Evolution, Homology, and Development of Tetrapod Limb Muscles
Abstract
1. Introduction
2. Limb Muscle Reconstructions in Extinct Tetrapods and Persistent Controversies
2.1. Evolution of Reconstruction Methodology
2.2. Stem Mammalia: “Mammal-Like Reptiles” and the Evolution of Mammalian Locomotion
2.3. Stem Amniota: “Bridging the Gap”
2.4. Stem Lissamphibia
2.5. Stem Reptilia
2.6. Stem Tetrapoda: The Fish Fin Meets the Tetrapod Limb
2.7. Controversies in Tetrapod Muscle Reconstruction
2.7.1. Evolution of the Deltoid Group
2.7.2. Was a True Subcoracoscapularis Present in Stem Mammals? In Stem Tetrapods?
2.7.3. Was a Teres Major Present Ancestrally in Amniotes?
2.7.4. How many Radial Extensors Were Present Ancestrally in Tetrapods?
2.7.5. Was Caudifemoralis Reduced in Stem Reptiles?
3. Development of Limb Muscles in Tetrapods
3.1. Morphogenesis and Developmental Patterns
3.2. The Fore-Hindlimb Enigma and the Origin of Pectoral and Pelvic Appendages
3.3. Atavisms, Variations, Anomalies, and Links between Ontogeny and Phylogeny
4. General Remarks and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Muscle Homology Tables
| Lissamphibia (Salamandra) | Squamata (Iguana) | Archosauria (Alligator) | Mammalia (Didelphis) | Eutheria (Homo) | |
|---|---|---|---|---|---|
| Superficial dorsomesial | Latissimus dorsi | Latissimus dorsi | |||
| Dorsoepitrochlearis | - | ||||
| Extensor digitorum (extensor digitorum communis, humerodorsalis, extensor digitorum longus) | |||||
| Extensor antebrachii et carpi radialis (supinator longus; tractor radii) | Extensor carpi radialis brevis | ||||
| Extensor carpi radialis longus + abductor radialis | Extensor carpi radialis longus | ||||
| Brachioradialis | |||||
| Supinator (supinator brevis) | |||||
| Triceps scapularis (triceps longus) | |||||
| Triceps humeralis lateralis | Triceps humeralis lateralis | Triceps humeralis lateralis | |||
| triceps humeralis posticum | |||||
| Triceps humeralis medialis | |||||
| Extensor antebrachii et carpi ulnaris (flexor ulnaris) | Extensor carpi ulnaris | ||||
| Anconeus | |||||
| Triceps coracoideus | - | ||||
| Deltoideus scapularis | Deltoideus | ||||
| Deep dorsomesial | Procoraco-humeralis | Deltoideus acromialis et clavicularis | Deltoideus acromialis et clavicularis | ||
| humeroradialis | - | ||||
| Scapulohumeralis anterior | - | Teres minor | |||
| Subcoraco-scapularis | Subcoracoscapularis | Subcoracoscapularis | Subscapularis | ||
| Teres major | |||||
| Scapulohumeralis posterior | - | ||||
| Abductor et extensor digit 1 (supinator manus) | Abductor pollicis longus * | Abductor pollicis longus | |||
| Extensor pollicis brevis | |||||
| Extensores digitorum breves | Extensores digitorum breves | Extensor digiti minimi | |||
| Extensor indicis | |||||
| Extensor pollicis longus | |||||
| Dorsometacarpales | - | ||||
| Superficial ventrolateral | Pectoralis | Pectoralis major | |||
| Pectoralis minor | |||||
| Panniculus carnosus | - | ||||
| Flexor antebrachii et carpi ulnaris | Flexor carpi ulnaris | ||||
| Epitrochleo-anconeus | - | Epitrochleo-anconeus | - | ||
| Coracobrachialis | Coracobrachialis longus | - | Coracobrachialis | ||
| Coracobrachialis brevis | |||||
| Flexor antebrachii et carpi radialis | Flexor carpi radialis | ||||
| Pronator teres | |||||
| Humero-antebrachialis | Brachialis * | ||||
| Flexor digitorum communis | Flexor digitorum longus (palmaris communis) | Flexor digitorum superficialis* | |||
