Feeding Habits of Predatory Thermophilic Fish Species and Species with Subtropical Affinity from Recently Extended Distributional Range in Northeast Adriatic Sea, Croatia
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area and Fish Sampling
2.2. Gut Content Analysis, Diet Overlap and Prey Importance
2.3. Statistical Analysis of the Fish Feeding Habits
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Scientific Name and Authority | Common Name | Abbreviation | Year | Sampling Month | Sampling Site | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Net | Dražice | Školjić | Kažela | |||||||||||||||||
| n | TL (cm) | W (g) | n | TL (cm) | W (g) | n | TL (cm) | W (g) | ||||||||||||
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |||||||||
| Seriola dumerili (Risso, 1810) | Greater amberjack | SeriDumr | 2017 | 9 | G | 2 | 26.0 | 27.2 | 213.7 | 238.1 | ||||||||||
| 10 | G | 7 | 22.9 | 35.1 | 129.6 | 539.0 | 15 | 29.5 | 36.7 | 319.9 | 626.3 | |||||||||
| 11 | G | 2 | 28.7 | 31.8 | 285.8 | 402.0 | 3 | 33.1 | 36.4 | 331.9 | 661.4 | |||||||||
| 2018 | 9 | G | 7 | 19.7 | 32.7 | 94.1 | 392.2 | |||||||||||||
| 10 | G | 2 | 27.9 | 32.1 | 314.4 | 377.7 | 7 | 29.9 | 34.7 | 298.9 | 568.8 | 7 | 34.7 | 41.0 | 458.2 | 774.0 | ||||
| 11 | G | 5 | 32.4 | 40.6 | 352.5 | 836.8 | ||||||||||||||
| 2019 | 8 | S | 9 | 19.5 | 23.2 | 70.1 | 152.4 | |||||||||||||
| 9 | G, S | 5 | 24.3 | 29.4 | 168.1 | 319.3 | 24 | 19.3 | 31.3 | 99.6 | 348.2 | |||||||||
| 10 | G | 4 | 31.7 | 35.8 | 352.1 | 538.4 | 18 | 26.1 | 39.9 | 194.9 | 662.1 | 30 | 30.1 | 38.3 | 322.3 | 651.3 | ||||
| Pomatomus saltatrix (Linnaeus, 1766) | Bluefish | - | 2017 | 6 | G | 1 | 34.1 | 350.0 | ||||||||||||
| 10 | G | |||||||||||||||||||
| 11 | G | 3 | 31.8 | 35.1 | 332.0 | 388.0 | ||||||||||||||
| 12 | G | 1 | 39.6 | 499.9 | ||||||||||||||||
| 2018 | 11 | G | 1 | 41.6 | 662.0 | |||||||||||||||
| 2019 | 1 | G | 1 | 30.5 | 220.0 | |||||||||||||||
| 10 | G | 1 | 39.3 | 490.4 | ||||||||||||||||
| Sphyraena sphyraena (Linnaeus, 1758) | Mediterranean barracuda | SphrSphr | 2018 | 2 | G | 1 | 38.6 | 213.3 | ||||||||||||
| 6 | G | 2 | 35.7 | 35.9 | 190.9 | 194.3 | ||||||||||||||
| 11 | G | 8 | 31.5 | 38.3 | 142.6 | 236.7 | ||||||||||||||
| 2019 | 8 | G, S | 21 | 13.4 | 35.3 | 8.3 | 150.4 | |||||||||||||
| 9 | G | 1 | 35.6 | 162.2 | ||||||||||||||||
| Lichia amia (Linnaeus, 1758) | Leerfish | LichAmia | 2017 | 10 | G | 2 | 27.9 | 34.9 | 253.8 | 359.8 | 2 | 31.6 | 36.1 | 213.1 | 447.0 | |||||
| 2018 | 10 | G | 9 | 35.2 | 44.9 | 432.4 | 830.0 | |||||||||||||
| 11 | G | 4 | 37.8 | 41.3 | 476.6 | 672.5 | ||||||||||||||
| 12 | G | 1 | 66.8 | 2850.3 | ||||||||||||||||
| 2019 | 8 | S | 2 | 24.0 | 26.9 | 124.5 | 174.0 | |||||||||||||
| 9 | S | 1 | 21.3 | 28.0 | ||||||||||||||||
| 10 | G | 1 | 34.2 | 338.0 | ||||||||||||||||
| Coryphaena hippurus Linnaeus, 1758 | Common dolphinfish | - | 2017 | 10 | G | 6 | 41.7 | 44.6 | 392.2 | 512.2 | ||||||||||
| Caranx crysos (Mitchill, 1815) | Blue runner | - | 2018 | 11 | G | 1 | 37.5 | 628.0 | ||||||||||||
| Trachinotus ovatus (Linnaeus, 1758) | Pompano | - | 2018 | 8 | S | 2 | 7.8 | 8.4 | 4.0 | 5.5 | ||||||||||
| 11 | G | 1 | 31.6 | 308.8 | ||||||||||||||||
References
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailiantis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [Green Version]
- Katsanevakis, S.; Coll, M.; Piroddi, C.; Steenbeek, J.; Lasram, F.B.R.; Zenetos, A.; Cardoso, A.C. Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Front. Mar. Sci. 2014, 1, 32. [Google Scholar] [CrossRef] [Green Version]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Pérez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Gambaiani, D.D.; Mayol, P.; Isaac, S.J.; Simmonds, M.P. Potential impacts of climate change and greenhouse gas emissions on Mediterranean marine ecosystems and cetaceans. J. Mar. Biol. Assoc. U.K. 2009, 89, 179–201. [Google Scholar] [CrossRef] [Green Version]
