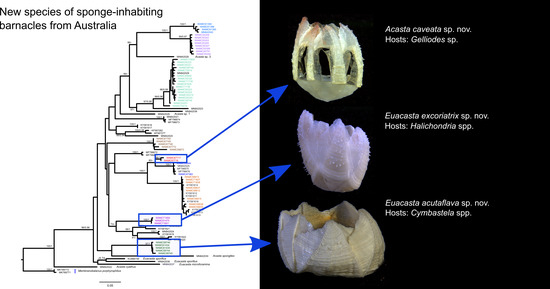
New Species and New Records of Sponge-Inhabiting Barnacles (Cirripedia, Balanidae, Acastinae) from Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Morphological Examination
2.2. Molecular Analysis
3. Results
3.1. Systematics
3.1.1. Acasta Leach, 1817 [8]
Acasta aspera Yu et al., 2017 [3]
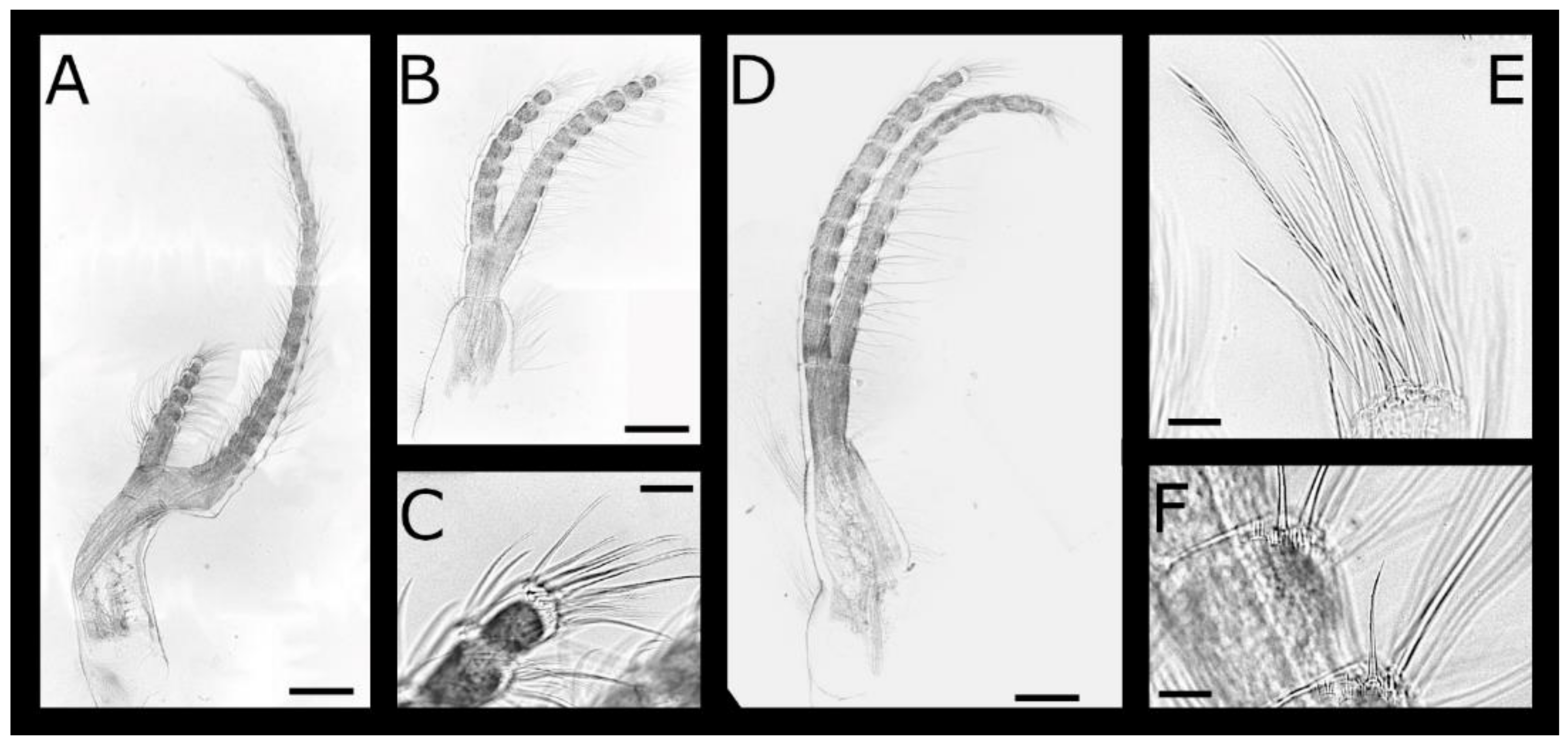
Acasta caveata sp. nov. (Figure 1, Figure 2, Figure 3 and Figure 4)




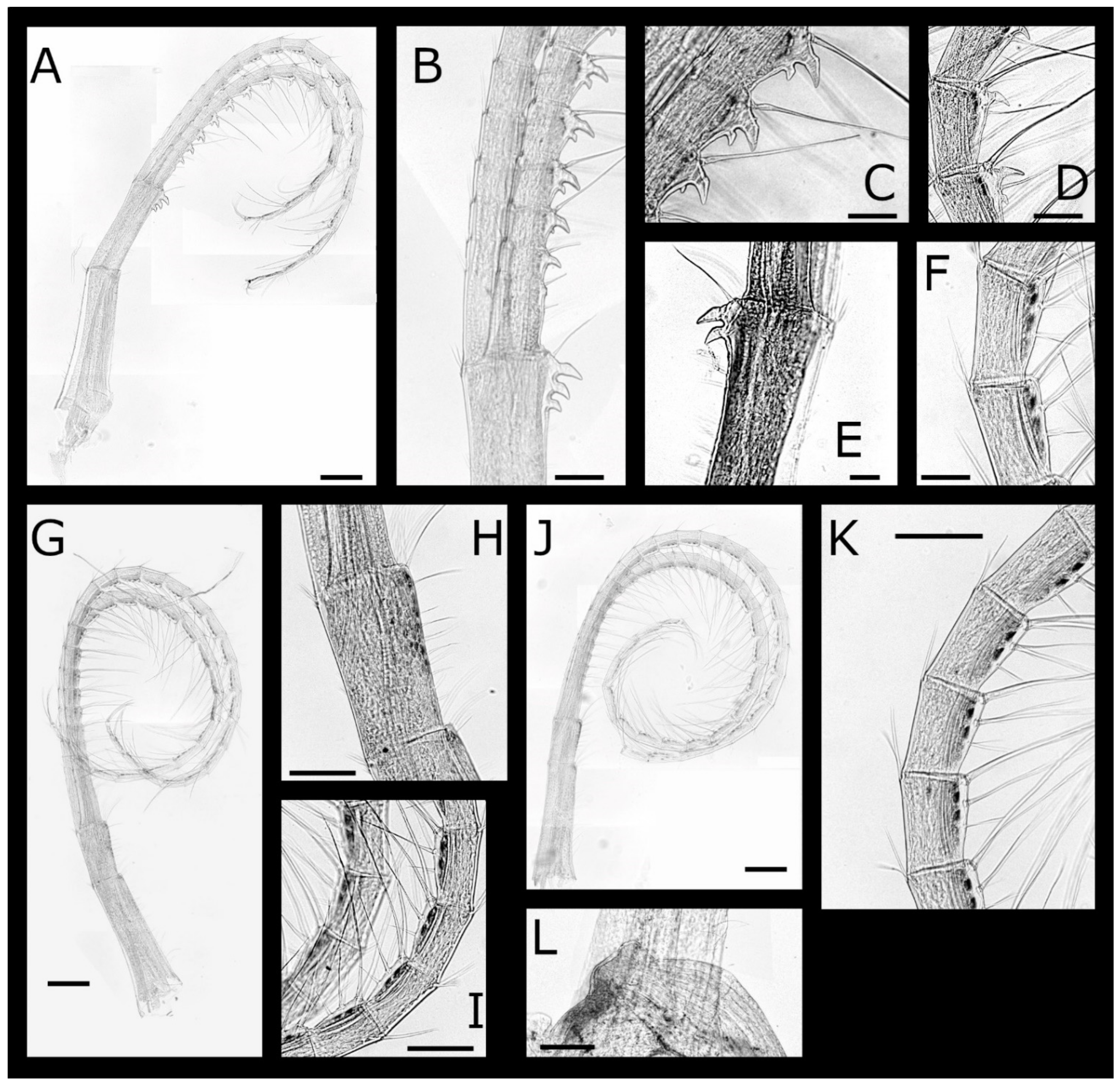
Acasta fenestrata Darwin, 1854 [38] (Figure 5, Figure 6, Figure 7 and Figure 8)




Acasta sandwichi Yu, Chan, Achituv & Kolbasov, 2017 [2]
3.1.2. Euacasta Kolbasov, 1993 [5]
Euacasta acutaflava sp. nov. (Figure 9, Figure 10, Figure 11 and Figure 12)


Euacasta excoriatrix sp. nov. (Figure 13, Figure 14, Figure 15 and Figure 16)




3.1.3. Pectinoacasta Kolbasov, 1993 [5]
Pectinoacasta cancellorum (Hiro, 1931) [69] (Figure 17, Figure 18, Figure 19 and Figure 20)




Pectinoacasta pectinipes (Pilsbry, 1912) [71] (Figure 21, Figure 22, Figure 23 and Figure 24)



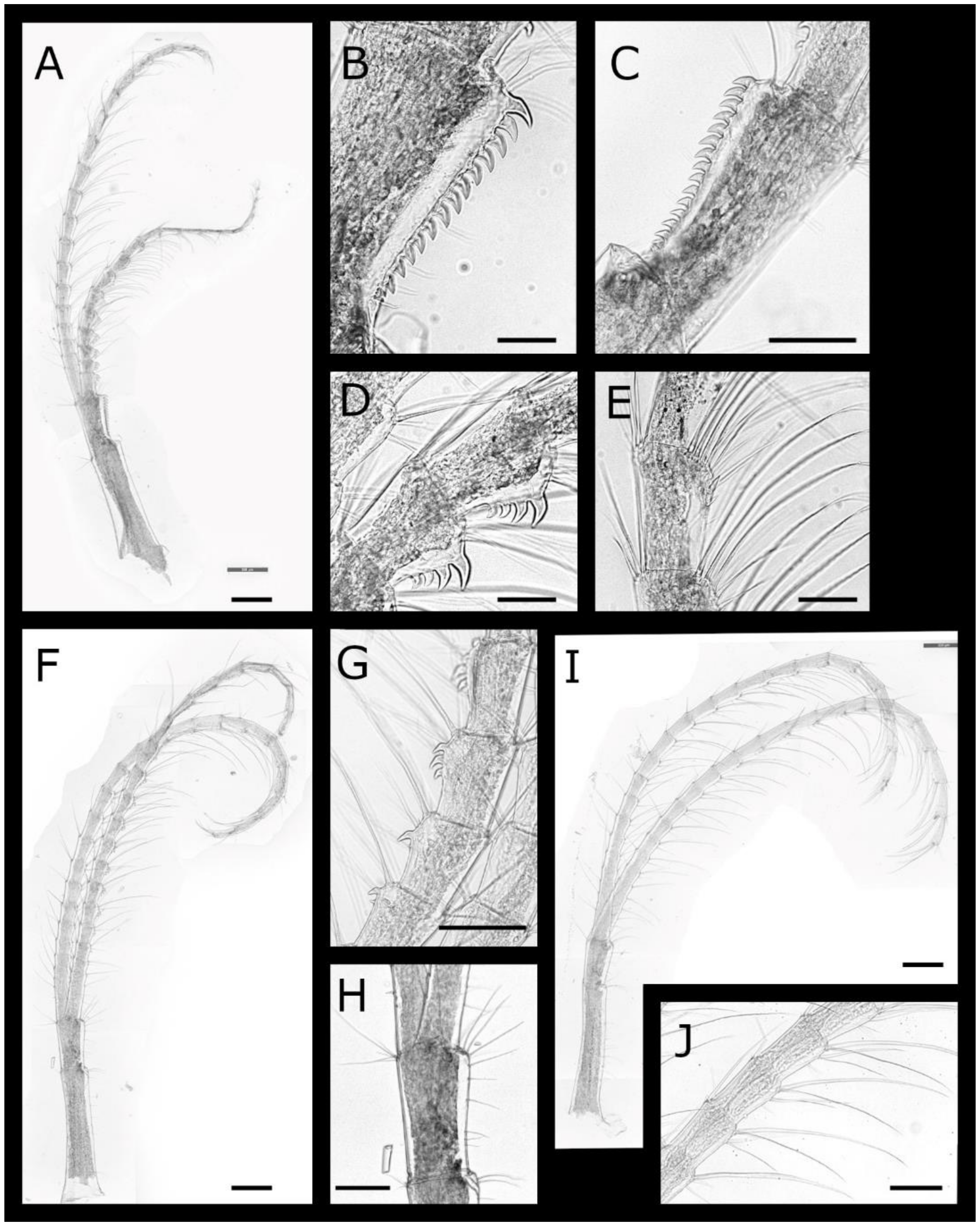
Pectinoacasta sculpturata (Broch, 1931) [6] (Figure 25, Figure 26, Figure 27 and Figure 28)
3.2. Molecular Results





4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Syoc, R.J.; Van Soest, R.W.; Xavier, J.R.; Hooper, J.N. A phylogenetic overview of sponge-inhabiting barnacles and their host specificity (Crustacea, Cirripedia). Proc. Calif. Acad. Sci. Ser. 2015, 4, 331–357. [Google Scholar]
- Yu, M.-C.; Chan, B.K.K.; Achituv, Y.; Kolbasov, G.A. Four new sponge-inhabiting barnacles of the genus Acasta (Thoracica: Archaeobalanidae: Acastinae) from the Indo-Pacific. Raffles Bull. Zool. 2017, 65, 585–615. [Google Scholar]
- Yu, M.-C.; Kolbasov, G.A.; Hosie, A.M.; Lee, T.-M.; Chan, B.K.K. Descriptions of four new sponge-inhabiting barnacles (Thoracica: Archaeobalanidae: Acastinae). Zootaxa 2017, 4277, 151–198. [Google Scholar] [CrossRef] [PubMed]
- Hosie, A.M.; Fromont, J.; Munyard, K.; Wilson, N.G.; Jones, D.S. Surveying keratose sponges (Porifera, demospongiae, Dictyoceratida) reveals hidden diversity of host specialist barnacles (Crustacea, Cirripedia, Balanidae). Mol. Phylogenetics Evol. 2021, 161, 107179. [Google Scholar] [CrossRef]
- Kolbasov, G.A. Revision of the genus Acasta Leach (Cirripedia: Balanoidea). Zool. J. Linn. Soc. 1993, 109, 395–427. [Google Scholar] [CrossRef]
- Broch, H. Papers from Dr. Th. Mortensen’s Pacific expédition 1914–1916. LVI. Indomalayan Cirripedia. Vidensk. Medd. Fra Dan. Nat. Foren. 1931, 91, 1–142. [Google Scholar]
- Hiro, F. Studies on cirripedian fauna of Japan II: Cirripeds found in the vicinity of the Seto marine biological laboratory. Mem. Coll. Sci. Kyoto Imp. University. Ser. B 1937, 12, 385–478. [Google Scholar]
- Leach, W.E. The Zoological Miscellany: Being Descriptions of New Or Interesting Animals; E. Nodder & Sons: London, UK, 1817; Volume 3. [Google Scholar]
- Say, T. An account of some marine shells of the United States. J. Acad. Nat. Sci. Phila. 1822, 2, 221–248; 302–325. [Google Scholar]
- Hoek, P.P.C. The Cirripedia of the Siboga-expedition. B. In Siboga-Expeditie Mongographes; E.J. Brill: Leyden, The Netherlands, 1913; Volume XXXIb, pp. 129–275. [Google Scholar]
