, J.Y.; Pironneau, E. Résultats de la campagne d’octobre 2011 de recherche et d’éradication de la Chlorobionte envahissante Caulerpa taxifolia (Vahl) C. Agardh dans les eaux du Parc national de Port-Cros. Sci. Rep. Port-Cros Natl. Park 2012, 26, 247–250. [Google Scholar]
- Barcelo, A.; Cottalorda, J.M.; Peirache, M.; Jaubert, R.; Bergere, H.; Esposito, G.; Formentin, J.Y.; Gillet, P.; Houard, T.; Jullian, E.; et al. Deux décennies d’amélioration des techniques de recherche et de contrôle du Chlorobionte invasif Caulerpa taxifolia (Vahl) C. Agardh dans les eaux du Parc national de Port-Cros (Méditerranée, France). Sci. Rep. Port-Cros Natl. Park 2013, 27, 437–450. [Google Scholar]
- Jaubert, R.; Cottalorda, J.M.; Barcelo, A.; Peirache, M.; Bergere, H.; Jullian, E.; Formentin, J.Y.; Pasqualini, B.; Badaire, C.; Pironneau, É.; et al. Résultats de la campagne 2014 de recherche et d’éradication du chlorobionte invasif Caulerpa taxifolia (Vahl) C. Agardh dans les eaux de l’île de Port-Cros, cœur du Parc national de Port-Cros (Var, France). Sci. Rep. Port-Cros Natl. Park 2015, 29, 255–258. [Google Scholar]
- Barcelo, A.; Cottalorda, J.M.; Peirache, M.; Abiven, T.; Gomez, M.C.; Viviani, R.A.; Bergere, H.; Baudin, E.; Jullian, E.; Moreau, S.; et al. Définition d’une politique et d’une stratégie globale de gestion concertées du chlorobionte invasif Caulerpa taxifolia à l’échelle des cœurs et de l’aire marine adjacente du Parc national de Port-Cros (Provence, France). Sci. Rep. Port-Cros Natl. Park 2016, 30, 45–64. [Google Scholar]
- Lascève, M. Premiers résultats de l’opération de limitation de la population de tortue de Floride sur le site des Vieux Salins, Hyères (Var, France). Sci. Rep. Port-Cros Natl. Park 2014, 28, 195–201. [Google Scholar]
- Berville, L.; Renucci, M.; Provost, E. Mise en place de protocoles de contrôle de la fourmi d’Argentine (Linepithema humile) sur les îles de Port-Cros et de Porquerolles (Var, France). Sci. Rep. Port-Cros Natl. Park 2012, 26, 91–108. [Google Scholar]
- Astruc, G.; Cheylan, M.; Couturier, T. Suivi de l’Implantation de la Tarente de Maurétanie Tarentola mauritanica (Linnaeus, 1758) sur l’île de Porquerolles. Impact sur l’Espèce Autochtone l’Hémidactyle Verruqueux Hemidactylus turcicus (Linnaeus, 1758); Parc National de Port-Cros: Hyères, France, 2008; pp. 1–15. [Google Scholar]
- Deso, G.; Gomez, M.C.; Priol, P.; Capoulade, F.; Duguet, R. Premières mentions de la tarente de Maurétanie Tarentola mauritanica (Linnaeus, 1758) et de la grenouille rieuse Pelophylax ridibundus (Pallas, 1771) sur l’île du Levant (îles d’Hyères, Var). Sci. Rep. Port-Cos Natl. Park 2008, 32, 237–240. [Google Scholar]
- Simberloff, D.; Holle, B.V. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Passetti, A.; Aboucaya, A.; Buisson, E.; Gauthier, J.; Médail, F.; Pascal, M.; Ponel, P.; Vidal, E. Restauration écologique de la Réserve intégrale de l’île de Bagaud (Parc national de Port-Cros, Var, France) et ‘état zéro’ des suivis scientifiques: Synthèse méthodologique. Sci Rep Port-Cros Natl Park 2012, 26, 149–171. [Google Scholar]
- Braschi, J.; Caceres, M.; Delcourt, N.; Tournier, F.; Ponel, P. Conséquences sur les communautés d’insectes volants de l’éradication simultanée du rat noir (Rattus rattus) et des griffes de sorcières (Carpobrotus spp.) dans le cadre du programme de restauration écologique de l’île de Bagaud (Parc national de Port-Cros, France): Résultats préliminaires. Sci. Rep. Port-Cros Natl. Park 2017, 31, 71–79. [Google Scholar]
- Buisson, É.; Aboucaya, A.; Affre, L.; Braschi, J.; Chenot, J.; Dailly, S.; Hess, M.; Passetti, A.; Pavon, D.; Ramone, H.; et al. Rétablissement des communautés végétales après éradication des griffes de sorcière (Carpobrotus sp.) dans le cadre du programme de restauration écologique de l’île de Bagaud (Parc national de Port-Cros, France): Résultats 5 ans après l’éradication. Sci. Rep. Port-Cros Natl. Park 2018, 32, 123–135. [Google Scholar]
