Genetic Distinctiveness but Low Diversity Characterizes Rear-Edge Thuja standishii (Gordon) Carr. (Cupressaceae) Populations in Southwest Japan
Abstract
1. Introduction
2. Materials and Methods
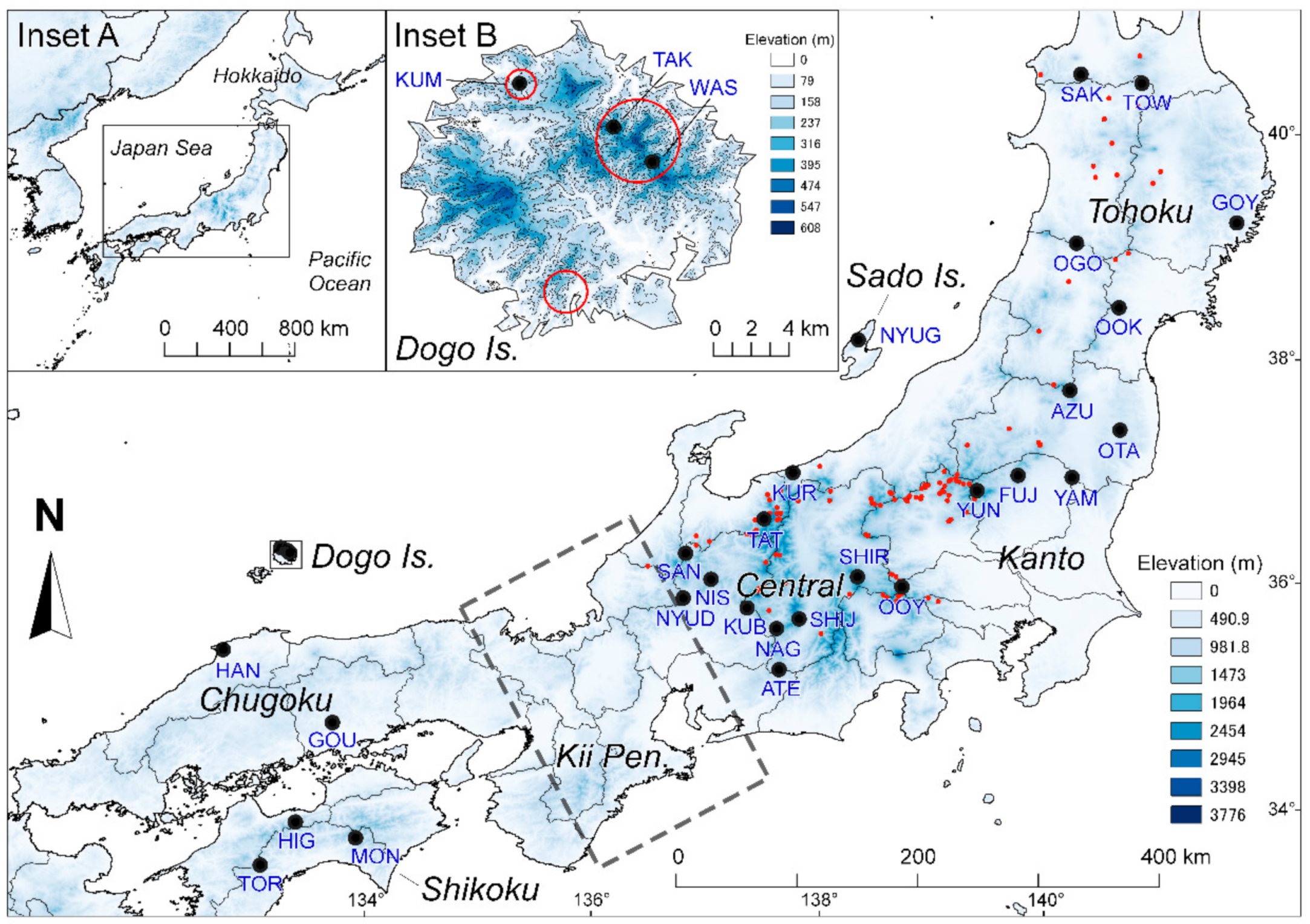
2.1. Population Sampling and Identification of Clones
2.2. Laboratory Work and Genotyping
2.3. Data Integrity
2.4. Isolation by Distance and Environment
2.5. Range-Wide Genetic Structure
2.6. Genetic Diversity and Bottlenecks
2.7. Past Demographic Change and Estimates of Migration
2.8. Species Distribution Modelling
3. Results
3.1. Data Integrity
3.2. Isolation by Distance and Environment
3.3. Range-Wide Genetic Structure
3.4. Genetic Diversity and Bottlenecks
3.5. Past Demographic Change and Estimates of Migration
3.6. Species Distribution Modelling
4. Discussion
4.1. High Diversity and Range Dynamics in Northeast Japan
4.2. Genetic Distinctiveness and Biogeographic History of Rear-Edge Populations in Shikoku and Chugoku
4.3. Distinct Genetic Lineage of T. standishii on Dogo Island
4.4. Conservation Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Provan, J.; Maggs, C.A. Unique genetic variation at a species’ rear edge is under threat from global climate change. Proc. R. Soc. B Boil. Sci. 2011, 279, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Vilà-Cabrera, A.; Premoli, A.C.; Jump, A.S. Refining predictions of population decline at species’ rear edges. Glob. Chang. Biol. 2019, 25, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Haond, S.; Teixeira, S.; Massa, S.I.; Billot, C.; Saenger, P.; Coupland, G.; Duarte, C.M.; Serrao, E. Genetic structure at range edge: Low diversity and high inbreeding in Southeast Asian mangrove (Avicennia marina) populations. Mol. Ecol. 2006, 15, 3515–3525. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef]
- Morales-Molino, C.; Tinner, W.; Perea, R.; Carrión, J.S.; Colombaroli, D.; Valbuena-Carabaña, M.; Valbuena-Carabaña, M.; Zafra, E.; Gil, L. Unprecedented herbivory threatens rear-edge populations of Betula in southwestern Eurasia. Ecology 2019, 100, e02833. [Google Scholar] [CrossRef]
- Lepais, O.; Muller, S.D.; Saad-Limam, S.B.; Benslama, M.; Rhazi, L.; Belouahem-Abed, D.; Gammar, A.M.; Ghrabi-Gammar, Z.; Valbuena-Carabaña, M.; Zafra, E.; et al. High genetic diversity and distinctiveness of rear-edge climate relicts maintained by ancient tetraploidisation for Alnus glutinosa. PLoS ONE 2013, 8, e75029. [Google Scholar] [CrossRef]
- Zardi, G.I.; Nicastro, K.R.; Serrão, E.A.; Jacinto, R.; Monteiro, C.A.; Pearson, G.A. Closer to the rear edge: Ecology and genetic diversity down the core-edge gradient of a marine macroalga. Ecosphere 2015, 6, 1–25. [Google Scholar] [CrossRef]
- Woolbright, S.A.; Whitham, T.G.; Gehring, C.A.; Allan, G.J.; Bailey, J.K. Climate relicts and their associated communities as natural ecology and evolution laboratories. Trends Ecol. Evol. 2014, 29, 406–416. [Google Scholar] [CrossRef]
- Pironon, S.; Papuga, G.; Villellas, J.; Angert, A.L.; García, M.B.; Thompson, J.D. Geographic variation in genetic and demographic performance: New insights from an old biogeographical paradigm. Biol. Rev. 2016, 92, 1877–1909. [Google Scholar] [CrossRef]
- Diekmann, O.E.; Serrão, E.A. Range-edge genetic diversity: Locally poor extant southern patches maintain a regionally diverse hotspot in the seagrass Zostera marina. Mol. Ecol. 2012, 21, 1647–1657. [Google Scholar] [CrossRef]
- Tzedakis, P.C.; Lawson, I.T.; Frogley, M.R.; Hewitt, G.M.; Preece, R.C. Buffered tree population changes in a Quaternary refugium: Evolutionary implications. Science 2002, 297, 2044–2047. [Google Scholar] [CrossRef]
