Abstract
The current work investigated the ecotoxicological effects induced by Titanium Dioxide (TiO2) nanoparticles (NPs), used at three different concentrations (C1 = 10 μg·L−1, C2 = 100 μg·L−1 and C3 = 1000 μg·L−1) in a laboratory experiment, on the freshwater mussel Unio ravoisieri. Biochemical analyses of gills and digestive glands revealed a stress-related disruption of the antioxidant system. The catalase activity and the rates of malonedialdehyde and hydrogen peroxide production were significantly higher in both organs following the exposure to TiO2 NPs and was concentration-dependent. In addition, based on the observed changes in acetylcholinesterase activity, it can be concluded that the disturbance threshold for the cholinergic system was less than 1 mg·L−1 of TiO2. Overall, the results suggest that the mussel Unio ravoisieri could be used as a sentinel species in monitoring surveys assessing the environmental impact of metallic nanoparticles in freshwater systems.
1. Introduction
Since the beginning of the industrial revolution, human activities have increased the negative impact on the environment with every technological advance []. In time, the nature of the contaminants emitted by humans has also changed. In the XXI century, a flourishing industry is the one of nanotechnology [,,]. Consequently, it is necessary for environmental survey programs to follow closely the emergence rate of such contaminants in order to prevent, where possible, their detrimental impact on ecosystems and human health [,]. Nanoparticles (NPs) are widely incorporated into many products. However, despite the obvious benefits for human wellbeing, the increasing demand of NPs inevitably entails their entry into the environment. Previous studies focused on the modelling of their distribution in various types of ecosystems (e.g., air, soil, and water) and targeted mostly the methodological practices that measure their concentrations in the environment [,,]. The nanoparticles, whether inadvertently or deliberately released into the environment, ultimately reach the aquatic habitats, leading to toxic effects on biota [].
Metallic nanoparticles (MNPs) are widespread because of their ease of synthesis and high demand. With the increase in their use in various types of industry, the environmental impacts of metallic nanoparticles became inevitable [,,]. These emergent products are detected routinely in aquatic habitats, where they accumulate in living organisms, potentially modifying their physiology and biochemical response and the proper functionality of aquatic ecosystems []. In ecotoxicology, one frequently measured endpoint is the assessment of changes in body markers. A preferred model group in such assays are the bivalves []. These organisms are abundant, of sufficient size to carry biochemical analyses, easy to sample and valuable from an economic and ecologic perspective. The fact that most bivalves are sessile or sedentary and are filter feeders make them ideal for laboratory bioassays [,]. The biomarkers cover several molecular, biochemical, and cellular changes, which reflect the health status of a studied population []. They can provide accurate and appropriate information on the impact of pollutants on the health of studied organisms [].
The current work focused on the response of the freshwater mussel Unio ravoisieri to TiO2 NPs exposure. We are aware of a single study, which used a hybrid (Au/TiO2) nanocomposite that has partially covered this topic (Dellali et al. []). In this study, the Au/TiO2 NPs comprised many-faceted quasi-spherical gold cores (10 nm) surrounded by closely packed titanium dioxide nanoparticles (25 nm) forming a pattern that facilitated good dispersion. The hybrid Au/TiO2 NPs had a different chemical composition and properties and was larger (Ø = 35 nm) compared to the TiO2 synthetized and used in the current experiment (Ø = 10 nm). Such differences are suspected to affect the effectiveness and spread of NPs entrance into bivalve tissues and consequently the response rates of biomarker activities. The current experiment aimed for a better understanding of the TiO2 NPs toxicity by employing a wide range of concentrations (i.e., 10, 100, and 1000 µg·L−1) and to choose relevant concentrations of the metallic alloy MNPs of Au/TiO2 (100 and 200 µg·L−1). The increasing contamination rate with nanoparticles (NPs) at concentrations ranging in concentration several orders of magnitude in surface and sewage treatment effluent waters [,] raises nowadays serious concerns on their potential toxic effects for aquatic ecosystems []. Moreover, the results could provide a useful tool for improving the current knowledge gap on the toxic effects of TiO2 NPs on aquatic life. The novelty of the current work is the fact that most previous experiments have focused on marine taxa [], despite the fact that lotic ecosystems are the primary receptacles of these xenobiotics. Therefore, we tried in the current study to fill this knowledge gap by answering to the following questions:
- (i)
- Are TiO2 NPs harmful for the freshwater mussel Unio ravoisieri?
- (ii)
- If positive, how do the biomarkers in Unio ravoisieri tissues respond?
- (iii)
- What are the organs targeted by TiO2 NPs, and what are the thresholds of their toxicity?
2. Materials and Methods
2.1. Sampling Area and Collection Site
The unionid mussels are of special interest, mainly the species Unio ravoisieri that is likely to be one the most threatened species of the Unio pictorum group [,].
The individuals of Unio ravoisieri used in the current experiment were sampled from ‘Wadi Sejnene’, a permanent and endorheic river, which flows into Lake Ichkeul (Bizerte, Northern Tunisia) (Figure 1). It covers an area of 372 km2 between ‘Cap Serrat’ and Ichkeul lake, with a length of 68.4 km []. The substrate from where the bivalves were collected (37°11.603′ N, 9°34.764′ E) was comprised mostly silt/clay, coarse sand, gravel, and rock blocks and the aquatic vegetation is dominated by reeds and Potamogeton spp. []. The number of individuals collected for the current experiment was within the safe range that does not jeopardize the viability of the local population [].
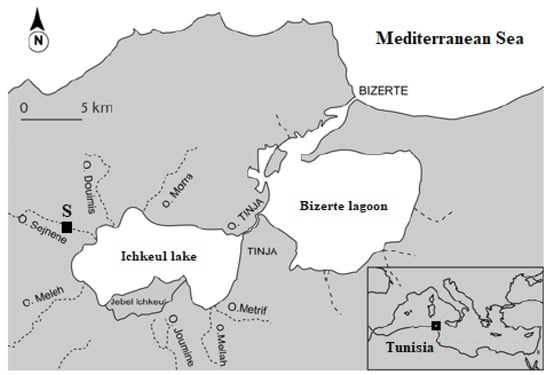
Figure 1.
Collection site (S) of the freshwater bivalve Unio ravoisieri. Oued (O).
2.2. Sampling and Laboratory Conditions
The collected individuals were partially buried in sediment at a depth of 0.5 m. On the sampling day (4 April 2017), three parameters were measured at the sediment–water interface: temperature (= 18 °C) and salinity (= 1.5 PSU) using a Microprocessor Conductivity meter (LF.196) and pH (= 8) using a pH-meter (WTW pH 196). The collected individuals were transferred to the laboratory in a cooler box. In the laboratory the animals were placed in aquaria (30 × 20 × 20 cm3) filled with filtered river water (0.7 µm pore-size Glas Microfibre GF/F, Whatman) []. Individuals of Unio ravoisieri were placed in glass aquaria, each filled with 1 L of water and containing five individuals. The animals were left for an acclimatization period of 7 days. The experiment design comprised the following: a control and three treatments with TiO2 (C1 = 10 μg·L−1, C2 = 100 μg·L−1, and C3 = 1000 μg·L−1), each set up in triplicates. Two time periods were selected before starting the experiment, 48 h and 7 days, the water being renewed every 48 h []. The experimental aquariums were constantly aerated through a continuous bubbling with an air-diffuser, and the photoperiod was set at 16h/8h of light/darkness cycle. The mussels were fed every two days (Algamac 82 protein plus, approximately 150,000 cells per individual) during the whole experiment, and no mortality was registered.
