Of Necks, Trunks and Tails: Axial Skeletal Diversity among Vertebrates
Abstract
:1. Introduction
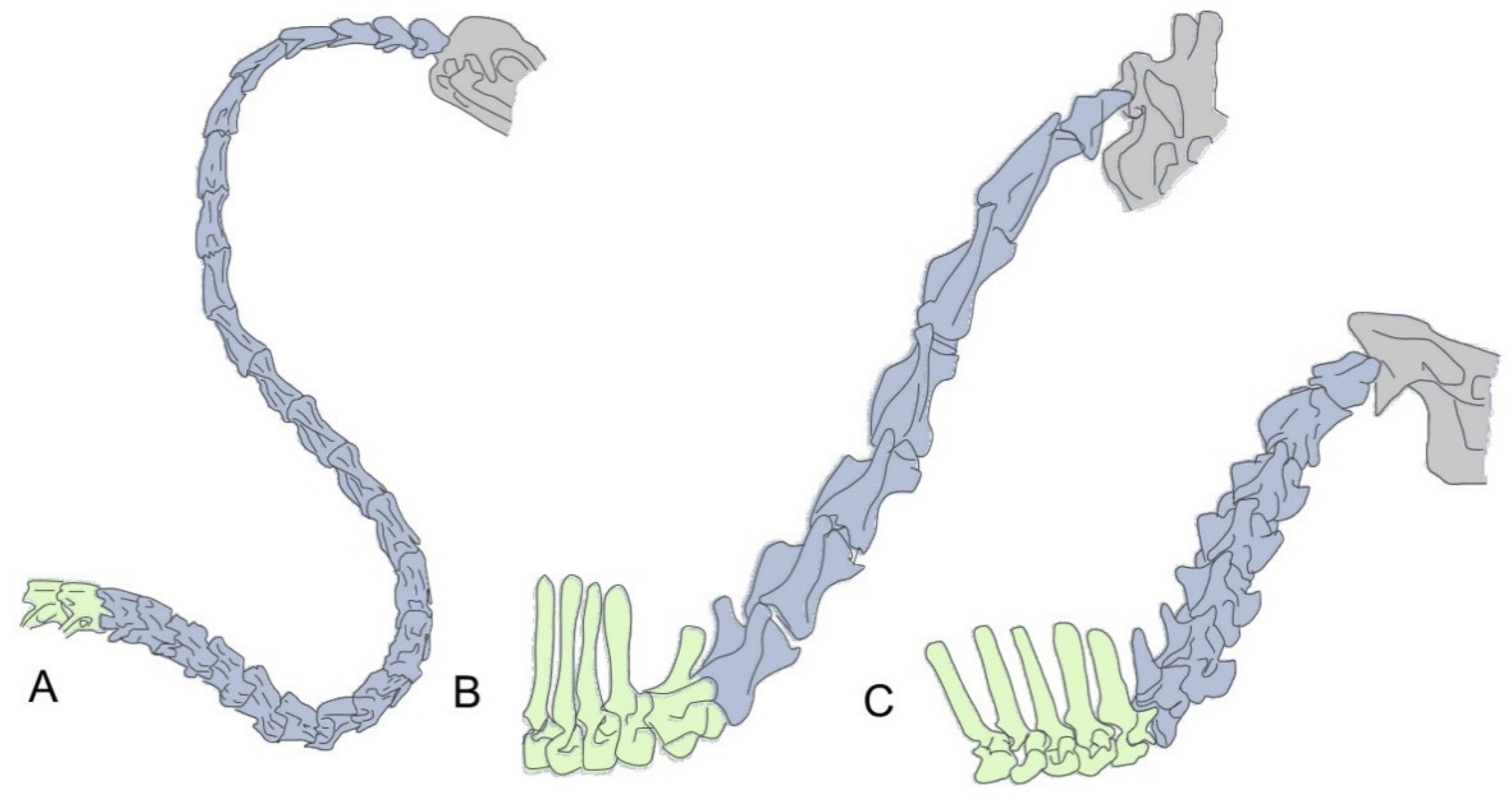
2. The Neck: Different Solutions to Similar Problems
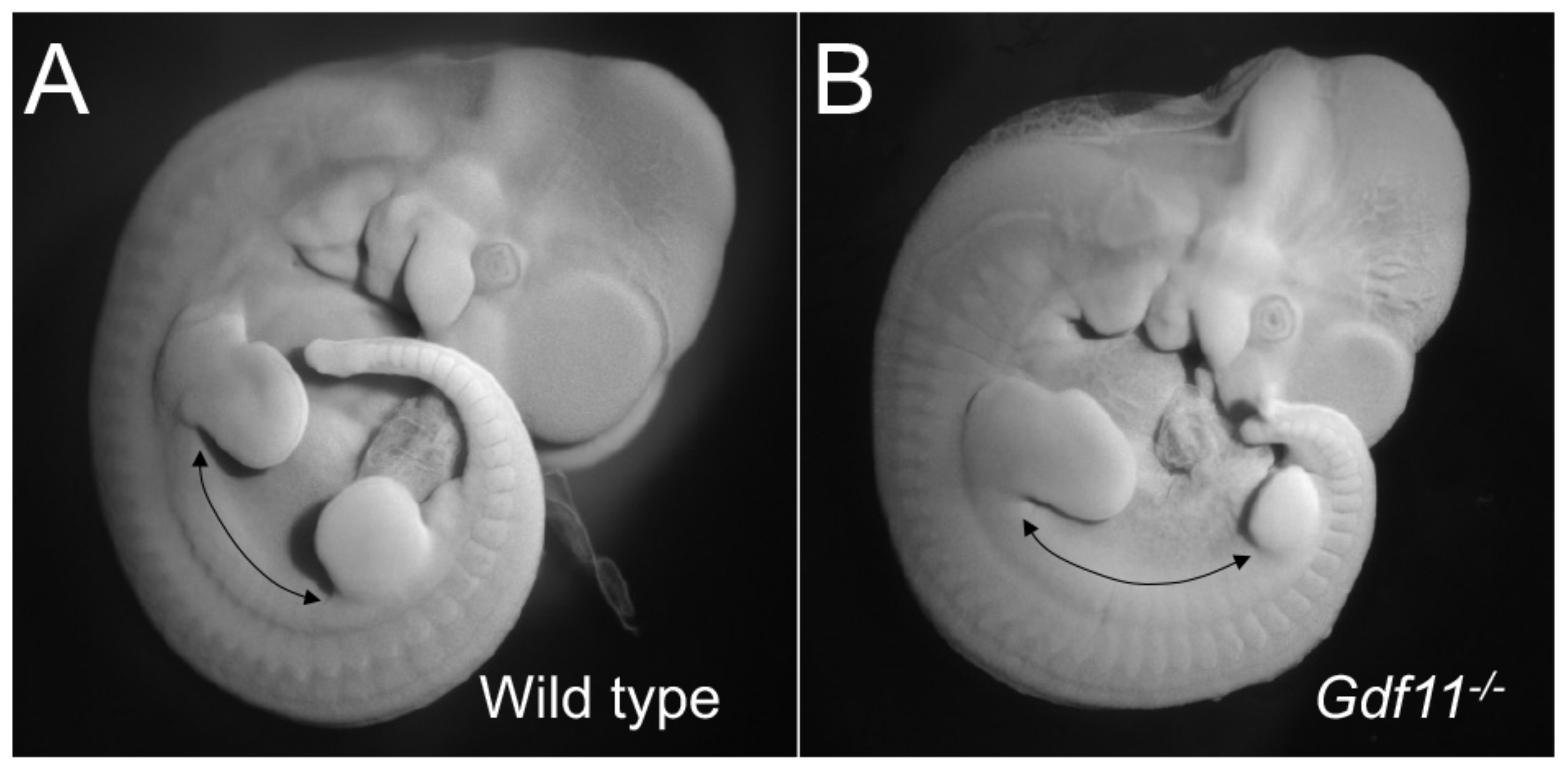
3. Trunk Size and Shape: Extreme Differences
4. The Tail Is Not Just a Tail
5. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallo, M. The Axial Musculoskeletal System. In Kaufman’s Atlas of Mouse Development Supplement; Baldock, R., Bard, J., Davidson, D.R., Morriss-Kay, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 165–175. ISBN 9780128000434. [Google Scholar]
- Bénazéraf, B.; Pourquié, O. Formation and Segmentation of the Vertebrate Body Axis. Annu. Rev. Cell Dev. Biol. 2013, 29, 1–26. [Google Scholar] [CrossRef]
- Gridley, T. The long and short of it: Somite formation in mice. Dev. Dyn. 2006, 235, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Hubaud, A.; Pourquie, O. Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 2014, 15, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Mallo, M. Revisiting the involvement of signaling gradients in somitogenesis. FEBS J. 2016, 283, 1430–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couly, G.F.; Coltey, P.M.; Le Douarin, N.M. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development 1993, 117, 409–429. [Google Scholar] [CrossRef]
- Handrigan, G.R.; Wassersug, R.J. The anuran Bauplan: A review of the adaptive, developmental, and genetic underpinnings of frog and tadpole morphology. Biol. Rev. 2007, 82, 1–25. [Google Scholar] [CrossRef]
- Morin-Kensicki, E.M.; Melancon, E.; Eisen, J.S. Segmental relationship between somites and vertebral column in zebrafish. Development 2002, 129, 3851–3860. [Google Scholar] [CrossRef]
- Mallo, M.; Vinagre, T.; Carapuço, M. The road to the vertebral formula. Int. J. Dev. Biol. 2009, 53, 1469–1481. [Google Scholar] [CrossRef] [Green Version]
- Mallo, M.; Wellik, D.M.; Deschamps, J. Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 2010, 344, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Wellik, D.M. Hox patterning of the vertebrate axial skeleton. Dev. Dyn. 2007, 236, 2454–2463. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, J.; Duboule, D. Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock. Genes Dev. 2017, 31, 1406–1416. [Google Scholar] [CrossRef] [Green Version]
- Krumlauf, R. Hox genes in vertebrate development. Cell 1994, 78, 191–201. [Google Scholar] [CrossRef]
- Amores, A.; Force, A.; Yan, Y.-L.; Joly, L.; Amemiya, C.; Fritz, A.; Ho, R.K.; Langeland, J.; Prince, V.; Wang, Y.-L.; et al. Zebrafish hox Clusters and Vertebrate Genome Evolution. Science 1998, 282, 1711–1714. [Google Scholar] [CrossRef]
- Burke, A.C.; Nelson, C.E.; Morgan, B.A.; Tabin, C. Hox genes and the evolution of vertebrate axial morphology. Development 1995, 121, 333–346. [Google Scholar] [CrossRef]
- Gomez, C.; Özbudak, E.M.; Wunderlich, J.; Baumann, D.; Lewis, J.; Pourquie, O. Control of segment number in vertebrate embryos. Nature 2008, 454, 335–339. [Google Scholar] [CrossRef]
