Diversity of Seahorse Species (Hippocampus spp.) in the International Aquarium Trade
Abstract
:1. Introduction
1.1. International Trade in Marine Ornamental Fishes
1.2. International Trade in Seahorses (Hippocampus spp.)
1.3. Captive Breeding
| Name | IUCN Status | First CITES Record | Maximum Length (cm) | |
|---|---|---|---|---|
| Live | Dead | |||
| H. hippocampus (Linnaeus, 1758) | DD | 1997 | 2003 | 15.0 [30] |
| H. erectus Perry, 1810 | VU | 1997 | 2011 | 19.0 [30] |
| H. trimaculatus Leach, 1814 | LC | 1997 | 2004 | 22.0 [70] |
| H. abdominalis Lesson, 1827 | LC | 1999 | 2007 | 35.0 [30] |
| H. guttulatus Cuvier, 1829 | DD | 1997 | 21.5 [71] | |
| H. comes Cantor, 1849 | VU | 2003 | 2004 | 18.7 [30] |
| H. coronatus Temminck & Schlegel, 1850 | DD | 1997 | 13.3 [72] | |
| H. kuda Bleeker, 1852 | VU | 1997 | 2003 | 17.0 [30] |
| H. mohnikei Bleeker, 1853 | VU | 1997 | 2003 | 8.0 [30] |
| H. camelopardalis Bianconi, 1854 | DD | 10.0 [30] | ||
| H. whitei Bleeker, 1855 | EN | 2002 | 13.0 [30] | |
| H. algiricus Kaup, 1856 | VU | 2004 | 2004 | 19.0 [30] |
| H. histrix Kaup, 1856 | VU | 1997 | 2003 | 17.0 [30] |
| H. lichtensteinii Kaup, 1856 | NE | 4.0 [30] | ||
| H. ingens Girard, 1858 | VU | 1998 | 2004 | 31.0 [30] |
| H. breviceps Peters, 1869 | LC | 2002 | 10.0 [30] | |
| H. angustus Günther, 1870 | LC | 1999 | 16.0 [30] | |
| H. tristis Castelnau, 1872 | NE | 18.0 [73] | ||
| H. subelongatus Castelnau, 1873 | DD | 2004 | 20.0 [30] | |
| H. planifrons Peters, 1877 | LC | 2011 | 11.6 [74] | |
| H. zosterae Jordan & Gilbert, 1882 | LC | 1998 | 2.5 [30] | |
| H. capensis Boulenger, 1900 | EN | 2000 | 12.0 [30] | |
| H. jayakari Boulenger, 1900 | LC | 1998 | 14.0 [30] | |
| H. kelloggi Jordan & Snyder, 1901 | VU | 2005 | 2003 | 28.0 [30] |
| H. sindonis Jordan & Snyder, 1901 | LC | 10.8 [72] | ||
| H. fisheri Jordan & Evermann, 1903 | LC | 8.0 [30] | ||
| H. barbouri Jordan & Richardson, 1908 | VU | 2002 | 2005 | 15.0 [30] |
| H. dahli Ogilby, 1908 | LC | 14.0 [46] | ||
| H. spinosissimus Weber, 1913 | VU | 1998 | 2004 | 17.2 [30] |
| H. reidi Ginsburg, 1933 | NT | 1997 | 2007 | 17.5 [30] |
| H. zebra Whitley, 1964 | DD | 2004 | 9.4 [30] | |
| H. bargibanti Whitley, 1970 | DD | 2.4 [30] | ||
| H. minotaur Gomon, 1997 | DD | 2007 | 5.0 [30] | |
| H. jugumus Kuiter, 2001 | DD | 4.4 [74] | ||
| H. colemani Kuiter, 2003 | DD | 2.7 [75] | ||
| H. denise Lourie & Randall, 2003 | DD | 2004 | 2.1 [30] | |
| H. patagonicus Piacentino & Luzzatto, 2004 | VU | 15.0 [76] | ||
| H. pusillus Fricke, 2004 | DD | 2.8 [77] | ||
| H. pontohi Lourie & Kuiter, 2008 | LC | 1.7 [75] | ||
| H. satomiae Lourie & Kuiter, 2008 | DD | 1.4 [75] | ||
| H. debelius Gomon & Kuiter, 2009 | DD | 2.4 [78] | ||
| H. tyro Randall & Lourie, 2009 | DD | 6.1 [79] | ||
| H. waleananus Gomon & Kuiter, 2009 | NE | 1.8 [80] | ||
| H. paradoxus Foster & Gomon, 2010 | DD | 6.5 [81] | ||
| H. casscsio Zhang et al., 2016 | DD | 13.3 [82] | ||
| H. haema Han et al., 2017 | NE | 11.4 [72] | ||
| H. japapigu Short et al., 2018 | NE | 1.6 [80] | ||
| H. nalu Short et al., 2020 | NE | 2.2 [83] | ||
1.4. Research Aim
2. The Natural History of Hippocampus Species
2.1. Species Discovery: Nominal and Valid Species
2.2. Body Size and Habitat Requirements
3. Hippocampus Species in the International Aquarium Trade
3.1. Hippocampus and CITES
| Species | C | F | W | U | Total |
|---|---|---|---|---|---|
| H. abdominalis (8) | 6748 | 0 | 243 | 156 | 7147 |
| H. barbouri (5) | 5167 | 906 | 8235 | 0 | 14,308 |
| H. comes (3) | 6972 | 61,867 | 9480 | 5 | 78,324 |
| H. erectus (7) | 179 | 1060 | 7684 | 160 | 9083 |
| H. hippocampus (12) | 200 | 20 | 86 | 82 | 388 |
| H. histrix (6) | 1037 | 100 | 8578 | 20 | 9735 |
| H. ingens (9) | 3968 | 0 | 3146 | 8 | 7122 |
| H. kelloggi (4) | 0 | 300 | 14,037 | 0 | 14,337 |
| H. kuda (1) | 54,951 | 231,079 | 103,337 | 1065 | 390,432 |
| H. reidi (2) | 105,357 | 1038 | 18,723 | 116 | 125,234 |
| H. spinosissimus (11) | 627 | 80 | 3424 | 0 | 4131 |
| H. zosterae (10) | 4 | 1448 | 2805 | 190 | 4447 |
| Hippocampus spp. | 5919 | 1607 | 6997 | 123 | 14,646 |
| Total | 191,129 | 299,505 | 186,775 | 1925 | 679,334 |
| Species | C | F | W | U | Total |
|---|---|---|---|---|---|
| H. abdominalis (4) | 26,732 | 60 | 165 | 828 | 27,785 |
| H. barbouri (5) | 6170 | 2354 | 14,630 | 0 | 23,154 |
| H. comes (3) | 30,690 | 110,986 | 13,185 | 0 | 154,861 |
| H. erectus (9) | 1509 | 505 | 9786 | 0 | 11,800 |
| H. hippocampus (12) | 24 | 0 | 20 | 0 | 44 |
| H. histrix (6) | 0 | 5650 | 14,637 | 0 | 20,287 |
| H. ingens (8) | 11,532 | 0 | 3216 | 0 | 14,748 |
| H. kelloggi (7) | 0 | 0 | 15,024 | 0 | 15,024 |
| H. kuda (1) | 71,009 | 367,645 | 60,372 | 0 | 499,026 |
| H. reidi (2) | 291,464 | 0 | 12,340 | 0 | 303,804 |
| H. spinosissimus (10) | 431 | 0 | 7029 | 0 | 7460 |
| H. zosterae (11) | 1644 | 194 | 1880 | 0 | 3718 |
| Hippocampus spp. | 3114 | 81 | 6547 | 324 | 10,066 |
| Total | 444,319 | 487,475 | 158,831 | 1152 | 1,091,777 |
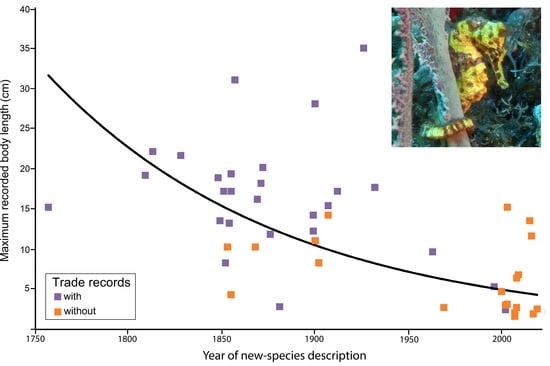
3.2. Threat Status and Body Size of Hippocampus Species in the Aquarium Trade
3.3. Life History Traits and Habitats of Hippocampus Species
3.4. Sources of Live Hippocampus Spp.
3.5. International Trade in Live Animals vs. Dead Bodies
4. Aquaculture of Hippocampus
4.1. Methods of Seahorse Aquaculture
4.2. Challenges in Seahorse Aquaculture
4.3. Aquaculture in Different Source Countries
4.4. Opportunities for Hippocampus Aquaculture
4.5. Use of Molecular Tools in Hippocampus Diversity Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wood, E. Collection of Coral Reef Fishes for Aquaria: Global Trade, Conservation Issues and Management Strategies; Marine Conservation Society: Ross-on-Wye, UK, 2001; p. 56. [Google Scholar]
- Wabnitz, C.; Taylor, M.; Green, E.; Razak, T. From Ocean to Aquarium: The Global Trade in Marine Ornamental Species; UNEP-WCMC Biodiversity Series 17; UNEP-WCMC: Cambridge, UK, 2003; p. 64. [Google Scholar]
- Rhyne, A.L.; Tlusty, M.F.; Schofield, P.J.; Kaufman, L.; Morris, J.A., Jr.; Bruckner, A.W. Revealing the appetite of the marine aquarium fish trade: The volume and biodiversity of fish imported into the United States. PLoS ONE 2012, 7, e35808. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Vaz, M.C.M.; Puga, J.; Rocha, R.J.M.; Brown, C.; Rosa, R.; Calado, R. Marine ornamental fish imports in the European Union: An economic perspective. Fish Fish. 2016, 17, 459–468. [Google Scholar] [CrossRef]
- Biondo, M.V.; Burki, R.P. Monitoring the trade in marine ornamental fishes through the European trade control and expert system TRACES: Challenges and possibilities. Mar. Policy 2019, 108, 103620. [Google Scholar] [CrossRef]
- Dee, L.E.; Karr, K.A.; Landesberg, C.J.; Thornhill, D.J. Assessing vulnerability of fish in the U.S. marine aquarium trade. Front. Mar. Sci. 2019, 5, 527. [Google Scholar] [CrossRef]
- Biondo, M.V.; Burki, R.P. A systematic review of the ornamental fish trade with emphasis on coral reef fishes—An impossible task. Animals 2020, 10, 2014. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.M. Essential fish habitat and the effective design of marine reserves: Application for marine ornamental fishes. Aquar. Sci. Conserv. 2001, 3, 135–150. [Google Scholar] [CrossRef]
- Zajicek, P.; Hardin, S.; Watson, C. A Florida marine ornamental pathway risk analysis. Rev. Fish. Sci. 2009, 17, 156–169. [Google Scholar] [CrossRef]
- Cohen, F.P.A.; Valenti, W.C.; Calado, R. Traceability issues in the trade of marine ornamental species. Rev. Fish. Sci. 2013, 21, 98–111. [Google Scholar] [CrossRef]
- Barber, C.V.; Pratt, V.R. Sullied Seas: Strategies for Combating Cyanide Fishing in Southeast Asia and Beyond; World Resources Institute: Washington, DC, USA, 1997. [Google Scholar]
- Lecchini, D.; Polti, S.; Nakamura, Y.; Mosconi, P.; Tsuchiya, M.; Remoissenet, G.; Planes, S. New perspectives on aquarium fish trade. Fish. Sci. 2006, 72, 40–47. [Google Scholar] [CrossRef]
- Shuman, C.S.; Hodgson, G.; Ambrose, R.F. Population impacts of collecting sea anemones and anemonefish for the marine aquarium trade in the Philippines. Coral Reefs 2005, 24, 564–573. [Google Scholar] [CrossRef]
- Jones, A.M.; Gardner, S.; Sinclair, W. Losing ‘Nemo’: Bleaching and collection appear to reduce inshore populations of anemonefishes. J. Fish Biol. 2008, 73, 753–761. [Google Scholar] [CrossRef]
- Morrisey, D.; Inglis, G.; Neil, K.; Bradley, A.; Fitridge, I. Characterization of the marine aquarium trade and management of associated marine pests in Australia, a country with stringent import biosecurity regulation. Environ. Conserv. 2011, 38, 89–100. [Google Scholar] [CrossRef]
- Betancur, R.R.; Hines, A.; Acero, P.A.; Ortí, G.; Wilbur, A.E.; Freshwater, D.W.; Betancur, R.R.; Hines, A.; Ortí, G.; Wilbur, A.E.; et al. Reconstructing the lionfish invasion: Insights into Greater Caribbean biogeography. J. Biogeogr. 2011, 38, 1281–1293. [Google Scholar] [CrossRef]
- Côté, I.M.; Green, S.J.; Hixon, M.A. Predatory fish invaders: Insights from Indo-Pacific lionfish in the western Atlantic and Caribbean. Biol. Conserv. 2013, 164, 50–61. [Google Scholar] [CrossRef]
- Padilla, D.K.; Williams, S.L. Beyond ballast water: Aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front. Ecol. Environ. 2004, 2, 131–138. [Google Scholar] [CrossRef]
- Ferreira, C.E.L.; Luiz, O.J.; Floeter, S.R.; Lucena, M.B.; Barbosa, M.C.; Rocha, C.R.; Rocha, L.A. First record of invasive lionfish (Pterois volitans) for the Brazilian coast. PLoS ONE 2015, 10, e0123002. [Google Scholar] [CrossRef]
- Dimitriou, A.C.; Chartosia, N.; Hall-Spencer, J.M.; Kleitou, P.; Jimenez, C.; Antoniou, C.; Hadjioannou, L.; Kletou, D.; Sfenthourakis, S. Genetic data suggest multiple introductions of the lionfish (Pterois miles) into the Mediterranean sea. Diversity 2019, 11, 149. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.D.; Clua, E.; Hair, C.A.; Galzin, R.; Doherty, P.J. The capture and culture of post-larval fish and invertebrates for the marine ornamental trade. Rev. Fish. Sci. 2009, 17, 223–240. [Google Scholar] [CrossRef]
- Foster, S.J.; Stanton, L.M.; Nellas, A.C.; Arias, M.M.; Vincent, A.C.J. The Catch and Trade of Seahorses in the Philippines Post-CITES. In Fisheries Centre Research Reports 27(2); University of British Columbia: Vancouver, BC, Canada, 2019; p. 45. [Google Scholar]
- Stocks, A.P.; Foster, S.J.; Bat, N.K.; Ha, N.M.; Vincent, A.C.J. Local fishers’ knowledge of target and incidental seahorse catch in southern Vietnam. Hum. Ecol. 2019, 47, 397–408. [Google Scholar] [CrossRef]
- Ballesteros, L.V.; Matthews, J.L.; Hoeksema, B.W. Pollution and coral damage caused by derelict fishing gear on coral reefs around Koh Tao, Gulf of Thailand. Mar. Pollut. Bull. 2018, 135, 1107–1116. [Google Scholar] [CrossRef]
- Olivier, K. World Trade in Ornamental Species. In Marine Ornamental Species: Collection, Culture and Conservation; Cato, J., Brown, B., Eds.; Iowa State Press: Ames, IA, USA, 2003; pp. 49–63. [Google Scholar]
- Livengood, E.J.; Chapman, F.A. The Ornamental Fish Trade: An Introduction with Perspectives for Responsible Aquarium Fish Ownership, Publication FA124; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2011; p. 7. [Google Scholar]
- Hodgson, G. A global assessment of human effects on coral reefs. Mar. Pollut. Bull. 1999, 38, 345–355. [Google Scholar] [CrossRef]
- Vincent, A.C.J.; Foster, S.J.; Koldewey, H.J. Conservation and management of seahorses and other Syngnathidae. J. Fish Biol. 2011, 78, 1681–1724. [Google Scholar] [CrossRef]
- Foster, S.J.; Vincent, A.C.J. Life history and ecology of seahorses: Implications for conservation and management. J. Fish Biol. 2004, 65, 1–61. [Google Scholar] [CrossRef]
- Lourie, S.A.; Foster, S.J.; Cooper, E.W.T.; Vincent, A.C.J. A Guide to the Identification of Seahorses; Project Seahorse; University of British Columbia: Vancouver, BC, Canada; TRAFFIC North America: Washington, DC, USA, 2004; p. 114. [Google Scholar]
- Rosa, I.L.; Defavari, G.R.; Alves, R.R.N.; Oliveira, T.P.R. Seahorses in Traditional Medicines: A global Overview. In Animals in Traditional Folk Medicine; Metzler, J.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 207–240. [Google Scholar]
- Lourie, S.A.; Pritchard, J.C.; Casey, S.P.; Truong, S.K.; Hall, H.J.; Vincent, A.C.J. The taxonomy of Vietnam’s exploited seahorses (family Syngnathidae). Biol. J. Linn. Soc. 1999, 66, 231–256. [Google Scholar] [CrossRef]
- Foster, S.; Wiswedel, S.; Vincent, A. Opportunities and challenges for analysis of wildlife trade using CITES data-seahorses as a case study. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 26, 154–172. [Google Scholar] [CrossRef]
- Zeng, L.; Armani, A.; Wen, J.; Lin, H.; Xu, Y.; Fan, S.; Sun, Y.; Yang, C.; Chen, Z.; Chen, D.; et al. Molecular identification of seahorse and pipefish species sold as dried seafood in China: A market-based survey to highlight the actual needs for a proper trade. Food Control. 2019, 103, 175–181. [Google Scholar] [CrossRef]
- Salin, K.R.; Yohannan, T.M.; Nair, C.M. Fisheries and trade of seahorses, Hippocampus spp., in Southern India. Fish. Manag. Ecol. 2005, 12, 269–273. [Google Scholar] [CrossRef]
- Baum, J.K.; Meeuwig, J.J.; Vincent, A.C.J. Bycatch of seahorse (Hippocampus erectus) in a Gulf of Mexico shrimp trawl fishery. Fish. Bull. 2003, 101, 721–731. [Google Scholar]
- Kuo, T.-C.; Laksanawimol, P.; Aylesworth, L.; Foster, S.J.; Vincent, A.C.J. Changes in the trade of bycatch species corresponding to CITES regulations: The case of dried seahorse trade in Thailand. Biodivers. Conserv. 2018, 27, 3447–3468. [Google Scholar] [CrossRef]
- Aylesworth, L.; Phoonsawat, R.; Vincent, A.C.J. Effects of indiscriminate fisheries on a group of small data-poor species in Thailand. ICES J. Mar. Sci. 2017, 75, 642–652. [Google Scholar] [CrossRef]
- Silveira, R.; Barcelos, B.T.; Oliveira, R.L.; Silva, J.S. Records of bycatch of Hippocampus patagonicus (Pisces: Syngnathidae) in commercial fishing in southern Brazil. Lat. Am. J. Aquat. Res. 2018, 46, 744–755. [Google Scholar] [CrossRef]
- National Marine Fisheries Service. Status Review Report: Dwarf Seahorse (Hippocampus Zosterae); Office of Protected Resources, National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2020; p. 84.
