Preliminary Study of Cave Sample Storage Conditions on Fungal Community Diversity
Abstract
:1. Introduction
2. Materials and Methods
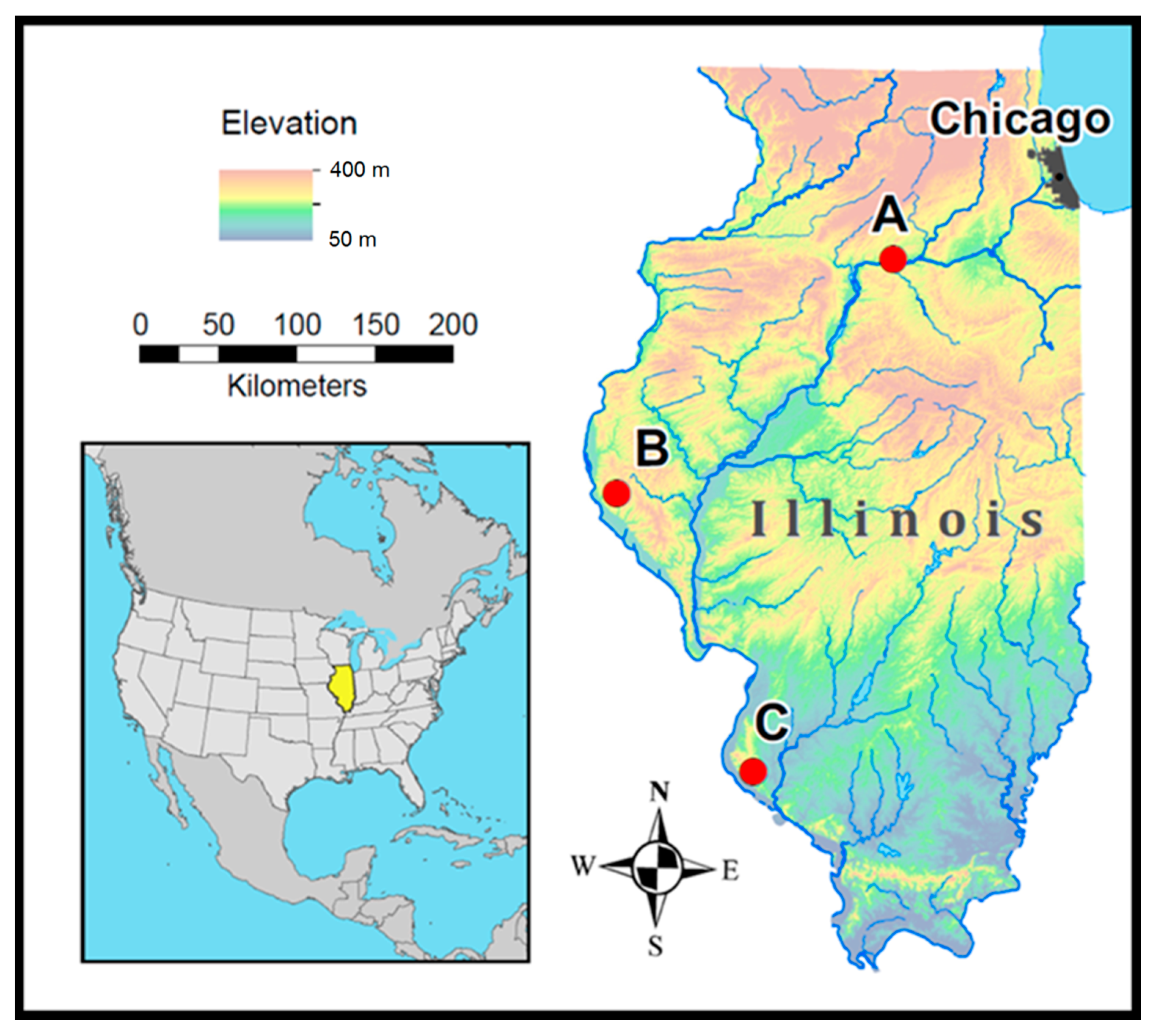
2.1. Study Area
2.2. Collection and Isolation
2.3. Culture Identification and Molecular Methods
2.4. Analysis
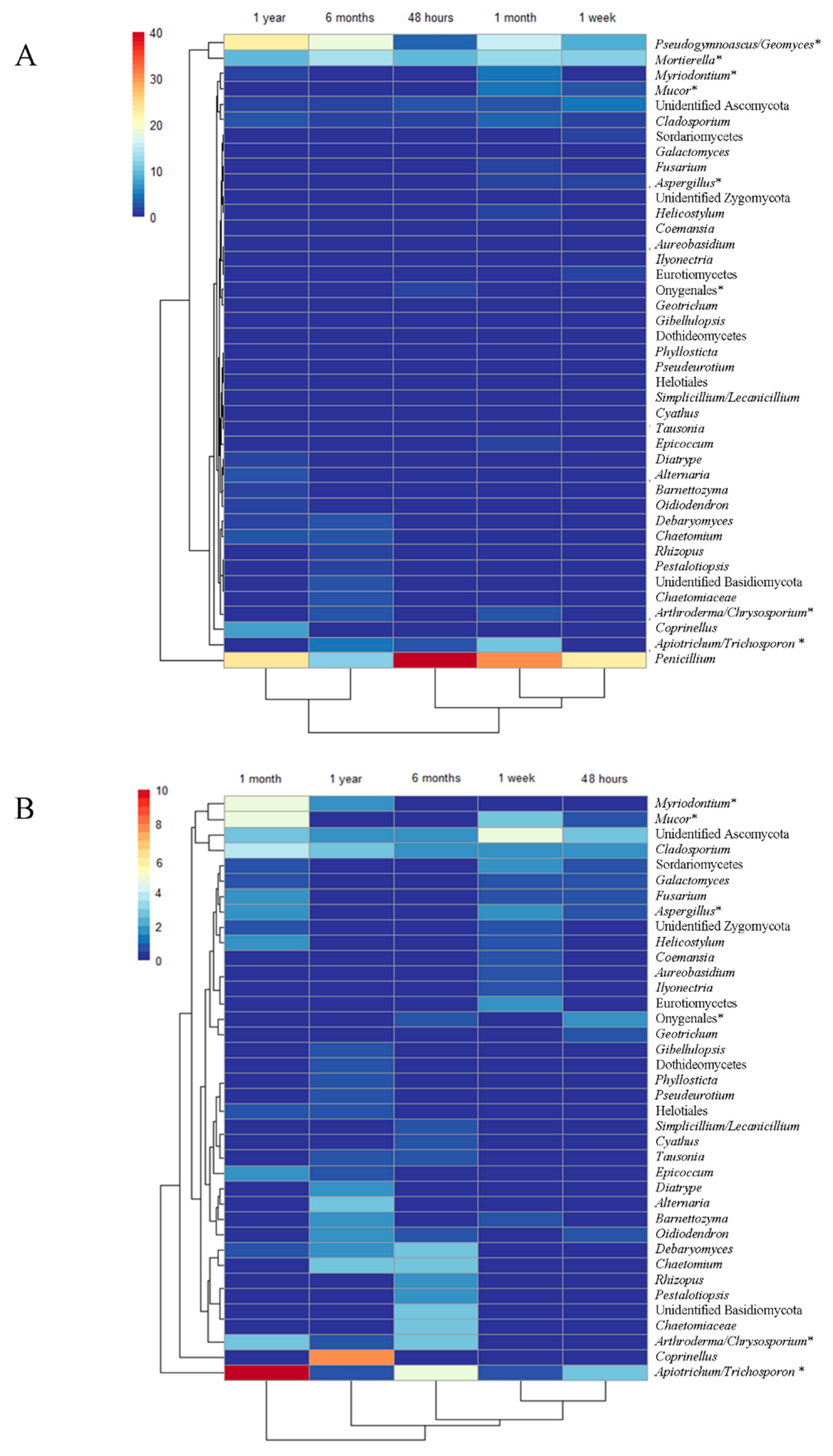
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panasenko, V.T. Ecology of Microfungi. Bot. Rev. 1967, 33, 189–215. [Google Scholar] [CrossRef]
- Ramos, C.L.; de Almeida, E.G.; Freire, A.L.; Schwan, R.F. Diversity of bacteria and yeast in the naturally fermented cotton seed and rice beverage produced by Brazilian Amerindians. Food Microbiol. 2011, 28, 1380–1386. [Google Scholar] [CrossRef] [Green Version]
- Al-Awadhi, H.; Dashti, N.; Khanafer, M.; Al-Mailem, D.; Ali, N.; Radwan, S. Bias problems in culture-independent analysis of environmental bacterial communities: A representative study on hydrocarbonoclastic bacteria. SpringerPlus 2013, 2, 369. [Google Scholar] [CrossRef] [Green Version]
- Shade, A.; Hogan, C.S.; Klimowicz, A.K.; Linske, M.; McManus, P.S.; Handelsman, J. Culturing captures members of the soil rare biosphere. Environ. Microbiol. 2012, 14, 2247–2252. [Google Scholar] [CrossRef]