- Aboucaya, A.; Cottaz, C.; Barcelo, A.; Buisson, É.; Ponel, P. Bilan du séminaire scientifique ‘Programme de restauration écologique de la réserve intégrale de l’île de Bagaud, Parc national de Port-Cros, résultats de dix années de suivi’. Hyères, 5 novembre 2019. Sci. Rep. Port-Cros Natl. Park 2020, 34, 289–292. [Google Scholar]
- Braschi, J. Conséquences du Contrôle D’espèces Exotiques Envahissantes sur la Dynamique des Assemblages d’Araignées et de Coléoptères de l’île de Bagaud (Parc National de Port-Cros). Cas de la Griffe de Sorcière (Carpobrotus) et du rat Noir (Rattus rattus). Ph.D. Thesis, Aix-Marseille University, Marseille, France, 2021; pp. 1–233. [Google Scholar]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Perez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T.; et al. Global imprint of climate change on marine life. Nat. Clim. Change 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Butt, N.; Possingham, H.P.; De Los Rios, C.; Maggini, R.; Fuller, R.A.; Maxwell, S.L.; Watson, J.E.M. Challenges in assessing the vulnerability of species to climate change to inform conservation actions. Biol. Conserv. 2016, 199, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Francour, P.; Boudouresque, C.F.; Harmelin, J.G.; Harmelin-Vivien, M.L.; Quignard, J.P. Are the Mediterranean waters becoming warmer? Information from biological indicators. Mar. Pollut. Bull. 1994, 28, 523–526. [Google Scholar] [CrossRef]
- Lejeusne, C.; Chevaldonné, P. Population structure and life history of Hemimysis margalefi (Crustacea: Mysidacea), a thermophilic cave-dwelling species benefiting from the warming of the NW Mediterranean. Mar. Ecol. Prog. Ser. 2005, 287, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, C.N.; Azzola, A.; Bertolino, M.; Betti, F.; Bo, M.; Cattaneo-Vietti, R.; Cocito, S.; Montefalcone, M.; Morri, C.; Oprandi, A.; et al. Consequences of the marine climate and ecosystem shift of the 1980-90s on the Ligurian Sea biodiversity (NW Mediterranean). Eur. Ecol. J. 2019, 86, 458–487. [Google Scholar] [CrossRef]
- Encarnação, J.; Morais, P.; Baptista, V.; Cruz, J.; Teodósio, M.A. New evidence of marine fauna tropicalization off the southwestern Iberian Peninsula (Southwest Europe). Diversity 2019, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Astruch, P.; Bonhomme, P.; Goujard, A.; Rouanet, É.; Boudouresque, C.F.; Harmelin, J.; Harmelin-Vivien, M. Provence and Mediterranean warming: The parrotfish Sparisoma cretense is coming. Rapp. Comm. Int. Mer Médit. 2016, 41, 362. [Google Scholar]
- Perez, T. Impact des Changements Climatiques sur la Biodiversité en mer Méditerranée; CAR/ASP: Tunis, Tunis, 2008; pp. 1–62. [Google Scholar]
- Boudouresque, C.F.; Bernard, G.; Pergent, G.; Shili, A.; Verlaque, M. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Bot. Mar. 2009, 52, 395–418. [Google Scholar] [CrossRef]
- Le Bourg, B.; Bănaru, D.; Saraux, C.; Nowaczyk, A.; Le Luherne, E.; Jadaud, A.; Bigot, J.L.; Richard, P. Trophic niche overlap of sprat and commercial small pelagic teleosts in the Gulf of Lions (NW Mediterranean Sea). J. Sea Res. 2015, 103, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Saraux, C.; Van Beveren, E.; Brosset, P.; Queiros, Q.; Bourdeix, J.H.; Dutto, G.; Gasset, E.; Jac, C.; Bonhommeau, S.; Fromentin, J.M. Small pelagic fish dynamics: A review of mechanisms in the Gulf of Lions. Deep Sea Res. Part II 2019, 259, 52–61. [Google Scholar] [CrossRef]
- Vergés, A.; Doropoulos, C.; Malcolm, H.A.; Skye, M.; Garcia-Pizá, M.; Marzinelli, E.M.; Campbell, A.H.; Ballesteros, E.; Hoey, A.S.; Vila-Concejo, A.; et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. USA 2016, 113, 13791–13796. [Google Scholar] [CrossRef] [Green Version]
- Paris Agreement. Adoption of the Paris Agreement. Conference of the Parties, 31st session, Paris, 30 November to 11 December 2015. United Nations, Framework Convention on Climate Change, FCCC/CP/2015/L.9/Rev. 2015, 1, 1–32.