2.3. Synthesis, Structural, and Optical Characterizations and Assessing Concentrations of TiO2 Nanoparticles
Titanium (IV) butoxide (Ti (OCH2CH2CH2CH3)4), Aldrich, AR grade) (5 mL) was dissolved in 50 mL of Dimethyl Sulfoxide (DMSO) ((CH3)2SO, Sigma) to prepare the NPs solutions. Then, the solution was heated to 190 °C and kept at this temperature for 2 h under mechanical agitation. Once the reaction was concluded, centrifugation was employed to separate the precipitate. In order to collect the TiO2 powder, the remnants were washed several times with ethanol/acetone (2:1), and dried afterwards in vacuum, at 50 °C for 12 h. The TiO2 NP was calcined at 400 °C. In a next step, hybrid Au/TiO2 NPs were synthetized using the same protocol as above, by adding hydrogen tetrachloroaurate (III) trihydrate (HAuCl4:3H2O) (from Sigma-Aldrich) as source of gold precursor. During the annealing process of TiO2 NPs the solvothermal treatment induces crystallinity, and thus the solvent molecules entrapped inside particles are removed [].
The morphology of the TiO2 NPs was observed using transmission electron microscopy (TEM) JEOL 2011 (JEOL Ltd, Tokyo Japan) microscope operating at 100 kV. The average size of the TiO2 nanoparticles was approximately 10 nm []. To confirm the formation of titanium dioxide (TiO2) nanomaterial, XRD measurement was performed. The shape of the obtained nanomaterial was also observed by transmission electron microscopy (TEM) and SEM microscopy using JEOL 2011 instrument working at 100 kV. To study the stability and the character of the particle surface of TiO2 NPs, zeta potential technique was performed at room temperature and the pH value was adjusted to 7.4.
The process of sedimentation was followed during 7 days from dispersion, with a PerkinElmer Lambda 650 UV–Vis spectrophotometer [,]. By measuring the absorbance at 269 nm of a range of TiO2 dispersions of known concentration the calibration curve was obtained. The concentration of suspended TiO2 was estimated after dispersion through absorbance at 0, 1, 2, 4, 6, and 24 h and 2, 3, 4, 5, 6, and 7 days. Each absorbance was measured three times, from the uppermost layers of dispersions (<1 cm from the surface). The volumes were left unmoved in 50 mL Falcon tubes at room temperature during the experiment to avoid perturbation and resuspension. The sedimentation profile was obtained by plotting normalized concentration values (i.e., C/C0, where C0 is the initial concentration at 0 h and C refers to specific time points) in time, expressed as means ± SD.
2.4. Biochemical Analyses
At the end of the experiment, the changes in four biomarkers response were measured to assess the toxic effects of TiO2 nanoparticles: (1) acetylcholinesterase (Ache), a neurotoxicity biomarker; (2) catalase (CAT), a defense biomarker against oxidative stress; (3) hydrogen peroxide (H2O2), an important Reactive Oxygen Species ‘ROS’ generated under oxidative stress conditions; and (4) malonedialdehyde (MDA), a frequently measured cellular damage biomarker resulting from the lipid peroxidation.
The mussels were frozen in liquid nitrogen, dissected by removing first the valves, following the incision of the adductive muscles with a scalpel. After dissection, the gills and digestive glands were extracted with a clamp and placed in pills for grinding. The grinding was performed using an Ultra Turrax (IKA) in 3 TBS buffer volumes (Tris 50 mM, NaCl 150 mM, pH 7.4). The homogenate obtained was then centrifuged at 9000 g for 30 min at 4 °C. The protein dosage was obtained based on Bradford’s method [].
The quantification of H2O2 was carried out using a Biomagreb kit (Tunisia). The concentrations were deduced from pre-known hydrogen peroxide concentrations, ranging from 0 to 1000 mM. After 15 min incubation at 37 °C, the optical density was read at 505 nm. Moreover, the catalase’ activity was measured according to the Aebi method [] and Beutler [], respectively. The amount of peroxidized lipids was estimated from the amount of MDA formed. The activity was measured according to the colorimetric method of Ellman et al. []. Finally, the AChE activity was evaluated according to Ellman et al. [] by determining the thiocholine produced after acetylthiocholine hydrolysis by AChE using Ellmann reagent or 5.5′-dithio-bis(2-nitrobenzoic (DTNB).
2.5. Statistical Processing
The statistical analysis of data was performed with the software SPSS v.10. The distribution of biological measures (i.e., the enzymatic activity) is known to be normally distributed. Data were first checked for the homogeneity of variances and Gaussian distribution with Bartlett and Kolmogorov–Smirnov tests, respectively. One-way ANOVA was used to check for general significant difference among treatments and control, followed by multiple comparisons with Tukey’s HSD ((honestly significant difference) test. The threshold of significance was set at p-value < 0.05.
3. Results
3.1. TiO2 nanoparticles Morphology
As shown in Figure 2, the diffractogram of the prepared sample revealed the formation of TiO2 (Figure 2); all diffractions peaks can be the anatase structure (JCPDS 01-084-1286). The powder appeared with high crystallinity and the broadening of peaks revealed the formation of TiO2 in the nanostructure form.
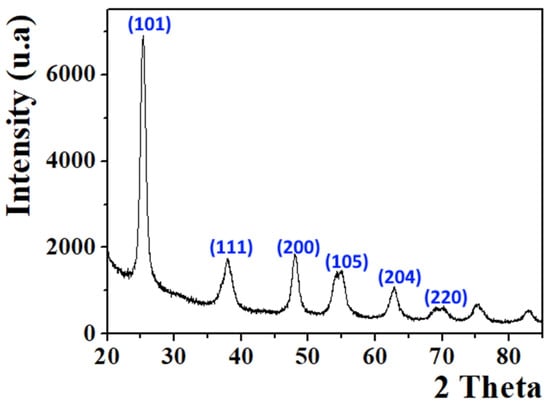
Figure 2.
XRD pattern of TiO2 nanoparticles.
Figure 3a displayed the TEM image of the sample. As shown, the majority of particles exhibited the spherical shape with the presence of some irregular particles with an average size of 10 nm (Figure 3b). Based on EDX spectrum (Figure 3c), the sample presented high purity; only Ti and O chemical elements were detected with no other addition impurity. The SEM image shown in Figure 4 confirms the morphology of the sample; most of particles revealed the spherical shape as shown previously by TEM images.