- Erichsen, E.O.; Budde, K.B.; Sagheb-Talebi, K.; Bagnoli, F.; Vendramin, G.G.; Hansen, O.K. Hyrcanian forests-Stable rear-edge populations harbouring high genetic diversity of Fraxinus excelsior, a common European tree species. Divers. Distrib. 2018, 24, 1521–1533. [Google Scholar] [CrossRef]
- Takahashi, T.; Tani, N.; Taira, H.; Tsumura, Y. Microsatellite markers reveal high allelic variation in natural populations of Cryptomeria japonica near refugial areas of the last glacial period. J. Plant Res. 2005, 118, 83–90. [Google Scholar] [CrossRef]
- Gugger, P.F.; González-Rodríguez, A.; Rodríguez-Correa, H.; Sugita, S.; Cavender-Bares, J. Southward Pleistocene migration of Douglas-fir into Mexico: Phylogeography, ecological niche modeling, and conservation of ‘rear edge’ populations. New Phytol. 2011, 189, 1185–1199. [Google Scholar] [CrossRef]
- Hampe, A.; Bairlein, F. Modified dispersal-related traits in disjunct populations of bird-dispersed Frangula alnus (Rhamnaceae): A result of its Quaternary distribution shifts? Ecography Cop. 2000, 23, 603–613. [Google Scholar] [CrossRef]
- Stojnić, S.; Suchocka, M.; Benito-Garzón, M.; Torres-Ruiz, J.M.; Cochard, H.; Bolte, A.; Cocozza, C.; Cvjetković, B.; De Luis, M.; Martínez-Vilalta, J.; et al. Variation in xylem vulnerability to embolism in European beech from geographically marginal populations. Tree Physiol. 2018, 38, 173–185. [Google Scholar] [CrossRef]
- Fuentes-Utrilla, P.; Valbuena-Carabaña, M.; Ennos, R.; Gil, L. Population clustering and clonal structure evidence the relict state of Ulmus minor Mill. in the Balearic Islands. Heredity 2014, 113, 21–31. [Google Scholar] [CrossRef]
- Migliore, J.; Baumel, A.; Juin, M.; Fady, B.; Roig, A.; Duong, N.; Médail, F. Surviving in mountain climate refugia: New insights from the genetic diversity and structure of the relict shrub Myrtus nivellei (Myrtaceae) in the Sahara Desert. PLoS ONE 2013, 8, e73795. [Google Scholar] [CrossRef][Green Version]
- Takahara, H.; Sugita, S.; Harrison, S.P.; Miyoshi, N.; Morita, Y.; Uchiyama, T. Pollen-based reconstructions of Japanese biomes at 0, 6000 and 18,000 14C yr bp. J. Biogeogr. 2000, 27, 665–683. [Google Scholar] [CrossRef]
- Worth, J.R.P. Current distribution and climatic range of the Japanese endemic conifer Thuja standishii (Cupressaceae). Bull. FFPRI 2019, 18, 275–288. [Google Scholar]
- Gansert, D. Treelines of the Japanese Alps – altitudinal distribution and species composition under contrasting winter climates. Flora-Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 143–156. [Google Scholar] [CrossRef]
- Tsumura, Y.; Suyama, Y. Seedling Transfer Guideline of Japanese Tree Spcies; Bun-ichi Co. Ltd.: Tokyo, Japan, 2015. (In Japanese) [Google Scholar]
- Kikuchi, R.; Jae-Hong, P.; Takahashi, H.; Maki, M. Disjunct distribution of chloroplast DNA haplotypes in the understory perennial Veratrum album ssp. oxysepalum (Melanthiaceae) in Japan as a result of ancient introgression. New Phytol. 2010, 188, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Kaneko, Y.; Ito, S.; Yamanaka, K.; Sakio, H.; Hoshizaki, K.; Suzuki, W.; Yamanaka, N.; Setoguchi, H. Phylogeography of Japanese horse chestnut (Aesculus turbinata) in the Japanese Archipelago based on chloroplast DNA haplotypes. J. Plant Res. 2010, 124, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Higashi, H.; Sakaguchi, S.; Ikeda, H.; Isagi, Y.; Setoguchi, H. Multiple introgression events and range shifts in Schizocodon (Diapensiaceae) during the Pleistocene. Bot. J. Linn. Soc. 2013, 173, 46–63. [Google Scholar] [CrossRef]