- Llausàs, A.; Vila-Subirós, J.; Puryo-Ros, J.; Fraguell, R.M. Carrying capacity as a tourism management strategy in a Marine Protected Area: A political ecology analysis. Conserv. Soc. 2019, 17, 366–376. [Google Scholar] [CrossRef]
- Law n° 2016-1087 of 8 August 2016 for the reconquest of biodiversity, nature and landscapes. Journal Officiel de la République Française, n° 0184 of 9 August 2016.
- Martin, G.J. Ecosystem-based approach and restoration of compensation for ecological damage. Vie Milieu/Life Environ. 2020, 70, 113–120. [Google Scholar]
- Martin, G.J. Les potentialités de l’obligation réelle environnementale. Droit De l’Environnement 2016, 249, 334–340. [Google Scholar]
- Comité Français de la Biodiversité. Avis Relatif à la Stratégie Nationale pour les Aires Protégées. Délibération n° 2020-01. Available online: http://www.avis-biodiversite.developpement-durable.gouv.fr/IMG/pdf/20209822 (accessed on 15 September 2021).
- Bermudez, G.M.A.; Lindemann-Matthies, P. ‘What matters is species richness’—High school students’understanding of the components of biodiversity. Res. Sci. Educ. 2020, 50, 3159–3187. [Google Scholar] [CrossRef]
- Simberloff, D. The role of science in the preservation of forest biodiversity. Forest. Ecol. Manag. 1999, 115, 101–111. [Google Scholar] [CrossRef]
- Enserink, M.; Vogel, G. The carnivore comeback. Science 2006, 314, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Branton, M.; Richardson, J.S. Assessing the value of the umbrella-species concept for conservation planning with meta-analysis. Conserv. Biol. 2010, 25, 9–20. [Google Scholar] [CrossRef]
- Tribot, A.S.; Carabeux, Q.; Deter, J.; Claverie, T.; Villéger, S.; Mouquet, N. Confronting species aesthetics with ecological functions in coral reef fish. Sci. Rep. 2018, 8, 1–7. [Google Scholar]
- Leleu, K.; Alban, F.; Pelletier, D.; Charbonnel, E.; Letourneur, Y.; Boudouresque, C.F. Fishers’ perceptions as indicators of performance of Marine Protected Areas (MPAs). Mar. Pol. 2012, 36, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Cadoret, A. Conflicts and acceptability of visitation management measures for a marine protected area: The case of Porquerolles, Port-Cros National Park. Ocean Coast. Manag. 2021, 204, 1–13. [Google Scholar] [CrossRef]
- Meur-Férec, C.; Favennec, J. The opening to the French public of ‘natural sites of coastal dunes: The choice between ‘over-visiting’ and ‘over-protection’. In Dunes and Estuaries 2005, Proceedings of the International Conference on Nature Restoration Practices in European Coastal Habitats, Koksijde, Belgium, 19–23 September 2005; Special Publication, VLIZ; Herrier, J.L., Mees, J., Salman, A., Seys, J., Van Nieuwenhuyse, H., Cobbelaere, I., Eds.; VLIZ: Oostende, Belgium, 2005; Volume 19, pp. i–xiv + 1–685. [Google Scholar]