- Fredj, G. Stockage et exploitation des données en écologie marine. A-un fichier sur ordinateur des invertebres macrobenthiques. Mem. De L’institut Oceanogr. (Monaco) 1974, 4, 1–61. [Google Scholar]
- Calvo, E.; Simó, R.; Coma, R.; Ribes, M.; Pascual, J.; Sabbatés, A.; Gili, J.M.; Pelejero, C. Effects of climate change on Mediterranean marine ecosystems: The case of the Catalan Sea. Clim. Res. 2011, 50, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Salat, J. Review of hydrographic environmental factors that may influence anchovy habitats in the northwestern Mediterranean. Sci. Mar. 1996, 60, 21–32. [Google Scholar]
- Bianchi, C.N.; Morri, C. Global sea warming and “tropicalisation” of the Mediterranean Sea: Situation, problems and prospects for future research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Francour, P.; Boudouresque, C.F.; Harmelin, J.G.; Harmelin-Vivien, M.L.; Quingard, J.P. Are the Mediterranean waters becoming warmer? Information from biological indicators. Mar. Pollut. Bull. 1994, 28, 523–526. [Google Scholar] [CrossRef]
- Morri, C.; Bianchi, C.N. Recent Changes in Biodiversity in the Ligurian Sea (NW Mediterranean): Is there a Climatic Forcing? In Mediterranean Ecosystems; Faranda, F.M., Guglielmo, L., Spezie, G., Eds.; Springer: Milano, Italy, 2001; pp. 375–384. [Google Scholar] [CrossRef]
- Stebbing, A.R.D.; Turk, S.M.T.; Wheeler, A.; Clarke, K.R. Immigration of southern fish species to south-west England linked to warming of the North Atlantic (1960–2001). J. Mar. Biol. Assoc. U.K. 2002, 82, 177–180. [Google Scholar] [CrossRef]
- Bodilis, P.; Crocetta, F.; Langeneck, J.; Francour, P. The spread of an Atlantic fish species, Pomadasys incisus (Bowdich, 1825) (Osteichtyes: Haemulidae), within the Mediterranean sea with new additional records from the French Mediterranean coast. Ital. J. Zool. 2013, 80, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, A. Assessing species invasions as a cause of extinction. Trends Ecol. Evol. 2004, 19, 619. [Google Scholar] [CrossRef]
- Galil, B.S. Loss or gain? Invasive aliens and biodiversity in the Mediterranean Sea. Mar. Pollut. Bull. 2007, 55, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Galil, B.S. Seeing Red: Alien species along the Mediterranean coast of Israel. Aquat. Invasions. 2007, 2, 281–312. [Google Scholar] [CrossRef]
- Azzurro, E. The advance of thermophilic fish in the Mediterranean Sea: Overview and methodological questions U. In Climate Warming and Related Changes in Mediterranean Marine Biota, CIESM Workshop Monographs; Briand, F., Ed.; CIESM: Monaco City, Monaco, 2008; Volume 35, pp. 39–46. [Google Scholar]
- Azzurro, E.; Sbragaglia, V.; Cerri, J.; Bariche, M.; Bolognini, L.; Souissi, J.B.; Busoni, G.; Coco, S.; Chryssanthi, A.; Garrabou, J.; et al. Climate change, biological invasions and shifting distribution of Mediterranean fishes: A large-scale survey based on ecological knowledge. Glob. Chang. Biol. 2019, 25, 2779–2792. [Google Scholar] [CrossRef]
- Bettoso, N.; Dulčić, J. First record of the oilfish Ruvettus pretiosus (Pisces: Gempylidae) in the northern Adriatic Sea. J. Mar. Biol. Assoc. U.K. 1999, 79, 1145–1146. [Google Scholar] [CrossRef]
- Parenti, P.; Bressi, N. First record of the orange-spotted grouper, Ephinephelus coioides (Perciformes: Serranidae) in the Northern Adriatic Sea. Cybium 2001, 25, 281–284. [Google Scholar]
- Sinovčić, G.; Franičević, M.; Zorica, B.; Čikeš-Keč, V. Length-weight and length-length relationships for 10 pelagic fish species from the Adriatic Sea (Croatia). J. Appl. Ichthyol. 2004, 20, 156–158. [Google Scholar] [CrossRef]