- Woltering, J.M.; Vonk, F.J.; Müller, H.; Bardine, N.; Tuduce, I.L.; de Bakker, M.A.G.; Knöchel, W.; Sirbu, I.O.; Durston, A.J.; Richardson, M.K. Axial patterning in snakes and caecilians: Evidence for an alternative interpretation of the Hox code. Dev. Biol. 2009, 332, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Narita, Y.; Kuratani, S. Evolution of the vertebral formulae in mammals: A perspective on developmental constraints. J. Exp. Zool. Part B Mol. Dev. Evol. 2005, 304, 91–106. [Google Scholar] [CrossRef]
- Gaunt, S.J. Conservation in the Hox code during morphological evolution. Int. J. Dev. Biol. 1994, 38, 549–552. [Google Scholar]
- Di-Poï, N.; Montoya-Burgos, J.I.; Miller, H.; Pourquie, O.; Milinkovitch, M.C.; Duboule, D. Changes in Hox genes’ structure and function during the evolution of the squamate body plan. Nature 2010, 464, 99–103. [Google Scholar] [CrossRef]
- Mansfield, J.H.; Abzhanov, A. Hox expression in the American alligator and evolution of archosaurian axial patterning. J. Exp. Zool. Part B Mol. Dev. Evol. 2010, 314B, 629–644. [Google Scholar] [CrossRef]
- Vinagre, T.; Moncaut, N.; Carapuço, M.; Nóvoa, A.; Bom, J.; Mallo, M. Evidence for a Myotomal Hox/Myf Cascade Governing Nonautonomous Control of Rib Specification within Global Vertebral Domains. Dev. Cell 2010, 18, 655–661. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, D.C.; Rakshit, S.; Yallowitz, A.R.; Loken, L.; Jeannotte, L.; Capecchi, M.R.; Wellik, D.M. Hox patterning of the vertebrate rib cage. Development 2007, 134, 2981–2989. [Google Scholar] [CrossRef] [Green Version]
- Rancourt, D.E.; Tsuzuki, T.; Capecchi, M.R. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev. 1995, 9, 108–122. [Google Scholar] [CrossRef] [Green Version]
- Noordermeer, D.; Leleu, M.; Schorderet, P.; Joye, E.; Chabaud, F.; Duboule, D. Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. eLife 2014, 2014, e02557. [Google Scholar] [CrossRef]
- Mallo, M.; Alonso, C.R. The regulation of Hox gene expression during animal development. Development 2013, 140, 3951–3963. [Google Scholar] [CrossRef] [Green Version]
- Soshnikova, N.; Duboule, D. Epigenetic Temporal Control of Mouse Hox Genes in Vivo. Science 2009, 324, 1320–1323. [Google Scholar] [CrossRef]
- Berlivet, S.; Paquette, D.; Du Mouchel, A.; Langlais, D.; Dostie, J.; Kmita, M. Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of HoxA Genes in Developing Limbs. PLoS Genet. 2013, 9, e1004018. [Google Scholar] [CrossRef] [Green Version]
- Narendra, V.; Rocha, P.P.; An, D.; Raviram, R.; Skok, J.A.; Mazzoni, E.O.; Reinberg, D. CTCF establishes discrete functional chromatin domains at theHoxclusters during differentiation. Science 2015, 347, 1017–1021. [Google Scholar] [CrossRef] [Green Version]
- Andrey, G.; Montavon, T.; Mascrez, B.; Gonzalez, F.; Noordermeer, D.; Leleu, M.; Trono, D.; Spitz, F.; Duboule, D. A Switch Between Topological Domains Underlies HoxD Genes Collinearity in Mouse Limbs. Science 2013, 340, 1234167. [Google Scholar] [CrossRef]