- Iwasaki, T.; Aoki, K.; Seo, A.; Murakami, N. Comparative phylogeography of four component species of deciduous broad-leaved forests in Japan based on chloroplast DNA variation. J. Plant Res. 2011, 125, 207–221. [Google Scholar] [CrossRef]
- Tsukada, M. Cryptomeria Japonica: Glacial refugia and Late-Glacial and postglacial migration. Ecology 1982, 63, 1091–1105. [Google Scholar] [CrossRef]
- Takahara, H.; Kitagawa, H. Vegetation and climate history since the last interglacial in Kurota Lowland, western Japan. Palaeogeogr. Palaeoclim. Palaeoecol. 2000, 155, 123–134. [Google Scholar] [CrossRef]
- Suzuki, K. Picea cone-fossils from Pleistocene strata of northeast Japan. Saito Ho-on Kai Mus. Nat. Hist. Res. Bull. 1991, 59, 1–41. [Google Scholar]
- Kamoi, Y.; Saito, M.; Fujita, H.; Kobayashi, I. Plant fossil assemblage of the Last Glacial age in the northern part of Niigata Prefecture, Central Japan. Quat. Res. (Daiyonki-Kenkyu) 1988, 27, 21–29. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research--an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Murray, M.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef]
- Worth, J.R.P.; Chang, K.S.; Ha, Y.-H.; Qin, A. Development of microsatellite markers for the Japanese endemic conifer Thuja standishii and transfer to other East Asian species. BMC Res. Notes 2019, 12, 1–5. [Google Scholar] [CrossRef]
- Raymond, M.; Rousset, F. GENEPOP (Version 1.2): Population Genetics Software for Exact Tests and Ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Dempster, A.P.; Laird, N.M.; Rubin, D.B. Maximum likelihood from incomplete data via the EM algorithm. J. R. Stat. Soc. Ser. B 1977, 39, 1–22. [Google Scholar]
- Chapuis, M.-P.; Estoup, A. Microsatellite Null Alleles and Estimation of Population Differentiation. Mol. Biol. Evol. 2006, 24, 621–631. [Google Scholar] [CrossRef]
- Ellis, J.R.; Burke, J.M. EST-SSRs as a resource for population genetic analyses. Heredity 2007, 99, 125–132. [Google Scholar] [CrossRef]
- Tiffin, P.; Hahn, M.W. Coding sequence divergence between two closely related plant species: Arabidopsis thaliana and Brassica rapa ssp. pekinensis. J. Mol. Evol. 2002, 54, 746–753. [Google Scholar] [CrossRef]
- Pettenkofer, T.; Finkeldey, R.; Müller, M.; Krutovsky, K.V.; Vornam, B.; Leinemann, L.; Gailing, O. Genetic variation of introduced red oak (Quercus rubra) stands in Germany compared to North American populations. Eur. J. For. Res. 2020, 139, 321–331. [Google Scholar] [CrossRef]
- Lind, J.F.; Gailing, O. Genetic structure of Quercus rubra L. and Quercus ellipsoidalis EJ Hill populations at gene-based EST-SSR and nuclear SSR markers. Tree Genet. Genomes 2013, 9, 707–722. [Google Scholar] [CrossRef]
- Woodhead, M.; Russell, J.; Squirrell, J.; Hollingsworth, P.M.; MacKenzie, K.; Gibby, M.; Powell, W. Comparative analysis of population genetic structure in Athyrium distentifolium (Pteridophyta) using AFLPs and SSRs from anonymous and transcribed gene regions. Mol. Ecol. 2005, 14, 1681–1695. [Google Scholar] [CrossRef]
- Foll, M.; Gaggiotti, O.E. A genome-scan method to Identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics 2008, 180, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Lotterhos, K.E.; Whitlock, M.C. Evaluation of demographic history and neutral parameterization on the performance of FST outlier tests. Mol. Ecol. 2014, 23, 2178–2192. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, H. The theory of probability. Nat. Cell Biol. 1922, 109, 132–133. [Google Scholar] [CrossRef]