- Vincent, A.C.; Meeuwig, J.J.; Pajaro, M.G.; Perante, N.C. Characterizing a small-scale, data-poor, artisanal fishery: Seahorses in the central Philippines. Fish. Res. 2007, 86, 207–215. [Google Scholar] [CrossRef]
- Teske, P.R.; Beheregaray, L.B. Evolution of seahorses’ upright posture was linked to Oligocene expansion of seagrass habitats. Biol. Lett. 2009, 5, 521–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, M.P.; Burhans, R.; Simões, N.; Planas, M. Seahorses and Pipefish. In Marine Ornamental Species Aquaculture; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 299–326. [Google Scholar]
- Teitelbaum, A.; Dubosc, J. Seahorses: Trade, aquaculture and their long-term outlook in New Caledonia. SPC Fish. Newsl. 2017, 152, 3233. [Google Scholar]
- Kuiter, R.H. Seahorses, Pipefishes and Their Relatives: A Comprehensive Guide to Syngnathiformes; TMC Publishing: Chorleywood, UK, 2000; p. 240. [Google Scholar]
- Kuiter, R.H. Seahorses and Their Relatives; Aquatic Photographics: Seaford, Australia, 2009; p. 333. [Google Scholar]
- Harasti, D. Declining seahorse populations linked to loss of essential marine habitats. Mar. Ecol. Prog. Ser. 2016, 546, 173–181. [Google Scholar] [CrossRef]
- Vincent, A.C.J.; De Mitcheson, Y.J.S.; Fowler, S.L.; Lieberman, S.S. The role of CITES in the conservation of marine fishes subject to international trade. Fish Fish. 2014, 15, 563–592. [Google Scholar] [CrossRef]
- Vincent, A.C.J.; Foster, S.J. Setting precedent in export regulations for marine fishes with seahorses. Fisheries 2017, 42, 40–43. [Google Scholar] [CrossRef]
- Kuo, T.-C.; Vincent, A. Assessing the changes in international trade of marine fishes under CITES regulations—A case study of seahorses. Mar. Policy 2018, 88, 48–57. [Google Scholar] [CrossRef]
- Koldewey, H.J.; Martin-Smith, K.M. A global review of seahorse aquaculture. Aquaculture 2010, 302, 131–152. [Google Scholar] [CrossRef]
- Foster, S.J. Seahorses (Hippocampus spp.) and the CITES Review of Significant Trade; Fisheries Centre Research Reports 24(4); University of British Columbia: Vancouver, BC, Canada, 2016; p. 48. [Google Scholar]
- Aylesworth, L.; Foster, S.J.; Vincent, A.C. Realities of offering advice to governments on CITES. Conserv. Biol. 2019, 34, 644–653. [Google Scholar] [CrossRef] [PubMed]
- CITES. List of Parties to the Convention. 2020. Available online: https://www.cites.org/eng/disc/parties/index.php (accessed on 30 September 2020).
- Job, S.; Do, H.; Meeuwig, J.; Hall, H. Culturing the oceanic seahorse, Hippocampus Kuda. Aquaculture 2002, 214, 333–341. [Google Scholar] [CrossRef]
- Vincent, A.C.J. The International Trade in Seahorses; TRAFFIC International: Cambridge, UK, 1996; p. 163. [Google Scholar]
- Olivotto, I.; Chemello, G.; Vargas, A.; Randazzo, B.; Piccinetti, C.C.; Carnevali, O. Marine ornamental species culture: From the past to “Finding Dory”. Gen. Comp. Endocrinol. 2017, 245, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Dawes, J. International Experience in Ornamental Marine Species Management. Part 1: Perspectives. In Workshop: Management Strategies for the Marine Ornate Species of the Gulf of California; La Paz: Baja California Sur, Mexico, 1998; pp. 18–19. [Google Scholar]
- Moe, M.A. Marine ornamentals: The industry and the hobby. Proc. Marine Ornam. Univ. Hawaii Sea Grant College Progr. 2001, 99, 53–63. [Google Scholar]
- Moe, M.A. Culture of Marine Ornamentals: For Love, for Money, and for Science. In Marine Ornamental Species: Collection, Culture & Conservation; Cato, J., Brown, C., Eds.; Iowa State Press: Ames, IA, USA, 2003; pp. 11–28. [Google Scholar]
- Moorhead, J.A.; Zeng, C. Development of captive breeding techniques for marine ornamental fish: A review. Rev. Fish. Sci. 2010, 18, 315–343. [Google Scholar] [CrossRef]
- Palmtag, M.R. The Marine Ornamental Species Trade. In Marine Ornamental Species Aquaculture; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 3–14. [Google Scholar]
- Woods, C.M.C. Factors affecting successful culture of the seahorse, Hippocampus abdominalis, Leeson, 1827. In Marine Ornamental Species. Collection, Culture & Conservation; Cato, J., Brown, C., Eds.; Iowa State Press: Ames, IA, USA, 2003; pp. 277–288. [Google Scholar]
- Payne, M.; Rippingale, R. Rearing West Australian seahorse, Hippocampus subelongatus, juveniles on copepod nauplii and enriched Artemia. Aquaculture 2000, 188, 353–361. [Google Scholar] [CrossRef]
- Job, S.; Buu, D.; Vincent, A. Growth and survival of the tiger tail seahorse, Hippocampus comes. J. World Aquac. Soc. 2006, 37, 322–327. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species. 2020. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=126224 (accessed on 30 September 2020).
- Froese, R.; Pauly, D.; FishBase. World Wide Web Electronic Publication. 2019. Available online: https://www.fishbase.org (accessed on 30 September 2020).
- IUCN Red List search for Hippocampus. 2020. Available online: https://www.iucnredlist.org/search?query=Hippocampus&searchType=species (accessed on 30 September 2020).
- CITES Trade Database. 2020. Available online: https://trade.cites.org (accessed on 30 September 2020).
- Kuiter, R.H.; Tonozuka, T. Pictorial guide to Indonesian Reef Fishes. Part 1. Eels—Snappers, Muraenidae—Lutjanidae; Zoonetics: Melbourne, Australia, 2001; p. 866. [Google Scholar]
- Curtis, J.M.R.; Vincent, A.C.J. Life history of an unusual marine fish: Survival, growth and movement patterns of Hippocampus guttulatus Cuvier 1829. J. Fish Biol. 2006, 68, 707–733. [Google Scholar] [CrossRef]
- Han, S.-Y.; Kim, J.-K.; Kai, Y.; Senou, H. Seahorses of the Hippocampus coronatus complex: Taxonomic revision, and description of Hippocampus haema, a new species from Korea and Japan (Teleostei, Syngnathidae). ZooKeys 2017, 712, 113–139. [Google Scholar] [CrossRef] [Green Version]
- Kuiter, R.H. Hippocampus tristis, a Lazarus species of seahorse (Teleostei: Syngnathidae) from Australia. J. Ocean Sci. Found. 2020, 35, 41–47. [Google Scholar] [CrossRef]
- Kuiter, R.H. Revision of the Australian seahorses of the genus Hippocampus (Syngnathiformes: Syngnathidae) with descriptions of nine new species. Rec. Austr. Mus. 2001, 53, 293–340. [Google Scholar] [CrossRef] [Green Version]
- Lourie, S.A.; Kuiter, R.H. Three new pygmy seahorse species from Indonesia (Teleostei: Syngnathidae: Hippocampus). Zootaxa 2008, 1963, 54–68. [Google Scholar] [CrossRef] [Green Version]
- González, R.; Dinghi, P.; Corio, C.; Medina, A.; Maggioni, M.; Storero, L.; Gosztonyi, A. Genetic evidence and new morphometric data as essential tools to identify the Patagonian seahorse Hippocampus patagonicus (Pisces, Syngnathidae). J. Fish Biol. 2014, 84, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Fricke, R. Review of the pipefishes and seahorses (Teleostei: Syngnathidae) of New Caledonia, with descriptions of five new species. Stutt. Beitr. Naturkd. Ser. A 2004, 668, 1–66. [Google Scholar]
- Gomon, M.F.; Kuiter, R.H. Two new pygmy seahorses (Teleostei: Syngnathidae: Hippocampus) from the Indo-West Pacific. Aqua Int. J. Ichthyol. 2009, 15, 37–44. [Google Scholar]
- Randall, J.E.; Lourie, S.A. Hippocampus tyro, a new seahorse (Gasterosteiformes: Syngnathidae) from the Seychelles. Smithiana Bull. 2009, 10, 19–21. [Google Scholar]
- Short, G.; Smith, R.; Motomura, H.; Harasti, D.; Hamilton, H. Hippocampus japapigu, a new species of pygmy seahorse from Japan, with a redescription of H. pontohi (Teleostei, Syngnathidae). ZooKeys 2018, 779, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.; Gomon, M.F. A new seahorse (Teleostei: Syngnathidae: Hippocampus) from south-western Australia. Zootaxa 2010, 2613, 61–68. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Qin, G.; Wang, X.; Lin, Q. A new species of seahorse (Teleostei: Syngnathidae) from the South China Sea. Zootaxa 2016, 4170, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Short, G.; Claassens, L.; Smith, R.; De Brauwer, M.; Hamilton, H.; Stat, M.; Harasti, D. Hippocampus nalu, a new species of pygmy seahorse from South Africa, and the first record of a pygmy seahorse from the Indian Ocean (Teleostei, Syngnathidae). ZooKeys 2020, 934, 141–156. [Google Scholar] [CrossRef]
- Gardner, T. The copepod/Artemia Tradeoff in the Captive Culture of Hippocampus erectus, a Vulnerable Species in Lower New York State. In Marine Ornamental Species. Collection, Culture & Conservation; Cato, J., Brown, C., Eds.; Iowa State Press: Ames, IA, USA, 2003; pp. 297–303. [Google Scholar]
- Payne, M.F. Rearing the Coral Seahorse, Hippocampus barbouri, on Live and Inert Prey. In Marine Ornamental Species. Collection, Culture & Conservation; Cato, J., Brown, C., Eds.; Iowa State Press: Ames, IA, USA, 2003; pp. 289–296. [Google Scholar]
- Olivotto, I.; Planas, M.; Simões, N.; Holt, G.J.; Avella, M.A.; Calado, R. Advances in breeding and rearing marine ornamentals. J. World Aquac. Soc. 2011, 42, 135–166. [Google Scholar] [CrossRef]
- Utter, F.; Epifanio, J. Marine aquaculture: Genetic potentialities and pitfalls. Rev. Fish Biol. Fish. 2002, 12, 59–77. [Google Scholar] [CrossRef]
- Primavera, J.H. Global voices of science: Mangroves, fishponds, and the quest for sustainability. Science 2005, 310, 57–59. [Google Scholar] [CrossRef] [Green Version]
- Pullin, R.; Sumaila, U.R. Aquaculture. In Fish for Life: Interactive Governance for Fisheries; Kooiman, J., Bavinck, M., Jentoft, S., Pullin, R., Eds.; Amsterdam University Press: Amsterdam, The Netherlands, 2005; pp. 93–108. [Google Scholar]
- Weir, L.K.; Grant, J.W. Effects of aquaculture on wild fish populations: A synthesis of data. Environ. Rev. 2005, 13, 145–168. [Google Scholar] [CrossRef] [Green Version]
- Tlusty, M. The benefits and risks of aquacultural production for the aquarium trade. Aquaculture 2002, 205, 203–219. [Google Scholar] [CrossRef]
- Project Seahorse. IUCN SSC Seahorse, Pipefish and Seadragon Specialist Group. Available online: https://www.iucn-seahorse.org/iucn-global-assessments (accessed on 30 September 2020).