- Psomadakis, P.N.; Scacco, U.; Vacchi, M. Recent findings of some uncommon fishes from the central Tyrrhenian Sea. Cybium 2006, 30, 297–304. [Google Scholar]
- Nerlović, V.; Mravinac, B.; Devescovi, M. Additional information on the blue runner, Caranx crysos (Mitill, 2013, 1815) from the Nothern Adriatic Sea: Meristic and molecular characterisations. Acta Adriat. 2015, 56, 309–318. [Google Scholar]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. In Biodiversity in Enclosed Seas and Artificial Marine Habitats. Developments in Hydrobiology; Relini, G., Ryland, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 193. [Google Scholar] [CrossRef]
- Psomadakis, P.N.; Bentivegna, F.; Giustino, S.; Travaglini, A.; Vacchi, M. Northward spread of tropical affinity fishes: Caranx crysos (Teleostea: Carangidae), a case study from the Mediterranean Sea. Ital. J. Zool. 2011, 78, 113–123. [Google Scholar] [CrossRef]
- Dulčić, J.; Dragičević, B.; Matić-Skoko, S.; Pavičić, M.; Vrdoljak, D. Bluefish Pomatomus saltatrix again in the fishing catches in the Noirthern Adriatic. In Proceedings of the Rapport Du 2019, 42e Congres De La CIESM/Frédéric Briand, Cascais, Portugal, 7–11 October 2019; p. 277. [Google Scholar]
- Dulčić, J. First record of larval Brama brama (Pisces: Bramidae) and Coryphaena hippurus (Pisces: Coryphaenidae) in the Adriatic Sea. J. Plankton Res. 1999, 21, 1171–1174. [Google Scholar] [CrossRef] [Green Version]
- Dragičević, B.; Dulčić, J.; Pallaoro, A.; Paladin, A.; Stagličić, N. First record of the dolphin-fish juveniles, Coryphaena hippurus (Linnaeus, 1758), in the eastern Adriatic Sea. Ann. Ser. Hist. Nat. 2010, 20, 157–160. [Google Scholar]
- Iveša, N.; Piria, M.; Gelli, M.; Mičić, M.; Gavrilović, A. Prisutnost i distribucija termofilnih vrsta riba u Medulinskom zaljevu. In Proceedings of the 2018, 53th Croatian & 13th International Symposium on Agriculture, Vodice, Croatia, 18–23 February 2020; pp. 360–364. [Google Scholar]
- Juanes, F.; Hare, J.A.; Miskiewicz, A.G. Comparing early life history strategies of Pomatomus saltatrix: A global approach. Mar. Freshw. Res. 1996, 47, 365–379. [Google Scholar] [CrossRef]
- Andalaro, F.; Pipitone, C. Food and feeding habits of the amberjack, Seriola dumerili, in the Central Mediterranean Sea during the spawning season. Cah. Biol. Mar. 1997, 38, 91–96. [Google Scholar]
- Aggrey-Fynn, J.; Fynn-Korsah, S.; Appiah, N. Length-weigth relationship and food preference of two costal marine fishes, Galeoides decadactylus (Polynemnidae) and Sphyraena sphyraena (Sphyrenidae) off Cape Coast, Ghana. West Afr. J. Appl. Ecol. 2013, 21, 81–96. [Google Scholar]
- Sley, A.; Taieb, A.H.; Jarboui, O.; Ghorbel, M.; Bouain, A. Feeding behavior of greater amberjack Seriola dumerili (Risoo, 1810) from Central Mediterranean (Gulf of Gabes, Tunisia). J. Mar. Biol. Assoc. U. K. 2016, 96, 1229–1234. [Google Scholar] [CrossRef]
- Battaglia, P.; Pedà, C.; Musolino, S.; Esposito, V.; Andaloro, F.; Romeo, T. Diet and first documented data on plastic ingestion of Trachinotus ovatus L. 1758 (Pisces: Carangidae) from the Strait of Messina (central Mediterranean Sea). Ital. J. Zool. 2016, 83, 121–129. [Google Scholar] [CrossRef]
- Bakran-Petricioli, T.; Antonić, O.; Bukovec, D.; Petricioli, D.; Janeković, I.; Križan, J.; Kušan, V.; Dujmović, S. Modelling spatial distribution of the Croatian marine benthic habitats. Ecol. Modell. 2006, 191, 96–105. [Google Scholar] [CrossRef]
- Bakran-Petricioli, T. Marine Habitats of the Region of Istria. Institute for Physical Planning Region of Istria. 2013. Available online: http://shape.istra-istria.hr/uploads/media/Morska_stanista_-_DVD_Book_ZA_TISAK_MPS_Konacno_Preview.pdf (accessed on 19 May 2020). (In Croatian).
