Interstitial Annelida
Abstract
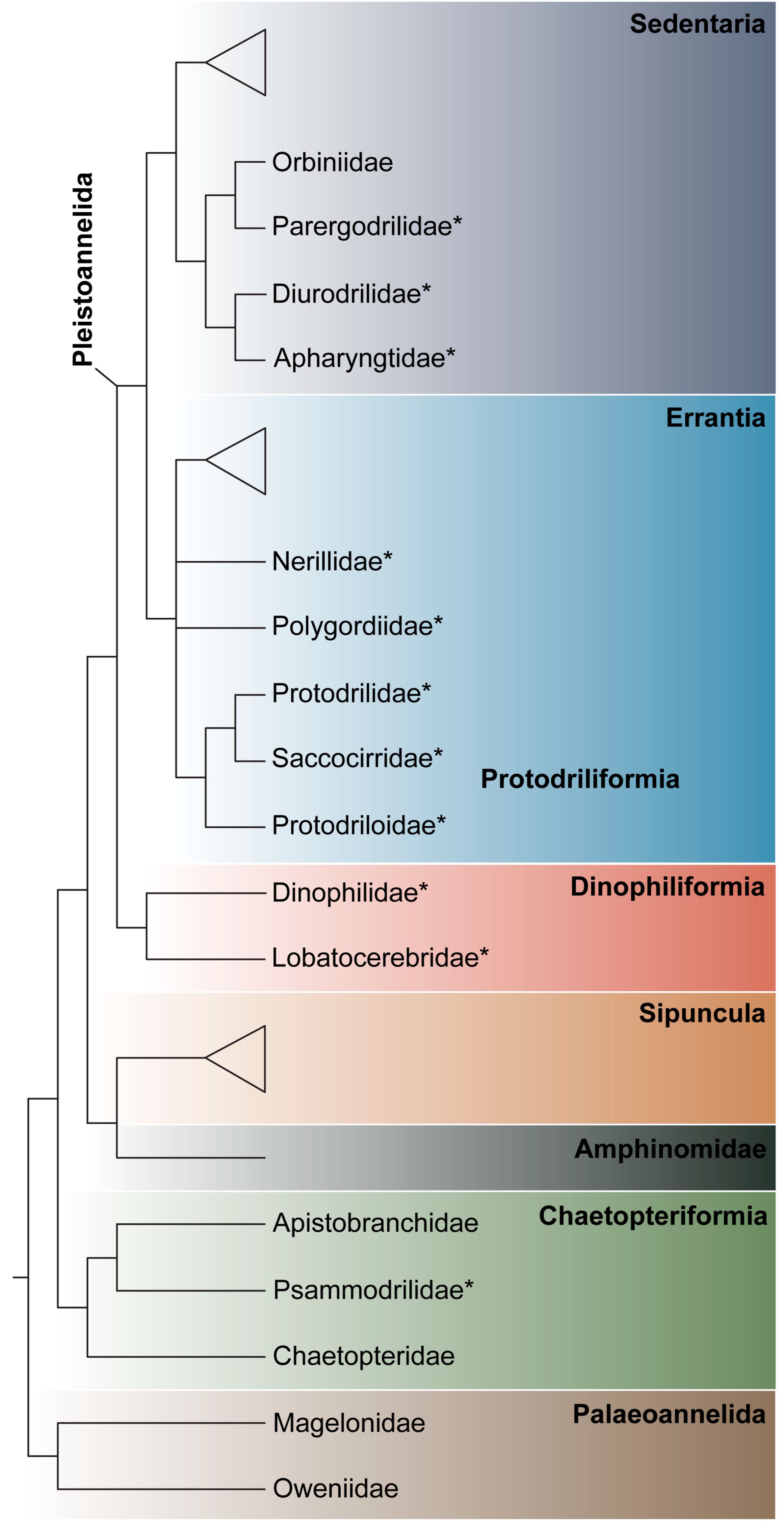
:1. Introduction
2. Materials and Methods
2.1. Extraction Methods
2.2. Fixation and Preservation Methods
2.3. Morphological and Molecular Methods for Species Identification
3. Results
3.1. Apharyngtidae n. fam
3.1.1. Phylogenetic Affinities
3.1.2. Morphology
3.1.3. Taxonomy
3.1.4. Distribution
3.1.5. Major Revisions and Most Important Literature
3.2. Dinophilidae Macalister, 1878
3.2.1. Phylogenetic Affinities
3.2.2. Morphology
3.2.3. Taxonomy
3.2.4. Distribution and Diversity
3.2.5. Major Revisions and Most Important Literature
3.3. Diurodrilidae Kristensen and Niilonen, 1982
3.3.1. Phylogenetic Affinities
3.3.2. Morphology
3.3.3. Taxonomy
3.3.4. Distribution and Diversity
3.3.5. Major Revisions and Most Important Literature
3.4. Lobatocerebridae Rieger, 1980
3.4.1. Phylogenetic Affinities
3.4.2. Morphology
3.4.3. Taxonomy
3.4.4. Distribution and Diversity
3.4.5. Major Revisions and Most Important Literature
3.5. Nerillidae Levinsen, 1883
3.5.1. Phylogenetic Affinities
3.5.2. Morphology
3.5.3. Taxonomy
Nine-Segmented Genera
Eight-Segmented Genera
Seven-Segmented Genera
3.5.4. Distribution and Diversity
3.5.5. Major Revisions and Most Important Literature
3.6. Polygordiidae Czerniavsky, 1881
3.6.1. Phylogenetic Affinities
3.6.2. Morphology
3.6.3. Taxonomy
3.6.4. Distribution and Diversity
3.6.5. Major Revisions and Most Important Literature
3.7. Protodrilidae Hatschek, 1888
3.7.1. Phylogenetic Affinities
3.7.2. Morphology
3.7.3. Taxonomy
3.7.4. Distribution and Diversity
3.7.5. Major Revisions and Most Important Literature
3.8. Protodriloididae Jouin, 1966
3.8.1. Phylogenetic Affinities
3.8.2. Morphology
3.8.3. Taxonomy
3.8.4. Distribution and Diversity
3.8.5. Major Revisions and Most Important Literature
3.9. Psammodrilidae Swedmark, 1952
3.9.1. Phylogenetic Affinities
3.9.2. Morphology
3.9.3. Taxonomy
3.9.4. Distribution and Diversity
3.9.5. Major Revisions
3.10. Saccocirridae Bobretzky, 1872
3.10.1. Phylogenetic Affinities
3.10.2. Morphology
3.10.3. Taxonomy
3.10.4. Distribution and Diversity
3.10.5. Major Revisions and Most Important Literature
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giere, O. Meiobenthology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Schmidt-Rhaesa, A. (Ed.) Guide to the Identification of Marine Meiofauna; Dr. Friedrich Pfeil Verlag: Munich, Germany, 2020. [Google Scholar]
- Worsaae, K. Annelida (excluding Clitellata and Sipuncula). In Guide to the Identification of Marine Meiofauna; Schmidt-Rhaesa, A., Ed.; Dr. Friedrich Pfeil Verlag: Munich, Germany, 2020; pp. 239–270. [Google Scholar]
- Bunke, D. Aeolosomatidae and Potamodrilidae. In Introduction to the Study of Meiofauna; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution: Washington, DC, USA, 1988; pp. 345–348. [Google Scholar]
- Hermans, C.O. The systematic position of the Archiannelida. Syst. Biol. 1969, 18, 85–102. [Google Scholar] [CrossRef]
- Hatschek, B. Studien über die Entwicklungsgeschichte der Anneliden: Ein Beitrag zur Morphologie der Bilaterien. Arb. Zool. Inst. Univ. Wien. 1878, 1, 277–404. [Google Scholar]
- Worsaae, K.; Kristensen, R.M. Evolution of interstitial Polychaeta (Annelida). Hydrobiologia 2005, 535/536, 319–340. [Google Scholar] [CrossRef]
- Laumer, C.E.; Bekkouche, N.; Kerbl, A.; Goetz, F.; Neves, R.C.; Sørensen, M.V.; Kristensen, R.M.; Hejnol, A.; Dunn, C.W.; Giribet, G.; et al. Spiralian phylogeny informs the evolution of microscopic lineages. Curr. Biol. 2015, 25, 2000–2006. [Google Scholar] [CrossRef] [Green Version]
- Helm, C.; Beckers, P.; Bartolomaeus, T.; Drukewitz, S.H.; Kourtesis, I.; Weigert, A.; Purschke, G.; Worsaae, K.; Struck, T.H.; Bleidorn, C. Convergent evolution of the ladder-like ventral nerve cord in Annelida. Front. Zool. 2018, 15, 36–17. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.C.S.; Novo, M.; Kawauchi, G.Y.; Worsaae, K.; Pleijel, F.; Giribet, G.; Rouse, G.W. Articulating “Archiannelids”: Phylogenomics and annelid relationships, with emphasis on meiofaunal taxa. Mol. Biol. Evol. 2015, 32, 2860–2875. [Google Scholar] [CrossRef] [PubMed]
- Struck, T.H.; Golombek, A.; Weigert, A.; Franke, F.A.; Westheide, W.; Purschke, G.; Bleidorn, C.; Halanych, K.M. The evolution of annelids reveals two adaptive routes to the interstitial realm. Curr. Biol. 2015, 25, 1993–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Durán, J.M.; Vellutini, B.C.; Marletaz, F.; Cetrangolo, V.; Cvetesic, N.; Thiel, D.; Henriet, S.; Grau-Bové, X.; Carrillo-Baltodano, A.M.; Gu, W.; et al. Conservative route to extreme genome compaction in a miniature annelid. Nat. Ecol. Evol. 2020. [Google Scholar]
- Chen, H.; Parry, L.A.; Vinther, J.; Zhai, D.; Hou, X.; Ma, X. A Cambrian crown annelid reconciles phylogenomics and the fossil record. Nature 2020, 583, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Worsaae, K.; Rouse, G.W. Is Diurodrilus an annelid? J. Morph. 2008, 269, 1426–1455. [Google Scholar] [CrossRef]
- Kerbl, A.; Bekkouche, N.; Sterrer, W.; Worsaae, K. Detailed reconstruction of the nervous and muscular system of Lobatocerebridae with an evaluation of its annelid affinity. BMC Evol. Biol. 2015, 15, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helm, C.; Vöcking, O.; Kourtesis, I.; Hausen, H. Owenia fusiformis—A basally branching annelid suitable for studying ancestral features of annelid neural development. BMC Evol. Biol. 2016, 16, 129. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.C. Polychaete nervous systems: Ground pattern and variations—cLS microscopy and the importance of novel characteristics in phylogenetic analysis. Integr. Comp. Biol. 2006, 46, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, N.; Shinozaki, A.; Fujiwara, Y. Neuroanatomy of the vestimentiferan tubeworm Lamellibrachia satsuma provides insights into the evolution of the polychaete nervous system. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Purschke, G. Male genital organs, spermatogenesis and spermatozoa in the enigmatic terrestrial polychaete Parergodrilus heideri (Annelida, Parergodrilidae). Zoomorphology 2002, 121, 125–138. [Google Scholar] [CrossRef]
- Purschke, G. Comparative electron microscopic investigation of the nuchal organs in Protodriloides, Protodrilus, and Saccocirrus (Annelida, Polychaeta). Can. J. Zool. 1990, 68, 325–338. [Google Scholar] [CrossRef]
- Worsaae, K.; Giribet, G.; Martínez, A. The role of progenesis in the diversification of the interstitial annelid lineage Psammodrilidae. Invertebr. Syst. 2018, 32, 774–793. [Google Scholar] [CrossRef]
- Bleidorn, C. The role of character loss in phylogenetic reconstruction as exemplified for the Annelida. J. Zool. Syst. Evol. Res. 2007, 45, 299–307. [Google Scholar] [CrossRef]
- Mock, H. Zur Kenntnis von Diurodrilus subterraneus (Polychaeta, Dinophilidae) aus dem Sandhang der Nordseeinsel Sylt. Helgol. Meeresunters. 1981, 34, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Rieger, R.M.; Rieger, G.E. Fine structure of the archiannelid cuticle and remarks on the evolution of the cuticle within the Spiralia. Acta Zool. 1976, 57, 53–68. [Google Scholar] [CrossRef]
- Worsaae, K.; Kristensen, R.M. Diurodrilidae Kristensen & Niilonen, 1982. In Handbook of Zoology: Annelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2020; Volume 3. [Google Scholar]
- Worsaae, K.; Kerbl, A.; Vang, Á.; Gonzalez, B.C. Broad North Atlantic distribution of a meiobenthic annelid—Against all odds. Sci. Rep. 2019, 9, 15497–13. [Google Scholar] [CrossRef] [Green Version]
- Gelder, S.R. A review of the zoogeography and habitat data of the genus Nerilla Schmidt, 1848 (Annelida: Archiannelida). J. Nat. Hist. 1974, 8, 631–643. [Google Scholar] [CrossRef]
