Diversity of Flower Visiting Beetles at Higher Elevations on the Yulong Snow Mountain (Yunnan, China)
Abstract
1. Introduction
2. Material and Methods
2.1. Study Sites and Plot Design
2.2. Data Collection
2.3. Network Construction and Analysis
- H2′: Network generalization describes the degree of specialization among plants and pollinators within the network [48] and ranges between 0 (extreme generalization) and 1 (extreme specialization).
- Weighted connectance refers to the realized proportion of possible links weighted by network size.
- Nestedness is based on overlap and decreasing fill (NODF): the extent to which specialists interact with a subset of species that also interact with generalists.
- Niche overlap of beetles refers to mean similarity in interaction patterns between flower visiting beetles.
- Extinction slope of beetles refers to the simulated secondary loss of beetle species following the extinctions of plant species.
- Robustness of beetles refers to the “fragility” of beetles to losses in the other level (plant).
- Functional complementarity of beetles refers to the extent of the sharing of interactions between beetles.
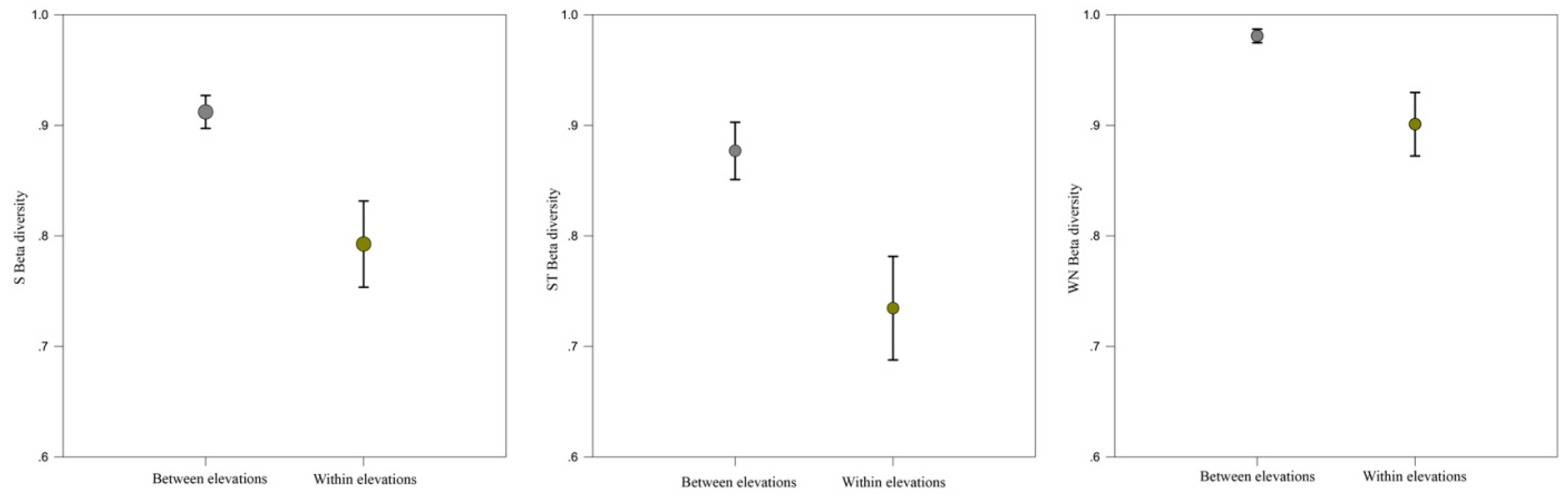
2.4. Network Dissimilarity (β-Diversity)
2.5. Statistical Analyses
3. Results
3.1. Species Identification and Community Composition
3.2. Network Structure of Plant-Flower Visiting Beetles
4. Discussion
4.1. Comparative Diversity of Flower Visiting Beetles in Sub-Alpine Versus Tropical Versus Mediterranean Communities
4.2. Coevolution or Foraging Opportunism of Flower Visiting Beetles and Plant and Plant Lineages?
4.3. Effect of Elevation and Habitat on Beetle Diversity on Montane Flora
4.4. Uncertain Roles of Flower Visiting Beetles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Slipinski, S.A.; Leschen, R.A.B.; Lawrence, J.F. Order Coleoptera Linneaus, 1758. In Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness; Zhang, Z.Q., Ed.; Magnolia Press: Auckland, New Zealand, 2011; Volume 3148, pp. 203–208. [Google Scholar]
- Bao, T.; Walczyska, K.S.; Moody, S.; Wang, B.; Rust, J. New family Apotomouridae fam. nov. (Coleoptera: Tenebrionoidea) from lower Cenomanian amber of Myanmar. Cretac. Res. 2018, 91, 14–19. [Google Scholar] [CrossRef]
- Wardhaugh, C.W. How many species of arthropods visit flowers? Arthropod-Plant Interact. 2015, 9, 547–565. [Google Scholar] [CrossRef]
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Bernhardt, P. Convergent evolution and adaptive radiation of beetle-pollinated angiosperms. Plant Syst. Evol. 2000, 222, 293–320. [Google Scholar] [CrossRef]
- Prance, G.; Arias, J. A study of the floral biology of Victoria amazonica (Poepp.) Sowerby (Nymphaeaceae). Acta Amaz. 1975, 6, 163–170. [Google Scholar] [CrossRef]
- Schneider, E.L.; Buchanan, J. Morphological studies of the Nymphaeaceae. XI. The floral biology of Nelumbo pentapetala. Amer. J. Bot. 1980, 67, 182–193. [Google Scholar] [CrossRef]