- Hyslop, E.J. Stomach contents analysis: A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Schoener, T.W. Non-synchronous spatial overlap of lizards in patchy enviroments. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, 5th ed.; Microcomputer Power: Ithaca, NY, USA, 2012; p. 496. [Google Scholar]
- Matallanas, J.; Casadevall, M.; Carrassón, M.; Boix, J.; Fernández, V. The food of Seriola dumerili (Pisces: Carangidae) in the Catalan Sea (western Mediterranean). J. Mar. Biol. Assoc. U.K. 1995, 75, 257–260. [Google Scholar] [CrossRef]
- Badalamenti, F.; D’Anna, G.; Lopiano, L.; Scilipoti, D.; Mazzola, A. Feeding habits of young-of-the-year greater amberjack Seriola dumerili (Risso, 1810) along the N/W Sicilian coast. Sci. Mar. 1995, 59, 317–323. [Google Scholar]
- Garcia-Gomez, A. Recent advantages in nutritional aspects of Seriola dumerili. Cah. Options Méditerranées 2000, 47, 249–257. [Google Scholar]
- Bakran-Petricioli, T. Manual for Determination of Marine Habitats in Croatia According to EU Habitat Directive, Državni Zavod Za Zaštitu Prirode. 2011. Available online: http://www.haop.hr/sites/default/files/uploads/publications/2018-01/Bakran-Petricioli%20-%20Prirucnik%20za%20morska%20stanista.pdf (accessed on 18 May 2020). (In Croatian).
- Carbonara, P.; Intini, S.; Modugno, E.; Maradonna, F.; Spedicato, M.T.; Lembo, G.; Zupa, W.; Carnevali, O. Reproductive biology characteristics of red mullet (Mullus barbatus L., 1758) in Southern Adriatic Sea and management implications. Aquat Living Resour 2015, 28, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Bello, G.; Vaglio, A.; Piscitelli, G. The reproductive cycle of Mothocya epimerica (Isopoda: Cymothoidae), a parasite of the sand smelt, Atherina boyeri (Osteichtyes: Atherinidae), in the Lesina Lagoon, Italy. J. Nat. Hist. 1997, 31, 1055–1066. [Google Scholar] [CrossRef]
- Marais, J.F.K. Feeding ecology of mayor carnivorous fish from eastern Cape estuaries. S. Afr. Zool. 1984, 19, 210–223. [Google Scholar] [CrossRef]
- Smale, M. The feeding habits of six pelagic and predatory teleosts in eastern Cape coastal waters (South Africa). J. Zool. 1986, 1, 357–409. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. Editors FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 15 December 2019).
- Jardas, I. Hrvatska Ihtiofauna; Školska knjiga: Zagreb, Croatiapp, 1996; p. 535. [Google Scholar]
- Karlou-Riga, C. Otolith morphology and age and growth of Trachurus mediterraneus (Steindachner) in the Eastern Mediterranean. Fish. Res. 2000, 46, 69–82. [Google Scholar] [CrossRef]
- Raya, V.; Sabatés, A. Diversity and distribution of early life stages of carangid fishes in the northwestern Mediterranean: Responses to environmental drivers. Fish. Oceanogr. 2015, 24, 118–134. [Google Scholar] [CrossRef]
- Viette, M.; Giulianini, P.G.; Ferrero, E.A. Reproductive biology of scad, Trachurus mediterraneus (Teleostei, Carangidae), from the Gulf of Trieste. ICES J. Mar. Sci. 1997, 54, 267–272. [Google Scholar] [CrossRef]
- D’Ambra, I.; Malej, A. Scyphomedusae of the Mediteranean: State of the art and future perspectives. Cent. Nerv. Syst. Agents. Med. Chem. 2015, 15, 81–94. [Google Scholar] [CrossRef]