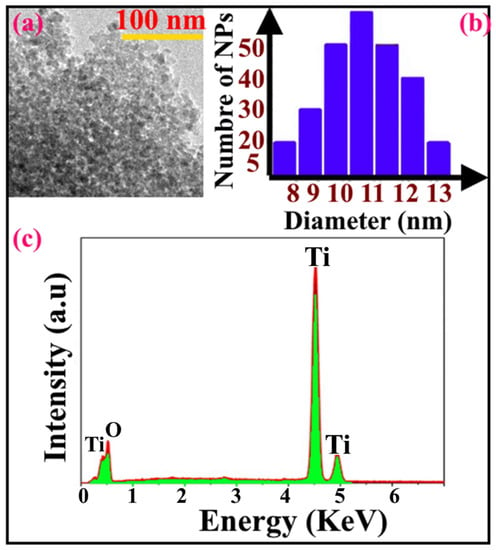
Figure 3.
(a) TEM image, (b) particle size distribution and (c) EDX spectrum of TiO2 nanoparticles.

Figure 4.
SEM image of TiO2 nanoparticles.
As shown in Figure 5, the zeta potential value reached −60 mV. This value implied that in solution, the TiO2 surface was saturated by negative oxygen molecules as shown in the inset of Figure 5. Thus, this comportment can enhance the potential stability of the suspension and consequently improve the stability of TiO2 NPs even in hard saline solution.
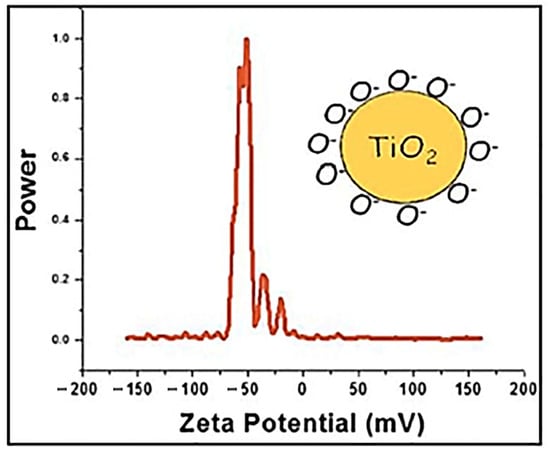
Figure 5.
Zeta potential plot of TiO2 NPs dispersed in water. The inset shows the behavior of TiO2 in the suspension.
The data presented in Table 1 show that the TiO2 concentrations did not change significantly between the targeted concentrations by the end of the experiment, for both time slots considered (i.e., 48 h and 7 days).

Table 1.
TiO2 concentrations (μg·L−1) measured in untreated control (Ut) and treated (C1-3) waters at the start of the experiment (T0) and after 48 h and 7 days. Undetected (UD). Different letters next to values indicate significant differences (log-transformed data, Tukey’s HSD test, p < 0.05).
3.2. Hydrogen Peroxide (H2O2) Content
The H2O2 content in gills were similar in C1 and controls, but significantly higher in C2 and C3 after 48 h and 7 days exposure of Unio ravoisieri to TiO2 NPs (Figure 6). In the digestive glands the mean rate of H2O2 production increased from about 5 times in C1 up to 20 times for C3 compared to control, a pattern similar after 48 h and 7 days exposure time, respectively (Figure 6).
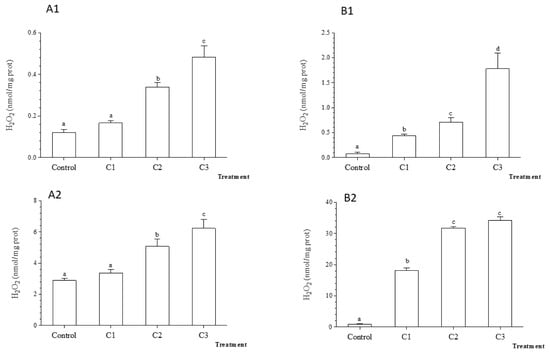
Figure 6.
Average hydrogen peroxide (H2O2) (±standard deviation) in the freshwater mussel Unio ravoiseri after exposure to Control and three concentrations of Titanium dioxide nanoparticles during 48 h and 7 days in gills, respectively (A1) and (A2), and in digestive gland (B1) and (B2). Bars represent the group means ± SE; a, b, c, d: data not sharing a common letter are significantly different using Tukey’s HSD test (p < 0.05).
3.3. Catalase (CAT) Activity
The catalase activity in gills and digestive glands was significantly higher in contaminated microcosms (Tukey’s HSD test, Figure 7; p < 0.05) compared to control. The exposure of Unio ravoisieri for 7 days to TiO2 nanoparticles showed a similar response of the CAT activity to that recorded after 48 h (Figure 7). Overall, the catalase activity was the highest in both glands in C2 treatment.
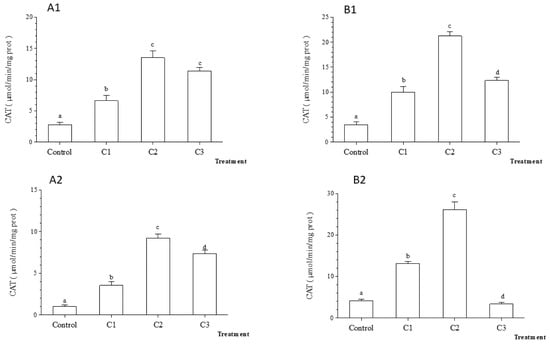
Figure 7.
Average catalase (CAT) activity (±standard deviation) in the freshwater mussel Unio ravoiseri after exposure to Control and three concentrations of Titanium dioxide nanoparticles during 48 h and 7 days in gills, respectively (A1) and (A2) and in digestive gland (B1) and (B2). Bars represent the group means ± SE; a, b, c, d: data not sharing a common letter are significantly different using Tukey’s HSD test (p < 0.05).
3.4. Malonedialdehyde (MDA) Content
After 48 h exposure to TiO2 nanoparticles, the MDA content in gills was significantly higher in C2 and C3 compared to C1 and control treatments, whereas in the digestive glands significant changes were detected only in C3 (Figure 8). A significant increase in MDA content in the digestive gland was recorded in C2, five times higher compared to control after 7 days (p < 0.001). However, following 7 days exposure, no significant changes were observed in MDA content in gills among treatments (Figure 8).
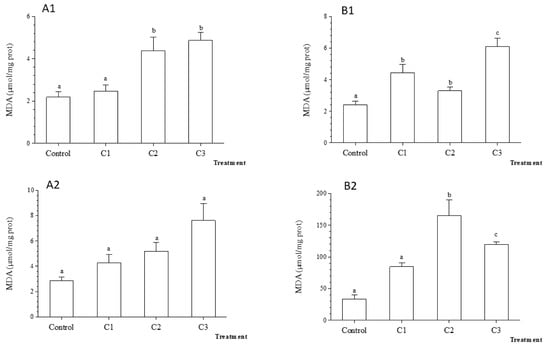
Figure 8.
Average Malondialdehyde (MDA) rate (±standard deviation) in the freshwater mussel Unio ravoiseri after exposure to Control and three concentrations of Titanium dioxide nanoparticles during 48 h and 7 days in gills, respectively (A1) and (A2) and in digestive gland (B1) and (B2). Bars represent the group means ± SE; a, b, c, d: data not sharing a common letter are significantly different using Tukey’s HSD test (p < 0.05).