- Bos, A.R.; Hoeksema, B.W. Cryptobenthic fishes and co-inhabiting shrimps associated with the mushroom coral Heliofungia actiniformis (Fungiidae) in the Davao Gulf, Philippines. Environ. Biol. Fishes 2014, 98, 1479–1489. [Google Scholar] [CrossRef]
- Bos, A.R.; Hoeksema, B.W. Mushroom corals (Fungiidae) in the Davao Gulf, Philippines, with records of associated fish and other cryptofauna. Raffles Bull. Zool. 2017, 65, 198–206. [Google Scholar]
- Brandl, S.J.; Goatley, C.H.R.; Bellwood, D.R.; Tornabene, L. The hidden half: Ecology and evolution of cryptobenthic fishes on coral reefs. Biol. Rev. 2018, 93, 1846–1873. [Google Scholar] [CrossRef]
- De Brauwer, M.; Hobbs, J.A.; Ambo-Rappe, R.; Jompa, J.; Harvey, E.S.; McIlwain, J.L. Biofluorescence as a survey tool for cryptic marine species. Conserv. Biol. 2018, 32, 706–715. [Google Scholar] [CrossRef]
- De Brauwer, M.; Hobbs, J.-P.A.; Jompa, J. Widespread low abundance despite habitat availability elevates extinction risk in pygmy seahorses. Coral Reefs 2020, 39, 847–852. [Google Scholar] [CrossRef] [Green Version]
- Lourie, S.A.; Randall, J.E. A new pygmy seahorse, Hippocampus denise (Teleostei: Syngnathidae), from the Indo-Pacific. Zool. Stud. 2003, 42, 284–291. [Google Scholar]
- Nishikawa, J.; Fitzpatrick, R.; Reimer, J.D.; Beaman, R.J.; Yamamoto, H.; Lindsay, D.J. In situ observation of Denise’s pygmy seahorse Hippocampus denise associated with a gorgonian coral Annella reticulata at Osprey Reef, Australia. Galaxea J. Coral Reef Stud. 2011, 13, 25–26. [Google Scholar] [CrossRef] [Green Version]
- Van Der Meij, S.E.; Reijnen, B.T.; Van Ofwegen, L.P. Fish, fans and hydroids: Host species of pygmy seahorses. ZooKeys 2011, 103, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Foster, R.; Bridge, T.C.L.; Bongaerts, P. The first record of Hippocamus denise (Syngnathidae) from Australia. Aqua Int. J. Ichthyol. 2012, 18, 55–57. [Google Scholar]
- Smith, R.E.; Grutter, A.S.; Tibbetts, I.R. Extreme habitat specialisation and population structure of two gorgonian-associated pygmy seahorses. Mar. Ecol. Prog. Ser. 2012, 444, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, B.; Wandell, M.; Ross, R. Mating, birth, larval development and settlement of Bargibant’s pygmy seahorse, Hippocampus bargibanti (Syngnathidae), in aquaria. AACL Bioflux 2017, 10, 1049–1063. [Google Scholar]
- Gomon, M.F. A remarkable new pygmy seahorse (Syngnathidae: Hippocampus) from southeastern Australia, with a redescription of H. bargibanti Whitley from New Caledonia. Mem. Mus. Vic. 1997, 56, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Hoeksema, B.W. The hidden biodiversity of tropical coral reefs. Biodiversity 2017, 18, 8–12. [Google Scholar] [CrossRef]
- Heard, J.; Chen, J.-P.; Wen, C.K.C. Citizen science yields first records of Hippocampus japapigu and Hippocampus denise (Syngnathidae) from Taiwan: A hotspot for pygmy seahorse diversity. ZooKeys 2019, 883, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; Tibbetts, I.R. Mating and birth of Denise’s pygmy seahorses (Hippocampus denise) observed in the wild. Coral Reefs 2008, 27, 617. [Google Scholar] [CrossRef]
- Freret–Meurer, N.; Fernández, T.; Okada, N.; Vaccani, A.; Úrsula, R.D.J.U.S. Population dynamics of the endangered seahorse Hippocampus reidi Ginsburg, 1933 in a tropical rocky reef habitat. Anim. Biodivers. Conserv. 2018, 41, 345–456. [Google Scholar] [CrossRef]
- Vincent, A.C. A role for daily greetings in maintaining seahorse pair bonds. Anim. Behav. 1995, 49, 258–260. [Google Scholar] [CrossRef]
- Moreau, M.-A.; Vincent, A.C.J. Social structure and space use in a wild population of the Australian short-headed seahorse Hippocampus breviceps Peters, 1869. Mar. Freshw. Res. 2004, 55, 231–239. [Google Scholar] [CrossRef]
- Bell, E.M.; Lockyear, J.F.; McPherson, J.M.; Marsden, A.D.; Vincent, A.C. First field studies of an endangered South African seahorse, Hippocampus capensis. Environ. Biol. Fishes 2003, 67, 35–46. [Google Scholar] [CrossRef]
- Vaccani, A.C.; Freret-Meurer, N.V.; Bertoncini, Á.A.; Santos, L.N. Shining in the dark: First record of biofluorescence in the seahorse Hippocampus reidi. PLoS ONE 2019, 14, e0220561. [Google Scholar] [CrossRef] [Green Version]
- Curtis, J.; Vincent, A. Distribution of sympatric seahorse species along a gradient of habitat complexity in a seagrass-dominated community. Mar. Ecol. Prog. Ser. 2005, 291, 81–91. [Google Scholar] [CrossRef]
- Vincent, A.C.J.; Evans, K.L.; Marsden, A.D. Home range behaviour of the monogamous Australian seahorse, Hippocampus whitei. Environ. Biol. Fishes 2005, 72, 1–12. [Google Scholar] [CrossRef]
- Teske, P.R.; Lockyear, J.F.; Hecht, T.; Kaiser, H. Does the endangered Knysna seahorse, Hippocampus capensis, have a preference for aquatic vegetation type, cover or height? Afr. Zool. 2007, 42, 23–30. [Google Scholar] [CrossRef]
- Choi, Y.-U.; Rho, S.; Park, H.-S.; Kang, D.-H. Population characteristics of two seahorses, Hippocampus coronatus and Hippocampus mohnikei, around seagrass beds in the southern coastal waters of Korea. Ichthyol. Res. 2012, 59, 235–241. [Google Scholar] [CrossRef]
- Aylesworth, L.; Lawson, J.M.; Laksanawimol, P.; Ferber, P.; Loh, T.-L. New records of the Japanese seahorse Hippocampus mohnikei in Southeast Asia lead to updates in range, habitat and threats. J. Fish Biol. 2016, 88, 1620–1630. [Google Scholar] [CrossRef]
- Pinault, M.; Wickel, J.; Nicet, J.-B.; Chenoz, M.; de Montgolfier, B.; Fricke, R. First record of the near threatened native seahorse Hippocampus reidi (Teleostei: Syngnathidae) in an ecosystem dominated by the invasive seagrass Halophila stipulacea in the Caribbean Sea. Cybium 2018, 42, 393–396. [Google Scholar] [CrossRef]
- Goffredo, S.; Piccinetti, C.; Zaccanti, F. Volunteers in marine conservation monitoring: A study of the distribution of seahorses carried out in collaboration with recreational scuba divers. Conserv. Biol. 2004, 18, 1492–1503. [Google Scholar] [CrossRef] [Green Version]
- Dias, T.L.; Rosa, I.L. Habitat preferences of a seahorse species, Hippocampus reidi (Teleostei: Syngnathidae) in Brazil. Aqua Int. J. Ichthyol. 2003, 6, 165–176. [Google Scholar]
- Rosa, I.L.; Oliveira, T.P.R.; Castro, A.L.C.; Moraes, L.E.D.S.; Xavier, J.H.A.; Nottingham, M.C.; Dias, T.L.P.; Bruto-Costa, L.V.; Araújo, M.E.; Birolo, A.B.; et al. Population characteristics, space use and habitat associations of the seahorse Hippocampus reidi (Teleostei: Syngnathidae). Neotrop. Ichthyol. 2007, 5, 405–414. [Google Scholar] [CrossRef]
- Aylesworth, L.A.; Xavier, J.H.; Oliveira, T.P.R.; Tenorio, G.D.; Diniz, A.F.; Rosa, I.L. Regional-scale patterns of habitat preference for the seahorse Hippocampus reidi in the tropical estuarine environment. Aquat. Ecol. 2015, 49, 499–512. [Google Scholar] [CrossRef]
- Harasti, D.; Martin-Smith, K.; Gladstone, W. Ontogenetic and sex-based differences in habitat preferences and site fidelity of White’s seahorse Hippocampus whitei. J. Fish Biol. 2014, 85, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Zhang, Y.; Huang, L.; Lin, Q. Effects of water current on swimming performance, ventilation frequency, and feeding behavior of young seahorses (Hippocampus erectus). J. Exp. Mar. Biol. Ecol. 2014, 461, 337–343. [Google Scholar] [CrossRef]
- Simpson, M.; Morris, R.L.; Harasti, D.; Coleman, R.A. Swimming nets have positive effects on populations of the endangered White’s seahorse Hippocampus whitei. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 60–73. [Google Scholar] [CrossRef]
- Correia, M.; Palma, J.; Koldewey, H.; Andrade, J.P. Can artificial holdfast units work as a habitat restoration tool for long-snouted seahorse (Hippocampus guttulatus Cuvier)? J. Exp. Mar. Biol. Ecol. 2013, 448, 258–264. [Google Scholar] [CrossRef]
- Correia, M.; Koldewey, H.; Andrade, J.P.; Palma, J. Effects of artificial holdfast units on seahorse density in the Ria Formosa lagoon, Portugal. J. Exp. Mar. Biol. Ecol. 2015, 471, 1–7. [Google Scholar] [CrossRef]
- Claassens, L. An artificial water body provides habitat for an endangered estuarine seahorse species. Estuar. Coast. Shelf Sci. 2016, 180, 1–10. [Google Scholar] [CrossRef]
- Claassens, L.; Hodgson, A.N. Monthly population density and structure patterns of an endangered seahorse Hippocampus capensis: A comparison between natural and artificial habitats. J. Fish Biol. 2018, 92, 2000–2015. [Google Scholar] [CrossRef] [PubMed]
- Claassens, L.; Booth, A.J.; Hodgson, A.N. An endangered seahorse selectively chooses an artificial structure. Environ. Biol. Fishes 2018, 101, 723–733. [Google Scholar] [CrossRef]
- Simpson, M.; Morris, R.L.; Harasti, D.; Coleman, R.A. The endangered White’s seahorse Hippocampus whitei chooses artificial over natural habitats. J. Fish Biol. 2019, 95, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.; Coleman, R.A.; Morris, R.L.; Harasti, D. Seahorse hotels: Use of artificial habitats to support populations of the endangered White’s seahorse Hippocampus whitei. Mar. Environ. Res. 2020, 157, 104861. [Google Scholar] [CrossRef]
- Evanson, M.; Foster, S.J.; Wisedel, S.; Vincent, A.C.J. Tracking the International Trade of Seahorses (Hippocampus Species); Fisheries Centre Research Reports 19; University of British Columbia: Vancouver, BC, Canada, 2011; p. 94. [Google Scholar]
- Zhang, Y.Y.; Ryu, B.-M.; Qian, Z.-J. A review—Biology, aquaculture and medical use of seahorse, Hippocampus spp. Annu. Res. Rev. Biol. 2017, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rosa, I.L.; Oliveira, T.P.R.; Osório, F.M.; Moraes, L.E.; Castro, A.L.C.; Barros, G.M.L.; Alves, R.R.N. Fisheries and trade of seahorses in Brazil: Historical perspective, current trends, and future directions. Biodivers. Conserv. 2011, 20, 1951–1971. [Google Scholar] [CrossRef]
- Sanaye, S.V.; Khandeparker, R.; Rayadurga, A.S.; Shivaramu, M.S.; Kankonkar, H.; Narvekar, J.; Gauthankar, M. Morphological and molecular evidence for first records and range extension of the Japanese seahorse, Hippocampus mohnikei (Bleeker, 1853) in a bay-estuarine system of Goa, central west coast of India. PLoS ONE 2020, 15, e0220420. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Zhang, Y.; Ho, A.L.F.C.; Zhang, Y.; Lin, Q. Seasonal distribution and reproductive strategy of seahorses. ICES J. Mar. Sci. 2017, 74, 2170–2179. [Google Scholar] [CrossRef]
- Han, S.-Y.; Kim, J.-K.; Tashiro, F.; Kai, Y.; Yoo, J.-T. Relative importance of ocean currents and fronts in population structures of marine fish: A lesson from the cryptic lineages of the Hippocampus mohnikei complex. Mar. Biodivers. 2017, 49, 263–275. [Google Scholar] [CrossRef]
- Lourie, S.A.; Vincent, A.C.J. A marine fish follows Wallace’s line: The phylogeography of the three-spot seahorse (Hippocampus trimaculatus, Syngnathidae, Teleostei) in Southeast Asia. J. Biogeogr. 2004, 31, 1975–1985. [Google Scholar] [CrossRef]
- Meeuwig, J.J.; Hoang, D.H.; Ky, T.S.; Job, S.D.; Vincent, A.C. Quantifying non-target seahorse fisheries in central Vietnam. Fish. Res. 2006, 81, 149–157. [Google Scholar] [CrossRef]
- Murugan, A.; Dhanya, S.; Sarcar, A.B.; Naganathan, V.; Rajagopal, S.; Balasubramanian, T. Fishery biology, demography of the three-spotted seahorse, Hippocampus trimaculatus inhabiting Gulf of Mannar region, southeast coast of India. Indian J. Geo-Mar. Sci. 2011, 40, 411–423. [Google Scholar]
- Laksanawimol, P.; Petpiroon, S.; Damrongphol, P. Trade of seahorses, Hippocampus spp. (Actinopterygii: Syngnathiformes: Syngnathidae), on the east coast of the gulf of Thailand. Acta Ichthyol. Piscat. 2013, 43, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.S.; Harun, M.F.; Yap, C.K. Captive breeding, rearing and closing of reproductive cycle of the three spot seahorse, Hippocampus trimaculatus (Leach, 1814) PDF. Acad. J. Life Sci. 2020, 6, 27–33. [Google Scholar] [CrossRef]
- Martin-Smith, K.M.; Vincent, A.C.J. Exploitation and trade of Australian seahorses, pipehorses, sea dragons and pipefishes (Family Syngnathidae). Oryx 2006, 40, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Parsons, E.C. Why IUCN should replace “Data Deficient” conservation status with a precautionary “Assume Threatened” status—A cetacean case study. Front. Mar. Sci. 2016, 3, 193. [Google Scholar] [CrossRef] [Green Version]
- Martin-Smith, K.M.; Samoilys, M.A.; Meeuwig, J.J.; Vincent, A.C. Collaborative development of management options for an artisanal fishery for seahorses in the central Philippines. Ocean Coast. Manag. 2004, 47, 165–193. [Google Scholar] [CrossRef]
- Bruckner, A.W.; Field, J.D.; Daves, N. (Eds.) The Proceedings of the International Workshop on CITES Implementation for Seahorse Conservation and Trade. NOAA Technical; Memorandum NMFS-OPR-36: Silver Spring, MD, USA, 2005; p. 171. [Google Scholar]
- Foster, S.J.; Vincent, A.C.J. Enhancing sustainability of the international trade in seahorses with a single minimum size limit. Conserv. Biol. 2005, 19, 1044–1050. [Google Scholar] [CrossRef]
- Carlson, J.K.; Horn, C.; Smith, K.L.; Bethea, D.M. Population viability analysis of the dwarf seahorse, Hippocampus zosterae, in Florida. NOAA Techn. Mem. 2019, NMFSSEFSC-SEFSC-739, 1–37. [Google Scholar] [CrossRef]
- Masonjones, H.D.; Rose, E.; Masonjones, M.C. Techniques used to increase recapture rates of dwarf seahorses (Hippocampus zosterae) in Tampa Bay: Implications for population estimates and movement patterns. Gulf Caribb. Res. 2019, 30, 10–19. [Google Scholar] [CrossRef]
- Giwojna, P. A Step-by-Step Book about Seahorses; T.F.H. Publications: Neptune City, NJ, USA, 1990; p. 64. [Google Scholar]
- Garrick-Maidment, N. Seahorses: Conservation and Care; T.F.H. Kingdom Books: London, UK, 1997; p. 48. [Google Scholar]
- Indiviglio, F. Seahorses. A Complete Pet Owner’s Manual; B.E.S. Publishing: Hauppauge, NY, USA, 2001; p. 96. [Google Scholar]