- Jouin, C. Recherches sur les Protodrilidae (Archiannélides). I. Étude morphologique et systématique du genre Protodrilus. Cah. Biol. Mar. 1970, 11, 367–434. [Google Scholar]
- Di Domenico, M.; Martínez, A.; Almeida, T.C.M.; Martins, M.O.; Worsaae, K.; Lana, P.C. Response of the meiofaunal annelid Saccocirrus pussicus (Saccocirridae) to sandy beach morphodynamics. Hydrobiologia 2014, 734, 1–16. [Google Scholar] [CrossRef]
- Sato-Okoshi, W.; Okoshi, K.; Fujiwara, Y. A new species of Protodrilus (Annelida, Protodrilidae), covering bone surfaces bright red, in whale-fall ecosystems in the northwest Pacific. Biol. Bull. 2015, 229, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Jouin, C. The ultrastructure of a gutless annelid, Parenterodrilus gen. nov. taenioides (=Astomus taenioides) (Polychaeta, Protodrilidae). Can. J. Zool. 1992, 70, 1833–1848. [Google Scholar] [CrossRef]
- Worsaae, K. Systematics of Nerillidae (Polychaeta, Annelida). Meiofauna Marina 2005, 14, 49–74. [Google Scholar]
- Worsaae, K.; Kristensen, R.M. A new species of Paranerilla (Polychaeta: Nerillidae) from Northeast Greenland Waters, Arctic Ocean. Cah. Biol. Mar. 2003, 44, 23–39. [Google Scholar]
- Morselli, I.; Sarto, M.; Mari, M. Troglochaetus beranecki Delachaux (Annelida, Polychaeta): Collecting methods and microscopy techniques for SEM and in vivo observations. Hydrobiologia 1998, 379, 213–216. [Google Scholar] [CrossRef]
- Sterrer, W.; Iliffe, T.M. Mesonerilla prospera, a new archiannelid from marine caves in Bermuda. Proc. Biol. Soc. Wash. 1982, 95, 509–514. [Google Scholar]
- Worsaae, K.; Martinez, A.; Núñez, J. Nerillidae (Annelida) from the Corona lava tube, Lanzarote, with description of Meganerilla cesari n. sp. Mar. Biodivers. 2009, 39, 195–207. [Google Scholar] [CrossRef]
- Worsaae, K.; Mikkelsen, M.D.; Martinez, A. Description of six new species of Mesonerilla (Nerillidae, Annelida) and an emended description of M. intermedia Wilke, 1953, from marine and cave environments. Mar. Biodivers. 2019, 49, 2141–2165. [Google Scholar] [CrossRef] [Green Version]
- Worsaae, K.; Rouse, G.W. Mesonerilla neridae, n. sp. (Nerillidae): First meiofaunal annelid from deep-sea hydrothermal vents. Zoosymposia 2009, 2, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.C.M.; Bernhard, J.M.; Jouin-Toulmond, C. A new member of Nerillidae (Annelida: Polychaeta), Xenonerilla bactericola gen. et sp. nov., collected off California, USA. Cah. Biol. Mar. 2001, 42, 203–217. [Google Scholar]
- Worsaae, K.; Sterrer, W.; Iliffe, T.M. Longipalpa saltatrix, a new genus and species of the meiofaunal family Nerillidae (Annelida: Polychaeta) from an anchialine cave in Bermuda. Proc. Biol. Soc. Wash. 2004, 117, 346–362. [Google Scholar]
- Martinez, A.; Kvindebjerg, K.; Iliffe, T.M.; Worsaae, K. Evolution of cave suspension feeding in Protodrilidae (Annelida). Zool. Scr. 2017, 46, 214–226. [Google Scholar] [CrossRef]
- Worsaae, K.; Gonzalez, B.C.; Kerbl, A.; Nielsen, S.H.; Jørgensen, J.T.; Armenteros, M.; Iliffe, T.M.; Martinez, A. Diversity and evolution of the stygobitic Speleonerilla nom. nov. (Nerillidae, Annelida) with description of three new species from anchialine caves in the Caribbean and Lanzarote. Mar. Biodivers. 2019, 49, 2167–2192. [Google Scholar] [CrossRef]
- Cerca, J.; Meyer, C.; Stateczny, D.; Siemon, D.; Wegbrod, J.; Purschke, G.; Dimitrov, D.; Struck, T.H. Deceleration of morphological evolution in a cryptic species complex and its link to paleontological stasis. Evolution 2020, 74, 116–131. [Google Scholar] [CrossRef] [PubMed]
- De Souza-Santos, L.P. An experimental approach to the ecophysiology of the interstitial polychaete Polygordius eschaturus (Annelida: Polygordiidae). Scimar 2017, 81, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Curini-Galletti, M.; Artois, T.; Delogu, V.; De Smet, W.H.; Fontaneto, D.; Jondelius, U.; Leasi, F.; Martinez, A.; Meyer-Wachsmuth, I.; Nilsson, K.S.; et al. Patterns of diversity in soft-bodied meiofauna: Dispersal ability and body size matter. PLoS ONE 2012, 7, e33801. [Google Scholar] [CrossRef]
- Martinez, A.; Di Domenico, M.; Leasi, F.; Curini-Galletti, M.; Todaro, M.A.; Zotto, M.D.; Gobert, S.; Artois, T.; Norenburg, J.; Jörger, K.M.; et al. Patterns of diversity and endemism of soft-bodied meiofauna in an oceanic island, Lanzarote, Canary Islands. Mar. Biodivers. 2019, 49, 2033–2055. [Google Scholar] [CrossRef] [Green Version]
- Tustison, C.A.; Ramey-Balci, P.A.; Rouse, G.W. More Knot Worms: Four new Polygordius (Annelida) species from the Pacific and Caribbean. Diversity 2020, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Cerca, J.; Meyer, C.; Purschke, G.; Struck, T.H. Delimitation of cryptic species drastically reduces the geographical ranges of marine interstitial ghost-worms (Stygocapitella; Annelida, Sedentaria). Mol. Phylogenet. Evol. 2020, 143, 106663. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Di Domenico, M.; Jörger, K.; Norenburg, J.; Worsaae, K. Description of three new species of Protodrilus (Annelida, Protodrilidae) from Central America. Mar. Biol. Res. 2013, 9, 676–691. [Google Scholar] [CrossRef]
- Martinez, A.; Di Domenico, M.; Rouse, G.W.; Worsaae, K. Phylogeny and systematics of Protodrilidae (Annelida) inferred with total evidence analyses. Cladistics 2015, 31, 250–276. [Google Scholar] [CrossRef]
- Di Domenico, M.; Martínez, A.; Lana, P.C.; Worsaae, K. Molecular and morphological phylogeny of Saccocirridae (Annelida) reveals two cosmopolitan clades with specific habitat preferences. Mol. Phylogenet. Evol. 2014, 75, 202–218. [Google Scholar] [CrossRef]
- Higgins, R.P.; Thiel, H. Introduction to the Study of Meiofauna, 1st ed.; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution: Washington, DC, USA, 1988. [Google Scholar]
- Westheide, W. Polychaetes: Interstitial Families, 2nd ed.; Crothers, J.H., Ed.; The Linnean Society of London (Field Studies Council Shrewsbury): London, UK, 2008. [Google Scholar]
- De Jonge, V.N.; Bouwman, L.A. A simple density separation technique for quantitative isolation of meiobenthos using the colloidal silica Ludox-TM. Mar. Biol. 1977, 42, 143–148. [Google Scholar] [CrossRef]
- Yoder, M.; De Ley, I.T.; King, I.W.; Mundo-Ocampo, M.; Mann, J.; Blaxter, M.; Poiras, L.; De Ley, P. DESS: A versatile solution for preserving morphology and extractable DNA of nematodes. Nematology 2006, 8, 367–376. [Google Scholar] [CrossRef]
- Raikova, O.I.; Meyer-Wachsmuth, I.; Jondelius, U. The plastic nervous system of Nemertodermatida. Org. Divers. Evol. 2016, 16, 85–104. [Google Scholar] [CrossRef] [Green Version]
- Kalt, M.R.; Tandler, B. A study of fixation of early amphibian embryos for electron microscopy. J. Ultrastruct. Res. 1971, 36, 633–645. [Google Scholar] [CrossRef]
- Cheng, P.C.; Walden, D.B.; Greyson, R.I. Improved plant microtechnique for TEM, SEM and LM specimen preparation. Natl. Sci. Counc. Month. Rep. China 1979, 7, 1000–1007. [Google Scholar]
- Westheide, W.; Purschke, G. Organism processing. In Introduction to the Study of Meiofauna; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution: Washington, DC, USA, 1988; pp. 146–160. [Google Scholar]
- Kerbl, A.; Vereide, E.H.; Gonzalez, B.C.; Rouse, G.W.; Worsaae, K. Two new meiofaunal species of Trilobodrilus (Dinophilidae, Annelida) from California, USA. Eur. J. Taxon. 2018, 421, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Worsaae, K.; Müller, M.C. Nephridial and gonoduct distribution patterns in Nerillidae (Annelida: Polychaeta) examined by tubulin staining and cLSM. J. Morphol. 2004, 261, 259–269. [Google Scholar] [CrossRef]