- Woltering, J.M.; Noordermeer, D.; Leleu, M.; Duboule, D. Conservation and Divergence of Regulatory Strategies at Hox Loci and the Origin of Tetrapod Digits. PLoS Biol. 2014, 12, e1001773. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nora, E.P.; Lajoie, B.R.; Schulz, E.G.; Giorgetti, L.; Okamoto, I.; Servant, N.; Piolot, T.; van Berkum, N.L.; Meisig, J.; Sedat, J.; et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 2012, 485, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narendra, V.; Bulajic, M.; Dekker, J.; Mazzoni, E.O.; Reinberg, D. CTCF-mediated topological boundaries during development foster appropriate gene regulation. Genes Dev. 2016, 30, 2657–2662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neijts, R.; Amin, S.; van Rooijen, C.; Deschamps, J. Cdx is crucial for the timing mechanism driving colinear Hox activation and defines a trunk segment in the Hox cluster topology. Dev. Biol. 2017, 422, 146–154. [Google Scholar] [CrossRef]
- Arnold, P.; Amson, E.; Fischer, M.S. Differential scaling patterns of vertebrae and the evolution of neck length in mammals. Evolution 2017, 71, 1587–1599. [Google Scholar] [CrossRef]
- Badlangana, N.L.; Adams, J.W.; Manger, P.R. The giraffe (Giraffa camelopardalis) cervical vertebral column: A heuristic example in understanding evolutionary processes? Zool. J. Linn. Soc. 2009, 155, 736–757. [Google Scholar] [CrossRef] [Green Version]
- Agaba, M.; Ishengoma, E.; Miller, W.C.; McGrath, B.C.; Hudson, C.N.; Reina, O.C.B.; Ratan, A.; Burhans, R.; Chikhi, R.; Medvedev, R.C.P.; et al. Giraffe genome sequence reveals clues to its unique morphology and physiology. Nat. Commun. 2016, 7, 11519. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Gao, J.; Cui, X.; Li, Z.; Chen, L.; Yuan, Y.; Zhang, Y.; Mei, L.; Zhao, L.; Cai, D.; et al. A towering genome: Experimentally validated adaptations to high blood pressure and extreme stature in the giraffe. Sci. Adv. 2021, 7, eabe9459. [Google Scholar] [CrossRef]
- Dubrulle, J.; McGrew, M.J.; Pourquie, O. FGF Signaling Controls Somite Boundary Position and Regulates Segmentation Clock Control of Spatiotemporal Hox Gene Activation. Cell 2001, 106, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Condie, B.G.; Capecchi, M.R. Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3 reveal synergistic interactions. Nature 1994, 370, 304–307. [Google Scholar] [CrossRef]
- Condie, B.G.; Capecchi, M.R. Mice homozygous for a targeted disruption of Hoxd-3 (Hox-4.1) exhibit anterior transformations of the first and second cervical vertebrae, the atlas and the axis. Development 1993, 119, 579–595. [Google Scholar] [CrossRef]
- Horan, G.S.B.; Ramírez-Solis, R.; Featherstone, M.S.; Wolgemuth, D.J.; Bradley, A.; Behringer, R.R. Compound mutants for the paralogous hoxa-4, hoxb-4, and hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes Dev. 1995, 9, 1667–1677. [Google Scholar] [CrossRef] [Green Version]
- Su, N.; Jin, M.; Chen, L. Role of FGF/FGFR signaling in skeletal development and homeostasis: Learning from mouse models. Bone Res. 2014, 2, 14003. [Google Scholar] [CrossRef] [Green Version]
- Regard, J.B.; Zhong, Z.; Williams, B.O.; Yang, Y. Wnt Signaling in Bone Development and Disease: Making Stronger Bone with Wnts. Cold Spring Harb. Perspect. Biol. 2012, 4, a007997. [Google Scholar] [CrossRef] [Green Version]
- Zieba, J.T.; Chen, Y.-T.; Lee, B.H.; Bae, Y. Notch Signaling in Skeletal Development, Homeostasis and Pathogenesis. Biomolecules 2020, 10, 332. [Google Scholar] [CrossRef] [Green Version]