- Tilves, U.; Sabatés, A.; Blázquez, M.; Raya, V.; Fuentes, V.L. Associations between fish and jellyfish in the NW Mediterranean. Mar. Biol. 2018, 165, 127. [Google Scholar] [CrossRef]
- Ramšak, A.; Stopar, K.; Malej, A. Comparative phylogeography of meroplanktonic species, Aurelia spp. and Rhizostoma pulmo (Cnidaria: Scyphozoa) in European Seas. In Jellyfish Blooms IV. Developments in Hydrobiology; Purcell, J., Mianzan, H., Frost, J.R., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 220, pp. 69–80. [Google Scholar] [CrossRef]
- Allam, S.M.; Faltas, S.N.; Ragheb, E. Food and feeding habits of barracudas in the Egyptian Mediterranean waters of Alexandria. Bull. Nat. Inst. Oceanogr. Fish. ARE 1999, 25, 395–410. [Google Scholar]
- Glamuzina, B.; Pešić, A.; Joksimović, A.; Glamuzina, L.; Matić-Skoko, S.; Conides, A.; Klaoudatos, D.; Zacharaki, P. Observations on the increase of wild gilthead seabream, Sparus aurata abundance in the eastern Adriatic sea: Problems and opportunities. Int. Aquat. Res. 2014, 6, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Kruschel, C.; Schultz, S.T. Aggressive predation drives assembley of Adriatic fish communities. Diversity 2020, 12, 130. [Google Scholar] [CrossRef] [Green Version]
- Rogdakis, Y.; Ramfos, A.; Koukou, K.; Dimitrou, E.; Katselis, G. Feeding habits and trophic level of sea bass (Dicentrarchus labrax) in the Messolonghi—Etoliko lagoons complex (Western Greece). J. Biol. Res. Thessal. 2010, 13, 13–26. [Google Scholar]
- Marengo, M.; Durieux, E.D.H.; Marchand, B.; Francour, P. A review of biology, fisheries and population structure of Dentex dentex (Sparidae). Rev. Fish Biol. Fish. 2014, 24, 1065–1088. [Google Scholar] [CrossRef]
- Dulčić, J.; Pallaoro, A.; Dragičević, B. First record of the blue runner, Caranx crysos (Mitchill, 1815), in the Adriatic Sea. J. Appl. Ichthyol. 2009, 25, 481–482. [Google Scholar] [CrossRef]
- Sinopoli, M.; Lauria, V.; Garofalo, G.; Maggio, T.; Cillari, T. Extensive use of fish aggregating devices together with environmental change influenced the spatial distribution of a tropical affinity fish. Sci. Rep. 2019, 9, 4934. [Google Scholar] [CrossRef]
- Dulčić, J.; Dragičević, B.; Antolović, N.; Sulić-Šprem, J.; Kožul, V.; Grgičević, R. Additional records of Lobotes surinamensis, Caranx crysos, Enchelicore anatina, Lagocephalus sceleratus (Actinopterygii) in the Adriatic sea. Acta Ichthyol. Piscat. 2014, 44, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Pavičić, M.; Šiljić, J.; Duganđžić, P.; Skaramuca, B. New records of blue runner, Caranx crysos (Mitchill, 1815) in the Adriatic Sea. Croat. J. Fish. 2014, 72, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Sley, A.; Jarboui, O.; Ghorbel, M.; Bouain, A. Food and feeding habits of Caranx crysos from the Goulf of Gabes (Tunisia). J. Mar. Biol. Assoc. U. K. 2009, 89, 1375–1380. [Google Scholar] [CrossRef]
- Sinopoli, M.; Castriota, L.; Vivona, P.; Gristina, M.; Andaloro, F. Assessing the fish assemblage associated with FADs (Fish Aggregating Devices) in the southern Tyrrhenian Sea using two different professional fishing gears. Fish. Res. 2012, 123, 56–61. [Google Scholar] [CrossRef]