- Kolbasov, G.A.; Chan, B.K.K.; Molodstova, T.N.; Achituv, Y. Revision of the coral-inhabiting genus Conopea (Cirripedia: Archaeobalanidae) with description of two new species of the genera Conopea and Acasta. Zootaxa 2016, 4178, 182–208. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Harp, M.; Høeg, J.T.; Achituv, Y.; Jones, D.; Watanabe, H.; Crandall, K.A. The tempo and mode of barnacle evolution. Mol. Phylogenetics Evol. 2008, 46, 328–346. [Google Scholar] [CrossRef]
- Tsang, L.M.; Chu, K.H.; Nozawa, Y.; Chan, B.K.K. Morphological and host specificity evolution in coral symbiont barnacles (Balanomorpha: Pyrgomatidae) inferred from a multi-locus phylogeny. Mol. Phylogenetics Evol. 2014, 77, 11–22. [Google Scholar] [CrossRef]
- Chan, B.K.K.; Dreyer, N.; Gale, A.S.; Glenner, H.; Ewers-Saucedo, C.; Pérez-Losada, M.; Kolbasov, G.A.; Crandall, K.A.; Høeg, J.T. The evolutionary diversity of barnacles, with an updated classification of fossil and living forms. Zool. J. Linn. Soc. 2021, zlaa160. [Google Scholar] [CrossRef]
- Yu, M.-C.; Kolbasov, G.; Chan, B.K.K. A new species of sponge inhabiting barnacle Bryozobia (Archaeobalanidae, Bryozobiinae) in the West Pacific. ZooKeys 2016, 571, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Van Syoc, R.J.; Winther, R. Sponge-inhabiting barnacles of the Americas: A new species of Acasta (Cirripedia, Archaeobalanidae), first record from the eastern Pacific, including discussion of the evolution of cirral morphology. Crustaceana 1999, 72, 467–486. [Google Scholar]
- Yu, M.-C.; Dreyer, N.; Kolbasov, G.A.; Høeg, J.T.; Chan, B.K.K. Sponge symbiosis is facilitated by adaptive evolution of larval sensory and attachment structures in barnacles. Proc. R. Soc. B 2020, 287, 20200300. [Google Scholar] [CrossRef]
- Hosie, A.M.; Fromont, J.; Munyard, K.; Jones, D.S. Description of a new species of Membranobalanus (Crustacea, Cirripedia) from southern Australia. ZooKeys 2019, 873, 25–42. [Google Scholar] [CrossRef]
- Pitriana, P.; Wessel, A.; Aschenbach, T.; Von Rintelen, K. Exploring sponge-inhabiting barnacles of eastern Indonesia using micro-CT scanning. Treubia 2020, 47, 77–98. [Google Scholar] [CrossRef]
- Fromont, J.; Abdul Wahab, M.A.; Gomez, O.; Ekins, M.; Grol, M.; Hooper, J.N.A. Patterns of sponge biodiversity in the Pilbara, Northwestern Australia. Diversity 2016, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Heyward, A.; Fromont, J.; Schönberg, C.H.L.; Colquhoun, J.; Radford, B.; Gomez, O. The sponge gardens of Ningaloo reef, Western Australia. Open Mar. Biol. J. 2010, 4, 3–11. [Google Scholar]
- Hooper, J.N.; Kennedy, J.A.; Quinn, R.J. Biodiversity ‘hotspots’, patterns of richness and endemism, and taxonomic affinities of tropical Australian sponges (Porifera). Biodivers. Conserv. 2002, 11, 851–885. [Google Scholar] [CrossRef]
- Chan, B.K.K.; Garm, A.; Høeg, J. Setal morphology and cirral setation of thoracican barnacle cirri: Adaptations and implications for thoracican evolution. J. Zool. 2008, 275, 294–306. [Google Scholar] [CrossRef]