- Buchholtz, E.A.; Bailin, H.G.; Laves, S.A.; Yang, J.T.; Drozd, L.E.; Chan, M.-Y. Fixed cervical count and the origin of the mammalian diaphragm. Evol. Dev. 2012, 14, 399–411. [Google Scholar] [CrossRef]
- Hautier, L.; Weisbecker, V.; Sanchez-Villagra, M.R.; Goswami, A.; Asher, R.J. Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proc. Natl. Acad. Sci. USA 2010, 107, 18903–18908. [Google Scholar] [CrossRef] [Green Version]
- Buchholtz, E.A. Crossing the frontier: A hypothesis for the origins of meristic constraint in mammalian axial patterning. Zoology 2014, 117, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Vasyutina, E.; Birchmeier, C. The development of migrating muscle precursor cells. Anat. Embryol. 2006, 211, 37–41. [Google Scholar] [CrossRef]
- Rommel, S.; Reynolds, J.E. Diaphragm structure and function in the Florida manatee (Trichechus manatus latirostris). Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2000, 259, 41–51. [Google Scholar] [CrossRef]
- Galis, F. Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. J. Exp. Zool. 1999, 285, 19–26. [Google Scholar] [CrossRef]
- Marek, R.D.; Falkingham, P.L.; Benson, R.B.J.; Gardiner, J.D.; Maddox, T.W.; Bates, K.T. Evolutionary versatility of the avian neck. Proc. R. Soc. B Boil. Sci. 2021, 288, 20203150. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 1999, 22, 260–264. [Google Scholar] [CrossRef]
- Szumska, D.; Pieles, G.; Essalmani, R.; Bilski, M.; Mesnard, D.; Kaur, K.; Franklyn, A.; El Omari, K.; Jefferis, J.; Bentham, J.; et al. VACTERL/caudal regression/Currarino syndrome-like malformations in mice with mutation in the proprotein convertase Pcsk5. Genes Dev. 2008, 22, 1465–1477. [Google Scholar] [CrossRef] [Green Version]
- Aires, R.; Jurberg, A.D.; Leal, F.; Nóvoa, A.; Cohn, M.J.; Mallo, M. Oct4 Is a Key Regulator of Vertebrate Trunk Length Diversity. Dev. Cell 2016, 38, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Jurberg, A.D.; Aires, R.; Varela-Lasheras, I.; Nóvoa, A.; Mallo, M. Switching Axial Progenitors from Producing Trunk to Tail Tissues in Vertebrate Embryos. Dev. Cell 2013, 25, 451–462. [Google Scholar] [CrossRef] [Green Version]
- McPherron, A.C.; Huynh, T.V.; Lee, S.-J. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev. Biol. 2009, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.P.; Li, E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997, 11, 1812–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.P.; Yeo, C.-Y.; Lee, Y.; Schrewe, H.; Whitman, M.; Li, E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002, 16, 2749–2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essalmani, R.; Zaid, A.; Marcinkiewicz, J.; Chamberland, A.; Pasquato, A.; Seidah, N.G.; Prat, A. In vivo functions of the proprotein convertase PC5/6 during mouse development: Gdf11 is a likely substrate. Proc. Natl. Acad. Sci. USA 2008, 105, 5750–5755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, O.; Reissmann, E.; Ibáñez, C.F. Growth differentiation factor 11 signals through the transforming growth factor-β receptor ALK5 to regionalize the anterior–posterior axis. EMBO Rep. 2006, 7, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, Y.; Hirasawa, T.; Egawa, S.; Hattori, A.; Suganuma, T.; Kohara, Y.; Nagai, T.; Tamura, K.; Kuratani, S.; Kuroiwa, A.; et al. Anatomical integration of the sacral–hindlimb unit coordinated by GDF11 underlies variation in hindlimb positioning in tetrapods. Nat. Ecol. Evol. 2017, 1, 1392–1399. [Google Scholar] [CrossRef]