The AChE activity reached the highest values in the control mussels after 7 days. The exposure to TiO2 nanoparticles was followed by a significant decrease in AChE activity in both organs compared to control (Figure 9, p < 0.001) such as in C2 for gills and C1 for the digestive gland (Figure 9). The results obtained after 7 days of contamination by TiO2 nanoparticles were overall similar to those found after 48 h.
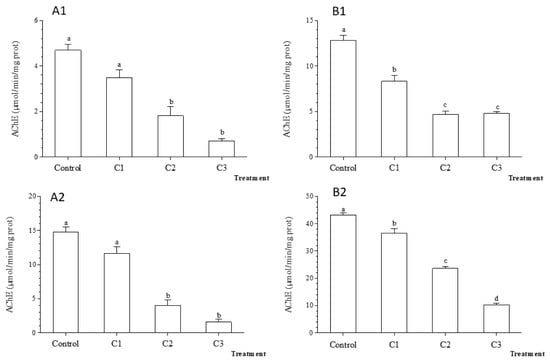
Figure 9.
Acetylcholinesterase (AChE) activity (±standard deviation) in the freshwater mussel Unio ravoiseri after exposure to Control and three concentrations of Titanium dioxide nanoparticles during 48 h and 7 days in gills, respectively (A1) and (A2) and in digestive gland (B1) and (B2). Bars represent the group means ± SE; a, b, c, d: data not sharing a common letter are significantly different using Tukey’s HSD test (p < 0.05).
4. Discussion
During last decades the nanotechnology industry flourished, including the TiO2 nanoparticles [,,]. The latter products are mainly used in cosmetic, food, and pharmaceutical industries, catalysis, water purification and in several construction materials []. The nanoparticles are tiny but a high surface/volume ratio, which makes them very responsive and with unique physicochemical properties compared to conventional forms, mainly related to their mobility and contamination potential of soils and drinking water sources []. Thus, the TiO2 NPs have the potential to become an important pollutant in wastewaters and effluents [,,]. The continuous input of pollutants into fresh waters and their permanence in the sediments can produce important acute and chronic effects to freshwater mussels, which have sedentary habits and long-lived life histories [,,,,]. Several properties make bivalves ideal for ecotoxicological risk assessment research [,,,,,]: they are sessile, easy to collected, filter feeders, and accumulate suspended particles from water, including pollutants, and are usually resilient to a wide array of environmental stressors, both in situ and in laboratory conditions.
The toxicity of nanoparticles was studied in molluscs, with advanced processes of internalization of nanoparticles through endocytosis []. In addition, the ability of bivalves to bioaccumulate toxic compounds makes this group a key component in the trophic transfer of environmental pollutants through food webs [,,]. Thus, bivalves could be considered vulnerable to the toxicity induced by nanoparticles [,,,] and are representative models for the monitoring of aquatic pollution. The literature on TiO2 NPs supports the hypothesis that they have negative effects on aquatic biota, but very little attention was given to freshwater compared to marine organisms.
For the current research, the concentrations of dissolved TiO2 NPs appeared to be constant throughout the experiment and comparable to those employed at the beginning of the assay (10, 100, and 1000 µg·L−1). This could be explained through the well-known catalytic properties of TiO2 NPs. The results showed that the TiO2 NPs induced an increase in the rate of H2O2 synthesis in gills and digestive glands of the freshwater mussel Unio ravoisieri. This result is in accordance with previous studies that used hybrid Au/TiO2 NPs (average size of 35 nm) during 48 h and 7 days [] or used other aquatic organisms [,]. The oxidative stress is one of the major effects induced by NPs on aquatic organisms []. Previous studies reported that TiO2 NPs induce oxidative stress by generating ROS in zebrafish [,], phytoplankton [], bacteria [], abalone [], and bivalves []. The ROS response induced by exposure to TiO2 NPs could induce oxidative damage, such as lipid peroxidation with the depletion of antioxidant enzymes like the superoxide dismutase and catalase.
According to Valko et al. [], the H2O2 acts in various biological processes at cellular level and their synthesis rate is controlled by catalase, an essential enzyme in detoxification processes and plays a crucial role in antioxidant mechanisms. This enzyme is located in peroxisomes, facilitating the conversion of hydrogen peroxide (H2O2) to molecular oxygen (O2) and water []. The increase in CAT activity observed in this experiment can therefore be linked to the increase in H2O2 production rate in gills and digestive glands.
The produced H2O2 is further catalyzed by the Fenton reaction [] in hydroxyl radicals that attacks lipids, proteins, and nucleic acids [,]. One of the first defense mechanisms for Unio ravoisieri to counteract the exposure to TiO2 NPs is the induction of the antioxidant defense system in the two targeted organs (i.e., gills and digestive gland).
Similar results were previously recorded for Unio ravoisieri following exposure to hybrid Au/TiO2 NPs (35 nm) [] and in the bivalve Scrobicularia plana following 3 mg·kg−1 of NPs of ZnO contamination [], as well as for the freshwater mussel Elliptio complanata exposedto 2 mg·L−1 of ZnO []. For the freshwater clam, Corbicula fluminea, Renault et al. [] and Cid et al. (2015) noticed also that the CAT activity increased directly with the concentration of the NPs of gold and diamond, respectively. Finally, Pan et al. [] showed that the CAT activity increased in the clam S. plana exposed to a concentration of 100 mg·L−1 of Au NPs. Our results are in accordance with the findings of Ali et al. [] who observed that the CAT activity in the digestive gland of the snail Lymnaea luteola exposed to the NPs of ZnO increased directly with employed concentrations and to those of Basha and Rani [] who reported similar responses for the freshwater fish Oreochromis mossambicus. The catalase activation seems to be a classic response following exposure to metallic nanoparticles [,,,].
Several authors have explained the variability of H2O2 and catalase activities found between gills and digestive glands, respectively, by the higher numbers of peroxisomes in the latter organ. This membrane-bound organelle holds the main source of hydrogen peroxide [,,]. In contrast, the inhibition of the catalase activity in the freshwater mussel Unio ravoisieri treated with 1000 μg·L−1 of TiO2 could be related to the flow rate of hydrogen peroxide, which was reported to act as an inhibitor of catalase []. Comparable results were reported by Zhu et al. [] who showed that 1 mg·L−1 of TiO2 NPs caused a significant increase in CAT activities in the marine abalone. However, the exposure to 5 mg·L−1 significantly inhibited the SOD and CAT activities of zebrafish []. Thus, it seems that beyond a certain threshold of NPs, the purifying capacity of gills against the entry of TiO2 NPs reaches a plateau, followed by the inactivation of the catalase activity []. Inevitably, such decline causes a general collapse of the physiological status in bodies of stressed bivalves [,]. A study on zebra fish [] showed also a similar inhibition rate of the catalase activity at high concentrations of NPs of ZnO, CuO, and TiO2. Finally, [] also showed that such inhibition of the catalase activity in the mussel Mytilus galloprovincialis is due to the interaction between silver NPs and the thiol group of the antioxidants (CAT, SOD).