- Meyer, C.P. Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol. J. Linn. Soc. 2003, 79, 401–459. [Google Scholar] [CrossRef] [Green Version]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2020, 1–12. [Google Scholar] [CrossRef]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. Mamm. Protein Metab. 1969, 3, 21–132. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.-T.; Von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Schwartz, T.; Hoover, P.; Yoshimoto, K.; Sivagnanam, S.; Majumdar, A. The CIPRES workbench: A flexible framework for creating science gateways. In Proceedings of the 2015 XSEDE Conference: Scientific Advancements Enabled by Enhanced Cyberinfrastructure, St. Louis, MO, USA, 26–30 July 2015; pp. 1–8. [Google Scholar]
- Van Syoc, R.; Carrison-Stone, D.; Madrona, L.; Williams, G. Barnacle symbionts of gorgonian sea fans, description of seven new species (Archaeobalanidae: Cirripedia) from the Philippines, Including a Key to the Western Pacific species of Conopea. In The Coral Triangle, The 2011 Hearst Philippine Biodiversity Expedition; Williams, G., Gosliner, T., Eds.; California Academy of Sciences: San Francisco, CA, USA, 2014; pp. 171–211. [Google Scholar]
- Pilsbry, H.A. The sessile barnacles (Cirripedia) contained in the US National Museum; including a monograph of the American species. Bull. United States Natl. Mus. 1916, 93, 1–366. [Google Scholar]
- Hentschel, E. Tetraxonida. 2. Teil. In Die Fauna Südwest-Australiens. Ergebnisse der Hamburger südwest-australischen Forschungsreise 1905; Michaelsen, W., Hartmeyer, R., Eds.; Gustav Fischer: Jena, Germany, 1911; Volume 3, pp. 279–393. [Google Scholar]
- Kolbasov, G.A. Acasta pertusa sp n (Cirripedia, Thoracica) from the Red Sea. Zool. Zhurnal 1990, 69, 142–145. [Google Scholar]
- Gravier, C. Sur deux espèces de Cirripèdes du genre Acasta Leach vivant a la côte française des Somalis. Bull. Muséum D’histoire Nat. Paris 1921, 353–357. [Google Scholar]
- Darwin, C. A Monograph on the Sub-Class Cirripedia; The Ray Society: London, UK, 1854. [Google Scholar]
- Kolbasov, G.A. Two new species of Acasta (Cirripedia, Thoracica) from southwest part of Indian Ocean. Zool. Zhurnal 1992, 71, 140–145. [Google Scholar]
- Weltner, W. Verzeichnis der bisher beschriebenen recenten Cirripedienarten. Arch. Naturgeschichte. 1897, 63, 227–280. [Google Scholar]
- Weltner, W. Cirripedia der deutschen Tiefsee-expedition. In Wissenschaftliche Ergebnisse der deutschen Tiefsee-Expedition auf dem Dampfer Valdivia 1898–1899; Gustav Fischer: Jena, Germany, 1922; Volume 23, pp. 59–112. [Google Scholar]
- Nilsson-Cantell, C.A. Cirripeds from the Indian Ocean in the collection of the Indian Museum, Calcutta. Mem. Indian Mus. 1938, 13, 1–81. [Google Scholar]
- Hiro, F. Studies on the cirripedian fauna of Japan. III. Supplementary notes on the cirripeds found in the vicinity of Seto. Mem. Coll. Sci. Kyoto Imp. Univ. Ser. B 1939, 15, 237–244. [Google Scholar]
- Newman, W.A.; Ross, A. Revision of the balanomorph barnacles, including a catalog of the species. Mem. San Diego Soc. Nat. Hist. 1976, 9, 1–108. [Google Scholar]
- Ren, X. Studies on Chinese Cirripedia (Crustacea) V. Genus Acasta. Studia Mar. Sin. 1984, 23, 183–214. [Google Scholar]
- Rosell, N.C. Thoracic Cirripeds from the MUSORSTOM 2 expedition. Mémoires du Muséum Natl. d’Histoire naturelle. Ser. A Zool. 1989, 144, 9–35. [Google Scholar]
- Liu, R.; Ren, X. Crustacea Cirripedia Thoracica; Science Press: Beijing, China, 2007; Volume 42. [Google Scholar]