- Goldblatt, P.; Bernhardt, P.; Manning, J.C. Pollination of petaloid geophytes by monkey beetles (Scarabaeidae: Rutelinae: Hopliini) in southern Africa. Ann. Missouri Bot. Gard. 1998, 85, 215–230. [Google Scholar] [CrossRef]
- Dieringer, G.; Cabrera, R.L.; Lara, M.; Loya, L.; Resyes-Castillo, P. Beetle pollination and floral thermogenicity in Magnolia tamaulipana (Magnoliaceae). Int. J. Plant Sci. 1999, 160, 64–71. [Google Scholar] [CrossRef]
- Sakai, S.; Kuniyasu, M.; Ymoto, T.; Kato, M.; Inoue, T. A new pollination system: Dung-beetle pollination discovered in Orchidantha inouei (Lowiaceae, Zingiberales) in Sarawak, Malaysia. Amer. J. Bot. 1999, 86, 56–61. [Google Scholar] [CrossRef]
- Corlett, R.T. Pollination in a degraded tropical landscape: A Hong Kong case study. J. Trop. Ecol. 2001, 17, 155–161. [Google Scholar] [CrossRef]
- Sayers, T.D.J.; Steinbauer, M.J.; Miller, R.E. Visitor or vector? The extent of rove beetle (Coleoptera: Staphylinidae) pollination and floral interactions. Arthropod-Plant Interact. 2019, 13, 685–701. [Google Scholar] [CrossRef]
- Kirmse, S.; Chaboo, C.S. Flowers are essential to maintain high beetle diversity (Coleoptera) in a Neotropical rainforest canopy. J. Nat. Hist. 2020, 54, 1661–1696. [Google Scholar] [CrossRef]
- Armstrong, J.E.; Irvine, A.K. Floral Biology of Myristica-Insipida (Myristicaceae), a Distinctive Beetle Pollination Syndrome. Am. J. Bot. 1989, 76, 86–94. [Google Scholar] [CrossRef]
- Hansman, D.J. Floral biology of dry rainforest in north Queensland and a comparison with adjacent savanna woodland. Aust. J. Bot. 2001, 49, 137–153. [Google Scholar] [CrossRef]
- Momose, K.; Yumoto, T.; Teruyoshi, N.; Kato, M.; Nagamasu, H.; Sakai, S.; Harrison, R.; Ttioka, T.; Hamid, A.; Inoue, T. Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. I. Characteristics of the plant-pollinator community in a lowland dipterocarp forest. Am. J. Bot. 1998, 85, 1477–1501. [Google Scholar] [CrossRef]
- Wardhaugh, C.W.; Stork, N.E.; Edwards, W.; Grimbacher, P.S. The overlooked biodiversity of flower-visiting invertebrates. PLoS ONE 2012, 7, e45796. [Google Scholar] [CrossRef]
- Gottsberger, G. The structure and function of the primitive angiosperm flower-a discussion. Acta Bot. Neerl. 1974, 23, 461–471. [Google Scholar] [CrossRef]
- Wardhaugh, C.W.; Edwards, W.; Stork, N.E. The specialization and structure of antagonistic and mutualistic networks of beetles on rainforest canopy trees. Biol. J. Linn. Soc. 2015, 114, 287–295. [Google Scholar] [CrossRef]
- Körner, C. Mountain biodiversity, its causes and function. AMBIO 2004, 33, 11–17. [Google Scholar] [CrossRef]
- Körner, C. Coldest places on earth with angiosperm plant life. Alp. Bot. 2011, 121, 11–22. [Google Scholar] [CrossRef]
- Körner, C.; Jetz, W.; Paulsen, J.; Payne, D.; Rudmann-Maurer, K.; Spehn, E.M. A global inventory of mountains for bio-geographical applications. Alp. Bot. 2017, 127, 1–15. [Google Scholar] [CrossRef]