The antioxidant enzymes play an active role in ROS catalysis. However, any remaining parts of ROS could result in lipid peroxidation. The MDA content was widely used as an indicator of oxidative damages []. Many studies reported that TiO2 can cause membrane damage after lipid peroxidation []. During this work, after 7 days of exposure, the MDA rate increased significantly with TiO2 concentrations in both organs of Unio ravoisieri. This emphasizes the sensitivity of these organs that represent the first barriers for xenobiotics []. In the case of contamination with hybrid Au/TiO2 (35 nm) [], Unio ravoisieri showed a similar response in digestive gland after 7 days of exposure. An in vitro study using the bivalve Aulacomya atra atra showed a great increase in the MDA rate in gills [], and the effects were most visible when the mussels were exposed to a concentration of TiO2 NPs of 100 μg·L−1. The results from this experiment are also in line with those of Cid et al. [] who reported that the MDA rate showed a significant increase for the Asian clam Corbicula fluminea after 14 days of exposure to different concentrations of nanodiamond (0.01-10 mg·L−1). In the case of the carp Cyprinus carpio juveniles, Hao and Chen [] observed that the MDA rate increased significantly in gills, liver, brain, and intestines after two weeks exposure to 50 mg·L−1 of ZnO NPs. Recently, Xia et al. [] showed that the contamination with TiO2 (1 mg·L−1 for 14 days) induced in the marine scallop Chlamys farreri significant increase in the MDA rates.
The results suggest that TiO2 NPs are able of inducing cell damage, and this is probably corroborated with excessive production of free-radicals. It is known that immediately after exposure to TiO2 NPs the antioxidant system (i.e., CAT activity and other antioxidants) is effective in preventing deleterious effects on lipids. However, the increased content of MDA in time seems to indicate that the antioxidant capacity was exceeded by the increasing production rate of TiO2-induced ROSs.
The regulation of acetylcholine is ensured by the acetylcholinesterase []. The hydrolysis reaction causes the formation of choline and acetate from acetylcholine. The 367 changes in enzymatic activity can reveal alterations of the behavior and muscular activity of exposed organisms to organic or metallic contaminants []. In this context, the acetyl-cholinesterase (AChE) is commonly defined as a neurotoxic biomarker in aquatic organisms [,].
The results obtained in the current study showed that the high employed concentrations of TiO2 NPs strongly inhibited the AChE activity in Unio ravoisieri without reaching the level of muscle tetanization, given that no mortality was recorded during the experiment. This is in accordance with Dellali et al. [] for the same species, following its exposure to hybrid Au/TiO2 MNPs (35 nm) and with those of Buffet et al. [] and Minetto et al. [], supporting the idea that the inhibition of AChE activity in the polychaeta Hediste diversicolor and Nereis diversicolor was related to the toxic effects induced by increasing concentrations of ZnO NPs. Similarly, it showed that the nanoparticles of CuO (75 μg·L−1) contributed to a significant inhibition of the AChE activity in the clam Ruditapes decussatus []. Katuli et al. [] and Gomes et al. [] observed a decrease in AChE activity in the zebrafish Danio rerio after exposure to Ag NPs and in the mussel Mytilus galloprovincialis following exposure to CuO NPs.
The inhibition of the AChE activity could be linked to the indirect effect of H2O2 generated under stress []. Indeed, hydrogen peroxide is responsible for the alteration of active site of the AChE [,].
The results obtained in the current study are comparable to those of Dellali et al. [] who used the same species exposed to 100 and 200 μg·L−1 of hybrid Au/TiO2 MNPs (35 nm). Our findings showed that significant effects were noticed in the gills of Unio ravoisieri following exposure to 100 μg TiO2 L−1 for the biomarkers H2O2, MDA, and AChE. The results seem to indicate that TiO2 (100 μg·L−1) was more toxic for Unio ravoisieri compared to the hybrid Au/TiO2 (100 μg·L−1), probably because of their lower size and consequently higher entrance rate during water filtration.
5. Conclusions
The aim of this study was to assess the toxicity of TiO2 NPs on the freshwater mussel Unio ravoisieri. Three concentrations of NPs (10, 100, and 1000 μg·L−1 of TiO2) were tested and their response on three biochemical biomarkers were measured after 48 h and 7 days, respectively. Our findings revealed an increase in the oxidative stress triggered by higher activities recorded for H2O and CAT concentrations in gills and digestive glands. The latter enzyme is also involved in the catabolism of H2O2. The simultaneous increase in the MDA rate in a concentration depended manner in both organs provided support for claiming that TiO2 NPs exceeded the defense capacity of the antioxidant enzymes for these mussels, leading to lipid peroxidation in cell membranes. The findings of the current experiment showed also that the AChE activity gradually decreased following exposure to TiO2 NPs. It can be concluded that these nanomaterials behave like a producer of free oxygen radicals and neurotoxic products.
The current study also emphasized the importance of biomarker use in detecting the toxic effects induced by emerging materials such as TiO2 NPs for freshwater organisms. The vulnerability of Unio ravoisieri allowed us to classify it as a useful sentinel species in predicting the detrimental effects of nanomaterials in freshwater systems.
Author Contributions
Conceptualization: Conceptualization, H.S. and A.K.; methodology, A.K.; software, M.D.; validation, F.B., H.B. and M.D.; formal analysis, M.B.A.; investigation, O.P.; resources, M.A.; data curation, H.S.; writing—original draft preparation, O.P., B.A. and A.H.; writing—review and editing, A.M.; visuali-zation, F.B. and A.M.; supervision, H.B.; project administration, H.B. and B.A.; funding acquisition, B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Tunisian Ministry of the High Education and Scientific Research. The authors are grateful to the Deanship of Scientific Research for funding this article by Taif University Research Supporting Project number (TURSP-2020/301), Taif University, Taif, Saudi Arabia. O.P. was supported by the National Core Program—Romanian Ministry of Research and Innovation Program, project 25 N 2019 BIODIVERS 19270103.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not shared due to restrictions, e.g., privacy and regulation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zaoui, M.; Sellami, B.; Boufahja, F.; Falodah, F.; Nahdi, S.; Alrezaki, A.; Alwasel, S.; Harrath, A.H. Effects of ferroelectric oxides of barium strontium titanate (Ba0.85Sr0.15TiO3) nanoparticles on Ruditapes decussatus assessed through chemical, physiological, and biochemical methods. Chemosphere 2021, 265, 129078. [Google Scholar] [CrossRef]