- Jörger, K.M.; Norenburg, J.L.; Wilson, N.G.; Schrödl, M. Barcoding against a paradox? Combined molecular species delineations reveal multiple cryptic lineages in elusive meiofaunal sea slugs. BMC Evol. Biol. 2012, 12, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Wachsmuth, I.; Curini-Galletti, M.; Jondelius, U. Hyper-cryptic marine meiofauna: Species complexes in Nemertodermatida. PLoS ONE 2014, 9, e107688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontaneto, D.; Flot, J.-F.; Tang, C.Q. Guidelines for DNA taxonomy, with a focus on the meiofauna. Mar. Biodivers. 2015, 45, 433–451. [Google Scholar] [CrossRef]
- Fujisawa, T.; Barraclough, T.G. Delimiting Species Using Single-Locus Data and the Generalized Mixed Yule Coalescent Approach: A Revised Method and Evaluation on Simulated Data Sets. Syst. Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef] [Green Version]
- Kapli, P.; Lutteropp, S.; Zhang, J.; Kobert, K.; Pavlidis, P.; Stamatakis, A.; Flouri, T. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 2017, 33, 1630–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Eibye-Jacobsen, D.; Kristensen, R.M. A new genus and species of Dorvilleidae (Annelida, Polychaeta) from Bermuda, with a phylogenetic analysis of Dorvilleidae, Iphitimidae and Dinophilidae. Zool. Scr. 1994, 23, 107–131. [Google Scholar] [CrossRef]
- Struck, T.H. Phylogeny. In Handbook of Zoology: Annelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2019; Volume 1, pp. 37–68. [Google Scholar]
- Westheide, W. Apharyngtus Westheide, 1971. In Handbook of Zoology: Annelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2019; Volume 1, pp. 234–237. [Google Scholar]
- Westheide, W. Apharyngtus punicus nov. gen. nov. spec., an aberrant archiannelid from the mesopsammal of the Tunesian Coast of the Mediterranean. Mikrofauna Meeresboden 1971, 6, 233–248. [Google Scholar]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riser, N.W. General observations on the intertidal interstitial fauna of New Zealand. Tane 1984, 30, 239–249. [Google Scholar]
- Westheide, W. Dinophilidae Verrill, 1892. In Handbook of Zoology: Annelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2019; Volume 1, pp. 217–233. [Google Scholar]
- Struck, T.H.; Westheide, W.; Purschke, G. Progenesis in Eunicida (“Polychaeta,” Annelida)—Separate evolutionary events? Evidence from molecular data. Mol. Phylogenet. Evol. 2002, 25, 190–199. [Google Scholar] [CrossRef]
- Remane, A. Diagnosen neuer Archianneliden. Zool. Anz. 1925, 65, 15–17. [Google Scholar]
- Kerbl, A.; Fofanova, E.G.; Mayorova, T.D.; Voronezhskaya, E.E.; Worsaae, K. Comparison of neuromuscular development in two dinophilid species (Annelida) suggests progenetic origin of Dinophilus gyrociliatus. Front. Zool. 2016, 13, 49. [Google Scholar] [CrossRef] [Green Version]
- Kerbl, A.; Conzelmann, M.; Jekely, G.; Worsaae, K. High diversity in neuropeptide immunoreactivity patterns among three closely related species of Dinophilidae (Annelida). J. Comp. Neurol. 2017, 525, 3596–3635. [Google Scholar] [CrossRef]
- Fofanova, E.G.; Mayorova, T.D.; Voronezhskaya, E.E. Paradoxical effect of serotonin on ciliary locomotion of the adult archiannelid worms. Zool. Bespozvon. 2017, 14, 114–120. [Google Scholar]
- Müller, M.C.M.; Westheide, W. Comparative analysis of the nervous systems in presumptive progenetic dinophilid and dorvilleid polychaetes (Annelida) by immunohistochemistry and cLSM. Acta Zool. 2002, 83, 33–48. [Google Scholar] [CrossRef]
- Purschke, G.; Arendt, D.; Hausen, H.; Müller, M.C.M. Photoreceptor cells and eyes in Annelida. Arthropod Struct. Dev. 2006, 35, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Eakin, R.M.; Martin, G.G.; Reed, C.T. Evolutionary significance of fine structure of archiannelid eyes. Zoomorphology 1977, 88, 1–18. [Google Scholar] [CrossRef]
- Kajihara, H.; Ikoma, M.; Yamasaki, H.; Hiruta, S.F. Trilobodrilus itoi sp. nov., with a re-description of T. nipponicus (Annelida: Dinophilidae) and a molecular phylogeny of the genus. Zool. Sci. 2015, 32, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Purschke, G. On the ground pattern of Annelida. Org. Divers. Evol. 2002, 2, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Fofanova, E.G.; Nezlin, L.P.; Voronezhskaya, E.E. Ciliary and nervous structures in juvenile females of the annelid Dinophilus gyrociliatus (O. Schmidt, 1848) (Annelida: Polychaeta). Russ. J. Mar. Biol. 2014, 40, 43–52. [Google Scholar] [CrossRef]
- Moore, A. Dinophilus gardineri (sp. nov.). Biol. Bull. 1899, 1, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Kerbl, A.; Tolstrup, E.W.; Worsaae, K. Nerves innervating copulatory organs show common FMRFamide, FVRIamide, MIP and serotonin immunoreactivity patterns across Dinophilidae (Annelida) indicating their conserved role in copulatory behaviour. BMC Zool. 2019, 4, 8. [Google Scholar] [CrossRef]
- Jennings, J.B.; Gelder, S.R. Feeding and digestion in Dinophilus gyrociliatus (Annelida: Archiannelida). J. Zool. 1969, 158, 441–451. [Google Scholar] [CrossRef]
- Beniash, E.A.; Yerlikova, N.N.; Yevdonin, L.A. Some characteristics of the Dinophilus vorticoides anatomy of the nervous system. In Explorations of the Fauna of the Seas; Bushinskaya, G.N., Ed.; Russian Academy of Sciences: St. Petersburg, Russia, 1992; Volume 43, pp. 5–9. [Google Scholar]
- Repiakhov, V.M. K anatomii i istorii razvitiia Dinophilus gyrociliatus, O. Schmidt. Zap. Novoross. Obshchestva Estestvoispyt. 1886, 77p. [Google Scholar]
- Mamkaev, Y.V. Dinophilida as a primitive group of Trochozoa. Investig. Mar. Fauna 1985, 34, 99–103. [Google Scholar]
- Müller, M.C.M.; Westheide, W. Das Nervensystem parapodienloser Polychaeten: Orthogonale Strukturen des Nervensystems juveniler Stadien und progenetischer Arten. Verh. Dtsch. Zool. Ges. 1997, 90, 209. [Google Scholar]
- Purschke, G. Annelida: Basal groups and Pleistoannelida. In Structure and Evolution of Invertebrate Nervous Systems; Schmidt-Rhaesa, A., Harzsch, S., Purschke, G., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 254–312. [Google Scholar]
- Kerbl, A.; Martín-Durán, J.M.; Worsaae, K.; Hejnol, A. Molecular regionalization in the compact brain of the meiofaunal annelid Dinophilus gyrociliatus (Dinophilidae). EvoDevo 2016, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fofanova, E.G.; Voronezhskaya, E.E. The structure of archiannelid Dinophilus gyrociliatus ventral nerve cords. Acta Biol. Hung. 2012, 63, 88–90. [Google Scholar] [CrossRef]
- Jägersten, G. Zur Kenntnis der Morphologie, Enzystierung und Taxonomie von Dinophilus. Kungl. Svenska Vetenskapsakad. Handl. 1944, 21, 1–50. [Google Scholar]
- Jennings, J.B.; Donworth, P.J. Observations on the life cycle and nutrition of Dinophilus taeniatus Harmer 1889 (Annelida: Polychaeta). Ophelia 1986, 25, 119–137. [Google Scholar] [CrossRef]
- Jägersten, G. Life cycle of Dinophilus, with special reference to the encystment and its dependence on temperature. Oikos 1951, 3, 143–165. [Google Scholar] [CrossRef]
- Donworth, P.J. A reappraisal and validation of the species Dinophilus taeniatus Harmer 1889 and of taxonomically significant features in monomorphic dinophilids (Annelida: Polychaeta). Zool. Anz. 1985, 216, 32–38. [Google Scholar]
- Windoffer, R.; Westheide, W. The nervous system of the male Dinophilus gyrociliatus (Annelida: Polychaeta). I. Number, types and distribution pattern of sensory cells. Acta Zool. 1988, 69, 55–64. [Google Scholar] [CrossRef]
- Windoffer, R.; Westheide, W. The nervous system of the male Dinophilus gyrociliatus (Polychaeta, Dinophilidae): II. Electron microscopical reconstruction of nervous anatomy and effector cells. J. Comp. Neurol. 1988, 272, 475–488. [Google Scholar] [CrossRef]
- Fauvel, P. Polychètes sédentaires. Addenda aux errantes, Arachniannélides, Myzostomaires. In Faune de France; Volume 16, Paul Lechevalier: Paris, France, 1927. [Google Scholar]
- Jones, E.R.; Ferguson, F.F. The genus Dinophilus (Archiannelida) in the United States. Am. Midl. Nat. 1957, 57, 440–449. [Google Scholar] [CrossRef]