- Chape, S.; Spalding, M.; Jenkins, M. The World’s Protected Areas: Status, Values and Prospects in the 21st Century; University of California Press: Berkeley, CA, USA, 2008. [Google Scholar]
- Arroyo, M.T.K.; Primack, R.; Armesto, J. Community studies in pollination ecology in the high temperate Andes of central Chile. I. Pollination mechanisms and altitudinal variation. Am. J. Bot. 1982, 69, 82–97. [Google Scholar] [CrossRef]
- Müller, H. The fertilisers of alpine flowers. Nature 1880, 21, 275. [Google Scholar] [CrossRef]
- Heithaus, E.R. The role of plant-pollinator interactions in determining community structure. Ann. Mo. Bot. Gard. 1974, 61, 675–691. [Google Scholar] [CrossRef]
- Moldenke, A.R. California pollination ecology and vegetation types. Phytologia 1976, 34, 305–361. [Google Scholar]
- Warren, S.D.; Harper, K.T.; Booth, G.M. Elevational distribution of insect pollinators. Am. Midl. Nat. 1988, 120, 325–330. [Google Scholar] [CrossRef]
- Medan, D.; Montaldo, N.H.; Devoto, M.; Maniese, A.; Vasellati, V.; Roitman, G.G.; Bartoloni, N.H. Plant-pollinator Relationships at Two Altitudes in the Andes of Mendoza, Argentina. Arct. Antarct. Alp. Res. 2002, 34, 233–241. [Google Scholar] [CrossRef]
- Lefebvre, V.; Villemant, C.; Fontaine, C.; Daugeron, C. Altitudinal, temporal and trophic partitioning of flower-visitors in Alpine communities. Sci. Rep. 2018, 8, 4706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Ren, Z.X.; Lázaro, A.; Wang, H.; Bernhardt, P.; Li, H.D.; Li, D.Z. Floral traits influence pollen vectors’ choices in higher elevation communities in the Himalaya-Hengduan Mountains. BMC Ecol. 2016, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Lázaro, A.; Ren, Z.X.; Zhou, W.; Li, H.D.; Tao, Z.B.; Xu, K.; Wu, Z.K.; Wolfe, L.M.; Li, D.Z.; et al. The topological differences between visitation and pollen transport networks: A comparison in species rich communities of the Himalaya-Hengduan Mountains. Oikos 2019, 128, 551–562. [Google Scholar] [CrossRef]
- Doré, M.; Fontaine, C.; Thébault, E. Relative effects of anthropogenic pressures, climate, and sampling design on the structure of pollination networks at the global scale. Glob. Chang. Biol. 2021, 27, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Adedoja, O.A.; Kehinde, T.; Samways, M.J. Insect-flower interaction networks vary among endemic pollinator taxa over an elevation gradient. PLoS ONE 2018, 13, e0207453. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M. Flower traits, habitat, and phylogeny as predictors of pollinator service: A plant community perspective. Ecol. Monogr. 2020, 90, e01402. [Google Scholar] [CrossRef]
- Herrera, C.M.; Otero, C. Plant phylogeny as a major predictor of flower visitation by nitidulid beetles, a lineage of ancestral angiosperm pollinators. J. Pollinat. Ecol. 2021, 28, 179–188. [Google Scholar] [CrossRef]
- Kantsa, A.; Raguso, R.A.; Dyer, A.G.; Olesen, J.M.; Tscheulin, T.; Petanidou, T. Disentangling the role of floral sensory stimuli in pollination networks. Nat. Commun. 2018, 9, 1041. [Google Scholar] [CrossRef]