- Gerber, J.D.; Rodewald, R.; Knoepfel, P. The sustainable management of the landscape. The lessons the new regional nature parks must draw from the experience of the old corporations. J. Alpine Res. 2007, 95, 1–12. [Google Scholar]
- Araújo, R.; Sousa-Pinto, I.; Serrão, E.A.; Åberg, P. Recovery after trampling disturbance in a canopy-forming seaweed population. Mar. Biol. 2012, 159, 697–707. [Google Scholar]
- Agius, K.; Chaperon, S. Stakeholder management and the imbalance of power: A central Mediterranean perspective on tourism in Marine Protected Areas. In Mediterranean Protected Areas in the Era of Overtourism; Mandić, A., Petrić, L., Eds.; Spinger Nature: Cham, Switzerland, 2021; pp. 117–135. [Google Scholar]
- Carreño, A.; Lloret, J. Environmental impacts of increasing leisure boating activity in Mediterranean coastal waters. Ocean Coast. Manag. 2021, 209, 1–13. [Google Scholar] [CrossRef]
- Deldrève, V.; Michel, C. La démarche de capacité de charge sur Porquerolles (Provence, Parc national de Port-Cros, France): De la prospective au plan d’action. Sci. Rep. Port-Cros Natl. Park 2019, 33, 63–100. [Google Scholar]
- Boudouresque, C.F.; Astruch, P.; Bănaru, D.; Blanfuné, A.; Belloni, B.; Changeux, T.; Chevaldonné, P.; Fernandez, C.; Harmelin, J.G.; Perez, T.; et al. Ecosystem-based quality indices: Valuable tools for environment management. Life Environ. 2020, 70, 2–15. [Google Scholar]
- Schnitzler, A.; Génot, J.C.; Wintz, M. Espaces protégés: De la gestion conservatoire vers la non intervention. Courr. Environ. INRA 2008, 56, 29–44. [Google Scholar]
- Meinesz, A. Le Phoque moine Monachus monachus. In Méditerranée mer Vivante, 20th ed.; Meinesz, A., Ed.; Lyons Clubs Nice Doyen: Nice, France, 2020; pp. 88–91. [Google Scholar]
- Lupp, G.; Konold, W.; Bastian, O. Landscape management and landscape changes toward more naturalness and wilderness: Effects on scenic qualities—The case of the Müritz National Park in Germany. J. Nat. Cons. 2013, 21, 10–21. [Google Scholar] [CrossRef]
- Venter, F.J.; Naiman, R.J.; Biggs, H.C.; Pienaar, D.J. The evolution of conservation management philosophy: Science, environmental change and social adjustments in Kruger National Park. Ecosystems 2008, 11, 173–192. [Google Scholar] [CrossRef]
- Haines, R.; Verstraeten, Y.; Papadopoulou, L.; Hattam, C.; Pantzar, M.; Russi, D.; Chaparro, L.; Hoffman, J.; Van Dijk, E.; Vindigni, G.; et al. Study on the Economic Benefits of Marine Protected Areas. Task 5 Case Studies—Final Report; European Commission: Brussels, Belgium, 2018; pp. 1–312. [Google Scholar]
- Guidetti, P.; Claudet, J. Comanagement practices enhance fisheries in marine protected areas. Conserv. Biol. 2009, 24, 312–318. [Google Scholar] [CrossRef]
- Beschta, R.L.; Ripple, W.J. Riparian vegetation recovery in Yellowstone: The first two decades after wolf reintroduction. Biol. Conserv. 2016, 198, 93–103. [Google Scholar] [CrossRef]
- Landry, J.M. Le Loup; Delachaux and Niestlé: Paris, France, 2001; pp. 1–240. [Google Scholar]