- Ax, P. Das Fortpflanzungsverhalten von Trilobodrilus (Archiannelida, Dinophilidae). Mar. Biol. 1968, 1, 330–335. [Google Scholar] [CrossRef]
- Harmer, S.F. Notes on the anatomy of Dinophilus. Proc. Cambridge Philos. Soc. 1889, 6, 119–143. [Google Scholar] [CrossRef] [Green Version]
- Westheide, W. Die Gattung Trilobodrilus (Archiannelida, Polychaeta) von der deutschen Nordseeküste. Helgol. Meeresunters. 1967, 16, 207–215. [Google Scholar] [CrossRef]
- Du Bois-Reymond Marcus, E. Further archiannelids from Brazil. Com. Zool. Mus. 1948, 48, 1–17. [Google Scholar]
- Westheide, W.; Nordheim, H. Interstitial Dorvilleidae (Annelida, Polychaeta) from Europe, Australia and New Zealand. Zool. Scr. 1985, 14, 183–199. [Google Scholar] [CrossRef]
- Kristensen, R.M.; Niilonen, T. Structural Studies on Diurodrilus Remane (Diurodrilidae fam.n.), with description of Diurodrilus westheidei sp.n. from the Arctic Interstitial Meiobenthos, W. Greenland. Zool. Scr. 1982, 11, 1–12. [Google Scholar] [CrossRef]
- Westheide, W. The systematic position of the Dinophilidae and the archiannelid problem. In The Origins and Relationships of Lower Invertebrates; Conway Morris, S., George, J.D., Gibson, R., Platt, H.M., Eds.; Systematic Association: Oxford, UK, 1985; Volume 28, pp. 310–326. [Google Scholar]
- Golombek, A.; Tobergte, S.; Nesnidal, M.P.; Purschke, G.; Struck, T.H. Mitochondrial genomes to the rescue—Diurodrilidae in the myzostomid trap. Mol. Phylogenet. Evol. 2013, 68, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, H.; Ikoma, M.; Yamasaki, H.; Hiruta, S.F. Diurodrilus kunii sp. nov. (Annelida: Diurodrilidae) and a molecular phylogeny of the genus. Zool. Sci. 2019, 36, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, R.M.; Eibye-Jacobsen, D. Ultrastructure of spermiogenesis and spermatozoa in Diurodrilus subterraneus (Polychaeta, Diurodrilidae). Zoomorphology 1995, 115, 117–132. [Google Scholar] [CrossRef]
- Schmidt, P.; Westheide, W. Interstitielle Fauna von Galàpagos. XVII. Polygordiidae, Saccocirridae, Protodrilidae, Nerillidae, Dinophilidae (Polychaeta). Mikrofauna Meeresbod. 1977, 62, 1–38. [Google Scholar]
- Paxton, H. Family Diurodrilidae. In Polychaetes & Allies: The Southern Synthesis; Beesley, P.L., Ross, G.J.B., Glasby, C.J., Eds.; Csiro Publishing: Clayton, Australia, 2000; pp. 104–105. [Google Scholar]
- Villora-Moreno, S. Diurodrilus benazzi Gerlach, 1952 (Diurodrilida) and Dinophilus gyrociliatus O. Schmidt, 1857 (Dinophilida): First record of two orders of interstitial polychaetes on the Iberian Peninsula. Oceanogr. Lit. Rev. 1998, 7, 1186. [Google Scholar]
- Rieger, R.M. A new group of interstitial worms, Lobatocerebridae nov. fam. (Annelida) and its significance for metazoan phylogeny. Zoomorphology 1980, 95, 41–84. [Google Scholar] [CrossRef]
- Kerbl, A.; Worsaae, K. Lobatocerebridae Rieger, 1980. In Handbook of Zoology: Annelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2019; Volume 1, pp. 201–216. [Google Scholar]
- Haszprunar, G.; Rieger, R.M.; Schuchert, P. Extant “problematica” within or near the Metazoa. In The Early Evolution of Metazoa and the Significance of Problematic Taxa; Simonetta, A.M., Conway Morris, S., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 99–105. [Google Scholar]
- Zrzavý, J. Gastrotricha and metazoan phylogeny. Zool. Scr. 2003, 32, 61–81. [Google Scholar] [CrossRef]
- Rieger, R.M. Comparative ultrastructure and the Lobatocerebridae: Keys to understand the phylogenetic relationship of Annelida and the acoelomates. In The Ultrastructure of Polychaeta; Westheide, W., Hermans, C.O., Eds.; John Wiley & Sons: Stuttgart, Germany, 1988; Volume 4, pp. 373–382. [Google Scholar]
- Tzetlin, A.B.; Filippova, A.V. Muscular system in polychaetes (Annelida). Hydrobiologia 2005, 535/536, 113–126. [Google Scholar] [CrossRef]
- Rieger, R.M. Fine structure of the body wall, nervous system, and digestive tract in the Lobatocerebridae Rieger and the organization of the gliointerstitial system in Annelida. J. Morph. 1981, 167, 139–165. [Google Scholar] [CrossRef]
- Rieger, R.M. Neue Organisationstypen aus der Sandlückenraumfauna: Die Lobatocerebriden und Jennaria pulchra. Verh. Dtsch. Zool. Ges. 1991, 84, 247–259. [Google Scholar]
- Westheide, W. Progenesis as a principle in meiofauna evolution. J. Nat. Hist. 1987, 21, 843–854. [Google Scholar] [CrossRef]
- Westheide, W.; Purschke, G. Leptonerilla diplocirrata, a new genus and species of interstitial polychaetes from the island of Hainan, south China (Nerillidae). Proc. Biol. Soc. Wash. 1996, 109, 586–590. [Google Scholar]
- Worsaae, K.; Nygren, A.; Rouse, G.W.; Giribet, G.; Persson, J.; Sundberg, P.; Pleijel, F. Phylogenetic position of Nerillidae and Aberrantia (Polychaeta, Annelida), analyzed by direct optimization of combined molecular and morphological data. Zool. Scr. 2005, 34, 313–328. [Google Scholar] [CrossRef]
- Boaden, P.J.S. Meganerilla swedmarki, nov. gen., nov. spec., an archiannelid of the family Nerillidae. Arkiv Zool. 1961, 13, 553–559. [Google Scholar]
- Ax, P. Thalassochaetus palpifoliaceus nov. gen., nov. spec. (Archiannelida, Nerillidae), ein marine Verwandter von Troglochaetus beranecki Delachaux. Zool. Anz. 1954, 153, 64–75. [Google Scholar]
- Tzetlin, A.B.; Saphonov, M.V. Trochonerilla mobilis gen. et sp. n., a meiofaunal nerillid (Annelida, Polychaeta) from a marine aquarium in Moscow. Zool. Scr. 1992, 21, 251–254. [Google Scholar] [CrossRef]
- Delachaux, T. Un polychète d’eau douce cavernicole: Troglochaetus beranecki nov. gen. nov. spec. Bull. Soc. Neuchatel. Sci. Nat. 1921, 45, 1–11. [Google Scholar]
- Müller, M.C.M. Aristonerilla: A new genus (Annelida: Polychaeta) with description of Aristonerilla (Micronerilla) brevis comb. nov. from a seawater aquarium. Cah. Biol. Mar. 2002, 43, 131–139. [Google Scholar]
- Kirsteuer, E. Zur Kenntnis der Archianneliden des Roten Meeres. Zool. Anz. 1966, 177, 288–296. [Google Scholar]
- Worsaae, K. Nerillidae Levinsen, 1883. In Handbook of Zoology: Annelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2020; Volume 3. [Google Scholar]
- Müller, M.C.M.; Worsaae, K. CLSM analysis of the phalloidin-stained muscle system in Nerilla antennata, Nerillidium sp. and Trochonerilla mobilis (Polychaeta; Nerillidae). J. Morph. 2006, 267, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Purschke, G. Ultrastructure of nuchal organs in polychaetes (Annelida)—New results and review. Acta Zool. 1997, 78, 123–143. [Google Scholar] [CrossRef]
- Gelder, S.R.; Uglow, R.F. Feeding and gut structure in Nerilla antennata (Annelida: Archiannelida). J. Zool. 1973, 171, 225–237. [Google Scholar] [CrossRef]
- Purschke, G. Anatomy and ultrastructure of ventral pharyngeal organs and their phylogenetic importance in Polychaeta (Annelida). Zoomorphology 1985, 105, 223–239. [Google Scholar] [CrossRef]
- Tzetlin, A.B.; Zarvarzina, E.G.; Saphonov, M.V. Functional morphological analysis of the pharynx in some annelids. Dokl. Akad. Nauk SSSR 1987, 294, 1008–1011. [Google Scholar]
- Tzetlin, A.B.; Purschke, G.; Westheide, W.; Saphonov, M.V. Ultrastructure of enteronephridia and general description of the alimentary canal in Trochonerilla mobilis and Nerillidium troglochaetoides (Polychaeta, Nerillidae). Acta Zool. 1992, 73, 163–176. [Google Scholar] [CrossRef]
- Worsaae, K. Functional morphology of two mud-dwelling meiofana polychaetes of the family Nerillidae (Annelida). In Arctic Biology Field Course, Qeqertarsuaq; University of Copenhagen: Copenhagen, Denmark, 2002; pp. 119–128. [Google Scholar]
- Müller, M.C.M. Das Nervensystem der Polychaeten Untersuchungen an ausgewählten taxa. Ph.D. Thesis, University of Osnabrück, Osnabrück, Germany, 1999; pp. 1–207. [Google Scholar]