- Eldington, E. Seahorses as Pets. Seahorse Complete Owner’s Manual. Seahorse Care, Health, Tank, Costs and Feeding; Imb Publishing: San Bernardino, CA, USA, 2015; p. 132. [Google Scholar]
- Horsnby, T. So You Want to Keep Seahorses; CreateSpace Independent Publishing Platform: Charleston, SC, USA, 2016; p. 52. [Google Scholar]
- French, M. Seahorses for Beginners, 2nd ed.; CreateSpace Independent Publishing Platform: Charleston, CA, USA, 2017; p. 165. [Google Scholar]
- Abbott, A.W. The Complete Guide to Dwarf Seahorses in the Aquarium; T.F.H. Publications: Neptune City, NJ, USA, 2003; p. 144. [Google Scholar]
- Horsnby, T. So You Want to Keep Dwarf Seahorses; CreateSpace Independent Publishing Platform: Charleston, CA, USA, 2016; p. 54. [Google Scholar]
- French, M. Dwarf Seahorses for Beginners; CreateSpace Independent Publishing Platform: Charleston, CA, USA, 2019; p. 66. [Google Scholar]
- Koldewey, H. (Ed.) Syngnathid Husbandry in Public Aquariums; Manual; Project Seahorse: Vancouver, BC, Canada; Zoological Society of London: London, UK, 2005; p. 137. [Google Scholar]
- De Brauwer, M.; Gordon, L.M.; Shalders, T.C.; Saunders, B.J.; Archer, M.; Harvey, E.S.; Collin, S.P.; Partridge, J.C.; McIlwain, J.L. Behavioural and pathomorphological impacts of flash photography on benthic fishes. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giglio, V.J.; Ternes, M.L.F.; Kassuga, A.D.; Ferreira, C.E.L. Scuba diving and sedentary fish watching: Effects of photographer approach on seahorse behavior. J. Ecotourism 2018, 18, 142–151. [Google Scholar] [CrossRef]
- Scales, H. Advances in the ecology, biogeography and conservation of seahorses (genus Hippocampus). Prog. Phys. Geogr. Earth Environ. 2010, 34, 443–458. [Google Scholar] [CrossRef]
- Rose, E.; Simmonds, M.; Hayashida-Boyles, A.L.; Masonjones, H.D. Seasonal and spatial variation in the reproductive biology of the dwarf seahorse Hippocampus zosterae. J. Fish Biol. 2019, 95, 357–366. [Google Scholar] [CrossRef]
- Lourie, S.A.; Pollom, R.A.; Foster, S.J. A global revision of the seahorses Hippocampus rafinesque 1810 (Actinopterygii: Syngnathiformes): Taxonomy and biogeography with recommendations for further research. Zootaxa 2016, 4146, 1–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subburaman, S.; Murugan, A.; Goutham, S.; Kaul, R.; Jothi, P.V.R.P.; Balasubramanian, T. First distributional record of the giraffe seahorse, Hippocampus camelopardalis Bianconi 1854 (Family: Syngnathidae) from Gulf of Kachchh waters, North west coast. Indian J. Geo-Mar. Sci. 2014, 43, 408–411. [Google Scholar]
- Kim, S.Y.; Kweon, S.M.; Choi, S.H. First record of Hippocampus sindonis (Syngnathiformes: Syngnathidae) from Korea. Korean J. Ichthyol. 2013, 25, 41–44. [Google Scholar]
- Szabo, Z.; Kimokeo, B.K.; Toonen, R.J.; Randall, J.E. On the status of the Hawaiian seahorses Hippocampus hilonis, H. histrix and H. fisheri (Syngnathidae). Mar. Biol. Res. 2011, 7, 701–709. [Google Scholar] [CrossRef]
- Brittain, K. Fishers’ seahorse, Hippocampus fisheri. In Syngnathid Husbandry in Public Aquariums; Manual; Koldewey, H., Ed.; Project Seahorse: Vancouver, BC, Canada; Zoological Society of London: London, UK, 2005; pp. 62–63. [Google Scholar]
- Vincent, A.C.J.; Koldewey, H.J. An uncertain future for seahorse aquaculture in conservation and economic contexts. In Proceedings of the Regional Technical Consultation on Stock Enhancement for Threatened Species of International Concern, Iloilo City, Philippines, 13–15 July 2005; Primavera, J.H., Quinitio, E.T., Eguia, M.R.R., Eds.; Aquaculture Department, Southeast Asian Fisheries Development Center: Tigbauan, Philippines, 2006; pp. 71–84. [Google Scholar]
- Alford, K.; Grist, C. Dwarf Seahorse, Hippocampus zosterae. In Syngnathid Husbandry in Public Aquariums; Manual; Koldewey, H., Ed.; Project Seahorse: Vancouver, BC, Canada; Zoological Society of London: London, UK, 2005; pp. 88–90. [Google Scholar]
- Fedrizzi, N.; Stiassny, M.L.J.; Boehm, J.T.; Dougherty, E.R.; Amato, G.; Mendez, M. Population genetic structure of the dwarf seahorse (Hippocampus zosterae) in Florida. PLoS ONE 2015, 10, e0132308. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, X.; Huang, B. The genus Hippocampus—A review on traditional medicinal uses, chemical constituents and pharmacological properties. J. Ethnopharmacol. 2015, 162, 104–111. [Google Scholar] [CrossRef]
- Kumaravel, K.; Ravichandran, S.; Balasubramanian, T.; Sonneschein, L. Seahorses—A source of traditional medicine. Nat. Prod. Res. 2012, 26, 2330–2334. [Google Scholar] [CrossRef]
- Louw, S.; Bürgener, M. Seahorse trade dynamics from Africa to Asia. TRAFFIC Bull. 2020, 32, 37–44. [Google Scholar]
- Nijman, V. An overview of international wildlife trade from Southeast Asia. Biodivers. Conserv. 2009, 19, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Foster, S.J.; Kuo, T.-C.; Wan, A.K.Y.; Vincent, A.C. Global seahorse trade defies export bans under CITES action and national legislation. Mar. Policy 2019, 103, 33–41. [Google Scholar] [CrossRef]
- Lam, J.T.; Koldewey, H.J.; Yasue, M.; Vincent, A.C. Comparing interview and trade data in assessing changes in the seahorse Hippocampus spp. trade following CITES listing. Oryx 2014, 50, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Wright, K.A.; Woods, C.M.C.; Gray, B.E.; Lokman, P.M. Recovery from acute, chronic and transport stress in the pot-bellied seahorse Hippocampus abdominalis. J. Fish Biol. 2007, 70, 1447–1457. [Google Scholar] [CrossRef]
- Cohen, F.P.; Planas, M.; Valenti, W.C.; Lillebø, A.; Calado, R. Optimizing packing of live seahorses for shipping. Aquaculture 2018, 482, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Cohen, F.P.A.; Valenti, W.C.; Planas, M.; Calado, R. Seahorse aquaculture, biology and conservation: Knowledge gaps and research opportunities. Rev. Fish. Sci. Aquac. 2016, 25, 100–111. [Google Scholar] [CrossRef]
- Fioravanti, M.L.; Florio, D. Common diseases in marine ornamental fishes. Marine Ornam. Species Aquac. 2017, 347–380. [Google Scholar]
- Dzyuba, B.; Van Look, K.J.W.; Cliffe, A.; Koldewey, H.J.; Holt, W.V. Effect of parental age and associated size on fecundity, growth and survival in the yellow seahorse Hippocampus kuda. J. Exp. Biol. 2006, 209, 3055–3061. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, M.; Okubo, R.; Harada, A.; Miyasaka, K.; Takada, K.; Hiroi, J.; Yasumasu, S. Morphology of brood pouch formation in the pot-bellied seahorse Hippocampus abdominalis. Zool. Lett. 2017, 3, 19. [Google Scholar] [CrossRef]
- Stölting, K.N.; Wilson, A.B. Male pregnancy in seahorses and pipefish: Beyond the mammalian model. BioEssays 2007, 29, 884–896. [Google Scholar] [CrossRef]
- Wilson, A.B.; Vincent, A.; Ahnesjö, I.; Meyer, A. Male pregnancy in seahorses and pipefishes (Family Syngnathidae): Rapid diversification of paternal brood pouch morphology inferred from a molecular phylogeny. J. Hered. 2001, 92, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Whittington, C.M.; Friesen, C.R. The evolution and physiology of male pregnancy in syngnathid fishes. Biol. Rev. 2020, 95, 1252–1272. [Google Scholar] [CrossRef]
- Planas, M.; Quintas, P.; Chamorro, A.; Silva, C.N.S.; Oliver, M.P.; Planas, M. Female maturation, egg characteristics and fatty acids profile in the seahorse Hippocampus guttulatus. Anim. Reprod. Sci. 2010, 122, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Planas, M.; Quintas, P.; Chamorro, A.; Oliver, M.P. Maturation of Hippocampus guttulatus and Hippocampus hippocampus females by manipulation of temperature and photoperiod regimes. Aquaculture 2013, 388–391, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, R.L.; Musick, J.A. Reproduction and food habits of the lined seahorse, Hippocampus erectus (Teleostei: Syngnathidae) of Chesapeake bay, Virginia. Braz. J. Biol. 2001, 61, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Woods, C.M.C. Natural diet of the seahorse Hippocampus abdominalis. N. Z. J. Mar. Freshw. Res. 2002, 36, 655–660. [Google Scholar] [CrossRef]
- Kitsos, M.-S.; Tzomos, T.; Anagnostopoulou, L.; Koukouras, A. Diet composition of the seahorses, Hippocampus guttulatus Cuvier, 1829 and Hippocampus hippocampus (L., 1758) (Teleostei, Syngnathidae) in the Aegean Sea. J. Fish Biol. 2008, 72, 1259–1267. [Google Scholar] [CrossRef]
- Storero, L.P.; González, R.A. Prey selectivity and trophic behavior of the patagonian seahorse, Hippocampus patagonicus, in captivity. J. World Aquac. Soc. 2009, 40, 394–401. [Google Scholar] [CrossRef]
- Gurkan, S.; Taskavak, E.; Sever, T.M.; Akalin, S. Gut contents of two European seahorses Hippocampus hippocampus and Hippocampus guttulatus in the Aegean Sea, Coasts of Turkey. Pak. J. Zool. 2011, 43, 1197–1201. [Google Scholar]
- Valladares, S.; Soto, D.X.; Planas, M. Dietary composition of endangered seahorses determined by stable isotope analysis. Mar. Freshw. Res. 2017, 68, 831. [Google Scholar] [CrossRef] [Green Version]
- Nenciu, M.I.; Harcota, G.E.; Totoiu, A.; Bisinicu, E.; Filimon, A.; Nita, V.N. Prey preference of the long-snouted seahorse (Hippocampus guttulatus Cuvier, 1829) at the Romanian Black Sea coast. Sci. Pap. Ser. D. Anim. Sci. 2018, 61, 348–355. [Google Scholar]
- Castro, A.L.D.C.; Diniz, A.D.F.; Martins, I.Z.; Vendel, A.L.; De Oliveira, T.P.R.; Rosa, I.M.D.L. Assessing diet composition of seahorses in the wild using a non-destructive method: Hippocampus reidi (Teleostei: Syngnathidae) as a study-case. Neotrop. Ichthyol. 2008, 6, 637–644. [Google Scholar] [CrossRef]
- Francesca, A.; Corriero, G.; Mirto, S.; Pierri, C.; Lazic, T.; Gristina, M. Trophic flexibility and prey selection of the wild long-snouted seahorse Hippocampus guttulatus Cuvier, 1829 in three coastal habitats. Estuar. Coast. Shelf Sci. 2019, 224, 1–10. [Google Scholar] [CrossRef]
- Bergert, B.A.; Wainwright, P.C. Morphology and kinematics of prey capture in the syngnathid fishes Hippocampus erectus and Syngnathus floridae. Mar. Biol. 1997, 127, 563–570. [Google Scholar] [CrossRef]
- Roos, G.; Van Wassenbergh, S.; Herrel, A.; Aerts, P.; Tresguerres, M.; Katoh, F.; Fenton, H.; Jasinska, E.; Goss, G.G. Kinematics of suction feeding in the seahorse Hippocampus reidi. J. Exp. Biol. 2009, 212, 3490–3498. [Google Scholar] [CrossRef] [Green Version]
- Roos, G.; Van Wassenbergh, S.; Herrel, A.; Adriaens, D.; Aerts, P. Snout allometry in seahorses: Insights on optimisation of pivot feeding performance during ontogeny. J. Exp. Biol. 2010, 213, 2184–2193. [Google Scholar] [CrossRef] [Green Version]
- Roos, G.; Van Wassenbergh, S.; Aerts, P.; Herrel, A.; Adriaens, D. Effects of snout dimensions on the hydrodynamics of suction feeding in juvenile and adult seahorses. J. Theor. Biol. 2011, 269, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Gemmell, B.J.; Sheng, J.; Buskey, E.J. Morphology of seahorse head hydrodynamically aids in capture of evasive prey. Nat. Commun. 2013, 4, 2840. [Google Scholar] [CrossRef]
- Blanco, A.; Planas, M. Mouth growth and prey selection in juveniles of the European long-snouted seahorse, Hippocampus guttulatus. J. World Aquac. Soc. 2015, 46, 596–607. [Google Scholar] [CrossRef]
- Manning, C.G.; Foster, S.J.; Vincent, A.C.J. A review of the diets and feeding behaviours of a family of biologically diverse marine fishes (Family Syngnathidae). Rev. Fish Biol. Fish. 2019, 29, 197–221. [Google Scholar] [CrossRef]
- Olivotto, I.; Avella, M.; Sampaolesi, G.; Piccinetti, C.; Ruiz, P.N.; Carnevali, O. Breeding and rearing the longsnout seahorse Hippocampus reidi: Rearing and feeding studies. Aquaculture 2008, 283, 92–96. [Google Scholar] [CrossRef]
- Hora, M.D.S.C.D.; Joyeux, J.-C. Closing the reproductive cycle: Growth of the seahorse Hippocampus reidi (Teleostei, Syngnathidae) from birth to adulthood under experimental conditions. Aquaculture 2009, 292, 37–41. [Google Scholar] [CrossRef]
- Murugan, A.; Dhanya, S.; Sreepada, R.; Rajagopal, S.; Balasubramanian, T. Breeding and mass-scale rearing of three spotted seahorse, Hippocampus trimaculatus Leach under captive conditions. Aquaculture 2009, 290, 87–96. [Google Scholar] [CrossRef]
- Planas, M.; Olivotto, I.; González, M.J.; Laurà, R.; Zarantoniello, M. A Multidisciplinary experimental study on the effects of breeders diet on newborn seahorses (Hippocampus guttulatus). Front. Mar. Sci. 2020, 7, 638. [Google Scholar] [CrossRef]
- Planas, M. Carry-over effects of pre-breeding diets on seahorse (Hippocampus reidi) reproductive success. Aquaculture 2021, 533, 736148. [Google Scholar] [CrossRef]
- Qin, G.; Lin, Q.; Gu, N.; Lin, J.; Huang, L. Effect of broodstock origin, background and substrate color on skin coloration of three-spotted seahorses Hippocampus trimaculatus Leach, 1814. J. Exp. Mar. Biol. Ecol. 2012, 416–417, 129–134. [Google Scholar] [CrossRef]
- Segade, A.; Robaina, L.; Romero, J.G.; Domínguez, L.M.; Otero-Ferrer, F. Effects of the diet on seahorse (Hippocampus hippocampus) growth, body colour and biochemical composition. Aquac. Nutr. 2014, 21, 807–813. [Google Scholar] [CrossRef]
- Fonseca, T.; David, F.S.; Ribeiro, F.A.S.; Wainberg, A.A.; Valenti, W.C. Technical and economic feasibility of integrating seahorse culture in shrimp/oyster farms. Aquac. Res. 2015, 48, 655–664. [Google Scholar] [CrossRef]
- Adams, M.B.; Powell, M.D.; Purser, G.J. Effect of acute and chronic ammonia and nitrite exposure on oxygen consumption and growth of juvenile big bellied seahorse. J. Fish Biol. 2001, 58, 848–860. [Google Scholar] [CrossRef]
- Chang, M.; Southgate, P.C. Effects of varying dietary fatty acid composition on growth and survival of seahorse, Hippocampus sp., juveniles. Aquar. Sci. Conserv. 2001, 3, 205–214. [Google Scholar] [CrossRef]
- Project Seahorse. Seahorse Aquaculture. Position Statements. 20 April 2009. Available online: https://www.projectseahorse.org/conservation-tools/2015/9/23/seahorse-aquaculture (accessed on 28 September 2020).