- Ho, D.M.; Yeo, C.-Y.; Whitman, M. The role and regulation of GDF11 in Smad2 activation during tailbud formation in the Xenopus embryo. Mech. Dev. 2010, 127, 485–495. [Google Scholar] [CrossRef]
- Wong, S.F.L.; Agarwal, V.; Mansfield, J.H.; Denans, N.; Schwartz, M.G.; Prosser, H.M.; Pourquie, O.; Bartel, D.P.; Tabin, C.J.; McGlinn, E. Independent regulation of vertebral number and vertebral identity by microRNA-196 paralogs. Proc. Natl. Acad. Sci. USA 2015, 112, E4884–E4893. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Yan, Y.-L.; Eberhart, J.K.; Herpin, A.; Wagner, T.U.; Schartl, M.; Postlethwait, J.H. miR-196 regulates axial patterning and pectoral appendage initiation. Dev. Biol. 2011, 357, 463–477. [Google Scholar] [CrossRef] [Green Version]
- Asli, N.S.; Kessel, M. Spatiotemporally restricted regulation of generic motor neuron programs by miR-196-mediated repression of Hoxb8. Dev. Biol. 2010, 344, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Mallo, M. Reassessing the Role of Hox Genes during Vertebrate Development and Evolution. Trends Genet. 2018, 34, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Galis, F.; Carrier, D.R.; van Alphen, J.; van der Mije, S.D.; van Dooren, T.J.M.; Metz, J.A.J.; Broek, C.M.A.T. Fast running restricts evolutionary change of the vertebral column in mammals. Proc. Natl. Acad. Sci. USA 2014, 111, 11401–11406. [Google Scholar] [CrossRef] [Green Version]
- Nickel, R.; Schummer, A.; Seiferle, E.; Siller, W.G.; Wight, P.A.L. Anatomy of the Domestic Birds; Springer: Heidelberg/Berlin, Germany, 1977. [Google Scholar]
- Shine, R. Vertebral numbers in male and female snakes: The roles of natural, sexual and fecundity selection. J. Evol. Biol. 2000, 13, 455–465. [Google Scholar] [CrossRef]
- Wellik, D.M.; Capecchi, M.R. Hox10 and Hox11 Genes Are Required to Globally Pattern the Mammalian Skeleton. Science 2003, 301, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Carapuço, M.; Nóvoa, A.; Bobola, N.; Mallo, M. Hox genes specify vertebral types in the presomitic mesoderm. Genes Dev. 2005, 19, 2116–2121. [Google Scholar] [CrossRef] [Green Version]
- Head, J.J.; Polly, P.D. Evolution of the snake body form reveals homoplasy in amniote Hox gene function. Nature 2015, 520, 86–89. [Google Scholar] [CrossRef]
- Guerreiro, I.; Nunes, A.; Woltering, J.M.; Casaca, A.; Nóvoa, A.; Vinagre, T.; Hunter, M.E.; Duboule, D.; Mallo, M. Role of a polymorphism in a Hox/Pax-responsive enhancer in the evolution of the vertebrate spine. Proc. Natl. Acad. Sci. USA 2013, 110, 10682–10686. [Google Scholar] [CrossRef] [Green Version]
- Denans, N.; Iimura, T.; Pourquié, O. Hox genes control vertebrate body elongation by collinear Wnt repression. eLife 2015, 4, 4379. [Google Scholar] [CrossRef]
- Hirasawa, T.; Pascual-Anaya, J.; Kamezaki, N.; Taniguchi, M.; Mine, K.; Kuratani, S. The evolutionary origin of the turtle shell and its dependence on the axial arrest of the embryonic rib cage. J. Exp. Zool. Part B Mol. Dev. Evol. 2015, 324, 194–207. [Google Scholar] [CrossRef]