- Fritts, S.H.; Bangs, E.E.; Fontaine, J.A.; Johnson, M.R.; Phillips, M.K.; Koch, E.D.; Gunson, J.R. Planning and implementing a reintroduction of wolves to Yellowstone National Park and Central Idaho. Restor. Ecol. 1997, 5, 7–27. [Google Scholar] [CrossRef] [Green Version]
- Ripple, W.J.; Beschta, R.L. Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biol. Conserv. 2012, 145, 205–213. [Google Scholar] [CrossRef]


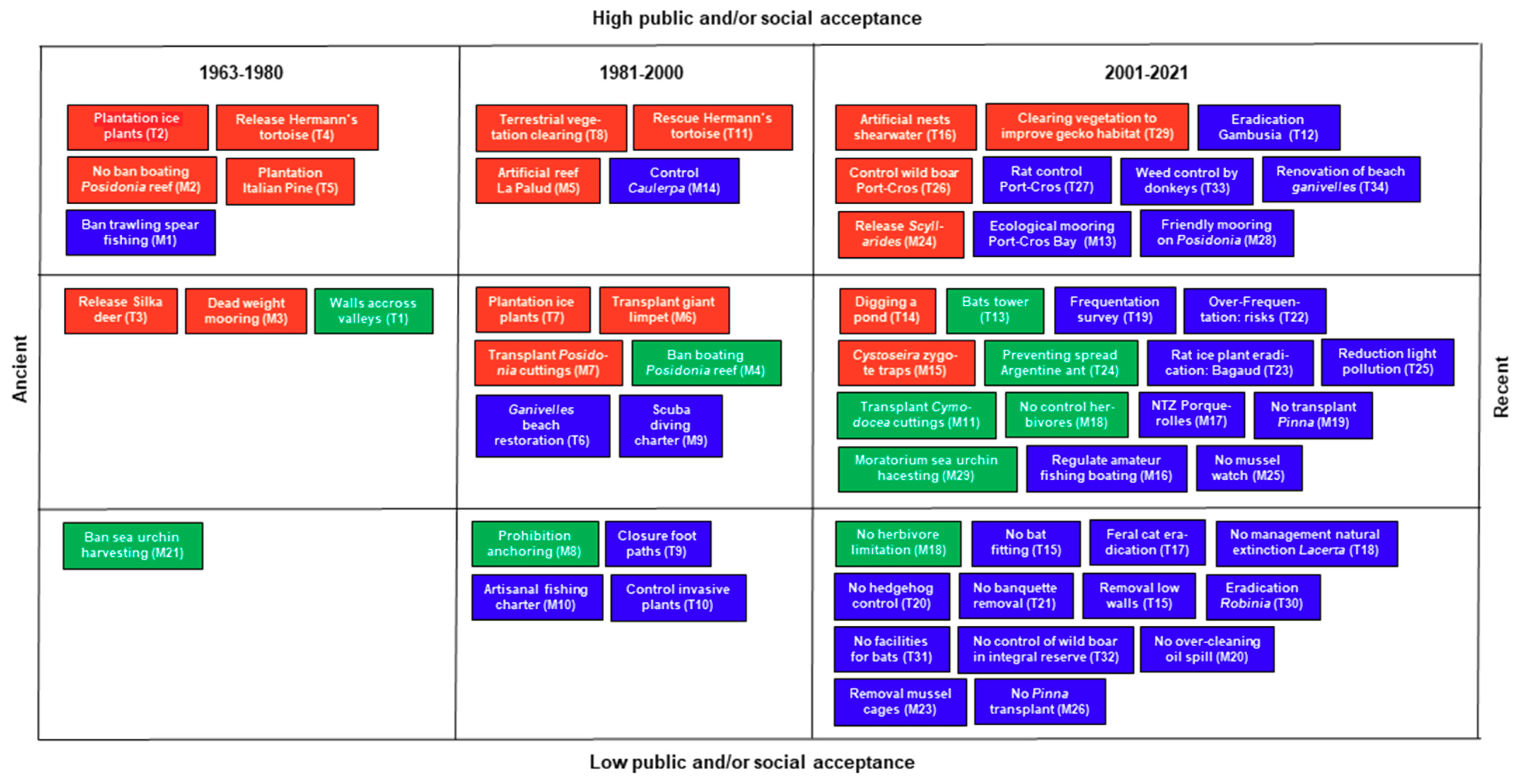
| No, Dates | Management Issues | Responses, Results and Comments | References |
|---|---|---|---|
| T27 2004 to present | Cyclical outbreaks of the non-native black rat Rattus rattus threaten seabirds and are a nuisance for residents (PC) | Setting up traps around the village and seabird nesting sites | Hervé Bergère (pers. comm.) |
| T28 2000s | Low walls across valleys built in the 1970s to favour the Tyrrhenian painted frog Discoglossus sardus (T1) were ineffective and inappropriate (PC) | Removal of the low walls | Élodie Debize (pers. comm.) |
| T29 2002–2005 | Decline of the European leaf-toed gecko Euleptes europaea (PC) | Clearing and thinning of the vegetation of Vallon de la Solitude. Renewed once, then discontinued | Élodie Debize (pers. comm.) |
| T30 2011–2012 | Presence of the invasive black locust Robinia pseudo-acacia near the Vallon de la Solitude dam (PC) | Successful eradication | Élodie Debize (pers. comm.) |
| T31 2011–2013 | Restoration of the La Sardinière farm, evidence of the agricultural past of PC | The SC rejected the proposal from bat specialists to install facilities for bats (shelters, waterers) | Avis 9/2010 of the SC |
| T32 2020 | Natural arrival of the wild boar Sus scrofa (swimming) in the integral reserve of Bagaud Island (PC) | The wild boar is native and a key species in Mediterranean ecosystems: no reason to eradicate it | Avis 5/2020 of the SC |
| T33 2021 | Weed control and fire risk, Plage d’Argent (PQ) | Use of four donkeys for environment-friendly weed control | Secrétariat connaissance du patrimoine, PCNP |
| T34 2021 | Aging of the split stake fences (ganivelles) installed in 1982 (T6) to protect the vegetation of the back beach (Plage du Sud) (PC) | Renovation of the split stake fences | Élodie Debize (pers. comm.) |
| M21 1963 to present | Over-exploitation of sea urchins (Paracentrotus lividus) (PC) | Ban on sea urchin harvesting | Philippe Robert (pers. comm.) |
| M22 1984–1995 | Rescue of the critically endangered Mediterranean monk seal Monachus monachus | Project of breeding in captivity of individuals from Cap Blanc (Mauretania), then release of the calves in an enclosed Port-Cros bay | Simon and Moutou [101], Boudouresque [102] |
| M23 2000s | Monitoring of water quality via Mytilus galloprovincialis (‘mussel watch’) | Cages containing mussels, placed without PCNP authorization, removed and destroyed by wardens | Andral et al. [103] |