| Deep ventrolateral | Flexor accessorius medialis | palmaris longus | Palmaris longus | ||
| Flexor accessorius lateralis | Palmaris longus internus | ||||
| Contrahentium caput longum | Flexor digitorum profundus | Flexor digitorum profundus | |||
| Flexor pollicis longus | |||||
| Supracoracoideus | Infraspinatus | ||||
| Supraspinatus | Supraspinatus | ||||
| Cleidoacromialis | |||||
| Coracoradialis | Biceps brachii * | ||||
| Palmaris profundus 1 | - | ||||
| Pronator quadratus (flexor palmaris profundus) | Pronator quadratus | - | Pronator quadratus | ||
| Pronator accessorius | - | ||||
| Intrinsic autopodial | Flexores digitorum minimi | - | |||
| Interphalangeaus | - | ||||
| Abductor digiti minimi | - | Abductor digiti minimi | |||
| - | Abductor pollicis brevis (abductor metacarpi 1) | ||||
| Flexores breves superficiales | Flexores breves superficiales (flexor digitorum brevis superficialis) | Flexor brevis digitorum manus | - | ||
| - | Palmaris brevis | ||||
| Lumbricales | |||||
| Contrahentes digitorum | Transversus palmaris + flexor digiti quinti? | Contrahentes digitorum | - | ||
| - | Adductor pollicis | ||||
| - | Adductor pollicis accessorius | ||||
| Intermetacarpales (“interossei”) | Interossei | ||||
| Flexores breves profundii | Flexores breves profundii | ||||
| Flexor pollicis brevis | Flexor pollicis brevis | ||||
| Opponens pollicis | |||||
| Flexor digiti minimi brevis | Flexor digiti minimi brevis | ||||
| Opponens digiti minimi | |||||
| Lissamphibia (Ambystoma) | Squamata (Timon) | Archosauria (Alligator) | Mammalia (Didelphis) | Eutheria (Homo) | |
|---|---|---|---|---|---|
| Axial | Caudofemoralis | Caudofemoralis longus | - | ||
| Caudofemoralis brevis | |||||
| Superficial dorsomesial | Iliotibialis | Extensor iliotibialis | Femorococcygeus | - | |
| Gluteus maximus | |||||
| Rectus femoris | |||||
| Femorotibialis | Vastus lateralis | ||||
| - | Vastus intermedius | ||||
| Vastus medialis | |||||
| Ambiens (sartorius) | |||||
| Extensor cruris tibialis | Tibialis anterior | ||||
| Extensor tarsi tibialis | |||||
| Extensor digitorum longus | Extensor hallucis longus | ||||
| Extensor digitorum longus | Extensor digitorum longus | ||||
| Fibularis tertius | |||||
| Iliofibularis (tenuissimus) | Biceps femoris (part) | ||||
| Extensor cruris et tarsi fibularis | Fibularis longus | ||||
| Fibularis brevis | |||||
| Deep dorsomesial | Puboischiofemoralis internus | Iliopsoas (iliacus + psoas major) | |||
| pectineus | |||||
| Iliofemoralis | Gluteus medius + minimus | ||||
| Piriformis | |||||
| Scansorius | - | ||||
| - | Tensor fasciae latae | ||||
| Abductor et extensor digit 1 (adductor et extensor hallucis et indicis; adductor hallucis dorsalis) | Extensor hallucis brevis * | ||||
| Extensores digitorum breves | Extensor digitorum brevis + adductor et extensor hallucis et indicus + abductor digiti 4 | Extensor digitorum brevis | Extensor digitorum brevis | ||
| Fibularis digiti quinti | |||||
| Superficial ventrolateral | Puboischiotibialis (gracilis) | ||||
| Pubotibialis (?) | Pubotibialis (?) | - | Adductor longus | ||
| Femorofibularis (?) | |||||
| Ischioflexorius | Flexor tibialis internus | Semimembranosus | |||
| Biceps femoris (part) | |||||
| Flexor tibialis externus | Semitendinosus * | ||||
| Flexor digitorum communis | Gastrocnemius internus | Gastrocnemius | |||
| Gastrocnemius externus | Gastrocnemius externus | ||||
| Soleus | |||||
| Plantaris | |||||