- Wibowo, R.A.; Prabowo, R.E.; Nuryanto, A. Biodiversitas teritip yang hidup pada spons di Perairan Pantai Kepulauan Karimunjawa. Semin. Nas. I Mataki 2011, 20, 219–235. [Google Scholar]
- Sulistiono, S.; Kawaroe, M.; Madduppa, H.; Prabowo, R. Karakteristik morfologi teritip spons Indonesia. DEPIK J. Ilmu-Ilmu Perair. Pesisir Dan Perikan. 2014, 3, 178–186. [Google Scholar]
- Jones, D.S.; Hosie, A.M. A checklist of the barnacles (Cirripedia: Thoracica) of Singapore and neighbouring waters. Raffles Bull. Zool. 2016, 34, 241–311. [Google Scholar]
- Utinomi, H. Studies on the Cirripedian fauna of Japan VII. Cirripeds from Sagami Bay. Publ. Seto Mar. Lab. 1958, 6, 281–311. [Google Scholar] [CrossRef] [Green Version]
- Rosell, N. Some barnacles (Cirripedia Thoracica) of Puerto Galera found in the vicinity of the UP Marine Biological Laboratory. Nat. Appl. Sci. Bull. Univ. Philipp. 1972, 24, 143–285. [Google Scholar]
- Foster, B.A.; Buckeridge, J.S. Barnacles (Cirripedia, Thoracica) of seas off Reunion Island and the east indies. Bull. Muséum Natl. D’histoire Nat. Sect. A Zool. Biol. Écologie Anim. 1994, 16, 345–382. [Google Scholar]
- Thiele, J. Studien über pazifische Spongien. II. Ueber einige Spongien von Celebes. Zoologica. Orig. Abh. Aus Dem Gesamtgeb. Zoologie. Stuttg. 1899, 24, 1–33. [Google Scholar]
- Döderlein, L. Studien an japanischen Lithistiden. Z. Fürwissenschaftliche Zool. 1884, 40, 62–104. [Google Scholar]
- Ridley, S.O.; Dendy, A. Preliminary report on the Monaxonida collected by H.M.S. Challenger. Part I. Ann. Mag. Nat. Hist. 1886, 18, 470–493. [Google Scholar] [CrossRef]
- Lamarck, J.-B.P.A. Histoire Naturelle des Animaux sans Vertèbres; Deterville: Paris, France, 1818; Volume 5, p. 612. [Google Scholar]
- Lamarck, J.-B.P.A. Histoire Naturelle des Animaux sans Vertèbres Précédée d’une Introduction Offrant la Détermination des Caractères Essentiels de L’animal, sa Distinction du Végétal et des Autres Corps Naturels, enfin, L’exposition des Principes Fondamentaux de la Zoologie; Verdière: Paris, France, 1815; Volume 7. [Google Scholar]
- Bergquist, P.R.; Tizard, C.A. Australian intertidal sponges from the Darwin area. Micronesica 1967, 3, 175–202. [Google Scholar]
- Hooper, J.N.A.; Bergquist, P.R. Cymbastela, a new genus of lamellate coral reef sponges. Mem. Qld. Mus. 1992, 32, 99–137. [Google Scholar]
- Kolbasov, G.A. New species of the genus Acasta (Cirripedia, Thoracica) from western Pacific. Zool. Žurnal 1991, 70, 32–38. [Google Scholar]
- Nilsson-Cantell, C.A. Cirripeden-studien zur kenhtnis der biologie, anatomie und systematik dieser gruppe. Zool. Bidr. Från Upps. 1921, 7. [Google Scholar]
- Krüger, P. Beitrage zur Cirripedienfauna Ostasiens. Konglige Bayer. Akad. Der Wiss. Munich Math. Phys. Klasse. Abh. Suppl. 1911, 2, 1–72. [Google Scholar]
- Rosell, N.C. A gorgonacean inhabiting barnacle Genus Acasta from Cebu. Nat. Appl. Sci. Bull. 1970, 22, 103–111. [Google Scholar]
- Kolbasov, G.A. Acasta tabachniki sp. nov. (Cirripedia, Thoracica) from South China Sea. Zool. Zhurnal 1990, 69, 135–137. [Google Scholar]
- Hiro, F. Report on the Cirripedia collected in the Malayan waters by the ship “Zuihomaru”. Jpn. J. Zool. 1936, 6, 621–636. [Google Scholar]