- Li, H.; Sun, H.; Bai, X.; Lin, Q.; Liu, X.; Wang, Y.; Wang, L.; Yan, D. HC2 of Pseudomonas sp. Induced enteritis in Hippocampus japonicus. Aquac. Res. 2014, 47, 2027–2030. [Google Scholar] [CrossRef]
- Xie, J.; Bu, L.; Jin, S.; Wang, X.; Zhao, Q.; Zhou, S.; Xu, Y. Outbreak of vibriosis caused by Vibrio harveyi and Vibrio alginolyticus in farmed seahorse Hippocampus kuda in China. Aquaculture 2020, 523, 735168. [Google Scholar] [CrossRef]
- Alcaide, E.; Gil-Sanz, C.; Sanjuan, E.; Esteve, D.; Amaro, C.; Silveira, L.; Gil-Sanz, C. Vibrio harveyi causes disease in seahorse, Hippocampus sp. J. Fish Dis. 2001, 24, 311–313. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, N.; Huang, H.; Feng, H.; Li, Y.; Han, B. Short communication: Recovery of Vibrio vulnificus from head ulceration in seahorse (Hippocampus kuda). Aquac. Int. 2019, 28, 653–660. [Google Scholar] [CrossRef]
- Lin, T.; Zhang, D.; Liu, X.; Xiao, D. Variations of immune parameters in the lined seahorse Hippocampus erectus after infection with enteritis pathogen of Vibrio parahaemolyticus. Fish Shellfish Immunol. 2016, 50, 247–254. [Google Scholar] [CrossRef]
- Shao, P.; Yong, P.; Wang, X.; Xie, S.; Fan, Y.; Zang, L.; Cui, L.; Sun, J. Isolation, identification, and histopathological analysis of Vibrio tubiashii from lined seahorse Hippocampus erectus. Dis. Aquat. Org. 2019, 133, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, X.; Xu, Y. Altered intestinal microbiota composition associated with enteritis in yellow seahorses Hippocampus kuda (Bleeker, 1852). Curr. Microbiol. 2020, 77, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Wang, X.; Tan, S.; Lin, Q. A bacterial infection by Vibrio harveyi causing heavy reduction of cultured lined seahorse Hippocampus erectus. J. Fish Dis. 2016, 40, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Zhang, Y.; Zhang, B.; Wang, X.; Yin, J.; Lin, Q. Seahorse TLR5 gene responses to Vibrio vulnificus infection, which in combination with scuticociliates causes heavy reductions in seahorse aquaculture. J. Fish Dis. 2018, 41, 1933–1936. [Google Scholar] [CrossRef]
- Planas, M.; Chamorro, A.; Quintas, P.; Vilar, A. Establishment and maintenance of threatened long-snouted seahorse, Hippocampus guttulatus, broodstock in captivity. Aquaculture 2008, 283, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Palma, J.; Bureau, D.P.; Andrade, J.P. The effect of diet on ontogenic development of the digestive tract in juvenile reared long snout seahorse Hippocampus guttulatus. Fish Physiol. Biochem. 2013, 40, 739–750. [Google Scholar] [CrossRef]
- Tanu; Deobagkar, D.D.; Khandeparker, R.; Sreepada, R.A.; Sanaye, S.V.; Pawar, H.B. A study on bacteria associated with the intestinal tract of farmed yellow seahorse, Hippocampus kuda (Bleeker, 1852): Characterization and extracellular enzymes. Aquac. Res. 2012, 43, 386–394. [Google Scholar] [CrossRef]
- Li, F.; Wang, K.; Luo, W.; Huang, L.; Lin, Q. Comparison of the intestinal bacterial flora in healthy and intestinal-diseased seahorses Hippocampus trimaculatus, Hippocampus erectus, and Hippocampus spinosissimus. J. World Aquac. Soc. 2015, 46, 263–272. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Qin, G.; Luo, W.; Lin, Q. A novel pathogenic bacteria (Vibrio fortis) causing enteritis in cultured seahorses, Hippocampus erectus Perry, 1810. J. Fish Dis. 2015, 39, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.L.; Janetski, N.; Abbott, J.; Blankenhorn, S.; Cheng, B.; Crafton, R.E.; Hameed, S.O.; Rapi, S.; Trockel, D. Ornamental marine species culture in the coral triangle: Seahorse demonstration project in the Spermonde Islands, Sulawesi, Indonesia. Environ. Manag. 2014, 54, 1342–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, S.J.; Aylesworth, L.; Do, H.H.; Bat, N.K.; Vincent, A.C.J. Seahorse Exploitation and Trade in Vietnam; Fisheries Centre Research Reports 25(2); University of British Columbia: Vancouver, BC, Canada, 2017; p. 53. [Google Scholar]
- Hettiarachchi, H.A.S.U.; Edirisinghe, U. Captive breeding of Hippocampus reidi Ginsburg, 1933 (Longsnout Seahorse) in Sri Lanka under artificial conditions. Trop. Agric. Res. 2017, 29, 77. [Google Scholar] [CrossRef]
- Ferrol-Schulte, D.; Wolff, M.; Ferse, S.; Glaser, M. Sustainable livelihoods approach in tropical coastal and marine social–ecological systems: A review. Mar. Policy 2013, 42, 253–258. [Google Scholar] [CrossRef]
- Cohen, F.P.; Valenti, W.C. Opportunities and constraints for developing low-cost aquaculture of seahorses in mangrove estuaries. Aquaculture 2019, 502, 121–127. [Google Scholar] [CrossRef]
- Robertson, A.I.; Blaber, S.J.M. Plankton, Epibenthos and Fish Communities. In Tropical Mangrove Ecosystems; Robertson, A.I., Alongi, D.M., Eds.; American Geophysical Union: Washington, DC, USA, 1992; pp. 173–224. [Google Scholar]
- Yip, M.; Lim, A.; Chong, V.; Lawson, J.; Foster, S. Food and feeding habits of the seahorses Hippocampus spinosissimus and Hippocampus trimaculatus (Malaysia). J. Mar. Biol. Assoc. U.K. 2014, 95, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.M.B.; Hilomen-Garcia, G.V.; Celino, F.T.; Gonzales, T.T.; Maliao, R.J. Diet composition and feeding periodicity of the seahorse Hippocampus barbouri reared in illuminated sea cages. Aquaculture 2012, 358–359, 1–5. [Google Scholar] [CrossRef]
- Xu, Y.; Mu, J. Study on an integrated eco-aquaculture system for rearing the yellow seahorse, Hippocampus kuda Bleeker. Aquac. Res. 2016, 48, 2868–2875. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, J.; Chen, S. Polyculture of the lined seahorse, Hippocampus erectus Perry, 1810 with two species of macroalgae in aquaria. Acta Oceanol. Sin. 2010, 29, 26–32. [Google Scholar] [CrossRef]
- Job, S. Integrating marine conservation and sustainable development: Community-based aquaculture of marine aquarium fish. SPC Life Reef Fish Info Bull. 2005, 13, 24–29. [Google Scholar]
- Vaidyanathan, T.; Zhang, X.; Balakrishnan, R.; Vincent, A. Catch and trade bans for seahorses can be negated by non-selective fisheries. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 43–59. [Google Scholar] [CrossRef]
- Gurjão, L.M.; Barros, G.M.L.; Lopes, D.P.; Machado, D.A.N.; Lotufo, T.M.C. Illegal trade of aquarium species through the Brazilian postal service in Ceará State. Mar. Freshw. Res. 2018, 69, 178–185. [Google Scholar] [CrossRef]
- De Gurjão, L.M.; Lotufo, T.M.D.C. Native species exploited by marine aquarium trade in Brazil. Biota Neotrop. 2018, 18, 20170387. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.J.; Benítez-Díaz, H.; Clemente-Muñoz, M.Á.; Donaldson, J.; Hutton, J.M.; McGough, H.N.; Medellin, R.A.; Morgan, D.H.; O’Criodain, C.; Oldfield, T.E.; et al. Assessing the impacts of international trade on CITES-listed species: Current practices and opportunities for scientific research. Biol. Conserv. 2011, 144, 82–91. [Google Scholar] [CrossRef]
- Meeuwig, J.; Samoilys, M.; Erediano, J.; Koldewey, H. Fishers’ Perceptions on the Seahorse Fishery in Central Philippines: Interactive Approaches and an Evaluation of Results. In Fishers’ Knowledge in Fisheries Science and Management; Coastal Management Sourcebooks 4; Haggan, N., Neis, B., Baird, I.G., Eds.; UNESCO: Paris, France, 2003; pp. 179–201. [Google Scholar]
- Rosa, I.M.; Alves, R.R.; Bonifácio, K.M.; Mourão, J.S.; Osório, F.M.; Oliveira, T.P.; Nottingham, M.C. Fishers’ knowledge and seahorse conservation in Brazil. J. Ethnobiol. Ethnomed. 2005, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Ternes, M.L.F.; Gerhardinger, L.C.; Schiavetti, A. Seahorses in focus: Local ecological knowledge of seahorse-watching operators in a tropical estuary. J. Ethnobiol. Ethnomed. 2016, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ulfah, F.; Afrizal; Pratomo, A. Sustainability of seahorses: Lessons learned of local wisdom from Bintan Islands, Riau Islands Province. E3S Web Conf. 2018, 47, 07001. [Google Scholar] [CrossRef]
- Perry, A.L.; Vaidyanathan, T.; Giles, B.; Moreau, M.-A.; Picco, C.M.; Vincent, A.C.J. The Catch and Trade of Seahorses in India Pre-Ban; Fisheries Centre Research Reports 28(3); University of British Columbia: Vancouver, BC, Canada, 2020; p. 55. [Google Scholar]
- Hall, H.; Warmolts, D. The Role of Public Aquariums in the Conservation and Sustainability of the Marine Ornamentals Trade. In Marine Ornamental Species: Collection, Culture and Conservation; Cato, J., Brown, B., Eds.; Iowa State Press: Ames, IA, USA, 2003; pp. 307–323. [Google Scholar]
- Tlusty, M.F.; Rhyne, A.L.; Kaufman, L.; Hutchins, M.; Reid, G.M.; Andrews, C.; Boyle, P.; Hemdal, J.; McGilvray, F.; Dowd, S. Opportunities for public aquariums to increase the sustainability of the aquatic animal trade. Zoo Biol. 2012, 32, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tlusty, M.F.; Baylina, N.; Rhyne, A.L.; Brown, C.; Smith, M. Public Aquaria. In Marine Ornamental Species Aquaculture; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 611–622. [Google Scholar]
- Anderson, P.A. Acoustic characterization of seahorse tank environments in public aquaria: A citizen science project. Aquac. Eng. 2013, 54, 72–77. [Google Scholar] [CrossRef]
- Calado, R. The Role of Public and Private Aquaria in the Culture and Conservation of Marine Ornamentals. In Marine Ornamental Species Aquaculture; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 609–610. [Google Scholar]
- Pouil, S.; Tlusty, M.F.; Rhyne, A.L.; Metian, M. Aquaculture of marine ornamental fish: Overview of the production trends and the role of academia in research progress. Rev. Aquac. 2019, 12, 1217–1230. [Google Scholar] [CrossRef]
- Leger, J.S.; Violetta, G. Interaction Between Public and Private Aquaria. In Marine Ornamental Species Aquaculture; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 623–634. [Google Scholar]
- Pavitt, A.; Malsch, K.; King, E.; Chevalier, A.; Kachelriess, D.; Vannuccini, S.; Friedman, K. CITES and the Sea: Trade in Commercially Exploited CITES-Listed Marine Species; FAO Fisheries and Aquaculture Technical Paper No. 666; FAO: Rome, Italy, 2021; p. 100. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Staats, M.; Arulandhu, A.J.; Gravendeel, B.; Holst-Jensen, A.; Scholtens, I.; Peelen, T.; Prins, T.W.; Kok, E. Advances in DNA metabarcoding for food and wildlife forensic species identification. Anal. Bioanal. Chem. 2016, 408, 4615–4630. [Google Scholar] [CrossRef] [Green Version]
- Van der Walt, K.; Mäkinen, T.; Swartz, E.; Weyl, O. DNA barcoding of South Africa’s ornamental freshwater fish—Are the names reliable? Afr. J. Aquat. Sci. 2017, 42, 155–160. [Google Scholar] [CrossRef]
- Aubriot, X.; Lowry, P.P.; Cruaud, C.; Couloux, A.; Haevermans, T. DNA barcoding in a biodiversity hot spot: Potential value for the identification of Malagasy Euphorbia L. listed in CITES Appendices I and II. Mol. Ecol. Resour. 2013, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Gathier, G.; Van Der Niet, T.; Peelen, T.; Van Vugt, R.R.; Eurlings, M.C.; Gravendeel, B. Forensic identification of CITES protected slimming cactus (Hoodia) using DNA barcoding. J. Forensic Sci. 2013, 58, 1467–1471. [Google Scholar] [CrossRef]
- Eurlings, M.C.M.; Lens, F.; Pakusza, C.; Peelen, T.; Wieringa, J.J.; Gravendeel, B. Forensic identification of Indian snakeroot (Rauvolfia serpentina Benth. ex Kurz) using DNA barcoding. J. Forensic Sci. 2013, 58, 822–830. [Google Scholar] [CrossRef] [Green Version]
- De Boer, H.J.; Ghorbani, A.; Manzanilla, V.; Raclariu, A.-C.; Kreziou, A.; Ounjai, S.; Osathanunkul, M.; Gravendeel, B. DNA metabarcoding of orchid-derived products reveals widespread illegal orchid trade. Proc. R. Soc. B: Biol. Sci. 2017, 284, 20171182. [Google Scholar] [CrossRef]
- Veldman, S.; Kim, S.J.; van Andel, T.R.; Bello Font, M.; Bone, R.E.; Bytebier, B.; Chuba, D.; Gravendeel, B.; Martos, F.; Mpatwa, G.; et al. Trade in Zambian edible orchids-DNA barcoding reveals the use of unexpected orchid taxa for Chikanda. Genes 2018, 9, 595. [Google Scholar] [CrossRef] [Green Version]
- Vu, H.-T.; Nguyen, T.-D.; Tran, H.-D.; Vu, Q.-L.; Tran, N.; Nguyen, T.-C.; Luu, P.-N.; Tran, D.-D.; Nguyen, T.-K.; Le, L. Identification of Vietnamese Paphiopedilum species using DNA sequences. Biology 2020, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppin, L.; McEwing, R.; Carvalho, G.R.; Ogden, R. A DNA-based approach for the forensic identification of Asiatic black bear (Ursus thibetanus) in a traditional Asian medicine. J. Forensic Sci. 2008, 53, 1358–1362. [Google Scholar] [CrossRef]
- Meganathan, P.R.; Dubey, B.; Haque, I. Molecular identification of Indian crocodile species: PCR-RFLP method for forensic authentication. J. Forensic Sci. 2009, 54, 1042–1045. [Google Scholar] [CrossRef]