| Deep ventrolateral | Flexor accessorius medialis | Flexor digitorum longus | Flexor digitorum longus | Flexor digitorum longus | |
| Flexor accessorius lateralis | Flexor hallucis longus | ||||
| Contrahentium caput longum | Flexor accessorius (“flexor hallucis longus”) | Quadratus plantae | |||
| Pubofemoralis (adductor femoris) | Adductor magnus | ||||
| Adductor brevis | |||||
| Puboischiofemoralis externus | Quadratus femoris | ||||
| Obturator externus | |||||
| Ischiotrochantericus (ischiofemoralis) | Obturator internus | ||||
| Gemellus superior | |||||
| Gemellus inferior | |||||
| Tibialis posterior (pronator profundus) | |||||
| Interosseous cruris | Popliteus | - | Popliteus | ||
| Interosseous cruris | - | ||||
| Intrinsic autopodial | Flexores breves superficiales (flexores digitores breves) | Flexor digitorum brevis superficialis | Flexor digitorum brevis | ||
| Fibulocalcaneus | |||||
| - | Lumbricales | ||||
| - | Abductor hallucis (flexor hallucis) | - | Abductor hallucis | ||
| Contrahentes pedis | Flexores digiti 2,3,4 | Contrahentes pedis | Adductor hallucis | ||
| Adductor hallucis accessorius | |||||
| Flexores breves profundi | - | Abductor hallucis brevis | |||
| Flexores breves profundi (interossei plantares) | Flexor digiti minimi brevis | ||||
| Flexor hallucis brevis | |||||
| Flexor brevis profundus 2 | |||||
| Flexores breves profundi | Interossei plantares | ||||
| Intermetatarsales | Interossei dorsales | ||||
| Flexores digitorum minimi | - | ||||
| Interphalangeii | - | ||||
| Abductor digiti minimi | |||||
References
- Romer, A.S. The Locomotor Apparatus of Certain Primitive and Mammal-Like Reptiles; Columbia University Press: New York, NY, USA, 1922. [Google Scholar]
- Romer, A.S. The appendicular skeleton of the Permian embolomerous amphibian Archeria. Contrib. Mus. Pale-Ontol. Univ. Mich. 1957, 8, 103–159. [Google Scholar]
- Miner, R.W. The pectoral limb of Eryops and other primitive tetrapods. Bull. Am. Mus. Nat. Hist. 1925, L1, 148–309. [Google Scholar]
- Haines, R.W. A revision of the extensor muscles of the forearm in tetrapods. J. Anat. 1939, 73, 211–233. [Google Scholar] [PubMed]
- Holmes, R. The osteology and musculature of the pectoral limb of small captorhinids. J. Morphol. 1977, 152, 101–140. [Google Scholar] [CrossRef]
- Gregory, W.K.; Camp, C.L. Studies in Comparative Myology and Osteology; American Museum of Natural History: New York, NY, USA, 1918. [Google Scholar]
- Witmer, L.M. Functional Morphology in Vertebrate Paleontology; Cambridge University Press: Cambridge, MA, USA, 1995; Volume 1, pp. 19–33. [Google Scholar]
- Campbell, R.M.; Vinas, G.; Henneberg, M.; Diogo, R. Visual Depictions of Our Evolutionary Past: A Broad Case Study Concerning the Need for Quantitative Methods of Soft Tissue Reconstruction and Art-Science Collaborations. Front. Ecol. Evol. 2021, 9, 60. [Google Scholar] [CrossRef]
- Bishop, P.J. The humerus of Ossinodus pueri, a stem tetrapod from the Carboniferous of Gondwana, and the early evolution of the tetrapod forelimb. Alcheringa Australas. J. Palaeontol. 2013, 38, 209–238. [Google Scholar] [CrossRef]
- Molnar, J.L.; Diogo, R.; Hutchinson, J.R.; Pierce, S.E. Reconstructing pectoral appendicular muscle anatomy in fossil fish and tetrapods over the fins-to-limbs transition. Biol. Rev. 2017, 93, 1077–1107. [Google Scholar] [CrossRef]