- Kawashima-Ohya, Y.; Narita, Y.; Nagashima, H.; Usuda, R.; Kuratani, S. Hepatocyte growth factor is crucial for development of the carapace in turtles. Evol. Dev. 2011, 13, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Ohya, Y.K.; Usuda, R.; Kuraku, S.; Nagashima, H.; Kuratani, S. Unique features of Myf-5 in turtles: Nucleotide deletion, alternative splicing, and unusual expression pattern. Evol. Dev. 2006, 8, 415–423. [Google Scholar] [CrossRef]
- Hirasawa, T.; Nagashima, H.; Kuratani, S. The endoskeletal origin of the turtle carapace. Nat. Commun. 2013, 4, 2107. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.L.R. On the Tails of Birds. Bioscience 1997, 47, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Desvignes, T.; Carey, A.; Postlethwait, J.H. Evolution of caudal fin ray development and caudal fin hypural diastema complex in spotted gar, teleosts, and other neopterygian fishes. Dev. Dyn. 2018, 247, 832–853. [Google Scholar] [CrossRef]
- Lauder, G.V. Caudal Fin Locomotion in Ray-finned Fishes: Historical and Functional Analyses. Am. Zool. 1989, 29, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Metscher, B.D.; Ahlberg, P.E. Origin of the teleost tail: Phylogenetic frameworks for developmental studies. In Major Events in Early Vertebrate Evolution: Palaeontology, Phylogeny, Genetics and Development; Ahlberg, P.E., Ed.; Taylor & Francis: London, UK; New York, NY, USA, 2001; Volume 61, pp. 333–349. [Google Scholar]
- Bird, N.C.; Mabee, P.M. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev. Dyn. 2003, 228, 337–357. [Google Scholar] [CrossRef] [PubMed]
- Woltering, J.M.; Holzem, M.; Schneider, R.F.; Nanos, V.; Meyer, A. The skeletal ontogeny of Astatotilapia burtoni—A direct-developing model system for the evolution and development of the teleost body plan. BMC Dev. Biol. 2018, 18, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felts, W.J.L. Some functional and structural characteristics of cetacean flippers and flukes. In Whales, Dolphins, and Porpoises; Norris, K.S., Ed.; University of California Press: Berkeley, CA, USA, 1966; pp. 255–276. [Google Scholar]
- Flammang, B.E. The fish tail as a derivation from axial musculoskeletal anatomy: An integrative analysis of functional morphology. Zoology 2014, 117, 86–92. [Google Scholar] [CrossRef]
- Marí-Beffa, M.; Murciano, C. Dermoskeleton morphogenesis in zebrafish fins. Dev. Dyn. 2010, 239, 2779–2794. [Google Scholar] [CrossRef] [Green Version]
- Thewissen, J.G.M.; Cohn, M.J.; Stevens, L.S.; Bajpai, S.; Heyning, J.; Horton, W.E.; Thewissen, J.G.M.; Cohn, M.J.; Stevens, L.S.; Bajpai, S.; et al. Developmental basis for hind-limb loss in dolphins and origin of the cetacean bodyplan. Proc. Natl. Acad. Sci. USA 2006, 103, 8414–8418. [Google Scholar] [CrossRef] [Green Version]
- Thewissen, J.G.M. Highlights of Cetacean Embryology. Aquat. Mamm. 2018, 44, 591–602. [Google Scholar] [CrossRef]
- Ogawa, T. On the presence and disappearance of the hind limb in the cetacean embryos. Sci. Rep. Whales Res. Inst. 1953, 8, 127–132. [Google Scholar]
- Wilson, V.; Olivera-Martinez, I.; Storey, K.G. Stem cells, signals and vertebrate body axis extension. Development 2009, 136, 1591–1604. [Google Scholar] [CrossRef] [Green Version]