- Broch, H. Cirripedien. Results of Dr. E. Mjoberg’s Swedish scientific expedition to Australia, 1910–1913, No. 8. K. Sven. Vetensk. Handl. 1916, 52, 1–16. [Google Scholar]
- Yu, M.-C.; Chan, B.K.K.; Kolbasov, G.A.; Ganmanee, M. Biodiversity and host specificity of sponge-associated barnacles (Cirripedia: Thoracica) in Thailand. J. Crustacean Biol. 2020, 40, 839–865. [Google Scholar] [CrossRef]
- Hiro, F. Notes on some new Cirripedia from Japan. Mem. Coll. Sci. Kyoto Imp. Univ. 1931, 7, 143–158. [Google Scholar]
- Carter, H.J. Contributions to our knowledge of the Spongida. Ann. Mag. Nat. Hist. 1883, 12, 308–329. [Google Scholar] [CrossRef]
- Pilsbry, H.A. Diagnoses of new barnacles from the Philippine Archipelago and China Sea. Proc. United States Natl. Mus. 1912, 42, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Broch, H. Papers from Dr. Th. Mortensen’s Pacific expedition 1914-1916. X. Studies on Pacific cirripedes. Vidensk. Meddr. Dan. Naturh. Foren. Kbh. 1922, 73, 215–358. [Google Scholar]
- Barnard, K.H. Contributions to the Crustacean fauna of South Africa. N7. Cirripedia. Ann. S. Afr. Mus. 1924, 20, 1–103. [Google Scholar]
- Utinomi, H. Studies on the cirripedian fauna of Japan. VIII. Thoracic cirripeds from western Kyusyu. Publ. Seto Mar. Lab. 1962, 10, 211–239. [Google Scholar] [CrossRef] [Green Version]
- Zullo, V.A. Catalog of the Cirripedia named by Henry A. Pilsbry. Proc. Acad. Nat. Sci. Phila. 1968, 120, 209–235. [Google Scholar]
- Jones, D. A guide to the shallow-water barnacles (Cirripedia: Lepadomorpha, Balanomorpha) of the Shark Bay area, Western Australia. In Research in Shark Bay: Report of the France-Australe Bicentenary Expedition Committee; IUCN: Perth, Australia, 1990; pp. 209–229. [Google Scholar]
- Jones, D.S.; Anderson, J.T.; Anderson, D.T. Checklist of the Australian Cirripedia. Tech. Rep. Aust. Mus. 1990, 3, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.S.; Hewitt, M.A.; Sampey, A. A checklist of the Cirripedia of the South China Sea. Raffles Bull. Zool. 2000, 48, 233–308. [Google Scholar]
- Jones, D. The littoral and shallow-water barnacles (Crustacea: Cirripedia) of south-eastern Queensland. In Proceedings of the Thirteenth International Marine Biological Workshop, The Marine Fauna and Flora of Moreton Bay; Davie, P.J.F., Phillips, J.A., Eds.; Queensland Museum: Brisbane, Australia, 2010; pp. 199–233. [Google Scholar]
- Kadota, J. Observations of two new species of the genus Reniera of monaxonid sponges. Zool. Mag. 1922, 34, 700–711. [Google Scholar]
- Foster, B. Shallow water barnacles from Hong Kong. Proc. First Int. Mar. Biol. Work. Mar. Flora Fauna Hong Kong S. China 1980, 1, 207–232. [Google Scholar]
- Rosell, N.C. Crustacea Cirripedia Thoracica: MUSORSTOM 3 Philippine collection. Mémoires Muséum Natl. D’histoire Nat. Ser. A Zool. 1991, 152, 9–61. [Google Scholar]
- Hoshino, T. Description of two new species in the genus Agelas (Demospongia) from Zamami Island, the Ryukyus, Japan. Proc. Jpn. Soc. Syst. Zool. 1985, 30, 1–10. [Google Scholar]
- Hooper, J.N.A. Revision of the family Raspailiidae (Porifera: Demospongiae), with description of Australian species. Invertebr. Taxon. 1991, 5, 1179–1418. [Google Scholar] [CrossRef]