- Suaria, G.; Aliani, S. Floating debris in the Mediterranean Sea. Mar. Pollut. Bull. 2014, 86, 494–504. [Google Scholar] [CrossRef]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Tugores, M.P.; Giannoulaki, M.; Iglesias, M.; Bonanno, A.; Tičina, V.; Leonori, I.; Machias, A.; Tsagarakis, K.; Díaz, N.; Giráldez, A.; et al. Habitat suitability modelling for sardine Sardina pilchardus in a highly diverse ecosystem: The Mediterranean sea. Mar. Ecol. Prog. Ser. 2011, 443, 181–205. [Google Scholar] [CrossRef] [Green Version]
- Moltó, V.; Hernández, P.; Sinopoli, M.; Besbes-Benseddik, A.; Besbes, R.; Mariani, A.; Gambin, M.; Alemany, F.; Morales-Nin, B.; Grau, A.M.; et al. A global review on the biology of the dolphinfish (Coryphaena hippurus) and its fishery in the Mediterranean Sea: Advances in the last two decades. Rev. Fish. Sci. Aquac. 2020, 28, 376–420. [Google Scholar] [CrossRef]
- Campo, D.; Mostarda, E.; Castriota, L.; Scarabello, M.P.; Andaloro, F. Feeding habits of the Atlantic bonito, Sarda sarda (Bloch, 1793) in the southern Tyrrhenian sea. Fish. Res. 2006, 81, 169–175. [Google Scholar] [CrossRef]
- Moreno, A.; Boavida-Portugal, J.; Pimentel, M.; Pereira, J.; Rosa, R. Loligo vulgaris, European Squid. In Advances in Sqid Biolgy, Eclogy and Fisheies; Rosa, R., O’Dor, R., Pierce, G., Eds.; Nova Biomedical, Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 3–32. [Google Scholar]
- Pešić, A.; Đurović, M.; Joksimović, A.; Regner, S.; Simonović, P.; Glamuzina, B. Some reproductive patterns of the sardine, Sardina pilchardus (Walb, 1792), in Boka Kotorska Bay (Montenegro, southern Adriatic Sea). Acta Adriat. 2010, 52, 159–168. [Google Scholar]
- Batistić, M.; Tutman, P.; Bojanić, D.; Skaramuca, B.; Kožul, V.; Glavić, N.; Bartulović, V. Diet and diel feeding activity of juvenile pompano (Trachinotus ovatus) (Teleostei: Caranghidae) from the southern Adriatic, Croatia. J. Mar. Biol. Assoc. U.K. 2005, 85, 1533–1534. [Google Scholar] [CrossRef]
- Dulčić, J.; Pallaoro, A.; Kraljević, M. First record of pompano fingerling Trachinotus ovatus (Linnaeus, 1758) (Pisces: Carangidae) in the eastern middle Adriatic. Nat. Croat. 1997, 1, 61–65. [Google Scholar]
- Lipej, L.; Dulčić, J. The current status of Adriatic fish biodiversity. In Balkan Biodiversity: Pattern and Process in the European Hotspot; Griffiths, H.I., Kryštufek, B., Reed, J.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 291–306. [Google Scholar]
- Dulčić, J.; Pallaoro, A.; Kraljević, M. Occurrence of bluefish, Pomatomus saltator (Linnaeus, 1766), and butterfish, Stromateus fiatola (Linnaeus, 1758), juveniles in the eastern central Adriatic. Ann. Ser. Hist. Nat. 2000, 19, 19–22. [Google Scholar]
- Sabatés, A.; Martín, P.; Raya, V. Changes in life-history traits in relation to climate change: Bluefish (Pomatomus saltatrix) in the northwestern Mediterranean. ICES J. Mar. Sci. 2012, 69, 1000–1009. [Google Scholar] [CrossRef] [Green Version]
- Cengiz, O.; Ozekinci, U.; Oztekin, A.; Kumova, C. Growth parameters and mortality of bluefish (Pomatomus saltatrix Linneaus, 1766) from Gallipoli peninsula and Dardanelles (northeastern Mediterranean, Turkey). Mar. Sci. Tech. Bull. 2013, 2, 1–7. [Google Scholar]