- Memmott, J. The structure of a plant-pollinator food web. Ecol. Lett. 1999, 2, 276–280. [Google Scholar] [CrossRef]
- Alarcón, R. Congruence between visitation and pollen-transport networks in a California plant–pollinator community. Oikos 2010, 119, 35–44. [Google Scholar] [CrossRef]
- Xu, X.; Ren, Z.X.; Trunschke, J.; Kuppler, J.; Zhao, Y.H.; Knop, E.; Wang, H. Bimodal activity of diurnal flower visitation at high elevation. Ecol. Evol. 2021, 11, 13487–13500. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, Y.H.; Rafferty, N.E.; Ren, Z.X.; Zhong, L.; Li, H.D.; Li, D.Z.; Wang, H. Evolutionary and ecological factors structure a plant-bumblebee network in a biodiversity hotspot, the Himalaya-Hengduan Mountains. Funct. Ecol. 2021, 35, 2523–2535. [Google Scholar] [CrossRef]
- Wardhaugh, C.W. The importance of flowers for beetle biodiversity and abundance. In Treetops at Risk: Challenges of Global Canopy Ecology and Conservation; Lowman, M., Devy, M., Ganesh, S.T., Eds.; Springer: New York, NY, USA, 2013; pp. 275–288. [Google Scholar]
- Zheng, L.Y.; Gui, H. Insect Classification; Nanjing Normal University Press: Nanjing, China, 1999. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. R News 2008, 8, 8–11. [Google Scholar]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef]
- Beckett, S.J. Improved community detection in weighted bipartite networks. R Soc. Open Sci. 2016, 3, 140536. [Google Scholar] [CrossRef]
- Poisot, T.; Canard, E.; Mouillot, D.; Mouquet, N.; Gravel, D. The dissimilarity of species interaction networks. Ecol. Lett. 2012, 15, 1353–1361. [Google Scholar] [CrossRef]
- Schwarz, B.; Dormann, C.F.; Vázquez, D.P.; Fründ, J. Within-day dynamics of plant–pollinator networks are dominated by early flower closure: An experimental test of network plasticity. Oecologia 2021, 196, 781–794. [Google Scholar] [CrossRef]
- Cook, S.M.; Bartlet, E.; Murray, D.A.; Williams, I.H. The role of pollen odour in the attraction of pollen beetles to oilseed rape flowers. Entomol. Exp. Appl. 2002, 104, 43–50. [Google Scholar] [CrossRef]
- Jürgens, A.; Dötterl, S. Chemical composition of anther volatiles in Ranunculaceae: Genera-specific profiles in Anemone, Aquilegia, Caltha, Pulsatilla, Ranunculus, and Trollius species. Am. J. Bot. 2004, 91, 1969–1980. [Google Scholar] [CrossRef]
- Pyke, G.H.; Ren, Z.X.; Trunschke, J.; Lunau, K.; Wang, H. Salvage of floral resources through re-absorption before flower abscission. Sci. Rep. 2020, 10, 15960. [Google Scholar] [CrossRef]
- Lunau, K.; Ren, Z.X.; Fan, X.Q.; Pyke, G.H.; Trunschke, J.; Wang, H. Nectar mimicry: A new phenomenon. Sci. Rep. 2020, 10, 7039. [Google Scholar] [CrossRef] [PubMed]
- De Vega, C.; Álvarez-Pérez, S.; Albaladejo, R.G.; Steenhuisen, S.-L.; Lachance, M.-A.; Johnson, S.D.; Herrera, C.M. The role of plant–pollinator interactions in structuring nectar microbial communities. J. Ecol. 2021, in press. [Google Scholar]