- Jouin, C. Sexualité et biologie de la reproduction chez Mesonerilla Remane et Meganerilla Boaden. Cah. Biol. Mar. 1968, 9, 31–52. [Google Scholar]
- Magagnini, G. Reproduction in Nerilla antennata O. Schmidt (Archiannelida Nerillidae): Induction of spawning. Boll. Zool. 1982, 49, 283–286. [Google Scholar] [CrossRef]
- Fransen, M.E. Fine structure of the brooding apparatus of the archiannelid Mesonerilla intermedia: Maternal connections to brooded eggs. Trans. Am. Microsc. Soc. 1983, 102, 25. [Google Scholar] [CrossRef]
- Jouin, C. Mesonerilla biantennata n. sp. nouvelle archiannélide Nerillidae de la region de Roscoff. C. R. Acad. Sci. 1963, 257, 4057–4060. [Google Scholar]
- Jouin, C. Hermaphrodisme chez Nerillidopsis hyalina n. g., n. sp. et chez Nerillidium Remane, Archiannélides. C. R. Acad. Sci. 1966, 263, 412–415. [Google Scholar]
- Jouin, C. Sexualité chez Meganerilla Boaden et Mesonerilla Remane (Archiannélides Nerillidae) et modalités de reproduction chez ce dernier genre. C. R. Acad. Sci. 1967, 265, 150–153. [Google Scholar]
- Jouin, C. Étude morphologique et anatomique de Nerillidopsis hyalina Jouin et de quelques Nerillidium Remane (Archiannelides Nerillidae). Arch. Zool. Exp. Gen. 1967, 108, 97–110. [Google Scholar]
- Jouin, C. Nouvelles données sur Troglochaetus beranecki Delachaux (Archiannelida Nerillidae). Ann. Spéliol. 1973, 28, 575–579. [Google Scholar]
- Jouin, C.; Swedmark, B. Paranerilla limicola n. g., n. sp., Archiannélide Nerillidae du benthos vaseux marin. Cah. Biol. Mar. 1965, 6, 201–218. [Google Scholar]
- Goodrich, E.S. Nerilla an archiannelid. Quart. Journ. Micr. Sci. 1912, 57, 397–425. [Google Scholar]
- Pennak, R.W. A fresh-water archiannelid from the Colorado Rocky Mountains. Trans. Am. Microsc. Soc. 1971, 90, 372–375. [Google Scholar] [CrossRef]
- Pennak, R.W.; Ward, J.V. Interstitial faunal communities of the hyporheic and adjacent groundwater biotopes of a Colorado mountain stream. Arch. Hydrobiol. 1986, 74, 356–396. [Google Scholar]
- Särkkä, J.; Mäkelä, J. Troglochaetus beranecki Delachaux (Polychaeta, Archiannelida) in esker groundwaters of Finland: A new class of limnic animals for northern Europe. Hydrobiologia 1998, 379, 17–21. [Google Scholar] [CrossRef]
- Schmidt, H.; Westheide, W. RAPD-PCR experiments confirm the distinction between three morphologically similar species of Nerilla (Polychaeta: Nerillidae). Zool. Anz. 1998, 236, 277–285. [Google Scholar]
- Worsaae, K. Phylogeny of Nerillidae (Polychaeta, Annelida) as inferred from combined 18S rDNA and morphological data. Cladistics 2005, 21, 143–162. [Google Scholar] [CrossRef]
- Lovén, S. Observations on the Metamorphosis of an Annelide. Ann. Mag. Nat. 1843, 111, 43–45. [Google Scholar] [CrossRef]
- Agassiz, A. On the young stages of a few annelids. Ann. Mag. Nat. Hist. 1867, 19, 203–218. [Google Scholar] [CrossRef]
- Schneider, A. Monographie der Nematoden; Georg Reimer: Berlin, Germany, 1866. [Google Scholar]
- Schneider, A. Über Bau und Entwicklung von Polygordius. Arch. Anat. Physiol. Wiss. Med. 1868, 51–60. [Google Scholar]
- Ramey-Balcı, P.; Fiege, D.; Struck, T.H. Molecular phylogeny, morphology, and distribution of Polygordius (Polychaeta: Polygordiidae) in the Atlantic and Mediterranean. Mol. Phylogenet. Evol. 2018, 127, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Ramey-Balci, P.A.; Fiege, D.; Purschke, G. Polygordiidae Czerniavsky, 1881. In Handbook of Zoology: Annelida, V; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2020; Volume 3. [Google Scholar]
- Ramey, P.A.; Fiege, D.; Leander, B.S. A new species of Polygordius (Polychaeta: Polygordiidae): From the inner continental shelf and in bays and harbours of the north-eastern United States. J. Mar. Biol. Assoc. UK 2006, 86, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Wilkens, V.; Purschke, G. Central nervous system and sense organs, with special reference to photoreceptor-like sensory elements, in Polygordius appendiculatus (Annelida), an interstitial polychaete with uncertain phylogenetic affinities. Invertebr. Biol. 2009, 128, 46–64. [Google Scholar] [CrossRef]
- Rouse, G.W.; Fauchald, K. Cladistics and polychaetes. Zool. Scr. 1997, 26, 139–204. [Google Scholar] [CrossRef]
- Rota, E.; Carchini, G. A new Polygordius (Annelida: Polychaeta) from the Terra Nova Bay, Ross Sea, Antarctica. Polar Biol. 1999, 21, 201–213. [Google Scholar] [CrossRef]
- Avery, L.; Ramey, P.A.; Wilson, R.S. New Polygordiidae (Polychaeta) from the Australian region. Zootaxa 2009, 2068, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, B.G.M.; Rouse, G.W. The spermatozoa of the polychaeta (annelida): An ultrastructural review. Biol. Rev. 1989, 64, 93–157. [Google Scholar] [CrossRef]
- Grassle, J.F.; Ramey, P.A.; Petrecca, R.F. Temporal and spatial variation in infaunal community structure in physically active continental shelf sediments at a long-term ecosystem observatory (LEO-15) off New Jersey, USA. J. Mar. Res. 2009, 67, 869–897. [Google Scholar] [CrossRef]
- Ramey, P.A. Life history and population dynamics of a dominant polychaete, Polygordius jouinae, in inner continental shelf sands of the Mid-Atlantic Bight, USA. Mar. Biol. 2008, 154, 443–452. [Google Scholar] [CrossRef]
- Ramey, P.A.; Bodnar, E. Selection by a deposit-feeding polychaete, Polygordius jouinae, for sands with relatively high organic content. Limnol. Oceanogr. 2008, 54, 1512–1520. [Google Scholar] [CrossRef]
- Martínez, A.; Purschke, G.; Worsaae, K. Protodrilidae Hatschek, 1888. In Handbook of Zoology: Annelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2020; Volume 3. [Google Scholar]
- Zrzavý, J.; Říha, P.; Piálek, L.; Janouškovec, J. Phylogeny of Annelida (Lophotrochozoa): Total-evidence analysis of morphology and six genes. BMC Evol. Biol. 2009, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Czerniavsky, V. Materialia ad Zoographiam Ponticam comparatam Fasc. III. Vermes. Bull. Soc. Nat. Moscou 1881, 55, 213–334. [Google Scholar]
- Hatschek, B. Protodrilus leuckartii. Eine neue Gattung Archianneliden. Arb. Zool. Inst. Univ. Wien. 1881, 3, 79–92. [Google Scholar]
- Hatschek, B. Lehrbuch der Zoologie: Eine Morphologische Übersicht des Thierreiches zur Einführung in das Studium Dieser Wissenschaft; Gustav Fischer: Jena, Germany, 1888. [Google Scholar]
- Uljanina, V.N. Observations on Polygordius living in Sevastopol Bay (In Russian). Bull. Soc. Nat. Moscou 1877, 52, 53–96. [Google Scholar]
- Purschke, G. Structure of the prostomial appendages and the central nervous system in the Protodrilida (Polychaeta). Zoomorphology 1993, 113, 1–20. [Google Scholar] [CrossRef]
- Lam, H.J. Über den Bau und die Verwandschaft der Protoannelis meyeri, nov. gen., nov. spec., eine neue Archiannelide. Tijdschr. Nederl. Dierk. Ver. 1922, 18, 44–84. [Google Scholar]
- Jouin, C. Description of a free-living Polychaete without gut: Astomus taenioides n.gen., n.sp. (Protodrilidae, Archiannelida). Can. J. Zool. 1979, 57, 2448–2456. [Google Scholar] [CrossRef]
- Jouin-Toulmond, C.; Purschke, G. Ultrastructure of the spermatozoa of Parenterodrilus taenioides (Protodrilida: “Polychaeta”) and its phylogenetic significance. Zoomorphology 2004, 123, 139–146. [Google Scholar] [CrossRef]
- Jägersten, G. On the locomotion and attachment of Protodrilus: With remarks on the function of locomotory cilia. Zool. Bidr. Fran Upps. 1954, 31, 315–320. [Google Scholar]
- Martin, G.G. Ciliary gliding in lower invertebrates. Zoomorphology 1978, 91, 249–261. [Google Scholar] [CrossRef]
- Von Nordheim, H. Six new species of Protodrilus (Annelida, Polychaeta) from Europe and New Zealand, with a concise presentation of the genus. Zool. Scr. 1989, 18, 245–268. [Google Scholar] [CrossRef]
- Jouin, C. Anatomical and ultrastructural study of the pharyngeal bulb in Protodrilus (Polychaeta, Archiannelida). I. Muscles and myo-epithelial junctions. Tissue Cell 1978, 10, 269–287. [Google Scholar] [CrossRef]