- Handy, R.D.; Owen, R.; Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicologial 2008, 17, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Abbasi, F.; Ben Ali, M.; Hedfi, A.; Mezni, A.; Rehman, W.; Waseem, M.; Khan, A.R.; Shaheen, H. Green synthesis of cobalt oxide nanoparticles and the effect of annealing temperature on their physiochemical and biological properties. Mater. Res. Express. 2021, 8, 075009. [Google Scholar] [CrossRef]
- Haq, S.; Dildar, S.; Ben Ali, M.; Mezni, A.; Hedfi, A.; Imran Shahzad, M.; Shahzad, N.; Shah, A. Antimicrobial and antioxidant properties of biosynthesized of NiO nanoparticles using Raphanus sativus (R. sativus) extract. Mater. Res. Express. 2021, 8, 055006. [Google Scholar] [CrossRef]
- Delay, M.; Frimmel, F. Nanoparticles in aquatic systems. Anal. Bioanal. Chem. 2012, 402, 583–592. [Google Scholar] [CrossRef]
- Shah, A.; Tauseef, I.; Ben Ali, M.; Arfat Yameen, M.; Mezni, A.; Hedfi, A.; Haleem, K.S.; Haq, S. In-Vitro and In-Vivo Tolerance and Therapeutic Investigations of Phyto-Fabricated Iron Oxide Nanoparticles against Selected Pathogens. Toxics 2021, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Amiard-Triquet, C.; Amiard, J.C.; Mouneyrac, C. Aquatic Ecotoxicology Advancing Tools for Dealing with Emerging Risks; Elsevier: Boston, MA, USA, 2015; pp. 53–171. [Google Scholar]
- Bour, A.; Mouchet, F.; Cadarsi, S.; Silvestre, J.; Verneuil, L.; Baqué, D.; Chauvet, E.; Bonzom, J.M.; Pagnout, C.; Clivot, H.; et al. Toxicity of CeO2 nanoparticles on a freshwater experimental trophic chain A study in environmentally relevant conditions through the use of mesocosms. Nanotoxicology 2016, 10, 244–255. [Google Scholar]
- Melvin, S.D. Oxidative stress energy storage and swimming performance of Limnodynastes peronii tadpoles exposed to a sublethal pharmaceutical mixture throughout development. Chemosphere 2016, 150, 790–797. [Google Scholar] [CrossRef]
- Rittschof, D.; McClellan Green, P. Molluscs as multidisciplinary models in environment toxicology. Mar. Pollut. Bull. 2005, 50, 369–373. [Google Scholar] [CrossRef]
- Dellali, M.; Khallouli, A.; Harrath, A.H.; Falodah, F.; Alwasel, S.; Beyrem, H.; Gyedu-Ababio, T.; Rohal-Lupher, M.; Boufahja, F. Effects of Au/TiO2 metallic nanoparticles on Unio ravoisieri: Assessment through an oxidative stress and toxicity biomarkers. Environ. Sci. Pollut. Res. 2021, 28, 18176–18185. [Google Scholar] [CrossRef]
- Dellali, M.; Hedfi, A.; Ben Ali, M.; Noureldeen, A.; Darwish, H.; Beyrem, H.; Gyedu-Ababio, T.; Dervishi, A.; Karachle, P.K.; Boufahja, F. Multi-biomarker approach in Mytilus galloprovincialis and Ruditapes decussatus as a predictor of pelago-benthic responses after exposure to Benzo[a]Pyrene. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2021, 249, 109141. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment a review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, Z.; Li, Q.; Pan, Q.; Yan, C.; Liu, F. Spatial distribution, electron microscopy analysis of gtitanium and its correlation to heavy metals: Occurrence and sources of titanium nanomaterials in surface sediments from Xiam en Bay, China. J. Environ. Monit. 2011, 13, 1046–1052. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biolol. Rev. 2017, 92, 572–607. [Google Scholar] [CrossRef] [PubMed]
- Khalloufi, N.; Toledo, C.; Machordom, A.; Boumaïza, M.; Araujo, R. The unionids of Tunisia: Taxonomy and phylogenetic relationships, with redescription of Unio ravoisieri Deshayes, 1847 and U. durieui Deshayes, 1847. J. Mollu. Stud. 2011, 77, 103–115. [Google Scholar] [CrossRef]
- Khalloufi, N.; Boumaıza, M. Premiere note sur la présence d’Anodonta cygnea (Linnaeus, 1758) (Mollusca, Bivalva, Unionidae) en Tunisie. Zool. Baeti. 2005, 16, 21–29. (In French) [Google Scholar]
- Nakamura, K.; Cañete, J.; Vijuesca, D.; Guillén, N.; Sosa, C.; Sosa, C.; Mesquita-Joanes, F.; Sousa, R.; Ginés, E.; Sorribas, V. Sensitivity of Pseudunio auricularius to metals and ammonia: First evaluation. Hydrobiologia 2021, 848, 2977–2992. [Google Scholar] [CrossRef]
- Mezni, A.; Alghool, S.; Sellami, B.; Ben Saber, N.; Altalhi, T. Titanium dioxide nanoparticles: Synthesis, characterisations and aquatic ecotoxicity effects. Chem. Ecol. 2018, 34, 288–299. [Google Scholar] [CrossRef]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Della Torre, C.; Balbi, T.; Grassi, G.; Frenzilli, G.; Bernardeschi, M.; Smerilli, A.; Guidi, P.; Canesi, L.; Nigro, M.; Monaci, F.; et al. Titanium dioxide nanoparticles modulate the toxicological response to cadmium in the gills of Mytilus galloprovincialis. J. Hazard. Mat. 2015, 297, 92–100. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of proteindye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Beutler, E. Red Cell Metabolism: A Manual Biochemical Methods, 2nd ed.; Grune and Sraton: New York, NY, USA, 1975; p. 160. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Gelover, S. Titanium dioxide sol–gel deposited over glass and its application as a photocatalyst for water decontamination. J. Photochem. Photobiol. 2004, 165, 241–246. [Google Scholar] [CrossRef]
- Simonet, B.M.; Valcárcel, M. Monitoring nanoparticles in the environment. Anal. Bioanal. Chem. 2009, 393, 17–21. [Google Scholar] [CrossRef]
- Shi, H. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre. Toxicol. 2013, 10, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Hossain, F. Antimicrobial nanomaterials as water disinfectant applications, limitation and future perspectives. Sci. Total. Environ. 2014, 466, 1047–1059. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Hench, L.L.; Seal, S. The potential toxicity of nanomaterials—The role of surfaces. Jom 2006, 58, 77–82. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles-A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanos. Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Van Hassel, J.H.; Farris, J.L. A Review of the Use of Unionid Mussels as Biological Indicators of Ecosystem Health in Freshwater Bivalve Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2007; pp. 19–49. [Google Scholar]
- Frank, H.; Gerstmann, S. Declining populations of freshwater pearlmussels (Margaritifera margaritifera) are burdened with heavy metals and DDT/DDE. AMBIO J. Hum. Environ. 2007, 36, 571–574. [Google Scholar]