- Watanabe, S. A widely applicable Bayesian information criterion. J. Mach. Learn. Res. 2013, 14, 867–897. [Google Scholar]
- Jones, D.S. The shallow-water barnacles (Cirripedia: Lepadomorpha, Balanomorpha) of southern Western Australia. In Proceedings of the Third International Marine Biological Workshop: The Marine Flora and Fauna of Albany, Western Australia; Western Australian Museum: Perth, WA, Australia, 1990; pp. 332–437. [Google Scholar]
- Krüger, P. Cirripedia. In Die Fauna Südwest-Australiens. Ergebnisse der Hamburger Südwest-Australischen Forschungsreise 1905 Herausgegeben von Prof. Dr. W. Michaelsen und Dr. R. Hartmeyer.; Michaelsen, W., Ed.; Gustav Fischer: Jena, Germany, 1914; Volume 4, pp. 427–441. [Google Scholar]
| Species | Accession | Host Species | GenBank |
|---|---|---|---|
| Acasta aspera | SGI20-1 | Iotroata sp. | KY581615 [3] |
| Acasta aspera | SGI20-2 | Iotroata sp. | KY581616 [3] |
| Acasta aspera | SGI2-1 | Iotroata sp. | KY581614 [3] |
| Acasta aspera | WAM C55057 | Crella (Yvesia) spinulata | KY581613 [3] |
| Acasta aspera | WAM C58741 | Crella (Yvesia) spinulata | KY581610 [3] |
| Acasta aspera | WAM C58742 | Crella (Yvesia) spinulata | KY581611 [3] |
| Acasta aspera | WAM C58743 | Crella (Yvesia) spinulata | KY581612 [3] |
| Acasta aspera | WAM C58812 | Lissodendoryx (Acanthodoryx) KMB1 | MZ086820 |
| Acasta aspera | WAM C58813 | Lissodendoryx (Acanthodoryx) KMB1 | MZ086821 |
| Acasta aspera | WAM C58815 | Lissodendoryx (Acanthodoryx) KMB1 | MZ086822 |
| Acasta aspera | WAM C58835 | Hamigera PB1 | MZ086823 |
| Acasta aspera | WAM C58837 | Hamigera | MZ086824 |
| Acasta aspera | WAM C58838 | Hamigera | MZ086825 |
| Acasta aspera | WAM C71837 | Lissodendoryx (Acanthodoryx) KMB1 | MZ086844 |
| Acasta aspera | WAM C71838 | Lissodendoryx (Acanthodoryx) KMB1 | MZ086845 |
| Acasta caveata sp. nov. | WAM C67716 | Gelliodes KMB1 | MZ086833 |
| Acasta caveata sp. nov. | WAM C67717 | Gelliodes KMB1 | MZ086834 |
| Acasta crucibasis | MF796674 | Unknown | MF796674 |
| Acasta crucibasis | S32-1 | Xestospongia vansoesti | MN842021 [17] |
| Acasta cyathus | SGI265-1 | Xestospongia testudinaria | MN842022 [17] |
| Acasta daedalusa | SGI28-2 | Petrosia (Petrosia) sp. | MN842023 [17] |
| Acasta fenestrata | WAM C46669 | Neopetrosia chaliniformis | MZ086796 |
| Acasta fenestrata | WAM C55251 | Neopetrosia chaliniformis | MZ086800 |
| Acasta fenestrata | WAM C55319 | Neopetrosia chaliniformis | MZ086808 |
| Acasta fenestrata | WAM C55320 | Neopetrosia chaliniformis | MZ086809 |
| Acasta fenestrata | WAM C55321 | Neopetrosia chaliniformis | MZ086810 |
| Acasta fenestrata | WAM C55322 | Neopetrosia chaliniformis | MZ086811 |
| Acasta fenestrata | WAM C55323 | Neopetrosia chaliniformis | MZ086812 |
| Acasta fenestrata | WAM C55324 | Neopetrosia chaliniformis | MZ086813 |
| Acasta fenestrata | WAM C55325 | Neopetrosia chaliniformis | MZ086814 |
| Acasta fenestrata | WAM C58748 | Neopetrosia chaliniformis | MZ086818 |
| Acasta fenestrata | WAM C58749 | Neopetrosia chaliniformis | MZ086819 |
| Acasta fenestrata | WAM C67829 | Neopetrosia chaliniformis | MZ086839 |
| Acasta fenestrata | WAM C71738 | Neopetrosia chaliniformis | MZ086840 |
| Acasta fenestrata | WAM C71739 | Neopetrosia chaliniformis | MZ086841 |
| Acasta fenestrata | WAM C72909 | Neopetrosia chaliniformis | MZ086846 |