| M24 2005 | Specimens of the protected Scyllarides latus seized in commercial outlets (PC) | Released in the PCNP, La Palud Bay (Port-Cros Island) | Philippe Robert (pers. comm.) |
| M25 2010s | Monitoring of water quality via Mytilus galloprovincialis (‘mussel watch’) | Risk of contamination by non-indigenous species. Authorization refused | Avis 7/2018 of the SC |
| M26 2018 | Pinna nobilis transplant from shallow towards deep habitats in an attempt to thwart mass mortality due to Haplosporidium pinnae | Absence of a scientific basis for such transplantations. Authorization refused by the SC | Amalric and Leclerc [104] |
| M27 2020 | Disposal and exploding of bombs and mines from World War II | Avoid PCNP waters, prior scaring of wildlife, less than 10–15 m depth | Unnumbered Avis of the SC of 3 March 2020 |
| M28 2020 to present | Degradation of the Posidonia oceanica meadow by the anchors of pleasure boats (PC) | Setting up of a ZMEL (Zone de mouillages et d’équipements légers—environment-friendly mooring) in the Bagaud Pass: 68 mooring buoys | Arrêté interpréfectoral N° 039/2020 (PREMAR)/No DDTM/SML 001/2020 (PREF) |
| M29 2021 | Decline of the populations of Paracentrotus lividus and other echinoderms | A moratorium on P. lividus harvesting would be acceptable, under certain conditions | Avis 1/2021 of the SC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudouresque, C.-F.; Barcelo, A.; Blanfuné, A.; Changeux, T.; Martin, G.; Médail, F.; Perret-Boudouresque, M.; Ponel, P.; Ruitton, S.; Taupier-Letage, I.; et al. Biodiversity Management in a Mediterranean National Park: The Long, Winding Path from a Species-Centred to an Ecosystem-Centred Approach. Diversity 2021, 13, 594. https://doi.org/10.3390/d13110594
Boudouresque C-F, Barcelo A, Blanfuné A, Changeux T, Martin G, Médail F, Perret-Boudouresque M, Ponel P, Ruitton S, Taupier-Letage I, et al. Biodiversity Management in a Mediterranean National Park: The Long, Winding Path from a Species-Centred to an Ecosystem-Centred Approach. Diversity. 2021; 13(11):594. https://doi.org/10.3390/d13110594
Chicago/Turabian StyleBoudouresque, Charles-François, Alain Barcelo, Aurélie Blanfuné, Thomas Changeux, Gilles Martin, Frédéric Médail, Michèle Perret-Boudouresque, Philippe Ponel, Sandrine Ruitton, Isabelle Taupier-Letage, and et al. 2021. "Biodiversity Management in a Mediterranean National Park: The Long, Winding Path from a Species-Centred to an Ecosystem-Centred Approach" Diversity 13, no. 11: 594. https://doi.org/10.3390/d13110594
APA StyleBoudouresque, C. -F., Barcelo, A., Blanfuné, A., Changeux, T., Martin, G., Médail, F., Perret-Boudouresque, M., Ponel, P., Ruitton, S., Taupier-Letage, I., & Thibaut, T. (2021). Biodiversity Management in a Mediterranean National Park: The Long, Winding Path from a Species-Centred to an Ecosystem-Centred Approach. Diversity, 13(11), 594. https://doi.org/10.3390/d13110594