- Sepulveda, M.; Oliva, D. Interactions between South American sea lions Otaria flavescens (Shaw) and salmon farms in southern Chile. Aquac. Res. 2005, 36, 1062–1068. [Google Scholar] [CrossRef]
- Sanchez-Jerez, P.; Fernandez-Jover, D.; Bayle-Sempere, J.; Valle, C.; Dempster, T.; Tuya, F.; Juanes, F. Interactions between bluefish Pomatomus saltatrix (L.) and coastal sea-cage farms in the Mediterranean Sea. Aquaculture 2008, 282, 61–67. [Google Scholar] [CrossRef]
- Dulčić, J.; Kraljević, M.; Pallaoro, A.; Glamuzina, B. Unusual catch of bluefish Pomatomus saltatrix (Pomatomidae) in Tarska cove (northern Adriatic). Cybium 2005, 29, 207–208. [Google Scholar]
- Grati, F.; Scarcella, G.; Bolognini, L.; Fabi, G. Releasing of the European sea bass Dicentrarchus labrax (Linnaeus) in the Adriatic Sea: Large-volume versus intensively cultured juveniles. J. Exp. Mar. Biol. Ecol. 2011, 397, 144–152. [Google Scholar] [CrossRef]
- Whitfield, A.K. The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Rev. Fish. Biol. Fish. 2017, 27, 75–110. [Google Scholar] [CrossRef]
- Iveša, N.; Špelić, I.; Gelli, M.; Castellicchio, A.; Piria, M.; Gavrilović, A. Fish catch analysis of the “poponica” net in Bay of Medulin. In Proceedings of the 2020, 55th Croatian & 15th International Symposium on Agriculture, Vodice, Croatia, 17–21 February 2020; pp. 328–333. [Google Scholar]

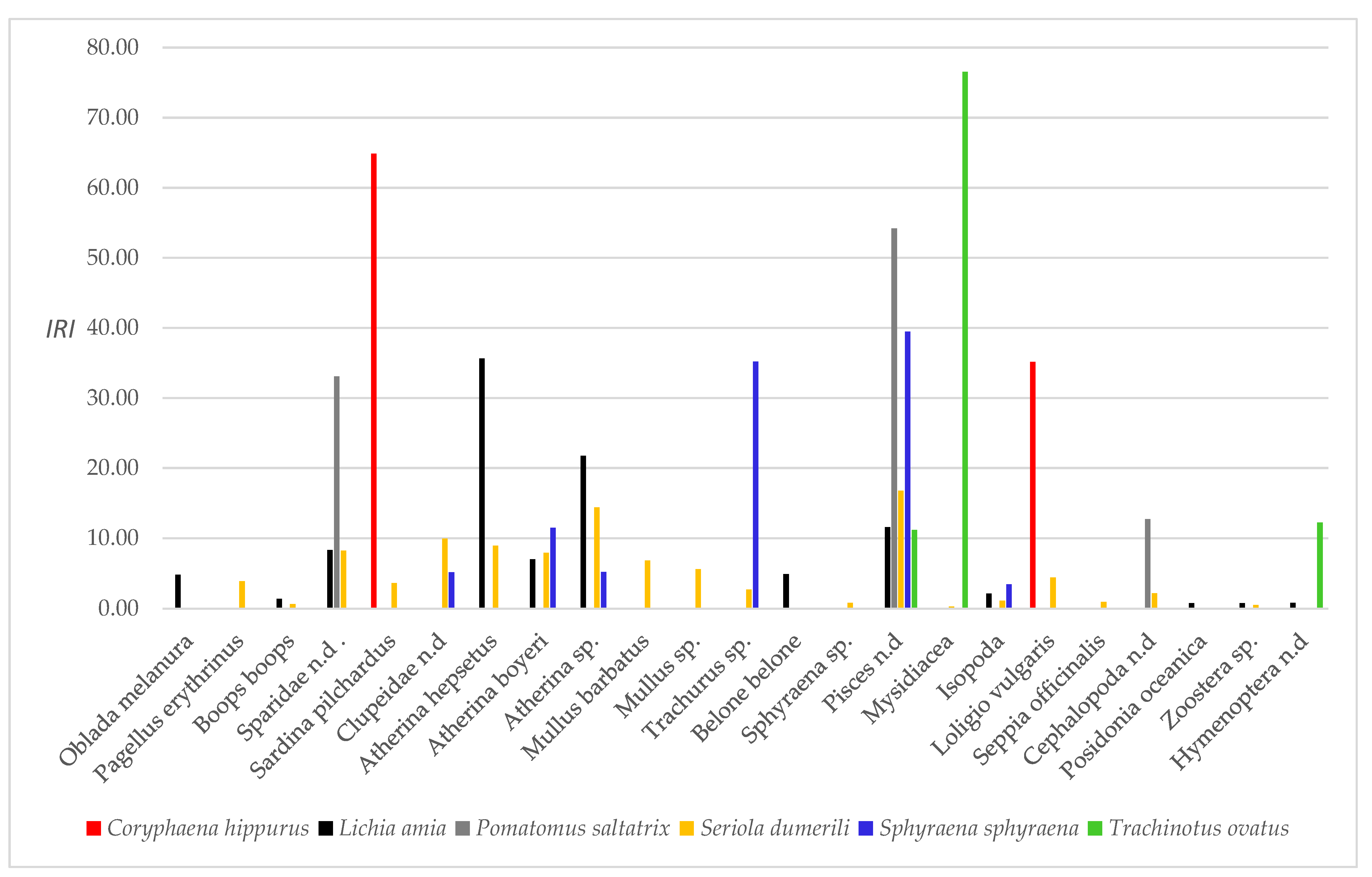
 —specimen size class, Δ—prey item). See Table 1 and Appendix A Table A1 for abbreviations.
—specimen size class, Δ—prey item). See Table 1 and Appendix A Table A1 for abbreviations.
 —specimen size class, Δ—prey item). See Table 1 and Appendix A Table A1 for abbreviations.
—specimen size class, Δ—prey item). See Table 1 and Appendix A Table A1 for abbreviations.
| Prey Item | Abbreviation | Coryphaena hippurus TL = 41.70–44.60 FI% = 1.55 ± 1.97 VI% = 33.33 MouthW = 3.3 MouthH = 4.3 NoBraRig = 9 n = 6 | Lichia amia TL = 24.00–66.80 FI% = 2.62 ± 2.81 VI% = 13.64 MouthW = 4.6 MouthH = 4.8 NoBraRig = 10 n = 22 | Pomatomus saltatrix TL = 30.50–41.60 FI% = 0.44 ± 0.45 VI% = 33.33 MouthW = 4.1 MouthH = 4.3 NoBraRig = 12 n = 8 | Seriola dumerili TL = 19.30–41.00 FI% = 1.55 ± 1.92 VI% = 36.05 MouthW = 4.1 MouthH = 3.9 NoBraRig = 16 n = 147 | Sphyraena sphyraena TL = 13.40–38.60 FI% = 1.38 ± 1.89 VI% = 54.55 MouthW = 1.6 MouthH = 2.9 NoBraRig = 0 n = 33 | Trachinotus ovatus TL = 7.85–66.80 FI% = 2.50 ± 0.94 VI% = 33.33 MouthW = 1.2 MouthH = 1.5 NoBraRig = 36 n = 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N% | F% | W% | N% | F% | W% | N% | F% | W% | N% | F% | W% | N% | F% | W% | N% | F% | W% | ||
| Oblada melanura | OblMel | 0.89 | 2.33 | 11.37 | |||||||||||||||
| Pagellus erythrinus | PagEry | 1.94 | 3.14 | 6.61 | |||||||||||||||
| Boops boops | BoopBop | 0.89 | 2.33 | 1.02 | 0.32 | 0.52 | 1.18 | ||||||||||||
| Sparidae n.d. | SparidND | 8.04 | 11.63 | 5.59 | 14.29 | 20.00 | 64.91 | 7.44 | 8.90 | 8.34 | |||||||||
| Sardina pilchardus | SardPilc | 50.00 | 50.00 | 94.55 | 1.62 | 1.57 | 7.67 | ||||||||||||