- Mallo, M. The vertebrate tail: A gene playground for evolution. Cell. Mol. Life Sci. 2020, 77, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Holmdahl, D.E. Experimentelle Untersuchungen uber die Lage der Grenze primarer und sekundarer Korperentwicklung beim Huhn. Anat. Anz. 1925, 59, 393–396. [Google Scholar]
- Hadzhiev, Y.; Lele, Z.; Schindler, S.; Wilson, S.W.; Ahlberg, P.; Strähle, U.; Müller, F. Hedgehog signaling patterns the outgrowth of unpaired skeletal appendages in zebrafish. BMC Dev. Biol. 2007, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.; Lozovska, A.; Wymeersch, F.J.; Nóvoa, A.; Binagui-Casas, A.; Sobral, D.; Martins, G.G.; Wilson, V.; Mallo, M. A Tgfbr1/Snai1-dependent developmental module at the core of vertebrate axial elongation. eLife 2020, 9, e56615. [Google Scholar] [CrossRef]
- Henrique, D.; Abranches, E.; Verrier, L.; Storey, K.G. Neuromesodermal progenitors and the making of the spinal cord. Development 2015, 142, 2864–2875. [Google Scholar] [CrossRef] [Green Version]
- Steventon, B.; Arias, A.M. Evo-engineering and the cellular and molecular origins of the vertebrate spinal cord. Dev. Biol. 2017, 432, 3–13. [Google Scholar] [CrossRef]
- Robinton, D.A.; Chal, J.; da Rocha, E.L.; Han, A.; Yermalovich, A.V.; Oginuma, M.; Schlaeger, T.M.; Sousa, P.; Rodriguez, A.; Urbach, A.; et al. The Lin28/let-7 Pathway Regulates the Mammalian Caudal Body Axis Elongation Program. Dev. Cell 2019, 48, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Aires, R.; de Lemos, L.; Nóvoa, A.; Jurberg, A.D.; Mascrez, B.; Duboule, D.; Mallo, M. Tail Bud Progenitor Activity Relies on a Network Comprising Gdf11, Lin28, and Hox13 Genes. Dev. Cell 2019, 48, 383–395.e8. [Google Scholar] [CrossRef] [Green Version]
- DeVeale, B.; Brokhman, I.; Mohseni, P.; Babak, T.; Yoon, C.; Lin, A.; Onishi, K.; Tomilin, A.; Pevny, L.; Zandstra, P.W.; et al. Oct4 Is Required∼E7.5 for Proliferation in the Primitive Streak. PLoS Genet. 2013, 9, e1003957. [Google Scholar] [CrossRef]
- Agathon, A.; Thisse, C.; Thisse, B. The molecular nature of the zebrafish tail organizer. Nature 2003, 424, 448–452. [Google Scholar] [CrossRef]
- Holley, S.A. Anterior-posterior differences in vertebrate segments: Specification of trunk and tail somites in the zebrafish blastula. Genes Dev. 2006, 20, 1831–1837. [Google Scholar] [CrossRef] [Green Version]
- Young, T.; Rowland, J.E.; van de Ven, C.; Bialecka, M.; Novoa, A.; Carapuco, M.; van Nes, J.; de Graaff, W.; Duluc, I.; Freund, J.-N.; et al. Cdx and Hox Genes Differentially Regulate Posterior Axial Growth in Mammalian Embryos. Dev. Cell 2009, 17, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Economides, K.D.; Capecchi, M.R. Hoxb13 is required for normal differentiation and secretory function of the ventral prostate. Development 2003, 130, 2061–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Z.; Kimelman, D. hox13 genes are required for mesoderm formation and axis elongation during early zebrafish development. Development 2020, 147, 185298. [Google Scholar] [CrossRef]