- Excoffier, L.; Hofer, T.; Foll, M. Detecting loci under selection in a hierarchically structured population. Heredity 2009, 103, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Corander, J.; Waldmann, P.; Sillanpää, M.J. Bayesian analysis of genetic differentiation between populations. Genetics 2003, 163, 367–374. [Google Scholar] [CrossRef]
- Wright, S. Isolation by distance. Genetics 1943, 28, 114. [Google Scholar] [CrossRef]
- Wang, I.J.; Glor, R.E.; Losos, J.B. Quantifying the roles of ecology and geography in spatial genetic divergence. Ecol. Lett. 2012, 16, 175–182. [Google Scholar] [CrossRef]
- Wang, I.J.; Summers, K. Genetic structure is correlated with phenotypic divergence rather than geographic isolation in the highly polymorphic strawberry poison-dart frog. Mol. Ecol. 2010, 19, 447–458. [Google Scholar] [CrossRef]
- Thibert-Plante, X.; Hendry, A.P. When can ecological speciation be detected with neutral loci? Mol. Ecol. 2010, 19, 2301–2314. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Keller, S.R. Ecological genomics meets community-level modelling of biodiversity: Mapping the genomic landscape of current and future environmental adaptation. Ecol. Lett. 2015, 18, 1–16. [Google Scholar] [CrossRef]
- Ferrier, S.; Manion, G.; Elith, J.; Richardson, K. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 2007, 13, 252–264. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Meirmans, P.G.; Van Tienderen, P.H. genotype and genodive: Two programs for the analysis of genetic diversity of asexual organisms. Mol. Ecol. Notes 2004, 4, 792–794. [Google Scholar] [CrossRef]
- Nei, M.; Tajima, F.; Tateno, Y. Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. J. Mol. Evol. 1983, 19, 153–170. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2005, 23, 254–267. [Google Scholar] [CrossRef]
- Kalinowski, S.T. Evolutionary and statistical properties of three genetic distances. Mol. Ecol. 2002, 11, 1263–1273. [Google Scholar] [CrossRef]
- Takezaki, N.; Nei, M. Genetic distances and reconstruction of phylogenetic trees from microsatellite DNA. Genetics 1996, 144, 389–399. [Google Scholar] [CrossRef]
- Kalinowski, S.T. How well do evolutionary trees describe genetic relationships among populations? Heredity 2009, 102, 506–513. [Google Scholar] [CrossRef]
- Mondin, L.A.D.C.; Machado, C.B.; De Resende, E.K.; Marques, D.K.S.; Galetti, P.M.J. Genetic pattern and demographic history of Salminus brasiliensis: Population expansion in the Pantanal Region during the Pleistocene. Front. Genet. 2018, 9, 1. [Google Scholar] [CrossRef]
- Latch, E.K.; Dharmarajan, G.; Glaubitz, J.C.; Rhodes, O.E. Relative performance of Bayesian clustering software for inferring population substructure and individual assignment at low levels of population differentiation. Conserv. Genet. 2006, 7, 295–302. [Google Scholar] [CrossRef]
- Provan, J.; Beatty, G.E.; Hunter, A.M.; McDonald, R.A.; McLaughlin, E.; Preston, S.J.; Wilson, S. Restricted gene flow in fragmented populations of a wind-pollinated tree. Conserv. Genet. 2007, 9, 1521–1532. [Google Scholar] [CrossRef]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.F.; Franco, F.F.; Bombonato, J.R.; Bonatelli, I.A.S.; Khan, G.; Romeiro-Brito, M.; Fegies, A.C.; Ribeiro, P.M.; Silva, G.A.R.; Moraes, E.M. Assessing population structure in the face of isolation by distance: Are we neglecting the problem? Divers. Distrib. 2018, 24, 1883–1889. [Google Scholar] [CrossRef]