- Molnar, J.L.; Diogo, R.; Hutchinson, J.R.; Pierce, S.E. Evolution of hindlimb muscle anatomy across the tetrapod water-to-land transition, including comparisons with forelimb anatomy. Anat. Rec. 2020, 303, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.L.; Hutchinson, J.R.; Diogo, R.; Clack, J.A.; Pierce, S.E. Evolution of forelimb musculoskeletal function across the fish-to-tetrapod transition. Sci. Adv. 2021, 7, eabd7457. [Google Scholar] [CrossRef]
- Diogo, R.; Wood, B. The broader evolutionary lessons to be learned from a comparative and phylogenetic analy-sis of primate muscle morphology. Biol. Rev. 2013, 88, 988–1001. [Google Scholar] [CrossRef]
- Dilkes, D.W.; Hutchinson, J.R.; Holliday, C.M.; Witmer, L.M. Reconstructing the Musculature of Dinosaurs. Complete Dinosaur; Indiana University Press: Bloomington, IN, USA, 2012; pp. 151–190. [Google Scholar]
- Bryant, H.N.; Russell, A.P. The role of phylogenetic analysis in the inference of unpreserved attributes of ex-tinct taxa. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1992, 337, 405–418. [Google Scholar]
- Bryant, H.N.; Seymour, K.L. Observations and comments on the reliability of muscle reconstruction in fossil vertebrates. J. Morphol. 1990, 206, 109–117. [Google Scholar] [CrossRef]
- Hall, B.K. Fins into Limbs: Evolution, Development, and Transformation; University of Chicago Press: Chicago, IL, USA, 2008. [Google Scholar]
- Gadow, H. Observations in Comparative Myology. J. Anat. Physiol. 1882, 16, 493–514. [Google Scholar]
- Romer, A.S. Pectoral limb musculature and shoulder girdle structure in fish and tetrapods. Anat. Rec. 1924, 27, 119–143. [Google Scholar] [CrossRef]
- Ahlberg, P.E. Humeral homology and the origin of the tetrapod elbow: A reinterpretation of the enigmatic specimens ANSP 21350 and GSM 104536. Stud. Foss. Tetrapods. 2011, 86, 17–29. [Google Scholar]
- Panchen, A.L. On the amphibian Crassigyrinus scoticus Watson from the Carboniferous of Scotland. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 309, 505–568. [Google Scholar]
- Sumida, S.S. The appendicular skeleton of the Early Permian genus Labidosaurus (Reptilia, Captorhinomorpha, Captorhinidae) and the hind limb musculature of captorhinid reptiles. J. Vertebr. Paleontol. 1989, 9, 295–313. [Google Scholar] [CrossRef]
- Watson, D.M.S. The Evolution of the Tetrapod Shoulder Girdle and Fore-limb. J. Anat. 1917, 52, 1–63. [Google Scholar] [PubMed]
- Fürbringer, M. Die Knochen und Muskeln der Extremitäten bei den Schlangenähnlichen Sauriern: Vergleichend-Anatomische Abhandlung; Wilhelm Engelmann: Leipzeg, Germany, 1870. [Google Scholar]
- Dilkes, D.W. Appendicular myology of the hadrosaurian dinosaur Maiasaura peeblesorum from the Late Creta-ceous (Campanian) of Montana. Earth Environ. Sci. Trans. R. Soc. Edinb. 1999, 90, 87–125. [Google Scholar] [CrossRef]
- Sewertzoff, A.N. Studien über die Entwickelung der Muskeln, Nerven und des Skeletts der Extremitäten der niederen Tetrapoda: Beiträge zu einer Theorie der pentadactylen Extremität der Wirbeltiere; Typo-lithogr. de la Société JN Kouchnéreff: Moscow, Russia, 1908. [Google Scholar]
- Brinkmann, H.; Venkatesh, B.; Brenner, S.; Meyer, A. Nuclear protein-coding genes support lungfish and not the coelacanth as the closest living relatives of land vertebrates. Proc. Natl. Acad. Sci. USA 2004, 101, 4900–4905. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, C.T.; Alföldi, J.; Lee, A.P.; Fan, S.; Philippe, H.; MacCallum, I.; Braasch, I.; Manousaki, T.; Schneider, I.; Rohner, N.; et al. The African coelacanth genome provides insights into tetrapod evolu-tion. Nature 2013, 496, 311–316. [Google Scholar] [CrossRef]