- Cope, W.G.; Bringolf, R.B.; Buchwalter, D.B.; Newton, T.J.; Ingersoll, C.G.; Wang, N.; Augspurger, T.; Dwyer, F.J.; Barnhart, M.C.; Neves, R.J.; et al. Differential exposure, duration, and sensitivity of unionoidean bivalve life stages to environmental contaminants. J. N. Am. Bentholo. Soc. 2008, 27, 451–462. [Google Scholar] [CrossRef]
- De Castro-Català, N.; Kuzmanovic, M.; Roig, N.; Sierra, J.; Ginebreda, A.; Barceló, D.; Pérez, S.; Petrovic, M.; Picó, Y.; Schuhmacher, M.; et al. Ecotoxcity of sediments in rivers: Invertebrate community, toxicity bioassays and the toxic unit approach as complementary assessment tools. Sci. Tot. Environ. 2016, 540, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Baudrimont, M.; Gonzalez, P.; Mesmer-Dudons, N.; Legeay, A. Sensitivity to cadmium of the endangered freshwater pearl mussel Margaritifera margaritifera from the Dronne River (France): Experimental exposure. Environ. Sci. Pollu. Res. 2020, 27, 3715–3725. [Google Scholar] [CrossRef]
- Viarengo, A.; Canesi, L. Mussels as biological indicators of pollution. Aquaculture 1991, 94, 225–243. [Google Scholar] [CrossRef]
- Dellali, M.; Gnassia Barelli, M.; Roméo, M.; Aissa, P. The use of acetylcholinesterase activity in Ruditapes decussatus and Mytilus galloprovincialis in the biomonitoring of Bizerta lagoon. Comp. Biochem. Physiol. C. 2001, 130, 227–235. [Google Scholar] [CrossRef]
- Garmendia, L.; Soto, M.; Ortiz Zarragoitia, M.; Orbea, A.; Cajaraville, M.P.; Marigómez, I. Application of a battery of biomarkers in mussel digestive gland to assess long-term effects of the prestige oil spill in Galicia and Bay of Biscay: Correlation and multivariate analysis. J. Environ. Monit. 2011, 13, 933–942. [Google Scholar] [CrossRef]
- Costa, P.M.; Carreira, S.; Costa, M.H.; Caeiro, S. Development of histopathological indices in a commercial marine bivalve (Ruditapes decussatus) to determine environmental quality. Aquat. Toxicol. 2013, 126, 442–454. [Google Scholar] [CrossRef]
- Carella, F.; Feist, S.W.; Bignell, J.P.; de Vico, G. Comparative pathology in bivalves: Aetiological agents and disease processes. J. Invertebr. Pathol. 2015, 131, 107–120. [Google Scholar] [CrossRef]
- Khessiba, A.; Hoarau, P.; Gnassia-Barelli, M.; Aissa, P.; Roméo, M. Biochemical response of the mussel Mytilus galloprovincialis from Bizerta (Tunisia) to chemical pollutant exposure. Arch. Environ. Contam. Toxicol. 2001, 40, 222–229. [Google Scholar] [CrossRef]
- Canesi, L. Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis. Aqua. Toxicol. 2014, 153, 53–65. [Google Scholar] [CrossRef]
- RMP Annual Results 2003: Bivalve Bioaccumulation Monitoring Results; San Francisco Estuary Regional Monitoring Program: Richmond, CA, USA, 2003.
- Villela, I.V. DNA damage and repair in haemolymph cells of golden mussel (Limnoperna fortune) exposed to environmental contaminants. Mutat. Res. 2006, 605, 78–86. [Google Scholar] [CrossRef]
- Alazemi, B.M.; Lewis, J.W.; Andrews, E.B. Gill damage in the freshwater fish Gnathonemus ptersii (Family: Mormyridae) exposed to selected pollutants: An ultra structural study. Environ. Technol. 1996, 17, 225–238. [Google Scholar] [CrossRef]
- Barmo, C. In vivo effects of n-TiO2 on digestive gland and immunefunction of the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2013, 132, 133–918. [Google Scholar]
- Libralato, G. Embryotoxicity of TiO2 nanoparticles to Mytilus galloprovincialis (Lmk). Mar. Environ. Res. 2013, 92, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, M.; Li, Q.; Li, J.; Lin, D.; Lu, W. Immune toxicity of TiO2 under hypoxia in the green lippedmussel Perna viridis based on flow cytometric analysis of hemocyteparameters. Sci. Total. Environ. 2014, 470, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Lapresta-Fernández, A.; Fernández, A.; Blasco, J. Nanoecotoxicity effects of engineered silver and gold nanoparticles in aquatic organisms. TrAC Trend. Anal. Chem. 2012, 32, 40–59. [Google Scholar] [CrossRef]
- Bhuvaneshwaria, M.; Iswaryaa, V.; Archanaab, S.; Madhuc, G.M.; Suraish Kumarb, G.K.; Nagarajand, R.; Chandrasekarana, N. Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions. Aqua. Toxicol. 2015, 162, 29–38. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish acute toxicity oxidative stress and oxidative damage. Sci. Tota. Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef]
- Faria, M.; Navas, J.M.; Raldua, D.; Soares, A.M.; Barata, C. Oxidative stress effects of titanium dioxide nanoparticle aggregates in zebrafish embryos. Sci. Total. Environ. 2014, 470, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.M.; Dalai, S.; Chandrasekaran, N.; Mukherjee, A. Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp. Ecotoxicol. Environ. Saf. 2011, 74, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free. Radical. Bio. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, J.; Cai, Z. TiO2 nanoparticles in the marine environment: Impact on the toxicity of tributyltin to abalone (Haliotis diversicolor supertexta) embryos. Environ. Sci. Technol. 2011, 45, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Fan, D.; Wang, L.; Shi, L.; Ding, J.; Chen, Y.; Shen, S. Effects of ZnO, CuO, Au, and TiO2 nanoparticles on Daphnia magna and early life stages of zebrafish Danio rerio. Environ. Prot. Eng. 2014, 40, 139–149. [Google Scholar]
- Mohanty, D.; Samanta, L. Multivariate analysis of potential biomarkers of oxidative stress in Notopterus notopterus tissues from Mahanadi River as a function of concentration of heavy metals. Chemosphere 2016, 155, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Hermes Lima, M. Oxygen in biology and biochemistry: Role of free radicals. In Functional Metabolism: Regulation and Adaptation; Storey, K.B., Ed.; Wiley-Liss: Hoboken, NJ, USA, 2005; pp. 319–368. [Google Scholar]
- Girardello, F.; Leite, C.C.; Branco, C.S.; Roesch-Ely, M.; Fernandes, A.N.; Salvador, M.; Henriques, J.A.P. Antioxidant defences and haemocyte internalization in Limnoperna fortunei exposed to TiO2 nanoparticles. Aqua. Toxicol. 2016, 176, 190–196. [Google Scholar] [CrossRef]
- Buffet, P.E.; Amiard Triquet, C.; Dybowska, A.; Risso de Faverney, C.; Guibboli ni, M.; Valsami-Jones, E.; Mouneyrac, C. Fate of isotopically labeled zinc oxide nanoparticles in sediment and effects on two endobenthic species, the clam Scrobicularia plana and the ragworm Hediste diversicolor. Ecotoxicol. Environ. Saf. 2012, 84, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; Turcotte, P.; Auclair, J.; Gagnon, C. The effects of zinc oxide nanoparticles on the metallome in freshwater mussels. Comp. Biochem. Physiol. 2013, 158, 22–28. [Google Scholar] [CrossRef]