- Ramasamy, R.K.; Ramasamy, S.; Bindroo, B.B.; Naik, V.G. STRUCTURE PLOT: A program for drawing elegant STRUCTURE bar plots in user friendly interface. SpringerPlus 2014, 3, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT (Version 1.2): A computer program to calculate F-statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Kalinowski, S.T. hp-rare 1.0: A computer program for performing rarefaction on measures of allelic diversity. Mol. Ecol. Notes 2005, 5, 187–189. [Google Scholar] [CrossRef]
- Luikart, G.; Allendorf, F.W.; Cornuet, J.-M.; Sherwin, W.B. Distortion of allele frequency distributions provides a test for recent population bottlenecks. J. Hered. 1998, 89, 238–247. [Google Scholar] [CrossRef]
- Piry, S.; Luikart, G.; Cornuet, J.M. BOTTLENECK: A computer program for detecting recent reductions in the effective population size using allele frequency data. J. Hered. 1999, 90, 502–503. [Google Scholar] [CrossRef]
- Di Rienzo, A.; Peterson, A.C.; Garza, J.C.; Valdes, A.M.; Slatkin, M.; Freimer, N.B. Mutational processes of simple-sequence repeat loci in human populations. Proc. Natl. Acad. Sci. USA 1994, 91, 3166–3170. [Google Scholar] [CrossRef]
- Chybicki, I.J.; Burczyk, J. Simultaneous estimation of null alleles and inbreeding coefficients. J. Hered. 2008, 100, 106–113. [Google Scholar] [CrossRef]
- Beaumont, M.A. Approximate Bayesian computation in evolution and ecology. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 379–406. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Estoup, A.; Jarne, P.; Cornuet, J.-M. Homoplasy and mutation model at microsatellite loci and their consequences for population genetics analysis. Mol. Ecol. 2002, 11, 1591–1604. [Google Scholar] [CrossRef]
- Setsuko, S.; Sugai, K.; Tamaki, I.; Takayama, K.; Kato, H.; Yoshimaru, H. Genetic diversity, structure, and demography of Pandanus boninensis (Pandanaceae) with sea drifted seeds, endemic to the Ogasawara Islands of Japan: Comparison between young and old islands. Mol. Ecol. 2020, 29, 1050–1068. [Google Scholar] [CrossRef]
- Excoffier, L.; Estoup, A.; Cornuet, J.-M. Bayesian analysis of an admixture model with mutations and arbitrarily linked markers. Genet. 2005, 169, 1727–1738. [Google Scholar] [CrossRef]
- Excoffier, L.; Foll, M. Fastsimcoal: A continuous-time coalescent simulator of genomic diversity under arbitrarily complex evolutionary scenarios. Bioinformatics 2011, 27, 1332–1334. [Google Scholar] [CrossRef]
- Pudlo, P.; Marin, J.-M.; Estoup, A.; Cornuet, J.-M.; Gauthier, M.; Robert, C.P. Reliable ABC model choice via random forests. Bioinformatics 2015, 32, 859–866. [Google Scholar] [CrossRef]
- Csilléry, K.; François, O.; Blum, M.G.B. abc: An R package for approximate Bayesian computation (ABC). Methods Ecol. Evol. 2012, 3, 475–479. [Google Scholar] [CrossRef]
- Blum, M.G.B.; François, O. Non-linear regression models for approximate Bayesian computation. Stat. Comput. 2010, 20, 63–73. [Google Scholar] [CrossRef]
- Plummer, M.; Best, N.; Cowles, K.; Vines, K. CODA: Convergence diagnosis and output analysis for MCMC. R News 2006, 6, 7–11. [Google Scholar]
- Lu, R.-S.; Chen, Y.; Tamaki, I.; Sakaguchi, S.; Ding, Y.-Q.; Takahashi, D.; Li, P.; Isaji, Y.; Chen, J.; Qiu, Y.-X. Pre-quaternary diversification and glacial demographic expansions of Cardiocrinum (Liliaceae) in temperate forest biomes of Sino-Japanese Floristic Region. Mol. Phylogenetics Evol. 2020, 143, 106693. [Google Scholar] [CrossRef]
- Iwauchi, A.; Hase, Y. Late Cenozoic vegetation and paleoenvironment of northern and central Kyushu, Japan - Part 5 Yoshino area (Middle Pleistocene). J. Geol. Soc. Japan 1992, 98, 205–221. [Google Scholar] [CrossRef][Green Version]