| Acasta fenestrata | WAM C72914 | Neopetrosia chaliniformis | MZ086847 |
| Acasta fenestrata | WAM C74419 | Neopetrosia chaliniformis | MZ086848 |
| Acasta huangi | SNE47-4 | Jaspis splendens | KY581621 [3] |
| Acasta radenta | SLQ32-1 | Jaspis splendens | MN842024 [17] |
| Acasta radenta | SNE47-1 | Jaspis splendens | KY581619 [3] |
| Acasta sandwichi | SGI208-1 | Xestospongia testudinaria | MN842025 [17] |
| Acasta sandwichi | SN77-1 | Unknown | MF796675 |
| Acasta sandwichi | SN77-2 | Unknown | MF796676 |
| Acasta sandwichi | WAM C47383 | Xestospongia testudinaria | MZ086797 |
| Acasta sandwichi | WAM C53344 | Xestospongia testudinaria | MZ086799 |
| Acasta sp. 1 MCY-2020 | SJP55-N1 | Mycale sp. | MN842026 [17] |
| Acasta sp. 2 MCY-2020 | SLQ20-2 | Haliclona sp. | MN842027 [17] |
| Acasta sp. 3 MCY-2020 | SN81-3 | Petrosia (Petrosia) sp. | MN842028 [17] |
| Acasta sp. 4 MCY-2020 | SDS4-2 | Unknown | MN842029 [17] |
| Acasta spongites | STI18-1 | Clathria sp. | MN842030 [17] |
| Acasta sulcata | SN48-1 | Callyspongia sp. | KY581617 [3] |
| Acasta sulcata | SN48-3 | Callyspongia sp. | KY581618 [3] |
| Acasta turriformis | SGI178-1 | Unknown | MF796671 |
| Acasta turriformis | SGI178-2 | Unknown | MF796672 |
| Acasta undulaterga | SNE47-3 | Jaspis splendens | KY581620 [3] |
| Acasta undulaterga | SNE47-5 | Jaspis splendens | KY581622 [3] |
| Acasta vipensis | CAS:IZ:187687A | Menella sp. | KF587277 [33] |
| Acasta vipensis | CAS:IZ:187764A | Menella sp. | KF587282 [33] |
| Euacasta acutaflava sp. nov. | WAM C58744 | Cymbastelastipitata | MZ086815 |
| Euacasta acutaflava sp. nov. | WAM C58745 | Cymbastelastipitata | MZ086816 |
| Euacasta acutaflava sp. nov. | WAM C58746 | Cymbastelastipitata | MZ086817 |
| Euacasta acutaflava sp. nov. | WAM C61434 | Cymbastela cf. stipitata | MZ086830 |
| Euacasta acutaflava sp. nov. | WAM C61435 | Cymbastela cf. stipitata | MZ086831 |
| Euacasta excoriatrix sp. nov. | WAM C61475 | Halichondria NW1 | MZ086832 |
| Euacasta excoriatrix sp. nov. | WAM C71800 | Halichondria BAR1 | MZ086842 |
| Euacasta excoriatrix sp. nov. | WAM C71801 | Halichondria BAR1 | MZ086843 |
| Euacasta dofleini | SGI168-2 | Haliclona sp. | MN842036 [17] |
| Euacasta microforamina | SJJ2-1 | Haliclona sp. | MN842037 [17] |
| Euacasta sporillus | RMNH:CRUS:C.10235 | Axinyssa sp. | KU986749 |
| Euacasta sporillus | S14-1 | Aaptos suberitoides | MN842038 [17] |
| Membranobalanus porphyrophilus | WAM C71853 | Spheciospongia purpurea | MK789771 [18] |
| Membranobalanus porphyrophilus | WAM C71881 | Spheciospongia purpurea | MK789772 [18] |
| Pecitnoacasta sculpturata | WAM C61394 | Amphinomiasulphurea | MZ086827 |
| Pecitnoacasta sculpturata | WAM C61395 | Amphinomiasulphurea | MZ086828 |
| Pecitnoacasta sculpturata | WAM C61396 | Amphinomiasulphurea | MZ086829 |
| Pectinoacasta cancellorum | WAM C50791 | Agelas KMB1 | MZ086798 |
| Pectinoacasta cancellorum | WAM C55302 | Agelas KMB1 | MZ086801 |
| Pectinoacasta cancellorum | WAM C55303 | Agelas KMB1 | MZ086802 |
| Pectinoacasta cancellorum | WAM C55304 | Agelas KMB1 | MZ086803 |
| Pectinoacasta cancellorum | WAM C55305 | Agelas KMB1 | MZ086804 |
| Pectinoacasta cancellorum | WAM C55306 | Agelas KMB1 | MZ086805 |