- Tsao, P.H. Selective media for isolation of pathogenic fungi. Annu. Rev. Phytopathol. 1970, 8, 157–186. [Google Scholar] [CrossRef]
- Frankland, J.C.; Dighton, J.; Boddy, L. Methods for studying fungi in soil and forest litter. In Methods in Microbiology; Grigorova, R., Norris, J.R., Eds.; Academic Press: New York, NY, USA, 1990; Volume 22, pp. 333–404. [Google Scholar]
- Fuller, M.S.; Fowles, B.E.; McLaughlin, D.J. Isolation and pure culture study of marine phycomycetes. Mycologia 1964, 56, 745–756. [Google Scholar] [CrossRef]
- Mueller, G.M.; Bills, G.F.; Foster, M.S. Biodiversity of Fungi: Inventory and Monitoring Methods; Elsevier Academic Press: San Diego, CA, USA, 2004; p. 612. [Google Scholar]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Ainsworth & Bisby’s Dictionary of the Fungi; CAB International: Wallingford, UK, 2008. [Google Scholar]
- van Etten, J.L.; Dahlberg, K.R.; Russo, G.M. Fungal spore germination. In Fungal Differentiation: A Contemporary Synthesis; Smith, J.E., Ed.; Marcel Dekker: New York, NY, USA, 1983; pp. 235–266. [Google Scholar]