- Fujii, S.; Nara, M.; Hatanaka, S.; Yamaguchi, G.; Suzuki, M.; Takeuti, S.; Yoshida, A.; Noshiro, S.; Muramoto, J.; Horiuchi, K.; et al. Submarine forests around Shimokita Peninsula, north end of Honshu, Japan. Earth Sci. (Chikyu Kagaku) 2006, 60, 375–387. (In Japanese) [Google Scholar]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, B.D.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Tsukada, M. Vegetation and climate during the Last Glacial Maximum in Japan. Quat. Res. 1983, 19, 212–235. [Google Scholar] [CrossRef]
- Ono, Y. Last Glacial paleoclimate reconstructed from glacial and periglacial landforms in Japan. Geogr. Rev. Jpn. Ser. B. 1984, 57, 87–100. [Google Scholar] [CrossRef][Green Version]
- Yoshida, A.; Akihiko, S.; Motonari, O.; Masataka, H.; Akifumi, I. Vegetation reconstruction during the Last Termination on Mt. Chokai, northeastern Japan and habitat of Larix gemelinii. Jpn. J. Histor. Bot. 2014, 23, 21–26. (In Japanese) [Google Scholar]
- Minaki, M.; Matsuba, C. Plant macrofossil assemblage from about 18,000 years ago in Tado-cho, Mie Prefecture, central Japan. Quat. Res. (Daiyonki-Kenkyu) 1985, 24, 51–55. [Google Scholar] [CrossRef][Green Version]
- Momohara, A.; Yoshida, A.; Kudo, Y.; Nishiuchi, R.; Okitsu, S. Paleovegetation and climatic conditions in a refugium of temperate plants in central Japan in the Last Glacial Maximum. Quat. Int. 2016, 425, 38–48. [Google Scholar] [CrossRef]
- Noshiro, S.; Terada, K.; Tsuji, S.-I.; Suzuki, M. Larix-Picea forests of the Last Glacial Age on the eastern slope of Towada Volcano in northern Japan. Rev. Palaeobot. Palynol. 1997, 98, 207–222. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Tsuyama, I.; Nakao, K.; Higa, M.; Matsui, T.; Shichi, K.; Tanaka, N. What controls the distribution of the Japanese endemic hemlock, Tsuga diversifolia? Footprint of climate in the glacial period on current habitat occupancy. J. For. Res. 2014, 19, 154–165. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Boyce, M.S.; Vernier, P.R.; Nielsen, S.E.; Schmiegelow, F.K. Evaluating resource selection functions. Ecol. Model. 2002, 157, 281–300. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Brier, G.W. Verification of forecasts expressed in terms of probability. Mon. Weather Rev. 1950, 78, 1–3. [Google Scholar] [CrossRef]
- Moreno-Amat, E.; Rubiales, J.M.; Morales-Molino, C.; García-Amorena, I. Incorporating plant fossil data into species distribution models is not straightforward: Pitfalls and possible solutions. Quat. Sci. Rev. 2017, 170, 56–68. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Fløjgaard, C.; Marske, K.A.; Nógues-Bravo, D.; Normand, S. Applications of species distribution modeling to paleobiology. Quat. Sci. Rev. 2011, 30, 2930–2947. [Google Scholar] [CrossRef]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.R.; Lawrence, D.M.; Neale, R.B.; Rasch, P.J.; Vertenstein, M.; et al. The Community Climate System Model Version. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef]
- Watanabe, S.; Hajima, T.; Sudo, K.; Nagashima, T.; Takemura, T.; Okajima, H.; Nozawa, T.; Kawase, H.; Abe, M.; Yokohata, T.; et al. MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model Dev. 2011, 4, 845–872. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P.; Liu, C. Modelling species distributions in Britain: A hierarchical integration of climate and land-cover data. Ecography 2004, 27, 285–298. [Google Scholar] [CrossRef]
- Hosner, S.; Lemeshow, D.W. Applied Logistic Regression; John Wiley & Son: New York, NY, USA, 1989. [Google Scholar]