- Hutson, J.; Hutson, K. A test of the validity of range of motion studies of fossil archosaur elbow mobility using repeated-measures analysis and the extant phylogenetic bracket. J. Exp. Biol. 2012, 215, 2030–2038. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Norton, J.M. Use of the Extant Phylogenetic Bracket (Epb) Concept in Reconstructing the Dinosaur Respiratory System. Paléontol. Soc. Spéc. Publ. 1996, 8, 293. [Google Scholar] [CrossRef][Green Version]
- Witmer, L.M. Homology of facial structures in extant archosaurs (birds and crocodilians), with special reference to paranasal pneumaticity and nasal conchae. J. Morphol. 1995, 225, 269–327. [Google Scholar] [CrossRef]
- Benton, M.J. Studying Function and Behavior in the Fossil Record. PLoS Biol. 2010, 8, e1000321. [Google Scholar] [CrossRef] [PubMed]
- Currie, P.J.C.J.; Eberth, D.A.E.A. On gregarious behavior in AlbertosaurusThis article is one of a series of papers published in this Special Issue on the theme Albertosaurus. Can. J. Earth Sci. 2010, 47, 1277–1289. [Google Scholar] [CrossRef]
- Andrews, S.M.; Westoll, T.S. The Postcranial Skeleton of Eusthenopteron foordi Whiteaves. Trans. R. Soc. Edinb. 1970, 68, 207–329. [Google Scholar] [CrossRef]
- Holmes, R. The Carboniferous amphibian Proterogyrinus Scheelei Romer, and the early evolution of tetrapods. Philos. Trans. R. Soc. B Biol. Sci. 1984, 306, 431–524. [Google Scholar] [CrossRef]
- Shubin, N.H.; Daeschler, E.B.; Coates, M.I. The Early Evolution of the Tetrapod Humerus. Science 2004, 304, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R.; Ziermann, J.M.; Molnar, J.L.; Siomova, N.; Abdala, V. Muscles of Chordates: Development, Homologies, and Evolution; Taylor & Francis: Boca Raton, FL, USA, 2018. [Google Scholar]
- Romer, A.S. The development of tetrapod limb musculature? The shoulder region of Lacerta. J. Morphol. 1944, 74, 1–41. [Google Scholar] [CrossRef]
- Russell, A.P.; Bauer, A.M. The appendicular locomotor apparatus of Sphenodon and normal-limbed squamates. Biol. Reptil. 2008, 21, 1–465. [Google Scholar]
- Diogo, R.; Abdala, V.; Aziz, M.; Lonergan, N.; Wood, B. From fish to modern humans–comparative anatomy, ho-mologies and evolution of the pectoral and forelimb musculature. J. Anat. 2009, 214, 694–716. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R.; Tanaka, E.M. Anatomy of the pectoral and forelimb muscles of wildtype and green fluorescent pro-tein-transgenic axolotls and comparison with other tetrapods including humans: A basis for regenerative, evo-lutionary and developmental studies. J. Anat. 2012, 221, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Francis, E. The Anatomy of the Salamander; Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Walker, W.F. Biology of the Reptilia; Academic Press: London, UK, 1974; Volume 4. [Google Scholar]
- Cheng, C.-C. The development of the shoulder region of the opossum, Didelphys virginiana, with special reference to the musculature. J. Morphol. 1955, 97, 415–471. [Google Scholar] [CrossRef]
- Walker, W.F. The development of the shoulder region of the turtle, Chrysemys picta marginata, with special reference to the primary musculature. J. Morphol. 1947, 80, 195–249. [Google Scholar] [CrossRef] [PubMed]