- Fromental-Ramain, C.; Warot, X.; Lakkaraju, S.; Favier, B.; Haack, H.; Birling, C.; Dierich, A.; Dolle, P.; Chambon, P. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development 1996, 122, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Warot, X.; Fromental-Ramain, C.; Fraulob, V.; Chambon, P.; Dolle, P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development 1997, 124, 4781–4791. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Bae, Y.-K.; Muraoka, O.; Hibi, M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev. Biol. 2005, 279, 125–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerschmidt, M.; Pelegri, F.; Mullins, M.C.; Kane, D.A.; Brand, M.; van Eeden, F.J.M.; Furutani-Seiki, M.; Granato, M.; Haffter, P.; Heisenberg, C.P.; et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish, Danio rerio. Development 1996, 123, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.J.; Kimelman, D. Interplay between FGF, one-eyed pinhead, and T-box transcription factors during zebrafish posterior development. Dev. Biol. 2003, 264, 456–466. [Google Scholar] [CrossRef] [Green Version]
- Carlson, M.R.J.; Komine, Y.; Bryant, S.V.; Gardiner, D.M. Expression of Hoxb13 and Hoxc10 in Developing and Regenerating Axolotl Limbs and Tails. Dev. Biol. 2001, 229, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Galbusera, F.; Bassani, T. The Spine: A Strong, Stable, and Flexible Structure with Biomimetics Potential. Biomimetics 2019, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Hayashi, H.; Garcia-Ojalvo, J.; Yoshioka-Kobayashi, K.; Kageyama, R.; Yamanaka, Y.; Ikeya, M.; Toguchida, J.; Alev, C.; Ebisuya, M. Species-specific segmentation clock periods are due to differential biochemical reaction speeds. Science 2020, 369, 1450–1455. [Google Scholar] [CrossRef]
- Rayon, T.; Stamataki, D.; Perez-Carrasco, R.; Garcia-Perez, L.; Barrington, C.; Melchionda, M.; Exelby, K.; Lazaro, J.; Tybulewicz, V.L.J.; Fisher, E.M.C.; et al. Species-specific pace of development is associated with differences in protein stability. Science 2020, 369, eaba7667. [Google Scholar] [CrossRef]
- Beccari, L.; Moris, N.; Girgin, M.; Turner, D.A.; Baillie-Johnson, P.; Cossy, A.-C.; Lutolf, M.P.; Duboule, D.; Arias, A.M. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 2018, 562, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Veenvliet, J.V.; Bolondi, A.; Kretzmer, H.; Haut, L.; Scholze-Wittler, M.; Schifferl, D.; Koch, F.; Guignard, L.; Kumar, A.S.; Pustet, M.; et al. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science 2020, 370, eaba4937. [Google Scholar] [CrossRef]
- O’Hara-Wright, M.; Gonzalez-Cordero, A. Retinal organoids: A window into human retinal development. Development 2020, 147, 189746. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallo, M. Of Necks, Trunks and Tails: Axial Skeletal Diversity among Vertebrates. Diversity 2021, 13, 289. https://doi.org/10.3390/d13070289
Mallo M. Of Necks, Trunks and Tails: Axial Skeletal Diversity among Vertebrates. Diversity. 2021; 13(7):289. https://doi.org/10.3390/d13070289
Chicago/Turabian StyleMallo, Moisés. 2021. "Of Necks, Trunks and Tails: Axial Skeletal Diversity among Vertebrates" Diversity 13, no. 7: 289. https://doi.org/10.3390/d13070289
APA StyleMallo, M. (2021). Of Necks, Trunks and Tails: Axial Skeletal Diversity among Vertebrates. Diversity, 13(7), 289. https://doi.org/10.3390/d13070289