- Dubey, B.; Meganathan, P.R.; Haque, I. Molecular identification of three Indian snake species using simple PCR-RFLP method. J. Forensic Sci. 2010, 55, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Jafar, S.; Raja, N.A.; Mahar, J. Use of DNA barcoding to control the illegal wildlife trade: A CITES case report from Pakistan. J. Bioresour. Manag. 2015, 2, 3. [Google Scholar] [CrossRef]
- Mendoza, Á.M.; Torres, M.F.; Paz, A.; Trujillo-Arias, N.; Alvarez, D.L.; Sierra, S.; Forero, F.; Gonzalez, M.A. Cryptic diversity revealed by DNA barcoding in Colombian illegally traded bird species. Mol. Ecol. Resour. 2016, 16, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.V.; Chan, C.-L.C.; Lin, O.; Hu, C.-S.; Chen, C.A. DNA barcoding of shark meats identify species composition and CITES-listed species from the markets in Taiwan. PLoS ONE 2013, 8, e79373. [Google Scholar] [CrossRef] [Green Version]
- Fields, A.T.; Abercrombie, D.L.; Eng, R.; Feldheim, K.; Chapman, D.D. A novel mini-DNA barcoding assay to identify processed fins from internationally protected shark species. PLoS ONE 2015, 10, e0114844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sembiring, A.; Pertiwi, N.P.D.; Mahardini, A.; Wulandari, R.; Kurniasih, E.M.; Kuncoro, A.W.; Cahyani, N.D.; Anggoro, A.W.; Ulfa, M.; Madduppa, H.; et al. DNA barcoding reveals targeted fisheries for endangered sharks in Indonesia. Fish. Res. 2015, 164, 130–134. [Google Scholar] [CrossRef]
- Chuang, P.-S.; Hung, T.-C.; Chang, H.-A.; Huang, C.-K.; Shiao, J.-C. The species and origin of shark fins in Taiwan’s fishing ports, markets, and customs detention: A DNA barcoding analysis. PLoS ONE 2016, 11, e0147290. [Google Scholar] [CrossRef] [PubMed]
- Steinke, D.; Bernard, A.M.; Horn, R.L.; Hilton, P.; Hanner, R.; Shivji, M.S. DNA analysis of traded shark fins and mobulid gill plates reveals a high proportion of species of conservation concern. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, L.M.; Martins, A.P.B.; Giarrizzo, T.; Macedo, W.; Monteiro, I.L.; Gemaque, R.; Nunes, J.L.S.; Gomes, F.; Schneider, H.; Sampaio, I.; et al. DNA-based identification reveals illegal trade of threatened shark species in a global elasmobranch conservation hotspot. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, B.J.; Ip, Y.C.A.; Neo, M.L.; Chang, J.J.M.; Gan, C.Z.; Clark-Shen, N.; Huang, D.; Rao, M. DNA barcoding of traded shark fins, meat and mobulid gill plates in Singapore uncovers numerous threatened species. Conserv. Genet. 2018, 19, 1393–1399. [Google Scholar] [CrossRef]
- Johri, S.; Doane, M.P.; Allen, L.; Dinsdale, E.A. Taking advantage of the genomics revolution for monitoring and conservation of chondrichthyan populations. Diversity 2019, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Hoeksema, B.W.; Arrigoni, R. DNA barcoding of a stowaway reef coral in the international aquarium trade results in a new distribution record. Mar. Biodivers. 2020, 50, 1–7. [Google Scholar] [CrossRef]
- Steinke, D.; Zemlak, T.S.; Hebert, P.D.N. Barcoding nemo: DNA-based identifications for the ornamental fish trade. PLoS ONE 2009, 4, e6300. [Google Scholar] [CrossRef]
- Galbusera, P.H.A.; Gillemot, S.; Jouk, P.; Teske, P.R.; Hellemans, B.; Volckaert, F.A.M.J. Isolation of microsatellite markers for the endangered Knysna seahorse Hippocampus capensis and their use in the detection of a genetic bottleneck. Mol. Ecol. Notes 2007, 7, 638–640. [Google Scholar] [CrossRef]
- Luo, W.; Qu, H.; Li, J.; Wang, X.; Lin, Q. A novel method for the identification of seahorses (genus Hippocampus) using cross-species amplifiable microsatellites. Fish. Res. 2015, 172, 318–324. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Zhang, H.; Lin, Q. Complete mitochondrial genome sequence of the Barbour’s seahorse Hippocampus barbouri Jordan & Richardson, 1908 (Gasterosteiformes: Syngnathidae). Mitochondrial DNA 2014, 26, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Nester, G.M.; De Brauwer, M.; Koziol, A.; West, K.M.; DiBattista, J.D.; White, N.E.; Power, M.; Heydenrych, M.J.; Harvey, E.; Bunce, M. Development and evaluation of fish eDNA metabarcoding assays facilitate the detection of cryptic seahorse taxa (family: Syngnathidae). Environ. DNA 2020, 2, 614–626. [Google Scholar] [CrossRef]
- Lai, M.; Sun, S.; Chen, J.; Lou, Z.; Qiu, F.; Zhang, G.; Cheng, R. Complete mitochondrial genome sequence for the seahorse adulteration Hippocampus camelopardalis Bianconi 1854. Mitochondrial DNA Part B 2019, 4, 432–433. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Zhu, L.; Chen, M.; Zhang, G.; Huang, Z.; Cheng, R. Complete mitochondrial genome sequence for the endangered Knysna seahorse Hippocampus capensis Boulenger 1900. Conserv. Genet. Resour. 2018, 10, 461–465. [Google Scholar] [CrossRef]
- Teske, P.R. Mitogenome reconstruction of an endangered African seahorse from a traditional Chinese medicine market was based on a misidentification. BioRxiv Prepr. 2020. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, H.-Y.; Jang-Liaw, N.-H.; Shao, K.-T.; Lin, Y.-S.; Ho, H.-C. The complete mitochondrial genome of the tiger tail seahorse, Hippocampus comes (Teleostei, Syngnathidae). Mitochondrial DNA 2013, 24, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Hanifaturahmah, F.; Perwitasari-Farajallah, D.; Butet, N.A.; Nurilmala, M.; Sudrajat, A.O.; Ochiai, Y. Morphological and molecular identification of seahorses (Hippocampus spp.) from the coast of Sumatra Island, Indonesia. Biodiversitas 2020, 21, 4116–4123. [Google Scholar] [CrossRef]
- Lin, Q.; Fan, S.; Zhang, Y.; Xu, M.; Zhang, H.; Yang, Y.; Lee, A.P.; Woltering, J.M.; Ravi, V.; Gunter, H.M.; et al. The seahorse genome and the evolution of its specialized morphology. Nat. Cell Biol. 2016, 540, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Luo, W.; Wan, S.; Gao, Z. De Novo transcriptome analysis of two seahorse species (Hippocampus erectus and H. mohnikei) and the development of molecular markers for population genetics. PLoS ONE 2016, 11, e0154096. [Google Scholar] [CrossRef]
- Lin, Q.; Qiu, Y.; Gu, R.; Xu, M.; Li, J.; Bian, C.; Zhang, H.; Qin, G.; Zhang, Y.; Luo, W.; et al. Draft genome of the lined seahorse, Hippocampus erectus. GigaScience 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Silveira, R.B.; Siccha-Ramirez, R.; Silva, J.R.S.; Oliveira, C. Morphological and molecular evidence for the occurrence of three Hippocampus species (Teleostei: Syngnathidae) in Brazil. Zootaxa 2014, 3861, 317–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, H.; Lin, Q.; Huang, L. Complete mitochondrial genome sequence of the lined seahorse Hippocampus erectus Perry, 1810 (Gasterosteiformes: Syngnathidae). Mitochondrial DNA 2013, 26, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-C.; Ma, Q.; Zhang, D.-C. Isolation and characterization of 100 SNP markers in lined seahorse (Hippocampus erectus) using RAD sequencing. Conserv. Genet. Resour. 2020, 12, 589–595. [Google Scholar] [CrossRef]
- Correia, M.; Campoy, A.; Madeira, C.; Andrade, J.P. Is filament clipping an effective tool for tissue sampling in Hippocampus guttulatus? Environ. Biol. Fishes 2018, 101, 1517–1523. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, L.; Chen, J.; Zhang, G.; Cheng, R.; Ge, Y. The complete mitochondrial genome of the short snouted seahorse Hippocampus hippocampus Linnaeus 1758 (Syngnathiformes: Syngnathidae) and its phylogenetic implications. Conserv. Genet. Resour. 2017, 10, 783–787. [Google Scholar] [CrossRef]
- Singh, K.V.; Gopalakrishnan, A.; Lakra, W.S.; Sobti, R.C. Microsatellite loci to determine population structure in the yellow seahorse (Hippocampus kuda) and the three-spotted seahorse (H. trimaculatus). Mar. Biodivers. 2012, 42, 481–488. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Lin, Q. Complete mitochondrial genome of the pacific seahorse Hippocampus ingens Girard, 1858 (Gasterosteiformes: Syngnathidae). Mitochondrial DNA 2013, 26, 755–756. [Google Scholar] [CrossRef]
- Cheng, R.; Fang, Y.; Ge, Y.; Liu, Q.; Zhang, G. Complete mitochondrial genome sequence of the Jayakar’s seahorse Hippocampus jayakari Boulenger, 1900 (Gasterosteiformes: Syngnathidae). Mitochondrial DNA Part B 2017, 2, 593–594. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, Y.; Zhang, H.; Qin, G.; Lin, Q. Complete mitochondrial genomes of eight seahorses and pipefishes (Syngnathiformes: Syngnathidae): Insight into the adaptive radiation of syngnathid fishes. BMC Evol. Biol. 2019, 19, 119. [Google Scholar] [CrossRef]
- Hue, N.T.; Tran, N.T.T. A DNA extraction method applied for living seahorses. IFMBE Proc. 2013, 49, 178–183. [Google Scholar] [CrossRef]
- Jahari, P.N.S.; Malik, N.F.A.; Shamsir, M.S.; Gilbert, M.T.P.; Salleh, F.M. The first complete mitochondrial genome data of Hippocampus kuda originating from Malaysia. Data Brief 2020, 31, 105721. [Google Scholar] [CrossRef]
- Leong, O.B.; Joseph, J.; Kuang, C.C. Genetic Mating System of Spotted Seahorse (Hippocampus kuda). In Proceedings of the 9th UMT In-ternational Symposium on Sustainability Science and Management (UMTAS); Universiti Malaysia Terengganu: Kuala Terengga-nu, Malaysia, 2010; Volume 2, pp. 1–6. [Google Scholar]
- Thangaraj, M.; Lipton, A.P. Assessment of genetic variation in closely related seahorse species (Genus: Hippocampus) using mtDNA marker. Indian J. Biotechnol. 2011, 10, 140–142. [Google Scholar]
- Yang, Q.-H.; Lu, Z.; Zheng, L.-Y.; Huang, Z.-C.; Lin, Q.; Wu, J.-N.; Zhou, C. The complete mitochondrial genome of the Japanese seahorse, Hipppocampus mohnikei (Syngnathiformes: Syngnathidae) and its phylogenetic implications. Conserv. Genet. Resour. 2017, 9, 613–617. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Wang, A.; Yu, X.; Li, Y.; Han, Z.; Fu, J.; Dong, J. Sequence variation of mtDNA control region in Hippocampus japonicus inhabiting Liaoning coast and its applicability as a marker for phylogenetic analysis. J. Fish. China 2017, 41, 1073–1082. (In Chinese) [Google Scholar] [CrossRef]
- González, R.; Dinghi, P.; Corio, C.; Medina, A.; Maggioni, M.; Storero, L.; Gosztonyi, A. Reply to Luzzatto et al. Genetic evidence and new morphometric data as essential tools to identify the Patagonian seahorse Hippocampus patagonicus (Pisces, Syngnathidae) by González et al. J. Fish Biol. 2014, 85, 1300–1302. [Google Scholar] [CrossRef]
- Luzzatto, D.C.; Sieira, R.; Pujol, M.G.; de Astarloa, J.M.D. The presence of the seahorse Hippocampus patagonicus in the Ar-gentine Sea based on the Cytochrome b sequence of mitochondrial DNA. Cybium 2012, 36, 329–333. [Google Scholar]
- Luzzatto, D.C.; Estalles, M.L.; De Astarloa, J.M.D. Comment on ‘Genetic evidence and new morphometric data as essential tools to identify the Patagonian seahorse Hippocampus patagonicus (Pisces, Syngnathidae) by González et al. (2014)’. J. Fish Biol. 2014, 85, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhang, H.; Meng, T.; Lin, Q. Complete mitochondrial genome sequence of the longsnout seahorse Hippocampus reidi (Ginsburg, 1933; Gasterosteiformes: Syngnathidae). Mitochondrial DNA 2014, 27, 1401–1402. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Zhang, Y.; Liu, S.; Lin, Q. Phylogenetic analysis and genetic structure of the seahorse, Hippocampus fuscus from the Arabian and Red Sea based on mitochondrial DNA sequences. Mitochondrial DNA Part A 2018, 30, 165–171. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Wang, C.; Lin, Q. Complete mitochondrial genome sequence of the hedgehog seahorse Hippocampus spinosissimus Weber, 1933 (Gasterosteiformes: Syngnathidae). Mitochondrial DNA Part A 2015, 27, 2767–2768. [Google Scholar] [CrossRef]
- Teske, P.R.; Lourie, S.A.; Matthee, C.A.; Green, D.M. Hippocampus queenslandicus Horne, 2001—A new seahorse species or yet another synonym? Aust. J. Zool. 2007, 55, 139–145. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Y.; Cheng, R.; Huang, Z.; Chen, Z.; Zhang, G. Sequencing and analysis of the complete mitochondrial genome of Hippocampus spinosissimus Weber, 1913 (Gasterosteiformes: Syngnathidae). Mitochondrial DNA Part A 2015, 28, 303–304. [Google Scholar] [CrossRef]
- Chang, C.-H.; Shao, K.-T.; Lin, Y.-S.; Liao, Y.-C. The complete mitochondrial genome of the three-spot seahorse, Hippocampus trimaculatus (Teleostei, Syngnathidae). Mitochondrial DNA 2013, 24, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Short, G.; Harasti, D.; Hamilton, H. Hippocampus whitei Bleeker, 1855, a senior synonym of the southern Queensland seahorse H. procerus Kuiter, 2001: Molecular and morphological evidence (Teleostei, Syngnathidae). ZooKeys 2019, 824, 109–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-H.; Jang-Liaw, N.-H.; Lin, Y.-S.; Fang, Y.-C.; Shao, K.-T. Authenticating the use of dried seahorses in the traditional Chinese medicine market in Taiwan using molecular forensics. J. Food Drug Anal. 2013, 21, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Hou, F.; Cao, J.; Wang, X.; Wang, S.; Peng, C.; Guo, J. Specific authentication of Hippocampus based on SNP markers. Biochem. Syst. Ecol. 2017, 71, 131–136. [Google Scholar] [CrossRef]