- Humphry, G.M. Art. XXIX.—Observations in Myology, including the Myology of Cryptobranch, Lepidosiren, Dog-Fish, Ceratodus, and Pseudopus Pallasii, with the nerves of Cryptobranch and Lepidosiren, and the disposition of muscles in Vertebrate Animals. Am. J. Med. Sci. 1873, 130, 520. [Google Scholar] [CrossRef]
- Abdala, V.; Manzano, A.S.; Herrel, A. The distal forelimb musculature in aquatic and terrestrial turtles: Phylog-eny or environmental constraints? J. Anat. 2008, 213, 159–172. [Google Scholar] [CrossRef]
- Lewis, O.J. Functional Morphology of the Evolving Hand and Foot; Oxford University Press: Oxford, MS, USA, 1989. [Google Scholar]
- Gatesy, S.M. Caudofemoral musculature and the evolution of theropod locomotion. Paleobiology 1990, 16, 170–186. [Google Scholar] [CrossRef]
- Romer, A.S. The development of the thigh musculature of the chick twelve figures. J. Morphol. 1927, 43, 347–385. [Google Scholar] [CrossRef]
- Romer, A.S. The development of tetrapod limb musculature? The thigh of Lacerta. J. Morphol. 1942, 71, 251–298. [Google Scholar] [CrossRef]
- Chen, H.-K. The development of the pectoral limb of Necturus maculosus; Illinois Biological Monographs; Urbana, Ill.,University of Illinois: Champaign County, IL, USA; p. 1935. [CrossRef][Green Version]
- Sullivan, G. Anatomy and embryology of the Wing Musculature of the domestic fowl (gallus). Aust. J. Zool. 1962, 10, 458–518. [Google Scholar] [CrossRef]
- Jones, C.L. The morphogenesis of the thigh of the mouse with special reference to tetrapod muscle homologies. J. Morphol. 1979, 162, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, S.; Tosney, K.W. Spatial and temporal patterns of muscle cleavage in the chick thigh and their value as criteria for homology. Am. J. Anat. 1991, 191, 325–350. [Google Scholar] [CrossRef]
- Bardeen, C.R. Development and variation of the nerves and the musculature of the inferior extremity and of the neighboring regions of the trunk in man. Am. J. Anat. 1906, 6, 259–390. [Google Scholar] [CrossRef]
- Čihák, R. Ontogenesis of the Skeleton and Intrinsic Muscles of the Human Hand and Foot; Springer: Berlin/Heidelberg, Germany, 1972; pp. 12–59. [Google Scholar]
- Diogo, R.; Tanaka, E.M. Development of fore-and hindlimb muscles in GFP-transgenic axolotls: Morphogenesis, the tetrapod bauplan, and new insights on the Forelimb-Hindlimb Enigma. J. Exp. Zoolog. B Mol. Dev. Evol. 2014, 322, 106–127. [Google Scholar] [CrossRef]
- Diogo, R.; Ziermann, J.M. Development of fore-and hindlimb muscles in frogs: Morphogenesis, homeotic trans-formations, digit reduction, and the forelimb–hindlimb enigma. J. Exp. Zoolog. B Mol. Dev. Evol. 2014, 322, 86–105. [Google Scholar] [CrossRef]
- Diogo, R.; Siomava, N.; Gitton, Y. Development of human limb muscles based on whole-mount immunostaining and the links between ontogeny and evolution. Development 2019, 146, dev180349. [Google Scholar] [CrossRef]
- Valasek, P.; Theis, S.; DeLaurier, A.; Hinits, Y.; Luke, G.N.; Otto, A.M.; Minchin, J.; He, L.; Christ, B.; Brooks, G. Cel-lular and molecular investigations into the development of the pectoral girdle. Dev. Biol. 2011, 357, 108–116. [Google Scholar] [CrossRef]
- Leal, F.; Cohn, M.J. Developmental, genetic, and genomic insights into the evolutionary loss of limbs in snakes. Genesis 2017, 56, e23077. [Google Scholar] [CrossRef]