| Pectinoacasta cancellorum | WAM C55307 | Agelas KMB1 | MZ086806 |
| Pectinoacasta cancellorum | WAM C55308 | Agelas KMB1 | MZ086807 |
| Pectinoacasta pectinipes | WAM C58873 | Tedania (Trachytedania) L1 | MZ086826 |
| Pectinoacasta pectinipes | WAM C67748 | Tedania (Trachytedania) MM1 | MZ086835 |
| Pectinoacasta pectinipes | WAM C67749 | Tedania (Trachytedania) MM1 | MZ086836 |
| Pectinoacasta pectinipes | WAM C67750 | Tedania (Trachytedania) MM1 | MZ086837 |
| Pectinoacasta pectinipes | WAM C67772 | Tedania (Trachytedania) MM1 | MZ086838 |
| Pectinoacasta sculpturata | SGI131-1 | Agelas nemoechinata | MN842042 [17] |
| Specimen | CI | C II | C III | C IV | C V | C VI | |
|---|---|---|---|---|---|---|---|
| Acasta caveata sp. nov. | |||||||
| WAM C67716 | L | 17, 8 | 11, 8 | 15, 13 | 23, 26 | 32, 34 | 34, 33 |
| R | 17, 9 | 11, 10 | 15, 12 | 24, 30 | 33, 38 | 34, 36 | |
| Acasta fenestrata | |||||||
| WAM C58749 | L | 16, 6 | 9, 8 | 12, 10 | 26, 27 | 17*, 26 | 32, 33 |
| R | 16, 6 | 8, 7 | 11, 9 | 27, 26 | 23, 23 | 34, 31 | |
| Euacasta acutaflava sp. nov. | |||||||
| WAM C58744 | L | 20, 6 | 10, 7 | 14, 12 | 17, 22 | 29, 28 | 28*, 31 |
| R | 20, 6 | *,* | 11, 11 | 17*, 21 | 27, 28 | 29, 29 | |
| WAM C74601 | L | 17, 6 | 9, 7 | 12, 11 | 18, 24 | 28, 29 | 11*, 26* |
| R | 17, 6 | 11, 6 | 12, 12 | 15*, 22 | 29, 31 | 31, 22* | |
| WAM C76451 | L | 17, 5 | 8, 6 | 10, 9 | 19, 18* | 29, 30 | 33, 34 |
| R | 18, 5 | 8, 6 | 10, 9 | 19, 26 | 31, 32 | 33, 12* | |
| Euacasta excoriatrix sp. nov. | |||||||
| WAM C71800 | L | 30, 7 | 13, 9 | 17, 14 | 27, 29 | 32,29* | 33, 34 |
| R | 30, 7 | 12, 8 | 16, 13 | 22, 30 | 25*,31 | 37, 26* | |
| WAM C61475 | L | 29, 7 | 12, 8 | 16, 14 | 20, 21* | 26*,28* | 17*, 23* |
| R | 28, 6 | 13, 9 | 14, 15 | 14*, 22* | 30,22* | 20*, 22* | |
| Pectinoacasta cancellorum | |||||||
| WAM C55305 | L | 20, 6 | 10, 8 | 13, 11 | 21*, 16* | 15*, 17* | 35, 33 |
| R | 20, 6 | 10, 7 | 13, 11 | 22, 21 | 28, 29 | 36, 32 | |
| Pectinoacasta pectinipes | |||||||
| WAM C58871 | L | 15, 7 | 9, 8 | 11, 11 | 17, 19 | 21*, 23 | 22, 23 |
| R | 12, 7* | 9, 7 | 9, 9 | 3*, 18 | 23, 22 | 23, 23 | |
| Pectinoacasta sculpturata | |||||||
| WAM C61395 | L | 22, 6 | 9, 7 | 12, 5* | 14*, 23 | 24, 2* | 24, 24 |
| R | *,* | 9, 7 | 11, 10 | 22, 17* | 24, 25 | 26, 24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosie, A.M.; Fromont, J.; Munyard, K.; Jones, D.S. New Species and New Records of Sponge-Inhabiting Barnacles (Cirripedia, Balanidae, Acastinae) from Australia. Diversity 2021, 13, 290. https://doi.org/10.3390/d13070290
Hosie AM, Fromont J, Munyard K, Jones DS. New Species and New Records of Sponge-Inhabiting Barnacles (Cirripedia, Balanidae, Acastinae) from Australia. Diversity. 2021; 13(7):290. https://doi.org/10.3390/d13070290
Chicago/Turabian StyleHosie, Andrew M., Jane Fromont, Kylie Munyard, and Diana S. Jones. 2021. "New Species and New Records of Sponge-Inhabiting Barnacles (Cirripedia, Balanidae, Acastinae) from Australia" Diversity 13, no. 7: 290. https://doi.org/10.3390/d13070290
APA StyleHosie, A. M., Fromont, J., Munyard, K., & Jones, D. S. (2021). New Species and New Records of Sponge-Inhabiting Barnacles (Cirripedia, Balanidae, Acastinae) from Australia. Diversity, 13(7), 290. https://doi.org/10.3390/d13070290