- Maguire, K.-C.; Nieto-Lugilde, D.; Blois, J.L.; Fitzpatrick, M.C.; Williams, J.W.; Ferrier, S.; Lorenz, D.J. Controlled comparison of species- and community-level models across novel climates and communities. Proc. R. Soc. B 2016, 283, 20152817. [Google Scholar] [CrossRef]
- Comps, B.; Gömöry, D.; Letouzey, J.; Thiébaut, B.; Petit, R.J. Diverging trends between heterozygosity and allelic richness during postglacial colonization in the European beech. Genetics 2001, 157, 389–397. [Google Scholar] [CrossRef]
- Iwaizumi, M.G.; Tsuda, Y.; Ohtani, M.; Tsumura, Y.; Takahashi, M. Recent distribution changes affect geographic clines in genetic diversity and structure of Pinus densiflora natural populations in Japan. For. Ecol. Manag. 2013, 304, 407–416. [Google Scholar] [CrossRef]
- Kimura, M.K.; Uchiyama, K.; Nakao, K.; Moriguchi, Y.; Jose-Maldia, L.S.; Tsumura, Y. Evidence for cryptic northern refugia in the last glacial period in Cryptomeria japonica. Ann. Bot. 2014, 114, 1687–1700. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Qiu, Y.-X.; Liu, Y.-H.; Qi, X.-S.; Kim, S.-H.; Han, J.; Takeuchi, Y.; Worth, J.R.P.; Yamasaki, M.; Sakurai, S.; et al. Climate oscillation during the Quaternary associated with landscape heterogeneity promoted allopatric lineage divergence of a temperate tree Kalopanax septemlobus (Araliaceae) in East Asia. Mol. Ecol. 2012, 21, 3823–3838. [Google Scholar] [CrossRef]
- Worth, J.R.P.; Williamson, G.J.; Sakaguchi, S.; Nevill, P.G.; Jordan, G.J. Environmental niche modelling fails to predict Last Glacial Maximum refugia: Niche shifts, microrefugia or incorrect palaeoclimate estimates? Glob. Ecol. Biogeogr. 2014, 23, 1186–1197. [Google Scholar] [CrossRef]
- Callahan, C.M.; Rowe, C.A.; Ryel, R.J.; Shaw, J.D.; Madritch, M.D.; Mock, K.E. Continental-scale assessment of genetic diversity and population structure in quaking aspen (Populus tremuloides). J. Biogeogr. 2013, 40, 1780–1791. [Google Scholar] [CrossRef]
- Cho, W.; So, S.; Han, E.K.; Myeong, H.H.; Park, J.S.; Hwang, S.H.; Hwang, S.-H.; Kim, J.-H.; Lee, J.-H. Rear-edge, low-diversity, and haplotypic uniformity in cold-adapted Bupleurum euphorbioides interglacial refugia populations. Ecol. Evol. 2020, 10, 10449–10462. [Google Scholar] [CrossRef]
- Dering, M.; Kosiński, P.; Wyka, T.P.; Pers-Kamczyc, E.; Boratyński, A.; Boratyńska, K.; Reich, P.B.; Romo, A.; Zadworny, M.; Żytkowiak, R.; et al. Tertiary remnants and Holocene colonizers: Genetic structure and phylogeography of Scots pine reveal higher genetic diversity in young boreal than in relict Mediterranean populations and a dual colonization of Fennoscandia. Divers. Distrib. 2017, 23, 540–555. [Google Scholar] [CrossRef]
- Worth, J.R.P.; Yokogawa, M.; Pérez-Figueroa, A.; Tsumura, Y.; Tomaru, N.; Janes, J.K.; Isagi, Y. Conflict in outcomes for conservation based on population genetic diversity and genetic divergence approaches: A case study in the Japanese relictual conifer Sciadopitys verticillata (Sciadopityaceae). Conserv. Genet. 2014, 15, 1243–1257. [Google Scholar] [CrossRef]
- Inanaga, M.; Hasegawa, Y.; Mishima, K.; Takata, K. Genetic diversity and structure of Japanese endemic genus Thujopsis (Cupressaceae) Using EST-SSR Markers. Forests 2020, 11, 935. [Google Scholar] [CrossRef]
- Burton, O.J.; Travis, J.M.J. Landscape structure and boundary effects determine the fate of mutations occurring during range expansions. Heredity 2008, 101, 329–340. [Google Scholar] [CrossRef] [PubMed]
- McInerny, G.; Turner, J.; Wong, H.; Travis, J.; Benton, T. How range shifts induced by climate change affect neutral evolution. Proc. R. Soc. B Boil. Sci. 2009, 276, 1527–1534. [Google Scholar] [CrossRef]