- Nagashima, H.; Sugahara, F.; Takechi, M.; Ericsson, R.; Kawashima-Ohya, Y.; Narita, Y.; Kuratani, S. Evolution of the Turtle Body Plan by the Folding and Creation of New Muscle Connections. Science 2009, 325, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, T.; Kuratani, S. Evolution of the muscular system in tetrapod limbs. Zool. Lett. 2018, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Grim, M.; Carlson, B.M. A comparison of morphogenesis of muscles of the forearm and hand during ontogene-sis and regeneration in the axolotl (Ambystoma mexicanum). Z. Für Anat. Entwicklungsgesch. 1974, 145, 149–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carlson, B.M. Muscle regeneration in amphibians and mammals: Passing the torch. Dev. Dyn. 2003, 226, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R.; Nacu, E.; Tanaka, E.M. Is salamander limb regeneration really perfect? Anatomical and morphoge-netic analysis of forelimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative, develop-mental, and evolutionary studies: Limb regeneration in salamanders. Anat. Rec. 2014, 297, 1076–1089. [Google Scholar]
- Diogo, R.; Murawala, P.; Tanaka, E.M. Is salamander hindlimb regeneration similar to that of the forelimb? An-atomical and morphogenetic analysis of hindlimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative and developmental studies. J. Anat. 2014, 224, 459–468. [Google Scholar]
- Kardon, G. Muscle and tendon morphogenesis in the avian hind limb. Development 1998, 125, 4019–4032. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Gauthier, J.A. 1,2,3 = 2,3,4: A solution to the problem of the homology of the digits in the avian hand. Proc. Natl. Acad. Sci. USA 1999, 96, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F. Developmental Biology, 8th ed.; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- Carlson, B.M. Tissue Engineering and Regeneration; Academic Press: London, UK, 2007; pp. 259–278. [Google Scholar] [CrossRef]
- Fröbisch, N.B.; Carroll, R.L.; Schoch, R.R. Limb ossification in the Paleozoic branchiosaurid Apateon (Temno-spondyli) and the early evolution of preaxial dominance in tetrapod limb development. Evol. Dev. 2007, 9, 69–75. [Google Scholar] [CrossRef]
- Fröbisch, N.B. Ossification patterns in the tetrapod limb—Conservation and divergence from morphogenetic events. Biol. Rev. 2008, 83, 571–600. [Google Scholar] [CrossRef] [PubMed]
- Fröbisch, N.B.; Shubin, N.H. Salamander limb development: Integrating genes, morphology, and fossils. Dev. Dyn. 2011, 240, 1087–1099. [Google Scholar] [CrossRef]
- Shubin, N.; Tabin, C.; Carroll, S. Fossils, genes and the evolution of animal limbs. Nature 1997, 388, 639–648. [Google Scholar] [CrossRef]
- Johanson, Z.; Joss, J.; Boisvert, C.A.; Ericsson, R.; Sutija, M.; Ahlberg, P.E. Fish fingers: Digit homologues in sar-copterygian fish fins. J. Exp. Zoolog. B Mol. Dev. Evol. 2007, 308B, 757–768. [Google Scholar] [CrossRef]
- Johanson, Z. Evolution of paired fins and the lateral somitic frontier. J. Exp. Zool. Part B Mol. Dev. Evol. 2010, 314, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.I. The origin of vertebrate limbs. Development 1994, 1994, 169–180. [Google Scholar] [CrossRef]
- Coates, M.I.; Cohn, M.J. Vertebrate axial and appendicular patterning: The early development of paired ap-pendages. Am. Zool. 1999, 39, 676–685. [Google Scholar] [CrossRef]
- Diogo, R.; Linde-Medina, M.; Abdala, V.; Ashley-Ross, M.A. New, puzzling insights from comparative myologi-cal studies on the old and unsolved forelimb/hindlimb enigma. Biol. Rev. 2013, 88, 196–214. [Google Scholar] [CrossRef]