- Lehto, T.; Brosinsky, A.; Heinonen-Tanski, H.; Repo, T. Freezing tolerance of ectomycorrhizal fungi in pure culture. Mycorrhiza 2008, 18, 385–392. [Google Scholar] [CrossRef]
- Morris, A.J.; Byrne, T.C.; Madden, J.F.; Reller, L.B. Duration of incubation of fungal cultures. J. Clin. Microbiol. 1996, 34, 1583–1585. [Google Scholar] [CrossRef] [Green Version]
- Sharon, A.; Shlezinger, N. Fungi infecting plants and animals: Killers, non-killers, and cell death. PLoS Pathog. 2013, 9, e1003517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Ahmed, S.A.; Danesi, P.; Guillot, J.; Gräser, Y. Distribution of pathogens and outbreak fungi in the fungal kingdom. In Emerging and Epizootic Fungal Infections in Animals; Seyedmousavi, S., de Hoog, G., Guillot, J., Verweij, P., Eds.; Springer: Cham, Switzerland, 2018; pp. 3–16. [Google Scholar]
- Seyedmousavi, S.; Bosco, S.D.M.; De Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. J. 2018, 56, S165–S187. [Google Scholar] [CrossRef]
- White-Nose Syndrome: The Devastating Disease of Hibernating Bats in North America. U.S. Fish and Wildlife Fact Sheet, Updated July 2019. Available online: https://www.whitenosesyndrome.org/mmedia-education/white-nose-syndrome-fact-sheet-june-2018 (accessed on 19 March 2021).