- Jouin, C. Anatomical and ultrastructural study of the pharyngeal bulb in Protodrilus (Polychaeta, Archiannelida) II. The stomodeal and its cuticle. Tissue Cell 1978, 10, 289–301. [Google Scholar] [CrossRef]
- Pierantoni, U. Genus Protodrilus. Fauna Flora Golf. Neapel. 1908, 31, 1–226. [Google Scholar]
- Pierantoni, U. Organi genitali e glandole salivari nei Protodrili. Boll. Soc. Nat. Napoli 1906, 20, 154–157. [Google Scholar]
- Jägersten, G. Zur Kenntnis der äusseren Morphologie, Entwicklung und Ökologie von Protodrilus rubropharyngeus n. sp. Ark. Zool. 1940, 32A, 1–19. [Google Scholar]
- Jägersten, G. Studies on the morphology, larval development and biology of Protodrilus. Zool. Bidr. Fran Upps. 1952, 29, 427–511. [Google Scholar]
- Von Nordheim, H. Vergleichende Ultrastrukturuntersuchungen der Eu-und Paraspermien von 13 Protodrilus-Arten (Polychaeta, Annelida) und ihre taxonomische und phylogenetische Bedeutung. Helgol. Meeresunters. 1989, 43, 113–156. [Google Scholar] [CrossRef] [Green Version]
- Von Nordheim, H. Ultrastructure and functional morphology of the female reproductive organs in Protodrilus (Polychaeta, Annelida). Helgol. Meeresunters. 1991, 45, 465–485. [Google Scholar] [CrossRef] [Green Version]
- Von Nordheim, H. Ultrastructure and functional morphology of male genital organs and spermatophore formation in Protodrilus (Polychaeta, Annelida). Zoomorphology 1991, 111, 81–94. [Google Scholar] [CrossRef]
- Bailey-Brock, J.H.; Jouin-Toulmond, C.; Brock, R.E. Protodrilidae (Annelida: Polychaeta) from the Hawaiian Islands and Comparison with Specimens from French Polynesia. Pac. Sci. 2010, 64, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Kirsteuer, E. Bredin-Archbold-Smithsonian Biological Survey of Dominica, 3: Marine Archiannelids from Dominica. Proc. U. S. Natl. Mus. 1967, 123, 1–6. [Google Scholar] [CrossRef]
- Pierantoni, U. Sopra un nuovo Protodrilus a’acqua dolce. Monit. Zool. Ital. 1903, 14, 324–327. [Google Scholar]
- Von Nordheim, H. Life histories of subtidal interstitial polychaetes of the families Polygordiidae, Protodrilidae, Nerillidae, Dinophilidae and Diurodrilidae from Helgoland (North Sea). Helgol. Meeresunters. 1984, 38, 1–20. [Google Scholar]
- Di Domenico, M.; Martinez, A.; da Cunha Lana, P.; Worsaae, K. Protodrilus (Protodrilidae, Annelida) from the southern and southeastern Brazilian coasts. Helgol. Meeresunters. 2013, 67, 733–748. [Google Scholar] [CrossRef] [Green Version]
- Kopiy, V. Some aspects of the biology and the present state of the population of Protodrilus flavocapitatus (Polychaeta: Protodrilidae) in the coastal zone of Crimea (the Black Sea). J. Black Sea Medit. Envirn. 2013, 19, 162–168. [Google Scholar]
- Martinez, A.; Eckert, E.M.; Artois, T.; Careddu, G.; Casu, M.; Curini-Galletti, M.; Gazale, V.; Gobert, S.; Ivanenko, V.N.; Jondelius, U.; et al. Human access impacts biodiversity of microscopic animals in sandy beaches. Commun. Biol. 2020, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lagos, A.M.; Leon, M.V.; Quiroga, S.Y.; Martinez, A.; Lagos, A.M.; Leon, M.V.; Quiroga, S.Y. Interstitial annelids from the Caribbean Coast of Colombia. Rev. Biol. Trop. 2018, 66, 658–673. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.; Gonzalez, B.C. Volcanic Anchialine Habitats of Lanzarote. In Cave Ecology; Analysis and Synthesis; Springer: Cham, Switzerland, 2018; Volume 235, pp. 399–414. [Google Scholar]
- Martinez, A.; Gonzalez, B.C.; Worsaae, K.; Wilkens, H.; Núñez, J.; Oromi, P.; Iliffe, T.M. Guide to the Anchialine Ecosystems of Túnel de la Atlántida; Medio Ambiente, Cabilde de Lanzarote: Lanzarote, Spain, 2016. [Google Scholar]
- Braby, C.E.; Rouse, G.W.; Johnson, S.B.; Jones, W.J.; Vrijenhoek, R.C. Bathymetric and temporal variation among Osedax boneworms and associated megafauna on whale-falls in Monterey Bay, California. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 1773–1791. [Google Scholar] [CrossRef]
- Langerhans, P. Über einige kanarische Anneliden. Nov. Act. Leop. 1881, 42, 93–124. [Google Scholar]
- Armenante, Z. Protodrilus hypoleucus n. sp. Monit. Zool. Ital. 1903, 14, 221–222. [Google Scholar]
- Jägersten, G. Die Abhängigkeit der Metamorphose vom Substrat des Biotops bei Protodrilus. Ark. Zool. 1940, 32, 1–12. [Google Scholar]
- Aiyar, G.; Alikunhi, K.H. On some archiannelids of the Madras coast. Proc. Indian Acad. Sci. Sect. B 1943, 67, 113–140. [Google Scholar]
- Alikunhi, K.H. On some archiannelids of the Krusadai Island. Proc. Nat. Inst. Sci. India 1948, 14, 373–383. [Google Scholar]
- Wieser, W. Archiannelids from the intertidal of Puget Sound. Trans. Am. Microsc. Soc. 1957, 76, 275–285. [Google Scholar] [CrossRef]
- Jouin, C. Archiannélides Interstitielles de Nouvelle-Caledónie. Expédition Francaise sur les Récifs Coralliens de la Nouvelle-Calédonie 1970, 4, 147–167. [Google Scholar]
- Von Nordheim, H. Systematics and ecology of Protodrilus helgolandicus sp.n., an Interstitial polychaete (Protodrilidae) from subtidal sands off Helgoland, German Bight. Zool. Scr. 1983, 12, 171–177. [Google Scholar] [CrossRef]
- Giard, M.A. Sur une faunule caractéristique des sables a diatomées d’ambleteuse (Pas-de-Calais). C. R. Seances Soc. Biol. Fil. 1904, 56, 295–298. [Google Scholar]
- Remane, A. Protodrilidae aus Ost- und Nordsee. Zool. Anz. 1926, 67, 119–125. [Google Scholar]
- Purschke, G.; Jouin, C. Anatomy and ultrastructure of the ventral pharyngeal organs of Saccocirrus and Protodriloides with remarks on the phylogenetic relationships within the Protodrilida (Annelida: Polychaeta). J. Zool. 1988, 215, 405–432. [Google Scholar] [CrossRef]
- Jouin, C. Morphologie et anatomie comparee de Protodrilus chaetifer Remane et Protodrilus symbioticus Giard; creation du nouveau genre Protodriloides (Archiannelides). Cah. Biol. Mar. 1966, 7, 139–155. [Google Scholar]
- Purschke, G.; Müller, M.C.M. Structure of prostomial photoreceptor-like sense organs in Protodriloides species (Polychaeta, Protodrilida). Cah. Biol. Mar. 1996, 37, 205–219. [Google Scholar]
- Martínez, A.; Worsaae, K.; Purschke, G. Protodriloididae Purschke & Jouin, 1988. In Handbook of Zoology; Annelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2020; Volume 3. [Google Scholar]
- Fransen, M.E. Ultrastructure of coelomic organization in annelids I. Archiannelids and other small polychaetes. Zoomorphology 1980, 95, 235–249. [Google Scholar] [CrossRef]
- Swedmark, B. Étude du développement larvaire et remarques sur la morphologie de Protodrilus symbioticus Giard (Archiannelides). Ark. Zool. 1954, 6, 511–522. [Google Scholar]
- Gray, J.S. The behaviour of Protodrilus symbioticus (Giard) in temperature gradients. J. Anim. Ecol. 1965, 34, 455–461. [Google Scholar] [CrossRef]
- Gray, J.S. Factors controlling the localizations of populations of Protodrilus symbioticus Giard. J. Anim. Ecol. 1966, 35, 435. [Google Scholar] [CrossRef]
- Gray, J.S. The response of Protodrilus symbioticus (Giard) (Archiannelida) to light. J. Anim. Ecol. 1966, 35, 55–64. [Google Scholar] [CrossRef]
- Sterrer, W. Paranerilla limicola Jouin & Swedmark (Archiannelida) von der Norwegischen und Adriatischen Küste. Sarsia 2011, 36, 65–68. [Google Scholar]
- Boaden, P.J.S. Soft meiofauna of sand from the Delta region of the Rhine, Meuse and Scheldt. Neth. J. Sea Res. 1976, 10, 461–471. [Google Scholar] [CrossRef]
- Besteiro, C.; Eugénio, W.; Carvalho, L.H.; Veiga, P.; Rubal, M. Contributions to the autoecology and biogeography of some psammic species of Annelida Polychaeta from Galicia (North-West Iberian Peninsula). Cah. Biol. Mar. 2016, 57, 253–259. [Google Scholar]
- Hamond, R. The non-polychaetous annelids of Norfolk, England, with additional notes on polychaetes. Cah. Biol. Mar. 1972, 13, 341–350. [Google Scholar]