- Duncan, C.J.; Worth, J.R.P.; Jordan, G.J.; Jones, R.C.; Vaillancourt, R.E. Genetic differentiation in spite of high gene flow in the dominant rainforest tree of southeastern Australia, Nothofagus cunninghamii. Heredity 2016, 116, 99–106. [Google Scholar] [CrossRef]
- Ledig, F.T. Human impacts on genetic diversity in forest ecosystems. Oikos 1992, 63, 87. [Google Scholar] [CrossRef]
- Aoki, K.; Ueno, S.; Kamijo, T.; Setoguchi, H.; Murakami, N.; Kato, M.; Tsumura, Y. Genetic differentiation and genetic diversity of Castanopsis (Fagaceae), the dominant tree species in Japanese broadleaved evergreen forests, revealed by analysis of EST-associated microsatellites. PLoS ONE 2014, 9, e87429. [Google Scholar] [CrossRef]
- Takahara, H.; Tanida, K.; Miyoshi, N. The full-Glacial refugium of Cryptomeria japonica in the Oki Islands, Western Japan. Jpn. J. Palynol. 2001, 47, 21–33. [Google Scholar]
- Inoue, K.; Mishima, M.; Fukaya, H.; Yahata, H.; Nobe, K. Distributions of several species of northern plants in Oki Islands, Shimane Prefecture. Bull. Shimane Nat. Mus. Mt Sanbe 2019, 17, 37–43. [Google Scholar]








| Source | df | SS | MS | Est. Var. | % |
|---|---|---|---|---|---|
| Among northeast and southwest Japan | 1 | 107.186 | 107.186 | 0.137 | 3.17 |
| Among Pops | 28 | 598.797 | 21.386 | 0.323 | 7.48 |
| Within Pops | 1604 | 6183.825 | 3.855 | 3.855 | 89.35 |
| Total | 1633 | 6889.808 | 4.314757 | 100 |
| Region | N | Na | Ne | Ho | He | Fis | Ar (43 ind.) | PAr (43 ind.) | No. of Private Alleles |
|---|---|---|---|---|---|---|---|---|---|
| Tohoku | 230 | 12.54 | 3.86 | 0.58 | 0.63 | 0.07 | 9.07 | 1.06 | 22 |
| Kanto | 122 | 10.77 | 3.40 | 0.58 | 0.61 | 0.05 | 8.82 | 0.8 | 8 |
| Japan Sea side/Central Honshu | 255 | 13.46 | 4.18 | 0.60 | 0.65 | 0.07 | 9.52 | 1.15 | 23 |
| Dogo Island | 71 | 8.15 | 3.63 | 0.57 | 0.62 | 0.07 | 7.36 | 0.2 | 2 |
| Mainland Chugoku | 44 | 5.00 | 2.65 | 0.40 | 0.54 | 0.25 | 4.98 | 0.09 | 1 |
| Shikoku | 95 | 7.69 | 3.26 | 0.49 | 0.62 | 0.21 | 6.91 | 0.24 | 1 |
| northeast Japan | 607 | 16.31 | 4.02 | 0.59 | 0.64 | 0.08 | 9.73 | 4.13 | 90 |
| southwest Japan | 210 | 9.69 | 3.85 | 0.50 | 0.63 | 0.20 | 7.7 | 2.17 | 4 |
| Total | 27.23 | 6.22 | 3.14 | 0.57 | 0.58 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worth, J.R.P.; Tamaki, I.; Tsuyama, I.; Harrison, P.A.; Sugai, K.; Sakio, H.; Aizawa, M.; Kikuchi, S. Genetic Distinctiveness but Low Diversity Characterizes Rear-Edge Thuja standishii (Gordon) Carr. (Cupressaceae) Populations in Southwest Japan. Diversity 2021, 13, 185. https://doi.org/10.3390/d13050185
Worth JRP, Tamaki I, Tsuyama I, Harrison PA, Sugai K, Sakio H, Aizawa M, Kikuchi S. Genetic Distinctiveness but Low Diversity Characterizes Rear-Edge Thuja standishii (Gordon) Carr. (Cupressaceae) Populations in Southwest Japan. Diversity. 2021; 13(5):185. https://doi.org/10.3390/d13050185
Chicago/Turabian StyleWorth, James R. P., Ichiro Tamaki, Ikutaro Tsuyama, Peter A. Harrison, Kyoko Sugai, Hitoshi Sakio, Mineaki Aizawa, and Satoshi Kikuchi. 2021. "Genetic Distinctiveness but Low Diversity Characterizes Rear-Edge Thuja standishii (Gordon) Carr. (Cupressaceae) Populations in Southwest Japan" Diversity 13, no. 5: 185. https://doi.org/10.3390/d13050185
APA StyleWorth, J. R. P., Tamaki, I., Tsuyama, I., Harrison, P. A., Sugai, K., Sakio, H., Aizawa, M., & Kikuchi, S. (2021). Genetic Distinctiveness but Low Diversity Characterizes Rear-Edge Thuja standishii (Gordon) Carr. (Cupressaceae) Populations in Southwest Japan. Diversity, 13(5), 185. https://doi.org/10.3390/d13050185