- Man, B.; Wang, H.; Xiang, X.; Wang, R.; Yun, Y.; Gong, L. Phylogenetic diversity of culturable fungi in the Heshang Cave, central China. Front. Microbiol. 2015, 6, 1158. [Google Scholar] [CrossRef]
- Vanderwolf, K.J.; Malloch, D.; McAlpine, D.F.; Forbes, G.J. A world review of fungi, yeasts, and slime molds in caves. Int. J. Speleol. 2013, 42, 77–92. [Google Scholar] [CrossRef]
- Christensen, M. Soil microfungi of dry to mesic conifer-hardwood forests in northern Wisconsin. Ecology 1969, 50, 9–27. [Google Scholar] [CrossRef]
- Paulus, B.; Gadek, P.; Hyde, K.D. Estimation of microfungal diversity in tropical rainforest leaf litter using particle filtration: The effects of leaf storage and surface treatment. Mycol. Res. 2003, 107, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.D.; Gunn, J.; Ellis, R.H.; Jenkins, N.E.; Moore, D. The effect of storage environment on the longevity of conidia of Beauveria bassiana. Mycol. Res. 2001, 105, 597–602. [Google Scholar] [CrossRef]
- Hong, T.D.; Ellis, R.H.; Moore, D. Development of a model to predict the effect of temperature and moisture on fungal spore longevity. Ann. Bot. 1997, 79, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Summerbell, R.C. Fungi associated with vertebrates. Biodivers. Fungi Invent. Monit. Methods 2004, 454–465. Available online: https://www.cabdirect.org/?target=%2fcabdirect%2fabstract%2f20198637227 (accessed on 19 March 2021).
- DauBach, C.; DauBach, P.; Taylor, S. Acquisition of the Paul Wightman Subterranean Nature Preserve. NSS News. 2015. Available online: https://www.inhs.illinois.edu/files/4114/4528/9003/DaubachEtAl_2015_AcquisitionofPWNSP_NSS-News_April_v73n4p14-16.pdf (accessed on 19 March 2021).
- Karunarathna, S.C.; Dong, Y.; Karasaki, S.; Tibpromma, S.; Hyde, K.D.; Lumyong, S.; Xu, J.; Sheng, J.; Mortimer, P.E. Discovery of novel fungal species and pathogens on bat carcasses in a cave in Yunnan Province, China. Emerg. Microbes. Infect. 2020, 9, 1554–1566. [Google Scholar] [CrossRef]
- Raudabaugh, D.B.; Miller, A.N. Morphogenetic effect of L-cysteine on Pseudogymnoascus destructans and related species. Mycosphere 2014, 5, 737–746. [Google Scholar] [CrossRef]
- Osmundson, T.W.; Eyre, C.A.; Hayden, K.M.; Dhillon, J.; Garbelotto, M.M. Back to basics: An evaluation of NaOH and alternative rapid DNA extraction protocols for DNA barcoding, genotyping, and disease diagnostics from fungal and oomycete samples. Mol. Ecol. Resour. 2013, 13, 66–74. [Google Scholar] [CrossRef] [PubMed]
- RStudio: Integrated Development for R; RStudio, Inc.: Boston, Massachusetts. 2015. Available online: http://www.rstudio.com/ (accessed on 19 March 2021).