| Clupeidae n.d | ClupeiND | 10.68 | 9.95 | 9.31 | 5.00 | 5.26 | 5.21 | ||||||||||||
| Atherina hepsetus | AtherHep | 44.64 | 23.26 | 39.87 | 10.36 | 9.95 | 6.52 | ||||||||||||
| Atherina boyeri | AtherBoy | 8.04 | 9.30 | 3.87 | 11.65 | 8.38 | 3.73 | 15.00 | 15.79 | 3.72 | |||||||||
| Atherina sp. | AtherSp | 24.11 | 18.60 | 23.10 | 16.83 | 17.28 | 9.17 | 5.00 | 5.26 | 5.29 | |||||||||
| Mullus barbatus | MullBarb | 7.12 | 2.09 | 11.25 | |||||||||||||||
| Mullus sp. | MullSp | 4.85 | 4.19 | 7.74 | |||||||||||||||
| Trachurus sp. | TrachuSp | 2.27 | 1.57 | 4.39 | 15.00 | 15.79 | 74.83 | ||||||||||||
| Belone belone | BelonBel | 3.57 | 9.30 | 1.96 | |||||||||||||||
| Sphyraena sp. | SphyraSp | 0.65 | 1.05 | 0.74 | |||||||||||||||
| Pisces n.d | PiscesND | 8.04 | 13.95 | 13.11 | 71.43 | 60.00 | 31.19 | 19.09 | 21.47 | 9.75 | 55.00 | 52.63 | 10.80 | 0.08 | 33.33 | 0.15 | |||
| Mysidiacea | Mysidiac | 0.32 | 0.52 | 0.02 | 99.52 | 33.34 | 96.79 | ||||||||||||
| Isopoda | Isopoda | 1.79 | 4.65 | 0.07 | 1.29 | 2.09 | 0.05 | 5.00 | 5.26 | 0.15 | |||||||||
| Loligio vulgaris | LoliVulg | 50.00 | 50.00 | 5.45 | 1.29 | 2.09 | 9.89 | ||||||||||||
| Seppia officinalis | SepOffic | 0.65 | 1.05 | 1.19 | |||||||||||||||
| Cephalopoda n.d | CephalND | 14.29 | 20.00 | 3.90 | 1.62 | 2.62 | 2.42 | ||||||||||||
| Posidonia oceanica | PosidOce | + | 2.33 | 0.03 | |||||||||||||||
| Zoostera sp. | ZoosterS | + | 2.33 | 0.01 | + | 1.57 | 0.02 | ||||||||||||
| Hymenoptera n.d | - | 0.40 | 33.33 | 3.05 | |||||||||||||||
| Species | Coryphaena hippurus | Lichia amia | Pomatomus saltatrix | Seriola dumerili | Sphyraena sphyraena | Trachinotus ovatus |
|---|---|---|---|---|---|---|
| Coryphaena hippurus | - | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 |
| Lichia amia | 0.00 | - | 0.20 | 0.52 | 0.26 | 0.12 |
| Pomatomus saltatrix | 0.00 | 0.19 | - | 0.27 | 0.39 | 0.11 |
| Seriola dumerili | 0.13 | 0.36 | 0.21 | - | 0.40 | 0.11 |
| Sphyraena sphyraena | 0.00 | 0.19 | 0.11 | 0.28 | - | 0.11 |
| Trachinotus ovatus | 0.00 | 0.13 | 0.01 | 0.01 | 0.01 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iveša, N.; Piria, M.; Gelli, M.; Trnski, T.; Špelić, I.; Radočaj, T.; Kljak, K.; Jug-Dujaković, J.; Gavrilović, A. Feeding Habits of Predatory Thermophilic Fish Species and Species with Subtropical Affinity from Recently Extended Distributional Range in Northeast Adriatic Sea, Croatia. Diversity 2021, 13, 357. https://doi.org/10.3390/d13080357
Iveša N, Piria M, Gelli M, Trnski T, Špelić I, Radočaj T, Kljak K, Jug-Dujaković J, Gavrilović A. Feeding Habits of Predatory Thermophilic Fish Species and Species with Subtropical Affinity from Recently Extended Distributional Range in Northeast Adriatic Sea, Croatia. Diversity. 2021; 13(8):357. https://doi.org/10.3390/d13080357
Chicago/Turabian StyleIveša, Neven, Marina Piria, Martina Gelli, Thomas Trnski, Ivan Špelić, Tena Radočaj, Kristina Kljak, Jurica Jug-Dujaković, and Ana Gavrilović. 2021. "Feeding Habits of Predatory Thermophilic Fish Species and Species with Subtropical Affinity from Recently Extended Distributional Range in Northeast Adriatic Sea, Croatia" Diversity 13, no. 8: 357. https://doi.org/10.3390/d13080357