- Hou, F.; Wen, L.; Peng, C.; Guo, J. Identification of marine traditional Chinese medicine dried seahorses in the traditional Chinese medicine market using DNA barcoding. Mitochondrial DNA Part A 2018, 29, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.G.; Cribbs, J.E.; Fienberg, H.G.; Hulburd, G.C.; Katz, L.S.; Palumbi, S.R. The tip of the tail: Molecular identification of seahorses for sale in apothecary shops and curio stores in California. Conserv. Genet. 2007, 9, 65–71. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, H.; Guo, J.; Hou, F. Morphology and molecular identification of the zoological origin of medicinal seahorses in Chinese herbal markets. Mitochondrial DNA Part A 2020, 31, 1–11. [Google Scholar] [CrossRef]
- Serite, C.R.; Ntshudisane, O.K.; Swart, E.; Simbine, L.; Jaime, G.L.M.; Teske, P.R. Limitations of DNA barcoding in deter-mining the origin of smuggled seahorses and pipefishes. BioRxiv Prepr. 2020. [Google Scholar] [CrossRef]
- López, A.; Vera, M.; Otero-Ferrer, F.; Pardo, B.G.; Martínez, P.; Molina, L.; Bouza, C. Species identification and genetic structure of threatened seahorses in Gran Canaria Island (Spain) using mitochondrial and microsatellite markers. Conserv. Genet. 2010, 11, 2431–2436. [Google Scholar] [CrossRef]
- López, A.; Vera, M.; Planas, M.; Bouza, C. Conservation genetics of threatened Hippocampus guttulatus in vulnerable habitats in NW spain: Temporal and spatial stability of wild populations with flexible polygamous mating system in captivity. PLoS ONE 2015, 10, e0117538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riquet, F.; Lieutard-Haag, C.; Serluca, G.; Woodall, L.; Claude, J.; Louisy, P.; Bierne, N. Effective population size and heterozygosity-fitness correlations in a population of the Mediterranean lagoon ecotype of long-snouted seahorse Hippocampus guttulatus. Conserv. Genet. 2019, 20, 1281–1288. [Google Scholar] [CrossRef] [Green Version]
- Van De Vliet, M.S.; Diekmann, O.E.; Serrão, E.T.A. Highly polymorphic microsatellite markers for the short-snouted seahorse (Hippocampus hippocampus), including markers from a closely related species the long-snouted seahorse (Hippocampus guttulatus). Conserv. Genet. Resour. 2009, 1, 93–96. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, B.; Mou, C.; Yang, W.; Wei, J.; Lu, L.; Zhu, J.; Du, J.; Wu, X.; Ye, L.; et al. Molecular profile of the unique species of traditional Chinese medicine, Chinese seahorse (Hippocampus kuda Bleeker). FEBS Lett. 2003, 550, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, H.; Saarman, N.; Short, G.; Sellas, A.B.; Moore, B.; Hoang, T.; Grace, C.L.; Gomon, M.; Crow, K.; Simison, W.B. Molecular phylogeny and patterns of diversification in syngnathid fishes. Mol. Phylogenet. Evol. 2017, 107, 388–403. [Google Scholar] [CrossRef] [Green Version]
- Koh, B.S.; Song, C.B. Molecular phylogeny of Syngnathiformes fishes inferred from mitochondrial cytochrome b DNA se-quences. J. Kor. Fish. Soc. 2004, 37, 405–413. [Google Scholar]
- Nurilmala, M.; Nurhayati, K.H.; Butet, N.A.; Sudrajat, A.O. Molecular marker based on 16S rRNA gene for seahorse (Hippocampus spp.) from Bintan Island-Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012054. [Google Scholar] [CrossRef]
- Nurilmala, M.; Sari, E.M.; Abdullah, A.; Hizbullah, H.H.; Butet, N.A.; Sudrajat, A.O. DNA barcoding for seahorse identification and its potential as antioxidant and stimulant indicator. IOP Conf. Ser. Earth Environ. Sci. 2019, 404, 012002. [Google Scholar] [CrossRef]
- Teske, P.R.; Cherry, M.I.; Matthee, C.A. The evolutionary history of seahorses (Syngnathidae: Hippocampus): Molecular data suggest a West Pacific origin and two invasions of the Atlantic Ocean. Mol. Phylogenet. Evol. 2004, 30, 273–286. [Google Scholar] [CrossRef]
- Wilson, A.B.; Ahnesjo, I.; Vincent, A.C.J.; Meyer, A. The dynamics of male brooding, mating patterns, and sex roles in pipefishes and seahorses (family Syngnathidae). Evolution 2003, 57, 1374–1386. [Google Scholar] [CrossRef]
- Wilson, A.B.; Orr, J.W. The evolutionary origins of Syngnathidae: Pipefishes and seahorses. J. Fish Biol. 2011, 78, 1603–1623. [Google Scholar] [CrossRef] [Green Version]
- Casey, S.P.; Hall, H.J.; Stanley, H.F.; Vincent, A.C. The origin and evolution of seahorses (genus Hippocampus): A phylogenetic study using the cytochrome b gene of mitochondrial DNA. Mol. Phylogenet. Evol. 2004, 30, 261–272. [Google Scholar] [CrossRef]
- Teske, P.R.; Hamilton, H.; Matthee, C.A.; Barker, N.P. Signatures of seaway closures and founder dispersal in the phylogeny of a circumglobally distributed seahorse lineage. BMC Evol. Biol. 2007, 7, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodall, L.C.; Koldewey, H.J.; Santos, S.V.; Shaw, P.W. First occurrence of the lined seahorse Hippocampus erectus in the eastern Atlantic Ocean. J. Fish Biol. 2009, 75, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Lourie, S.A. Spatial genetic patterns in the Hippocampus barbouri species group (Teleostei: Syngnathidae) across the Coral Triangle. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2003; pp. 478–484. [Google Scholar]
- Lourie, S.A.; Green, D.M.; Vincent, A.C.J. Dispersal, habitat differences, and comparative phylogeography of Southeast Asian seahorses (Syngnathidae: Hippocampus). Mol. Ecol. 2005, 14, 1073–1094. [Google Scholar] [CrossRef]
- Cheng, R.; Liao, G.; Ge, Y.; Yang, B.; Zhang, G. Complete mitochondrial genome of the great seahorse Hippocampus kelloggi Jordan & Snyder, 1901 (Gasterosteiformes: Syngnathidae). Mitochondrial DNA Part A 2015, 28, 227–228. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Qin, G.; Zhang, H.-X.; Wang, X.; Lin, Q. DNA barcoding reflects the diversity and variety of brooding traits of fish species in the family Syngnathidae along China’s coast. Fish. Res. 2017, 185, 137–144. [Google Scholar] [CrossRef]
- Teske, P.R.; Hamilton, H.; Palsbøll, P.J.; Choo, C.K.; Gabr, H.; Lourie, S.A.; Santos, M.; Sreepada, A.; Cherry, M.I.; Matthee, C.A. Molecular evidence for long-distance colonization in an Indo-Pacific seahorse lineage. Mar. Ecol. Prog. Ser. 2005, 286, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Song, H.Y.; Mabuchi, K.; Satoh, T.P.; Moore, J.A.; Yamanoue, Y.; Miya, M.; Nishida, M. Mitogenomic circumscription of a novel percomorph fish clade mainly comprising “Syngnathoidei” (Teleostei). Gene 2014, 542, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Ashe, J.L.; Wilson, A.B. Navigating the southern seas with small fins: Genetic connectivity of seahorses (Hippocampus abdominalis) across the Tasman Sea. J. Biogeogr. 2019, 47, 207–219. [Google Scholar] [CrossRef]
- Nickel, J.; Cursons, R. Genetic diversity and population structure of the pot-belly seahorse Hippocampus abdominalis in New Zealand. N. Z. J. Mar. Freshw. Res. 2012, 46, 207–218. [Google Scholar] [CrossRef]
- Mkare, T.K.; van Vuuren, B.J.; Teske, P.R. Conservation implications of significant population differentiation in an endangered estuarine seahorse. Biodivers. Conserv. 2017, 26, 1275–1293. [Google Scholar] [CrossRef]
- Teske, P.R.; Cherry, M.I.; Matthee, C. Population genetics of the endangered Knysna seahorse, Hippocampus capensis. Mol. Ecol. 2003, 12, 1703–1715. [Google Scholar] [CrossRef] [Green Version]
- Boehm, J.T.; Waldman, J.; Robinson, J.D.; Hickerson, M.J. Population genomics reveals seahorses (Hippocampus erectus) of the western mid-Atlantic coast to be residents rather than vagrants. PLoS ONE 2015, 10, e0116219. [Google Scholar] [CrossRef]
- Rose, E.; Masonjones, H.D.; Jones, A.G. A DNA-based assessment of the phylogenetic position of a morphologically distinct, anchialine-lake-restricted seahorse. J. Hered. 2016, 107, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Riquet, F.; Liautard-Haag, C.; Woodall, L.; Bouza, C.; Louisy, P.; Hamer, B.; Otero-Ferrer, F.; Aublanc, P.; Béduneau, V.; Briard, O.; et al. Parallel pattern of differentiation at a genomic island shared between clinal and mosaic hybrid zones in a complex of cryptic seahorse lineages. Evolution 2019, 73, 817–835. [Google Scholar] [CrossRef]
- Saarman, N.P.; Louie, K.D.; Hamilton, H. Genetic differentiation across eastern Pacific oceanographic barriers in the threatened seahorse Hippocampus ingens. Conserv. Genet. 2010, 11, 1989–2000. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, B.K.; Lakra, W.S.; Choudhary, M. Mitochondrial DNA phylogeny of Indian seahorse (Hippocampus kuda) based on cytochrome B gene sequences. Bioscan 2014, 9, 931–936. [Google Scholar]
- Goswami, M.; Thangaraj, K.; Chaudhary, B.K.; Bhaskar, L.V.S.K.; Gopalakrishnan, A.; Joshi, M.B.; Singh, L.; Lakra, W.S. Genetic heterogeneity in the Indian stocks of seahorse (Hippocampus kuda and Hippocampus trimaculatus) inferred from mtDNA cytochrome b gene. Hydrobiologia 2009, 621, 213–221. [Google Scholar] [CrossRef]
- Panithanarak, T.; Karuwancharoen, R.; Na-Nakorn, U.; Nguyen, T.T.T. Population genetics of the spotted seahorse (Hippocampus kuda) in Thai waters: Implications for conservation. Zool. Stud. 2010, 49, 564–576. [Google Scholar]
- Panithanarak, T. Phylogeography of three commercially important seahorses (genus Hippocampus) in Thai waters: An implication from collective sequence data. J. Fish. Environ. 2020, 44, 1–15. [Google Scholar]
- Panithanarak, T.; Karuwanjaroen, R. A preliminary study of genetic differentiation in the Japanese seahorse (Hippocampus mohnikei) populations: A possibility of cryptic species. Burapha Sci. J. 2019, 24, 124–137. (In Thai) [Google Scholar]
- Zhang, Y.; Pham, N.K.; Zhang, H.; Lin, J.; Lin, Q. Genetic variations in two seahorse species (Hippocampus mohnikei and Hippocampus trimaculatus): Evidence for middle Pleistocene population expansion. PLoS ONE 2014, 9, e105494. [Google Scholar] [CrossRef] [Green Version]
- Mobley, K.B.; Small, C.M.; Jones, A.G. The genetics and genomics of Syngnathidae: Pipefishes, seahorses and seadragons. J. Fish Biol. 2011, 78, 1624–1646. [Google Scholar] [CrossRef]
- Whittington, C.M.; Griffith, O.W.; Qi, W.; Thompson, M.B.; Wilson, A.B. Seahorse brood pouch transcriptome reveals common genes associated with vertebrate pregnancy. Mol. Biol. Evol. 2015, 32, 3114–3131. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.G.; Kvarnemo, C.; Moore, G.I.; Simmons, L.W.; Avise, J.C. Microsatellite evidence for monogamy and sex-biased recombination in the Western Australian seahorse Hippocampus angustus. Mol. Ecol. 1998, 7, 1497–1505. [Google Scholar] [CrossRef] [Green Version]
- Mukai, T.; Tsuihiji, T.; Sato, T.; Morisawa, M. Mitochondrial DNA divergence in the seahorse, Hippocampus coronatus (Syngnathiformes: Syngnathidae), collected from Sagami Bay. Jap. J. Ichthyol. 2000, 47, 139–143. (In Japanese) [Google Scholar]
- Ho, N.K.P.; Ho, A.; Underwood, G.D.; Underwood, A.; Zhang, D.; Lin, J. A simple molecular protocol for the identification of hybrid Western Atlantic seahorses, Hippocampus erectus × H. reidi, and potential consequences of hybrids for conservation. J. Zoo Aquar. Res. 2015, 3, 11–20. [Google Scholar] [CrossRef]
- Luo, W.; Wang, X.; Qu, H.; Qin, G.; Zhang, H.; Lin, Q. Genomic structure and expression pattern of MHC IIα and IIβ genes reveal an unusual immune trait in lined seahorse Hippocampus erectus. Fish Shellfish. Immunol. 2016, 58, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Luo, W.; Tan, S.; Zhang, B.; Ma, S.; Lin, Q. Dimorphism of sex and gonad-development-related genes in male and female lined seahorse, Hippocampus erectus, based on transcriptome analyses. Genomics 2019, 111, 260–266. [Google Scholar] [CrossRef]
- Qu, H.; Luo, W.; Lin, Q. Development of SNP markers in lined seahorse (Hippocampus erectus) based on transcriptome sequencing. Conserv. Genet. Resour. 2015, 8, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Qin, G.; Sun, J.; Zhang, B.; Lin, Q. The evolution and functional characterization of lined seahorse (Hippocampus erectus) CCKs involved in fasting and thermal stress response. Gen. Comp. Endocrinol. 2018, 255, 56–63. [Google Scholar] [CrossRef]
- Lazic, T.; Pierri, C.; Cardone, F.; Cariani, A.; Colangelo, P.; Corriero, G.; Ferrari, A.; Marzano, M.; Messinetti, S.; Pesole, G.; et al. Genetic structure of the long-snouted seahorse, Hippocampus guttulatus, in the Central-Western Mediterranean Sea. Biol. J. Linn. Soc. 2020, 130, 771–782. [Google Scholar] [CrossRef]
- Woodall, L.C.; Koldewey, H.J.; Shaw, P.W. Serial monogamy in the European long-snouted seahorse Hippocampus guttulatus. Conserv. Genet. 2011, 12, 1645–1649. [Google Scholar] [CrossRef] [Green Version]
- Woodall, L.C.; Koldewey, H.J.; Boehm, J.T.; Shaw, P.W. Past and present drivers of population structure in a small coastal fish, the European long snouted seahorse Hippocampus guttulatus. Conserv. Genet. 2015, 16, 1139–1153. [Google Scholar] [CrossRef] [Green Version]