- Blehert, D.S.; Hicks, A.C.; Behr, M.J.; Meteyer, C.U.; Berlowski-Zier, B.M.; Buckles, E.L.; Coleman, J.T.H.; Darling, S.R.; Gargas, A.; Niver, R.; et al. Bat white-nose syndrome: An emerging fungal pathogen? Science 2009, 323, 227-227. [Google Scholar] [CrossRef]
- Rubin, B.E.R.; Gibbons, S.M.; Kennedy, S.; Hampton-Marcell, J.; Owens, S.; Gilbert, J.A. Investigating the impact of storage conditions on microbial community composition in soil samples. PLoS ONE 2013, 8, e70460. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Koske, R.E. Room temperature isolations can bias against selection of low temperature microfungi in temperate forest soils. Mycologia 1992, 84, 886–900. [Google Scholar] [CrossRef]
- Vieira, F.C.S.; Nahas, E. Microbial counts of dark red latosol samples stored at different temperatures. Rev. Microbiol. 1998, 29. [Google Scholar] [CrossRef]
- Johnson, L.J.; Miller, A.N.; McCleery, R.A.; McClanahan, R.; Kath, J.A.; Lueschow, S.; Porras-Alfaroa, A. Psychrophilic and psychrotolerant fungi on bats and the presence of Geomyces spp. on bat wings prior to the arrival of white nose syndrome. Appl. Environ. Microbiol. 2013, 79, 5465–5471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderwolf, K.J.; McAlpine, D.F.; Malloch, D.; Forbes, G.J. Ectomycota associated with hibernating bats in eastern Canadian caves prior to the emergence of white-nose syndrome. Northeast. Nat. 2013, 20, 115–130. [Google Scholar] [CrossRef]
- Mok, W.Y.; Luizao, R.C.C.; Barreto da Silva, M.S. Isolation of fungi from bats of the Amazon Basin. Appl. Environ. Microbiol. 1982, 44, 570–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larcher, G.; Bouchara, J.P.; Pailley, P.; Montfort, D.; Beguin, H.; De Bièvre, C.; Chabasse, D. Fungal biota associated with bats in western France. J. Mycol. Med. 2003, 13, 29–34. [Google Scholar]
- Lorch, J.M.; Lindner, D.L.; Gargas, A.; Muller, L.K.; Minnis, A.M.; Blehert, D.S. A culture-based survey of fungi in soil from bat hibernacula in the eastern United States and its implications for detection of Geomyces destructans, the causal agent of bat white-nose syndrome. Mycologia 2013, 105, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Shabbir, M.Z.; Jamil, T.; Ali, A.A.; Ahmad, A.; Naeem, M.; Chaudhary, M.H.; Bilal, M.; Ali, M.A.; Muhammad, K.; Yaqub, T.; et al. Prevalence and distribution of soil-borne zoonotic pathogens in Lahore district of Pakistan. Front. Microbiol. 2015, 6, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, S.; Collins, T.; Marx, J.C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.A. The Geomyces fungi: Ecology and distribution. BioScience 2012, 62, 819–823. [Google Scholar] [CrossRef] [Green Version]
| Phylum | Class | Order | Family | Genus/Group | Number of Total Isolates Per Temperature (°C) | Isolates per Time Frame | |||
|---|---|---|---|---|---|---|---|---|---|
| −20 to −80 | 7 to 22 | 0 to 1M | 6M to 1Y | ||||||
| Ascomycota | |||||||||