- Millard, J.; Outhwaite, C.L.; Kinnersley, R.; Freeman, R.; Gregory, R.D.; Adedoja, O.; Gavini, S.; Kioko, E.; Kuhlmann, M.; Ollerton, J.; et al. Global effects of land-use intensity on local pollinator biodiversity. Nat. Commun. 2021, 12, 2902. [Google Scholar] [CrossRef] [PubMed]
- Spehn, E.M.; Rudmann-Maurer, K.; Körner, C.; Maselli, D. Mountain Biodiversity and Global Change; GMBA-DIVERSITAS: Basel, Switzerland, 2010. [Google Scholar]
- Ernakovich, J.G.; Hopping, K.A.; Berdanier, A.B.; Simpson, R.T.; Kachergis, E.J.; Steltzer, H.; Wallenstein, M.D. Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob. Chang. Biol. 2014, 20, 3256–3269. [Google Scholar] [CrossRef] [PubMed]




| Family | No. of Species | No. of Specimens | Family | No. of Species | No. of Specimens |
|---|---|---|---|---|---|
| Chrysomelidae | 42 | 955 | Eumolpididae | 3 | 6 |
| Nitidulidae | 34 | 1657 | Melyeridae | 3 | 6 |
| Curculionidae | 10 | 45 | Melolonthidae | 2 | 5 |
| Coccinellidae | 10 | 28 | Brentidae | 2 | 2 |
| Cantharidae | 7 | 141 | Scirtidae | 2 | 7 |
| Staphylinidae | 7 | 115 | Cetoniidae | 1 | 4 |
| Crioceridae | 5 | 173 | Omethidae | 1 | 4 |
| Elateridae | 5 | 55 | Attelabidae | 1 | 1 |
| Tenebrionidae | 5 | 12 | Buprestidae | 1 | 1 |
| Rutelidae | 3 | 72 | Corylophidae | 1 | 1 |
| Mordellidae | 3 | 66 | Cryptophagidae | 1 | 1 |
| Scraptiidae | 3 | 33 | Lycidae | 1 | 1 |
| Order | Family | No. of Plant Genus | No. of Plant Species | No. of Beetle Species | No. of Specimens | No. of Visiting Beetle Family |
|---|---|---|---|---|---|---|
| Asterales | Asteraceae | 12 | 21 | 57 | 442 | 13 |
| Ericales | Ericaceae | 1 | 2 | 37 | 462 | 10 |
| Rosales | Rosaceae | 5 | 5 | 30 | 264 | 12 |
| Apiales | Apiaceae | 2 | 3 | 27 | 336 | 14 |
| Fabales | Fabaceae | 6 | 9 | 21 | 34 | 7 |
| Dipsacales | Caprifoliaceae | 1 | 1 | 18 | 463 | 4 |
| Lamiales | Lamiaceae | 4 | 4 | 17 | 54 | 4 |
| Sapindales | Rutaceae | 1 | 2 | 14 | 39 | 5 |
| Ranunculales | Ranunculaceae | 4 | 9 | 15 | 84 | 5 |
| Lamiales | Oleaceae | 2 | 2 | 12 | 183 | 6 |
| Malpighiales | Hypericaceae | 1 | 2 | 12 | 307 | 7 |
| Gentianales | Gentianaceae | 3 | 3 | 12 | 25 | 6 |
| Asterales | Campanulaceae | 1 | 1 | 12 | 87 | 2 |
| Celastrales | Celastraceae | 1 | 1 | 10 | 246 | 9 |
| Dioscoreales | Nartheciaceae | 1 | 2 | 8 | 20 | 7 |
| Caryophyllales | Polygonaceae | 2 | 4 | 8 | 16 | 5 |
| Lamiales | Scrophulariaceae | 1 | 1 | 7 | 73 | 3 |
| Geraniales | Geraniaceae | 1 | 3 | 6 | 17 | 4 |
| Poales | Juncaceae | 1 | 1 | 5 | 47 | 3 |
| Dipsacales | Adoxaceae | 1 | 2 | 5 | 19 | 2 |
| Cornales | Hydrangeaceae | 1 | 1 | 5 | 8 | 2 |
| Poales | Eriocaulaceae | 1 | 1 | 4 | 41 | 4 |
| Caryophyllales | Caryophyllaceae | 1 | 1 | 4 | 34 | 2 |
| Ericales | Primulaceae | 1 | 1 | 3 | 65 | 2 |
| Boraginales | Boraginaceae | 1 | 1 | 3 | 4 | 3 |
| Saxifragales | Saxifragaceae | 1 | 2 | 2 | 10 | 2 |
| Lamiales | Orobanchaceae | 1 | 2 | 2 | 4 | 2 |
| Malvales | Thymelaeaceae | 1 | 1 | 1 | 5 | 1 |
| Asparagales | Orchidaceae | 1 | 1 | 1 | 1 | 1 |