- Renault, S.; Baudrimont, M.; Mesmer Dudons, N.; Gonzalez, P.; Mornet, S.; Brisson, A. Impacts of gold nanoparticle exposure on two freshwater species: A phytoplanktonic alga (Scenedesmus subspicatus) and a benthic bivalve (Corbicula fluminea). Gold. Bull. 2008, 41, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.F.; Buffet, P.E.; Poirier, L.; Amiard Triquet, C.; Gilliland, D.; Joubert, Y.; Pilet, P.; Guibbolini, M.; Risso de Faverney, C.; Roméo, M.; et al. Size dependent bioaccumulation and ecotoxicity of gold nanoparticles in an endobenthic invertebrate: The Tellinid clam Scrobicularia plana. Environ. Pollut. 2012, 168, 37–43. [Google Scholar] [CrossRef]
- Ali, D.; Alarifi, S.; Kumar, S.; Ahamed, M.; Siddiqui, M.A. Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola. Aqua. Toxicol. 2012, 124, 83–90. [Google Scholar] [CrossRef]
- Basha, P.S.; Rani, A.U. Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreo Chromismoss ambicus (Tilapia). Ecotoxicol. Environ. Saf. 2003, 56, 218–221. [Google Scholar] [CrossRef]
- Ringwood, A.H.; Levi Polyachenko, N.; Carroll, D.L. Fullerene exposures with oysters: Embryonic, adult, and cellular responses. Environ. Sci. Technol. 2009, 43, 7136–7141. [Google Scholar] [CrossRef]
- Zhang, H.; He, X.; Zhang, Z.; Zhang, P.; Li, Y.; Ma, Y.; Kuang, Y.; Zhao, Y.; Chai, Z. Nano-CeO2 exhibits adverse effects at environmental relevant concentrations. Environ. Sci. Technol. 2011, 45, 3725–3730. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.P.; Carroll, D.L.; Ringwood, A.H. Tissue specific responses of oysters Crassostrea virginica, to silver nanoparticles. Aquat. Toxicol. 2013, 138, 123–128. [Google Scholar] [CrossRef]
- Gomes, T.N.; Pinheiro, J.P.; Cancio, I.; Pereira, C.G.; Cardoso, C.; Bebianno, M.J.O. Effects of copper nanoparticles exposure in the mussel Mytilus galloprovincialis. Environ. Sci. Technol. 2011, 45, 9356–9362. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Pereira, C.G.; Cardoso, C.; Pinheiro, J.P.; Cancio, I.; Bebianno, M.J. Accumulation and toxicity of copper oxide nanoparticles in the digestive gland of Mytilus galloprovincialis. Aquat. Toxicol. 2012, 118, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Blickley, T.M.; Matson, C.W.; Vreeland, W.N.; Rittschof, D.; Giulio, R.T.D.; McClellan Green, P.D. Dietary CdSe/ZnS quantum dot exposure in estuarine fish: Bioavailability, oxidative stress responses, reproduction, and maternal transfer. Aqua. Toxicol. 2014, 148, 27–39. [Google Scholar] [CrossRef]
- Gomez Olivan, L.M.; Neri Cruz, N.; Galar Martínez, M.; Islas Flores, H.; García Medina, S. Binary mixtures of diclofenac with paracetamol, ibuprofen, naproxen, and acetylsalicylic acid and these pharmaceuticals in isolated form induce oxidative stress on Hyalella azteca. Environ. Monit. Assess. 2014, 186, 7259–7271. [Google Scholar] [CrossRef]
- Sayeed, I.; Parvez, S.; Pandey, S.; Bin Hafeez, B.; Haque, R.; Raisuddin, S. Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol. Environ. Saf. 2003, 56, 295–301. [Google Scholar] [CrossRef]
- Khazri, A.; Sellami, B.; Dellali, M.; Corcellas, C.; Eljarrat, E.; Barceló, D.; Mahmoudi, E. Acute toxicity of cypermethrin on the freshwater mussel Unio gibbus. Ecotoxicol. Environ. Saf. 2015, 115, 6266. [Google Scholar] [CrossRef] [Green Version]
- Giarratano, E.; Gil, M.N.; Malanga, G. Biomarkers of environmental stress in gills of ribbed mussel Aulacomya atra atra (Nuevo Gulf, Northern Patagonia). Ecotoxicol. Environ. Saf. 2014, 107, 111–119. [Google Scholar] [CrossRef]
- Cid, A.; Picado, A.; Correia, J.B.; Chaves, R.; Silva, H.; Caldeira, J.; de Matos, A.P.; Diniz, M.S. Oxidative stress and histological changes following exposure to diamond nanoparticles in the freshwater Asian clam Corbicula fluminea (Muller, 1774). J. Hazard. Mater. 2015, 284, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Chen, L. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol. Environ. Saf. 2012, 80, 103–110. [Google Scholar] [CrossRef]
- Xia, B.; Zhu, L.; Han, Q.; Sun, X.; Chen, B.; Qu, K. Effects of TiO2 nanoparticles at predicted environmental relevant concentration on the marine scallop Chlamys farreri: An integrated biomarker approach. Environ. Toxicol. Pharmacol. 2017, 50, 128–135. [Google Scholar] [CrossRef]
- Fulton, M.H.; Key, P.B. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ. Toxicol. Chem. 2001, 20, 37–45. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Umasankar, Y.; Chen, S.M. Nanomaterials-Acetylcholinesterase Enzyme Matrices for Organophosphorus Pesticides Electrochemical Sensors: A Review. Sensors 2009, 9, 4034–4055. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.L.; Gao, J.Q. Potential neurotoxicity of nanoparticles. Int. J. Pharm. 2010, 394, 115–121. [Google Scholar] [CrossRef]
- Czajka, M.; Sawicki, K.; Sikorska, K.; Popek, S.; Kruszewski, M.; Kapka Skrzypczak, L. Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol. Vitro. 2015, 29, 1042–1052. [Google Scholar] [CrossRef]
- Minetto, D.; Volpi, G.A.; Libralato, G. Saltwater ecotoxicology of Ag, Au, CuO, TiO2, ZnO and C60 engineered. nanoparticles: An overview. Environ. Internat. 2016, 9293, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Bebianno, M.J.; Geret, F.; Hoarau, P.; Serafim, M.A.; Coelho, M.R.; Gnassia Barelli, M.; Romeo, M. Biomarkers in Ruditapes decussatus: A potential bioindicator species. Biomark 2004, 9, 305–330. [Google Scholar] [CrossRef]
- Katuli, K.K.; Massarsky, A.; Hadadi, A.; Pourmehran, Z. Silver nanoparticles inhibit the gill Na+/K+-ATPase and erythrocyte AChE activities and induce the stress response in adult zebrafish Danio rerio. Ecotoxicol. Environ. Saf. 2014, 106, 173–180. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Gibbons, N.C.J.; Zothner, C.; Elwary, S.M.; Rokos, H.; Wood, J.M. Butyrylcholinesterase is present in the human epidermis and is regulated by H2O2: More evidence for oxidative stress in vitiligo. Biochem. Biophys. Res. Com. 2006, 349, 931–938. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Gibbons, N.C.J.; Elwary, S.M.; Parkin, S.M.; John, M.; Wood, J.M. Calcium–activated butyryl cholinesterase in human skin protects acetylcholinesterase against suicide inhibition by neurotoxic organophosphates. Biochem. Biophys. Res. Com. 2007, 355, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Gnatyshyna, L.; Fedoruk, O.; Sokolova, I.M.; Stoliar, M. Endocrine activities and cellular stress responses in the marsh frog Pelophylax ridibundus exposed to cobalt, zinc and their organic nanocomplexes. Aquat. Toxicol. 2016, 170, 62–71. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).