- Westheide, W. La faune des polychètes et des archiannelides dans les plages sableuses a ressac de la cote Méditerranéenne de la Tunisie. Bull. Inst. Oceanogr. Peche 1972, 2, 449–468. [Google Scholar]
- Boaden, P.J.S. 1962 Colonization of graded sand by an interstitial fauna. Cah. Biol. Mar. 1962, 3, 245–248. [Google Scholar]
- Jouin, C. Le développement larvaire de Protodrilus chaetifer Remane (Archiannelida). C. R. Acad. Sci. Paris 1962, 255, 3065–3067. [Google Scholar]
- Bellan, G. Remarques au sujet de la faune annélidienne “épibiote mobile” de quelques biotopes marins des côtes de Provence. Rapp. Proc. Verb. Réun. 1965, 18, 93–98. [Google Scholar]
- Renaud-Debyser, J. Note sur la faune interstitielle du bassin d“Arcachon et description d”un gastrotriche nouveau. Cah. Biol. Mar. 1964, 5, 111–123. [Google Scholar]
- Westheide, W. Zur Polychaetenfauna des Eulitorals der Nordseeinsel Sylt. Helgol. Meeresunters. 1966, 13, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, R.M.; Nørrevang, A. Description of Psammodrilus aedificator sp.n. (Polychaeta), with notes on the Arctic Interstitial Fauna of Disko Island, W. Greenland. Zool. Scr. 1982, 11, 265–279. [Google Scholar] [CrossRef]
- Mastepanova, E.A. Interstitial polychaetes of the seas of Russia. Zool. Bespozvon. 2004, 1, 59–64. [Google Scholar]
- Priyalakshmi, G.; Menon, N.R. Sediment transport and bioinvasion—Possible impact of Tsunami—Protodriloides chaetifer an example. J. Mar. Biol. Ass. India 2007, 49, 86–88. [Google Scholar]
- Swedmark, B. Psammodriloides fauveli n. gen. n. sp. et la famille des Psammodrilidae (Polychaeta, Sedentaria). Arkiv. Zool. 1958, 2, 55–65. [Google Scholar]
- Bartolomaeus, T.; Meyer, K. Development and phylogenetic significance of hooked setae in Arenicolidae (Polychaeta, Annelida). Invertebr. Biol. 1997, 116, 227. [Google Scholar] [CrossRef]
- Rouse, G.; Pleijel, F. Polychaetes; Oxford Univdersity Press: Oxford, UK, 2001. [Google Scholar]
- Parry, L.A.; Edgecombe, G.D.; Eibye-Jacobsen, D.; Vinther, J. The impact of fossil data on annelid phylogeny inferred from discrete morphological characters. Proc. R. Soc. B 2016, 283, 20161378–9. [Google Scholar] [CrossRef] [Green Version]
- Worsaae, K.; Sterrer, W. Description of two new interstitial species of Psammodrilidae (Annelida) from Bermuda. Mar. Biol. Res. 2006, 2, 431–445. [Google Scholar] [CrossRef]
- Worsaae, K.; Kvindebjerg, K.; Martinez, A. Morphology of a new interstitial Psammodrilus (Psammodrilidae, Annelida) from Sardinia, Italy. Zool. Anz. 2015, 259, 13–21. [Google Scholar] [CrossRef]
- Worsaae, K. Psammodrilidae Swedmark, 1952. In Handbook of Zoology; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2019; pp. 143–155. [Google Scholar]
- Swedmark, B. Recherches sur la morphologie le developpement et la biologie de Psammodrilus balanoglossoides, polychète sedentaire de la microfaune des sables. Arch. Zool. Exp. Gén. 1955, 92, 141–220. [Google Scholar]
- Tzetlin, A.B.; Saphonov, M.V. Interstitial polychaetes (Annelida) from the Kandalaksha Bay of the White Sea. Zool. Zhurnal. 2002, 81, 899–908. [Google Scholar]
- Brown, R. Saccocirridae (Annelida: Archiannelida) from the central coast of New South Wales. Austr. J. Mar. Freshw. Res. 1981, 32, 439–456. [Google Scholar] [CrossRef]
- Purschke, G. Ultrastructural investigation of presumed photoreceptive organs in two Saccocirrus species (Polychaeta, Saccocirridae). J. Morphol. 1992, 211, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Jouin-Toulmond, C.; Gambi, C. Description of Saccocirrus goodrichi sp. nov. (Annelida: Polychaeta: Saccocirridae), a new Mediterranean species and new data on the chaetae of S. papillocercus and S. Major. Cah. Biol. Mar. 2007, 48, 381–390. [Google Scholar]
- Di Domenico, M.; Martínez, A.; Worsaae, K. Saccocirridae (Annelida) from the Canary Islands with a description of Saccocirrus slateri sp. nov. Mar. Biodivers. 2019, 49, 2125–2139. [Google Scholar] [CrossRef]
- Weigert, A.; Bleidorn, C. Current status of annelid phylogeny. Org. Divers. Evol. 2016, 16, 345–362. [Google Scholar] [CrossRef]
- Jouin, C. Status of the knowledge of the systematics and ecology of Archiannelida. Smithson. Contr. Zool. 1971, 76, 47–56. [Google Scholar]
- Di Domenico, M.; Martínez, A.; Amaral, A.C.Z.; Lana, P.C.; Worsaae, K. Saccocirridae (Annelida) from the southern and southeastern Brazilian coasts. Mar. Biodivers. 2014, 44, 313–325. [Google Scholar] [CrossRef]
- Goodrich, E.S. On the structure and affinities of Saccocirrus. Q. J. Microsc. Sci. 1901, 44, 413–428. [Google Scholar]
- Salensky, W. Morphogenetische Studien an Würmern. Mem. Acad. Sci. St. Petersbourg 1907, 19, 1–349. [Google Scholar]
- Brown, S.; Rouse, G.; Hutchings, P.; Colgan, D. Assessing the usefulness of histone H3, U2 snRNA and 28S rDNA in analyses of polychaete relationships. Aust. J. Zool. 1999, 47, 499–516. [Google Scholar] [CrossRef]
- Purschke, G.; Tzetlin, A.B. Dorsolateral ciliary folds in the polychaete foregut: Structure, prevalence and phylogenetic significance. Acta Zool. 1996, 77, 33–49. [Google Scholar] [CrossRef]
- Tzetlin, A.B.; Purschke, G. Pharynx and intestine. Hydrobiologia 2005, 535/536, 199–225. [Google Scholar] [CrossRef]
- Du Bois-Reymond Marcus, E. On a new archiannelid, Saccocirrus gabriellae, from Brazil. Com. Zool. Mus. 1946, 37, 1–11. [Google Scholar]
- Villora-Moreno, S.; Capaccioni-Azzati, R.; Garcia-Carrascosa, A.M. Meiobenthos of sandy beaches from the Gulf of Valencia (Western Mediterranean): Ecology of interstitial polychaetes. Bull. Mar. Sci. 1991, 48, 376–385. [Google Scholar]
- Di Domenico, M.; Lana, P.C.; Garraffoni, A.R.S. Distribution patterns of interstitial polychaetes in sandy beaches of southern Brazil. Mar. Ecol. 2009, 30, 47–62. [Google Scholar] [CrossRef]
- Gray, J.S.A. A new species of Saccocirrus (Archiannelida) from the West Coast of North America. Pac. Sc. 1969, 23, 238–251. [Google Scholar]
- Bobretzky, N.V. Saccocirrus papillocerus, n. gen., n. sp. Tip’ novago semeistra annelid. Sravmitel’no-Anatomichskii obcherk. Kiev Odschestva Estest Zap. 1871, 2, 211–259. [Google Scholar]
- Pierantoni, U. Observazioni suilo sviluppo embrionale e larvale del Saccocirrus papillocercus Bobr. Mitt. Zool. Stat. Neapel 1906, 18, 46–72. [Google Scholar]
- Pierantoni, U. Il genere Saccocirrus Bobretzky e le sue specie. Ann. Ist. Mus. Zool. Univ. Napoli 1907, 2, 1–11. [Google Scholar]
- Boaden, P.J.S. The interstitial fauna of some North Wales beaches. J. Mar. Biol. Assoc. UK 1963, 43, 79–96. [Google Scholar] [CrossRef]
- Núñez, J.; Brito, M.C.; Docoito, J.R. Anélidos poliquetos de Canarias: Catálogo de especies, distribución y hábitats. Vieraea 2005, 33, 297–321. [Google Scholar]
- Bailey-Brock, J.H.; Dreyer, J.; Brock, R.E. Three new species of Saccocirrus (Polychaeta: Saccocirridae) from Hawai’i. Pac. Sc. 2003, 57, 463–478. [Google Scholar] [CrossRef] [Green Version]
- Di Domenico, M.; Worsaae, K.; Purschke, G. Saccocirridae Czerniavsky, 1881. In Handbook of Zoology: Annelida; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2020; Volume 3. [Google Scholar]















| Species of Diurodrilus | Intertidal (I)/Subtidal (S) | Primary Toes Longer than Secondary (2°) Toes | Shape of Primary Toes | Shape of Secondary Toes | Anal Cone | Ciliophores on Anterior Head | Dorsal Cuticular Plates |
|---|---|---|---|---|---|---|---|
| D. ankeli | I | yes | cylindrical | cone shaped | absent | 4 pairs + 1 | present |
| D. benazzii | I | no 2° toes | bottle-shaped | no 2° toes | absent | 3 pairs | absent |
| D. dohrni | I + S | yes | bottle-shaped | cone shaped | absent | unknown | unknown |
| D. kunii | I | yes | cylindrical | cone shaped | absent | 3 pairs | absent |
| D. minimus | I + S | equal | cylindrical | cone shaped | small | unknown | absent |