| Dothideomycetes | Capnodiales | Cladosporiaceae | Cladosporium | 10 | 3 | 8 | 5 | ||
| Dothideales | Saccotheciaceae | Aureobasidium | 1 | - | 1 | - | |||
| Pleosporales | Didymellaceae | Epicoccum | 2 | 1 | 2 | 1 | |||
| Pleosporaceae | Alternaria | 2 | 1 | - | 3 | ||||
| Incertae sedis | Phyllostictaceae | Phyllosticta | 1 | - | - | 1 | |||
| * | * | Unidentified | - | 1 | - | 1 | |||
| Eurotiomycetes | Eurotiales | Aspergillaceae | Penicillium | 51 | 83 | 98 | 36 | ||
| Aspergillus | 3 | 2 | 5 | - | |||||
| Onygenales | Arthrodermataceae | Arthroderma / Chrysosporium | - | 7 | 3 | 4 | |||
| * | Unidentified | 2 | 1 | 2 | 1 | ||||
| * | * | Unidentified | 1 | 1 | 2 | - | |||
| Incertae sedis | Incertae sedis | Incertae sedis | Myriodontium | 1 | 6 | 5 | 2 | ||
| Leotiomycetes | Helotiales | * | Unidentified | 1 | 1 | 1 | 1 | ||
| Myxotrichaceae | Oidiodendron | 1 | 3 | 1 | 3 | ||||
| Thelebolales | Pseudeurotiaceae | Pseudogymnoascus / Geomyces | 27 | 44 | 29 | 42 | |||
| Pseudeurotium | - | 1 | 1 | - | |||||
| Saccharomycetes | Saccharomycetales | Wickerhamomy- cetaceae | Barnettozyma | 3 | - | 1 | 2 | ||
| Saccharomycetaceae | Debaryomyces | 4 | 2 | 1 | 5 | ||||
| Dipodascaceae | Geotrichum | - | 1 | 1 | - | ||||
| Galactomyces | - | 3 | 3 | - | |||||
| Sordariomycetes | Amphisphaeriales | Pestalotiopsidaceae | Pestalotiopsis | - | 2 | - | 2 | ||
| Hypocreales | Cordycipitaceae | Simplicillium / Lecanicillium | 1 | - | - | 1 | |||
| Nectriaceae | Fusarium | 3 | 1 | 4 | - | ||||
| Incertae sedis | Ilyonectria | - | 1 | 1 | - | ||||
| Glomerellales | Plectosphaerellaceae | Gibellulopsis | 1 | - | - | 1 | |||
| Sordariales | Chaetomiaceae | Unidentified | 1 | 2 | - | 3 | |||
| Chaetomium | 1 | 5 | - | 6 | |||||
| Xylariales | Diatrypaceae | Diatrype | 2 | - | - | 2 | |||
| * | * | Unidentified | 2 | 2 | 4 | - | |||
| * | * | * | Unidentified | 4 | 11 | 11 | 4 | ||
| Basidiomycota | |||||||||
| Agaricomycetes | Agaricales | Incertae sedis | Cyathus | - | 2 | 2 | - | ||
| Psathyrellaceae | Coprinellus | 3 | 5 | - | 8 | ||||
| Tremellomycetes | Cystofilobasidiales | Mrakiaceae | Tausonia | - | 2 | - | 2 | ||
| Trichosporonales | Trichosporonaceae | Apiotrichum/Trichosporon | 8 | 13 | 15 | 6 | |||
| * | * | * | Unidentified | 1 | 2 | - | 3 | ||
| Kickxellomycota | |||||||||
| Kickxellomycetes | Kickxellales | Kickxellaceae | Coemansia | - | 1 | 1 | - | ||
| Mortierellomycota | |||||||||
| Mortierellomycetes | Mortierellales | Mortierellaceae | Mortierella | 24 | 35 | 35 | 24 | ||
| Mucoromycota | |||||||||
| Mucoromycetes | Mucorales | Mucoraceae | Helicostylum | 1 | 2 | 3 | - | ||
| Mucor | 4 | 5 | 9 | - | |||||
| Rhizopodaceae | Rhizopus | - | 2 | - | 2 | ||||
| zygomycotan fungi 1 | |||||||||
| * | * | * | Unidentified | - | 2 | 2 | - | ||
| Phylum | Class | Order | Species Epithet |
|---|---|---|---|
| Ascomycota | Dothideomycetes | Capnodiales | Cladosporium cladosporioides |