- Woodall, L.C.; Otero-Ferrer, F.; Correia, M.; Curtis, J.M.R.; Garrick-Maidment, N.; Shaw, P.W.; Koldewey, H.J. A synthesis of European seahorse taxonomy, population structure, and habitat use as a basis for assessment, monitoring and conservation. Mar. Biol. 2018, 165, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Woodall, L.C.; Koldewey, H.J.; Shaw, P.W. Historical and contemporary population genetic connectivity of the European short-snouted seahorse Hippocampus hippocampus and implications for management. J. Fish Biol. 2011, 78, 1738–1756. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-Y.; Rho, S.; Noh, G.E.; Kim, J.-K. Interspecific hybridization in seahorses: Artificially produced hybrid offspring of Hippocampus kuda and Hippocampus reidi. Fish. Aquat. Sci. 2018, 21, 11. [Google Scholar] [CrossRef] [Green Version]
- Rose, E.; Small, C.M.; Saucedo, H.A.; Harper, C.; Jones, A.G. Genetic evidence for monogamy in the dwarf seahorse, Hippocampus zosterae. J. Hered. 2014, 105, 828–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes, M.A.; Cardoso, M.L.V.; Neves, C.H.C.B.; Garcia, A.C.L.; Da Silva, J.C.; Silveira, R.B. Genetic diversity and populational structure of the seahorse Hippocampus reidi (Syngnathidae) in north-eastern Brazil: A conservationist approach. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1114–1122. [Google Scholar] [CrossRef]
- Song, H.; Mabuchi, K. Complete mitochondrial genome sequence of the thorny seahorse Hippocampus histrix (Gasterosteiformes: Syngnathidae). Mitochondrial DNA 2013, 25, 7–8. [Google Scholar] [CrossRef]
- Kawahara, R.; Miya, M.; Mabuchi, K.; Lavoué, S.; Inoue, J.G.; Satoh, T.P.; Kawaguchi, A.; Nishida, M.; Kawahara-Miki, R. Interrelationships of the 11 gasterosteiform families (sticklebacks, pipefishes, and their relatives): A new perspective based on whole mitogenome sequences from 75 higher teleosts. Mol. Phylogenet. Evol. 2008, 46, 224–236. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Zhao, L.; Song, N.; Gao, T. Complete mitochondrial DNA genome of Hippocampus mohnikei (Gasterosteiformes: Syngnathidae). Mitochondrial DNA Part A 2015, 28, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Panithanarak, T. Phylogeny of Thai seahorses inferred from mitochondrial DNA cytochrome b gene. In Proceedings of the Burapha University International Conference, Bangsaen, Chonburi, Thailand, 10–12 July 2015; pp. 1010–1023. [Google Scholar]
- Liu, Y.; Wu, Y.; Qin, G.; Chen, Y.; Wang, X.; Lin, Q. Bioaccumulation and reproductive toxicity of bisphenol A in male-pregnant seahorse (Hippocampus erectus) at environmentally relevant concentrations. Sci. Total Environ. 2021, 753, 141805. [Google Scholar] [CrossRef] [PubMed]
- Kleiber, D.; Blight, L.K.; Caldwell, I.R.; Vincent, A.C.J. The importance of seahorses and pipefishes in the diet of marine animals. Rev. Fish Biol. Fish. 2011, 21, 205–223. [Google Scholar] [CrossRef]
- Mastrangelli, A.; Silveira, R.B.; Burato, M.; Baldassin, P.; Werneck, M.R. First report of Lepidochelys olivacea feeding on Hippocampus patagonicus in Brazil. Mar. Turtle Newsl. 2019, 159, 26–27. [Google Scholar]
- Silveira, R.B. Presence of the seahorse Hippocampus reidi (Pisces: Syngnathidae) in diet of marine fish in northeastern Brazil. Oceanogr. Fish. Open Access J. 2020, 12, 1–3. [Google Scholar] [CrossRef]
- Huang, D. Threatened reef corals of the world. PLoS ONE 2012, 7, e34459. [Google Scholar] [CrossRef]
- Curnick, D.J.; Head, C.E.I.; Huang, D.; Crabbe, M.J.C.; Gollock, M.; Hoeksema, B.W.; Johnson, K.G.; Jones, R.; Koldewey, H.J.; Obura, D.O.; et al. Setting evolutionary-based conservation priorities for a phylogenetically data-poor taxonomic group (Scleractinia). Anim. Conserv. 2015, 18, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Isaac, N.J.B.; Pearse, W.D. The Use of EDGE (Evolutionary Distinct Globally Endangered) and EDGE-Like Metrics to Evaluate Taxa for Conservation. In Phylogenet. Diversity; Scherson, R., Faith, D., Eds.; Springer: Cham, Switzerland, 2018; pp. 27–39. [Google Scholar]
- Foster, S.; Vincent, A. Holding governments accountable for their commitments: CITES Review of Significant Trade for a very high-volume taxon. Glob. Ecol. Conserv. 2021, 27, e01572. [Google Scholar] [CrossRef]
- Pettier, J.-P. Re-enchanting the world: Seahorses’ magic and the global trade of affect for wildlife. Environ. Hist. 2019, 24, 711–717. [Google Scholar] [CrossRef]
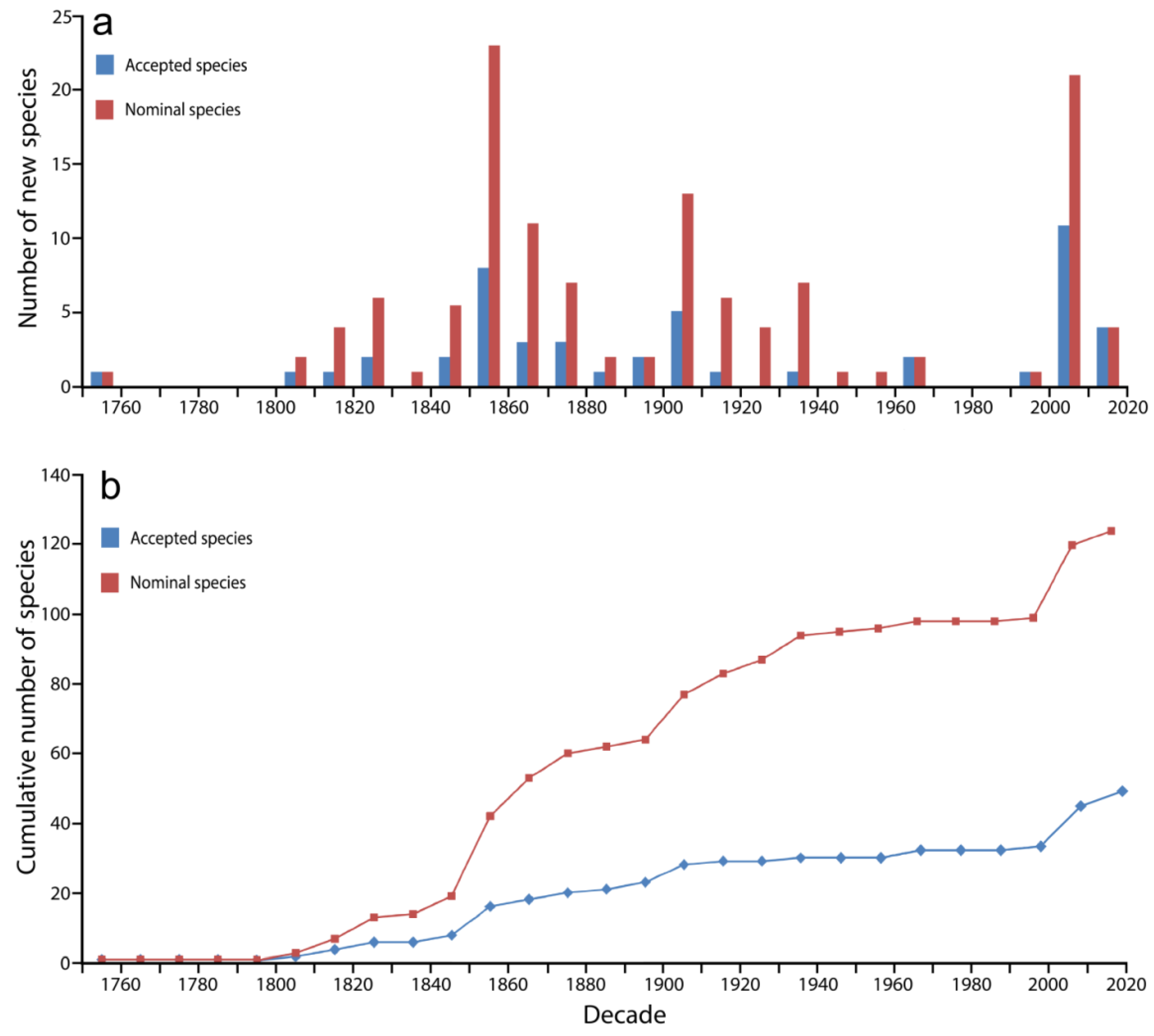






| Species | C | F | W | U | Total |
|---|---|---|---|---|---|
| H. abdominalis (6) | 0 | 0 | 769 | 0 | 769 |
| H. barbouri (11) | 40 | 0 | 0 | 0 | 0 |
| H. comes (10) | 0 | 0 | 35 | 6 | 41 |
| H. erectus (2) | 0 | 0 | 21,107 | 6632 | 27,739 |
| H. hippocampus (3) | 0 | 0 | 1727 | 3838 | 5565 |
| H. histrix (9) | 0 | 0 | 104 | 0 | 104 |
| H. ingens (5) | 0 | 0 | 1 | 1000 | 1001 |
| H. kelloggi (12) | 0 | 0 | 0 | 0 | 0 |
| H. kuda (1) | 0 | 0 | 29,285 | 22,156 | 51,441 |
| H. reidi (4) | 0 | 0 | 3557 | 1070 | 4627 |
| H. spinosissimus (8) | 40 | 0 | 70 | 58 | 168 |
| H. zosterae (7) | 0 | 0 | 233 | 0 | 233 |
| Hippocampus spp. | 40 | 0 | 40,409 | 10,254 | 50,703 |
| Total | 120 | 0 | 97,297 | 45,014 | 142,431 |
| Species Name | Geographical Range | Application of Molecular Analyses | ||||
|---|---|---|---|---|---|---|
| Barcoding/ Genomics | Forensics/ Conservation | Phylogeny Reconstructions | Phylogeography | Functional Genetics | ||
| H. abdominalis $¥ | SW Australia, New Zealand | 1 [283] | 1 [32] | 13 [186,287,305,308, | 2 [347,348] | 3 [128,361, |
| [165,347,348] | 309,323,331,332,333,334,335,336,337] | 362] | ||||
| H. algiricus $¥ | West Africa [39,165] | 1 [283] | 1 [320] | 11 [288,289,295,299,302, | 1 [339] | — |
| 323,333,335,338,339,340] | ||||||
| H. angustus $¥ | N Australia, South P.N.G. [165] | — | — | 3 [322,335,341] | — | 1 [363] |
| H. barbouri $¥ | Central Indonesia, | 3 [284,285,344] | 4 [321,322,323,324] | 24 [186,284,287,288,299, | 2 [341,342] | — |
| Philippines | 302,305,308,309,314, | |||||
| [165,341,342] | 315,317,322,323,333,334,335, | |||||
| 336,337,338,341,343,344,380] | ||||||
| H. bargibanti | Central Indo–West Pacific [165] | 1 [83] | — | 4 [42,331,334,335] | — | — |
| H. breviceps $ | Southern Australia [165] | 1 [286] | — | 5 [42,76,323,335,338] | — | — |
| H. camelopardalis | SW Africa [165] | 1 [287] | 1 [325] | 7 [287,295,305,323, | — | — |
| $¥ | 325,335,338] | |||||
| H. capensis | Southern tip of Africa | 4 [283,288, | 3 [320,324, | 11 [288,289,295,305, | 3 [339,349, | — |
| [165,339,349,350] | 289,350] | 325] | 309,323,325,335, | 350] | ||
| 338,339,345] | ||||||
| H. casscsio | South China Sea (SE China) [82] | 3 [82,289,344] | — | 3 [82,289,344] | — | — |
| H. colemani | Lord Howe Is., E Australia [165] | — | — | — | — | — |
| H. comes $¥ | Central Indo–West Pacific | 5 [284,290,291,292, | 4 [32,321, | 26 [76,186,284,287,288, | — | — |
| [165] | 344] | 322,324] | 299,302,305,308,309, | |||
| 314,315,317,322,323, | ||||||
| 333,334,335,336,337,338,341,343,344, | ||||||
| 380,381] | ||||||
| H. coronatus $ | Japan, South Korea [72,165] | — | — | 6 [72,76,323,333,335,338] | — | 1 [364] |
| H. dahlia | N, E Australia [165] | — | — | — | — | — |
| H. debelius | N Red Sea (Gulf of Suez) [78,165] | — | — | — | — | — |
| H. denise $ | Central Indo–West Pacific [165] | 2 [83,283] | 2 [331,334] | — | — | |
| H. erectus $¥ | W Atlantic, Caribbean Sea | 6 [284,293,294,295,296,297] | 1 [324] | 23 [76,186,284,287,288, | 4 [339,340, | 6 [365,366,367, |
| [165,339,340,351,352] | 295,299,302,305,311, | 351,352] | 368,369,382] | |||
| 314,315,317,322,323, | ||||||
| 332,333,335,337,338, | ||||||
| 339,343,380] | ||||||
| H. fisheri | Hawaii [165,168,339] | 1 [168] | — | 6 [308,309,335,336, | 1 [339] | — |
| 339,340] | ||||||
| H. guttulatus $¥ | W Europe, Mediterranean, | 1 [298] | 4 [326,327,328,329] | 8 [295,323,333,334,335, | 2 [339,353] | 4 [370,371,372,373] |
| Black Sea [165,339,353] | 338,339,340] | |||||
| H. haema | Japan, South Korea [72] | — | 2 [72,333] | — | — | |
| H. hippocampus | E Atlantic, Mediterranean | 1 [299] | 2 [326,329] | 12 [76,295,299,305,311, | 1 [339] | 2 [373,374] |
| $¥s | [165,339] | 323,333,334,335,338,339,340] | ||||
| H. histrix $¥ | Indo–West Pacific [165] | 5 [168,284,300, | 4 [32,320, | 20 [284,287,288,299,302, | — | — |
| 344,378] | 323,324] | 305,309,314,315,317, | ||||
| 323,332,333,334,335,338,343, | ||||||
| 344,380,381] | ||||||
| H. ingens $¥ | East Pacific | 4 [284,301, | 5 [321,322,323,324,325] | 27 [42,284,287,288,289, | 2 [339,354] | — |
| [165] | 325,344] | 299,305,308,309,314,315, | ||||
| 317,322,323,325,331,332,333,334,335, | ||||||
| 337,338,339,343,344,345,380] | ||||||
| H. japapigu | Japan [80] | 2 [80,83] | — | — | — | — |
| H. jayakari $ | Red Sea, NW Indian Ocean [165] | 1 [302] | 1 [324] | 4 [287,302,305,333] | — | — |
| H. jugumus | Lord Howe Is., Eastern | — | — | — | — | — |
| Australia [165] | ||||||
| H. kelloggi $¥ | Indian Ocean, | 3 [284,303, | 6 [32,34,320,321, | 21 [284,287,288,299, | 1 [339] | — |
| West Pacific | 344] | 322,324] | 302,303,305,308,316, | |||
| [165,339] | 317,322,323,332,333,334,335, | |||||
| 338,339,343,344,381] | ||||||
| H. kuda $¥ | Indo–West Pacific | 9 [168,283, | 7 [32,320,321, | 35 [76,82,186,284,287,288, | 8 [300,339, | 1 [375] |
| [165] | 284,291,304,305, | 322,324, | 289,299,302,307,308,309, | 342,345, | ||
| 306,307,344] | 325,330] | 314,315,316,317,322,323,325, | 355,356,357,358] | |||
| 331,332,333,334,335,336,337,338,339,343,344,345,346, | ||||||
| 379,380,381] | ||||||
| H. lichtensteinii | Red Sea [30] | — | — | — | — | — |
| H. minotaur $ | SE Australia [165] | — | — | — | — | — |
| H. mohnikei $¥ | East, South, SE Asia | 7 [284,293,303, | 4 [32,321, | 20 [81,284,287,288,299, | 4 [136,138, | — |
| [136,138,165,359,360] | 308,309, | 322,324] | 302,303,305,308,309, | 359,360] | ||
| 344,380] | 322,323,325,333,334, | |||||
| 335,338,344,380,381] | ||||||
| H. nalu | E South Africa [83] | 1 [83] | — | — | — | — |
| H. paradoxus | SW Australia [81,165] | — | — | — | — | — |
| H. patagonicus | SW Atlantic [165] | 5 [76,295,310,311,312] | — | 4 [76,295,311,334] | — | — |
| H. planifrons $ | Western tip of Australia [165] | — | — | — | — | — |
| H. pontohi | Central Indo–West Pacific [165] | 2 [80,83] | — | 1 [331] | — | — |
| H. pusillus | New Caledonia [165] | — | — | — | — | — |
| H. reidi $¥ | W Atlantic, Caribbean Sea | 4 [283,295,313, | 2 [324,377] | 25 [42,76,287,288, | 1 [339] | 2 [365,375] |
| [165,339] | 314] | 289,295,299,302,305,308, | ||||
| 309,314,317,323,325,331, | ||||||
| 333,334,335,337,338,339, | ||||||
| 343,345,380] | ||||||
| H. satomiae | Eastern Indonesia [165] | — | — | — | — | — |
| H. sindonis | Japan, South Korea [72,163] | — | — | 5 [72,287,305,333,335] | — | — |
| H. spinosissimus | Central Indo–West Pacific | 4 [284,315,316,317, | 6 [32,34,320,321, | 17 [284,287,288,299,302, | 3 [339,342, | — |
| $¥ | [165] | 344] | 322,324] | 305,315,316,317,323,333,334, | 358] | |
| 335,338,339,344,381] | ||||||
| H. subelongatus $ | SW Australia [165] | 1 [286] | — | 6 [308,314,323,335,338,341] | — | 1 [361] |
| H. trimaculatus | Central Indo–West Pacific | 4 [284,307,318, | 6 [32,34,320,321, | 24 [284,287,288,299,302, | 6 [139,300,342, | — |
| $¥ | [139,165,300,342,356,358,360] | 344] | 322,324] | 305,307,308,309,314,315, | 356,358,360] | |
| 317,322,323,325,331, | ||||||
| 333,334,335,338,343, | ||||||
| 344,380,381] | ||||||
| H. tristis | Southern Australia [73] | — | — | — | — | — |
| H. tyro | Seychelles [165] | — | — | — | — | — |
| H. waleananus | Eastern Indonesia [78] | — | — | — | — | — |
| H. whitei $¥ | S, E Australia, P.N.G., | 2 [283,319] | — | 6 [323,331,334,335, | — | — |
| Solomon Is. [165] | 338,341] | |||||
| H. zebra $¥ | Northern Australia [165] | — | — | — | — | — |
| H. zosterae $ | Gulf of Mexico, E Florida, | 1 [283] | — | 10 [42,76,186,311, | 2 [172,339] | 1 [376] |
| Bahamas, Bermuda | 323,331,335,336,337,338,339] | |||||
| [164,165,172,339] | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koning, S.; Hoeksema, B.W. Diversity of Seahorse Species (Hippocampus spp.) in the International Aquarium Trade. Diversity 2021, 13, 187. https://doi.org/10.3390/d13050187
Koning S, Hoeksema BW. Diversity of Seahorse Species (Hippocampus spp.) in the International Aquarium Trade. Diversity. 2021; 13(5):187. https://doi.org/10.3390/d13050187
Chicago/Turabian StyleKoning, Sasha, and Bert W. Hoeksema. 2021. "Diversity of Seahorse Species (Hippocampus spp.) in the International Aquarium Trade" Diversity 13, no. 5: 187. https://doi.org/10.3390/d13050187
APA StyleKoning, S., & Hoeksema, B. W. (2021). Diversity of Seahorse Species (Hippocampus spp.) in the International Aquarium Trade. Diversity, 13(5), 187. https://doi.org/10.3390/d13050187