| Commelinales | Commelinaceae | 1 | 1 | 1 | 1 | 1 |
| Index | Overall (n = 12) | High Elevation Forest (n = 3) | High Elevation Meadow (n = 3) | Low Elevation Forest (n = 3) | Low Elevation Meadow (n = 3) | between Elevations (F Value) | between Habitats (F Value) | Interaction |
|---|---|---|---|---|---|---|---|---|
| Network size | 43.08 ± 4.25 | 37 ± 6.93 | 30.33 ± 1.76 | 58.67 ± 7.75 | 46.33 ± 7.69 | F = 8.329, p = 0.020 | F = 2.119, p = 0.184 | F = 0.189, p = 0.676 |
| No. of interaction | 282.58 ± 45.44 | 188.00 ± 40.99 | 244.33 ± 101.15 | 500.33 ± 17.27 | 197.68 ± 26.27 | F = 5.472, p = 0.047 | F = 4.704, p = 0.062 | F = 9.991, p = 0.013 |
| No. of species HL | 26.25 ± 3.11 | 27 ± 5.77 | 18 ± 2.08 | 36 ± 5.51 | 24 ± 7.77 | F = 1.753, p = 0.222 | F = 3.436, p = 0.101 | F = 0.0701, p = 0.798 |
| No. of species LL | 16.83 ± 1.90 | 10 ± 1.16 | 12.33 ± 2.03 | 22.67 ± 2.91 | 22.33 ± 0.33 | F = 36.698, p < 0.001 | F = 0.286, p = 0.608 | F = 0.508, p = 0.496 |
| NODF | 21.93 ± 2.74 | 26.10 ± 2.89 | 27.75 ± 9.58 | 16.44 ± 2.29 | 17.42 ± 3.07 | F = 3.482, p = 0.099 | F = 0.0598, p = 0.813 | F = 0.00397, p = 0.951 |
| Weighted connectance | 0.08 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.05 ± 0.00 | 0.06 ± 0.01 | F = 39.214, p < 0.001 | F = 0.471, p = 0.512 | F = 1.340, p = 0. 280 |
| H2′ | 0.56 ± 0.03 | 0.56 ± 0.04 | 0.44 ± 0.05 | 0.57 ± 0.06 | 0.68 ± 0.01 | F = 7.191, p = 0.028 | F = 0.00320, p = 0.956 | F = 5.668, p = 0.044 |
| Niche overlap HL | 0.21 ± 0.04 | 0.38 ± 0.03 | 0.23 ± 0.02 | 0.15 ± 0.01 | 0.07 ± 0.01 | F = 100.330, p < 0.001 | F = 34.279, p < 0.001 | F = 3.113, p = 0.116 |
| Extinction slope HL | 1.96 ± 0.12 | 2.20 ± 0.24 | 1.67 ± 0.18 | 1.87 ± 0.08 | 2.09 ± 0.38 | F = 0.0337, p = 0.859 | F = 0.397, p = 0.546 | F = 2.34, p = 0.165 |
| Robustness HL | 0.65 ± 0.01 | 0.68 ± 0.02 | 0.62 ± 0.03 | 0.65 ± 0.01 | 0.66 ± 0.03 | F = 0.038, p = 0.850 | F = 0.909, p = 0.368 | F = 2.229, p = 0.174 |
| Functional complementarity HL | 264.91 ± 51.33 | 134.12 ± 15.63 | 244.10 ± 112.07 | 511.58 ± 32.44 | 169.84 ± 19.99 | F = 6.448, p = 0.035 | F = 3.768, p = 0.088 | F = 14.313, p = 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.-Q.; Ren, Z.-X.; Li, Q. Diversity of Flower Visiting Beetles at Higher Elevations on the Yulong Snow Mountain (Yunnan, China). Diversity 2021, 13, 604. https://doi.org/10.3390/d13110604
Li K-Q, Ren Z-X, Li Q. Diversity of Flower Visiting Beetles at Higher Elevations on the Yulong Snow Mountain (Yunnan, China). Diversity. 2021; 13(11):604. https://doi.org/10.3390/d13110604
Chicago/Turabian StyleLi, Kai-Qin, Zong-Xin Ren, and Qiang Li. 2021. "Diversity of Flower Visiting Beetles at Higher Elevations on the Yulong Snow Mountain (Yunnan, China)" Diversity 13, no. 11: 604. https://doi.org/10.3390/d13110604
APA StyleLi, K.-Q., Ren, Z.-X., & Li, Q. (2021). Diversity of Flower Visiting Beetles at Higher Elevations on the Yulong Snow Mountain (Yunnan, China). Diversity, 13(11), 604. https://doi.org/10.3390/d13110604