| Cladosporium sp. | |||
| Dothideales | Aureobasidium pullulans | ||
| Incertae sedis | Phyllosticta citricarpa | ||
| Pleosporales | Alternaria alternata | ||
| Alternaria sp. | |||
| Epicoccum sp. | |||
| Epicoccum tobaicum | |||
| Eurotiomycetes | Eurotiales | Aspergillus sp. | |
| Penicillium atramentosum | |||
| Penicillium bialowiezense | |||
| Penicillium commune | |||
| Penicillium crustosum | |||
| Penicillium expansum | |||
| Penicillium glandicola | |||
| Penicillium sp. | |||
| Penicillium vulpinum | |||
| Incertae sedis | Incertae sedis | Myriodontium keratinophilum | |
| Leotiomycetes | Helotiales | Oidiodendron sp. | |
| Thelebolales | Pseudeurotium sp. | ||
| Pseudogymnoascus pannorum | |||
| Pseudogymnoascus sp. | |||
| Saccharomycetes | Saccharomycetales | Barnettozyma californica | |
| Debaryomyces hansenii | |||
| Galactomyces sp. | |||
| Geotrichum candidum | |||
| Sordariomycetes | Amphisphaeriales | Pestalotiopsis sp. | |
| Glomerellales | Gibellulopsis nigrescens | ||
| Hypocreales | Fusarium sp. | ||
| Ilyonectria robusta | |||
| Sordariales | Chaetomium crispatum | ||
| Chaetomium sp. | |||
| Xylariales | Diatrype stigma | ||
| Basidiomycota | Agaricomycetes | Agaricales | Coprinellus micaceus |
| Cyathus ibericus | |||
| Tremellomycetes | Cystofilobasidiales | Tausonia pullulans | |
| Trichosporonales | Apiotrichum dulcitum | ||
| Arthroderma quadrifidum | |||
| Kickxellomycota | Kickxellomycetes | Kickxellales | Coemansia sp. |
| Mortierellomycota | Mortierellomycetes | Mortierellales | Mortierella alpina |
| Mortierella elongata | |||
| Mortierella hyalina | |||
| Mortierella polycephala | |||
| Mortierella sp. | |||
| Mucoromycota | Mucoromycetes | Mucorales | Helicostylum pulchrum |
| Helicostylum sp. | |||
| Mucor aligarensis | |||
| Mucor flavus | |||
| Mucor nidicola | |||
| Mucor racemosus | |||
| Mucor sp. | |||
| Rhizopus oryzae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raudabaugh, D.B.; Rivera, N.A.; Anchor, G.C.; Bach, E.; Miller, A.N.; Mateus-Pinilla, N.E. Preliminary Study of Cave Sample Storage Conditions on Fungal Community Diversity. Diversity 2021, 13, 188. https://doi.org/10.3390/d13050188
Raudabaugh DB, Rivera NA, Anchor GC, Bach E, Miller AN, Mateus-Pinilla NE. Preliminary Study of Cave Sample Storage Conditions on Fungal Community Diversity. Diversity. 2021; 13(5):188. https://doi.org/10.3390/d13050188
Chicago/Turabian StyleRaudabaugh, Daniel B., Nelda A. Rivera, Gretchen C. Anchor, Elizabeth Bach, Andrew N. Miller, and Nohra E. Mateus-Pinilla. 2021. "Preliminary Study of Cave Sample Storage Conditions on Fungal Community Diversity" Diversity 13, no. 5: 188. https://doi.org/10.3390/d13050188
APA StyleRaudabaugh, D. B., Rivera, N. A., Anchor, G. C., Bach, E., Miller, A. N., & Mateus-Pinilla, N. E. (2021). Preliminary Study of Cave Sample Storage Conditions on Fungal Community Diversity. Diversity, 13(5), 188. https://doi.org/10.3390/d13050188