| D. subterrraneus | I | yes, slightly | cone shaped | cone shaped | absent | 5 pairs | present |
| D. westheidei | S | yes | cylindrical | cylindrical | large | 3 pairs | absent |
| Genus | # segm | Palp Shape | Lateral Antennae | Median Ant | Chaetae | Chaetae segm I | IC segm I | Other segm with IC | IC Shape | Pygidial cirri | Eyes | Reproduction | Spermioduct Openings (* Fused) in segm # | # of Species |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nerilla | 9 | short-club | long-ann | long-ann | capil | + | long-ann | 2–8 | short or long-cf | long ann | 0 or 4 pigm | gono | 6 and 7 and 8 * | 11 |
| Meganerilla | 9 | long-cf | 0 | 0 or short | capil | + | +/− | 2–9 | short- leaf-s | short leaf-s or long-cf | 0 or 2 | gono | 7 | 5 |
| Mesonerilla ”luederitzi- intermedia” group | 9 | club | long | long | comp | + | + | 2–9 | long- bottle-s | long-cf | 0 or 2 cil | gono (+/- br-hood) | 5 * or 5 * and 6 * | 10 |
| Mesonerilla ”biantennata” group | 9 | club | short | 0 | comp | + | + | 2–9 | short-cf | short-bottle-s | 0 | gono | 5 * | 2 |
| Mesonerilla ”roscovita” group | 9 | club | long | short | comp | +/− | + | 2–9 | short-cf | long-cf, long-bottle-s | 0 | herma | 6 and 7 | 3 |
| Leptonerilla | 9 | club | long | long | comp | + | + | 2–9, | long-cf, double | long-cf | 2 pigm | gono | 8 | 3 |
| Speleonerilla isa | 9 | very long-cf | short | short | comp | + | +, rudi | 2–9 + nc-lobes | short, bottle-s | long-cf + cil. lobes | 0 | herma | 6 * and 7* | 1 |
| Speleonerilla (excl. S. isa) | 8 | very long-cf | short | short | comp | + | −/+, rudi | 3–8 + nc-lobes | short, bottle-s | long-cf + cil. lobes | 0 | herma | 7 | 3 |
| Genus 1 Japan** | 8 | long-cf | long | long | comp | + | + | 2–9 | long-cf | long-cf | 0 | gono | 7 and 8 | - |
| Genus 2 Japan** | 8 | long-cf | long | long | comp | + | − | 2–7? | very long-cf | long-cf? | 0 | herma | 7 | - |
| Micronerilla | 8 | club | long-wr | long-wr | comp | + | − | 2–7 | long-cf, +/− double | long-cf-wr | 2 pigm | gono | 7 and 8 | 1 |
| Thalassochaetus | 8 | club | 0 | 0 | comp | + | − | 2–7 | rudi | short- bottle-s | 0 | ? | ? | 1 |
| Trochonerilla | 8 | club | short | short | capil | + | − | 2–7 | long-cf | long-cf-wr | 2 pigm | gono | 7 and 8 | 1 |
| Troglochaetus | 8 | club | 0 | 0 | capil | +/− | +/− | 2–7 or 2–8 | rudi | short lobes or bulbs | 0 | herma | 6 * | 2 |
| Nerillidium | 8 | club | short- medium | 0 | capil | + | +/− | 2–7 | rudi or short-cf | long-cf or bottle-s | 0 or 2 mv | herma | 6 or 6 * | 10 |
| Nerillidopsis | 8 | long-cf | long | long | capil and comp | + | − | 2–7 | long-cf | long-cf | 0 | herma | 6 * | 1 |
| Aristonerilla | 7 | club | long-wr | long-wr | comp | + | − | 2–7 | long-cf | long-cf-wr | 2 red-black | gono | 7 | 1 |
| Psammoriedlia | 7 | club | 0 | 0 | capil | + | + | 2–6/7 | short-cf | long-cf | 0 | ? | ? | 2 |
| Paranerilla | 7 | lateral horns | 0 | 0 | capil | + | + | 2–7 | rudi | long-cf | 0 | gono | 5 | 2 |
| Species | Locality | Max. Body Length (mm) | Max. Body Width (mm) | Prostomial Sacs | Pharyngeal Musculature | Polygonal Collar Cells | Thoracic Cirri (Pairs) | Max. # Abdom. Segm. | Max. # Uncini/ Ramus |
|---|---|---|---|---|---|---|---|---|---|
| P. aedificator | Disko, W Greenland | 8.1 | 0.19 | Present | Well developed | ca. 10 rows | 3 long, 3 short | 20 | 3(5) |
| P. balanoglossoides | North Atlantic; White Sea; New Zealand | 5–6 | 0.15 | Present | Well developed | >25 rows | 2 long, 1 medium, 3 short | 31 | 16 |
| P. didomenicoi | Napoli, Italy | 1.9 | 0.13 | Present | Well developed | ca. 7–8 rows | 2 short, 3 rud. | 11 | 5 |
| P. fauveli | NE Atlantic | 1 | 0.13 | Absent | Absent | absent | 3 medium, 1 short, 2 rud. or none | 10 | 1 |
| P. moebjergi | Bermuda | 2.2 | 0.11 | Present | Absent | 2 lateral clusters | 2 medium, 1 short, 3 rud. | 11 | 4 |
| P. norenburgi | Bocas del Toro, Panama | 3.5 | 0.18 | Present | Well developed | ca. 25 rows | 3 long (or 2 long + 1 medium), 2–3 short | ? | 5 |
| P. swedmarki | Bermuda | 1.6 | 0.06 | Absent | Absent | Absent | 3 long, 1–2 rud. or none | 7 | 2 |
| Species | Max L. (mm) | Max W. (µm) | Max # Segm. | Max # pyg. adh. Ridges | Pharyngeal Bulb | Gonads Fem. | # Fertile Segm. | Gonads Males | Ciliary Groove | Ciliary Patches Mouth | Longest Chaetae | Prongs Length |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saccocirrus | ||||||||||||
| S. slateri | 25 | 730 | 155 | 22 | absent | bilateral | 72 | bilateral | absent | absent | forked | equal |
| S. papilocercus | 30 | 400 | 150 | 8 | absent | bilateral | 120 | bilateral | absent | absent | forked | equal |
| S. major | 70 | 1000 | 200 | 14 | absent | bilateral | 175 | bilateral | absent | absent | forked | equal |
| S. minor | 15 | 200 | 100 | absent | absent | bilateral | 40 | bilateral | absent | absent | forked | equal |
| S. orientalis | 12 | ? | 170 | 4 | absent | bilateral | 60 | bilateral | absent | absent | forked | equal |
| S. pussicus | 30 | 400 | 120 | 12 | absent | bilateral | 36 | bilateral | absent | absent | forked | unequal |
| S. heterochaetus | 9 | 300 | 74 | absent | absent | bilateral | 20 | bilateral | absent | absent | forked | unequal |
| S. parvus | 3 | 280 | 70 | absent | absent | bilateral | ? | bilateral | absent | absent | forked | unequal |
| S. oahuensis | 10.5 | 400 | 119 | 6 | absent | bilateral | ? | bilateral | absent | absent | forked | unequal |
| S. waianaensis | 10 | 450 | 210 | absent | absent | bilateral | ? | bilateral | absent | absent | forked | unequal |
| S. cirratus | 45 | ? | 200 | absent | present | bilateral | 115 | bilateral | absent | present | lyrid | unequal |
| Pharyngocirrus | ||||||||||||
| P. archiboldi | 6 | 200 | 84 | absent | present | ? | ? | ? | absent | present | spatulated | ? |
| P. krusadensis | 25 | 400 | 150 | 9 | present | left | 80 | left | absent | present | lyrid | unequal |
| P. gabriellae | 30 | 400 | 160 | 15 | present | right | 100 | left | present | present | lyrid | equal |
| P. eroticus | 22 | 300 | 125 | 22 | present | right | 110 | left | present | present | lyrid | equal |
| P. labilis | 14 | 250 | 133 | 9 | present | left | 100 | left | present | present | lyrid | unequal |
| P. sonomacus | 25 | 330 | 140 | 12 | present | right | 55 | left | absent | present | lyrid | equal |
| P. jouinae | 20 | 550 | 120 | ? | present | unilateral | ? | unilateral | present | present | lyrid | unequal |
| P. tridentiger | 20 | 600 | 100 | 14 | present | unilateral | ? | unilateral | to segm. 8 | present | lyrid | unequal |
| P. uchidai | 20 | 350 | 146 | 23 | present | left | 100 | left | present | present | lyrid | equal |
| P. goodrichi | 15 | 300 | 130 | 7 | present | left | 35 | unilateral | absent | absent | lyrid | unequal |
| P. alanhongi | 3.4 | 300 | 47 | 6 | present | unilateral | ? | unilateral | segm.1 to 3 | present | lyrid | unequal |
| P. burchell | 20 | 1000 | 200 | 10 | present | unilateral | 180 | unilateral | absent | present | absent? | unequal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worsaae, K.; Kerbl, A.; Domenico, M.D.; Gonzalez, B.C.; Bekkouche, N.; Martínez, A. Interstitial Annelida. Diversity 2021, 13, 77. https://doi.org/10.3390/d13020077
Worsaae K, Kerbl A, Domenico MD, Gonzalez BC, Bekkouche N, Martínez A. Interstitial Annelida. Diversity. 2021; 13(2):77. https://doi.org/10.3390/d13020077
Chicago/Turabian StyleWorsaae, Katrine, Alexandra Kerbl, Maikon Di Domenico, Brett C. Gonzalez, Nicolas Bekkouche, and Alejandro Martínez. 2021. "Interstitial Annelida" Diversity 13, no. 2: 77. https://doi.org/10.3390/d13020077
APA StyleWorsaae, K., Kerbl, A., Domenico, M. D., Gonzalez, B. C., Bekkouche, N., & Martínez, A. (2021). Interstitial Annelida. Diversity, 13(2), 77. https://doi.org/10.3390/d13020077