- Sears, K.E.; Capellini, T.D.; Diogo, R. On the serial homology of the pectoral and pelvic girdles of tetrapods. Evolution 2015, 69, 2543–2555. [Google Scholar] [CrossRef]
- Miyashita, T.; Diogo, R. Evolution of serial patterns in the vertebrate pharyngeal apparatus and paired append-ages via assimilation of dissimilar units. Front. Ecol. Evol. 2016, 4, 71. [Google Scholar] [CrossRef]
- Esteve-Altava, B.; Pierce, S.E.; Molnar, J.L.; Johnston, P.; Diogo, R.; Hutchinson, J.R. Evolutionary parallelisms of pectoral and pelvic network-anatomy from fins to limbs. Sci. Adv. 2019, 5, eaau7459. [Google Scholar] [CrossRef]
- Shubin, N.; Tabin, C.; Carroll, S. Deep homology and the origins of evolutionary novelty. Nature 2009, 457, 818–823. [Google Scholar] [CrossRef]
- Diogo, R.; Molnar, J. Comparative Anatomy, Evolution, and Homologies of Tetrapod Hindlimb Muscles, Comparison with Forelimb Muscles, and Deconstruction of the Forelimb-Hindlimb Serial Homology Hypothesis. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2014, 297, 1047–1075. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R. Cranial or postcranial—Dual origin of the pectoral appendage of vertebrates combining the fin-fold and gill-arch theories? Dev. Dyn. 2020, 249, 1182–1200. [Google Scholar] [CrossRef] [PubMed]
- Thacher, J.K. Median and Paired Fins, a Contribution to the History of Vertebrate Limbs; Connecticut Academy of Arts and Sciences: New Haven, CT, USA, 1874; Volume 3. [Google Scholar]
- Mivart, S.G. Notes on the fins of Elasmobranchs, with Considerations on the Nature and Homologues of Ver-tebrate Limbs. Trans. Zool. Soc. Lond. 1879, 10, 439–484. [Google Scholar] [CrossRef]
- Balfour, F.M. On the development of the skeleton of the paired fins of elasmobranchii, considered in relation to its bearings on the nature of the limbs of the vertebrata. In Proceedings of the Zoological Society of London; Blackwell Publishing Ltd: Oxford, UK, 1881; Volume 49. [Google Scholar]
- Gegenbaur, C. Grundzüge der vergleichenden Anatomie. Wilhelm Engelmann: Leipzig, Germany, 1870. [Google Scholar]
- Freitas, R.; Zhang, G.; Cohn, M.J. Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature 2006, 442, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Dahn, R.D.; Davis, M.C.; Pappano, W.N.; Shubin, N.H. Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature 2006, 445, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.; Dahn, R.D.; Shubin, N.H. Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc. Natl. Acad. Sci. USA 2009, 106, 5720–5724. [Google Scholar] [CrossRef]
- Gillis, J.A.; Hall, B.K. A shared role for sonic hedgehog signalling in patterning chondrichthyan gill arch ap-pendages and tetrapod limbs. Development 2016, 143, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.K.; Mahon, V.; Diogo, R. Muscles Lost in Our Adult Primate Ancestors Still Imprint in Us: On Muscle Evolution, Development, Variations, and Pathologies. Curr. Mol. Biol. Rep. 2020, 6, 32–50. [Google Scholar] [CrossRef]
- Diogo, R.; Ziermann, J.M.; Linde-Medina, M. Is evolutionary biology becoming too politically correct? A reflec-tion on the scala naturae, phylogenetically basal clades, anatomically plesiomorphic taxa, and ‘lower’ animals: Is evolutionary biology too politically correct? Biol. Rev. 2015, 90, 502–521. [Google Scholar]
- Diogo, R.; Smith, C.M.; Ziermann, J. Evolutionary developmental pathology and anthropology: A new field linking development, comparative anatomy, human evolution, morphological variations and defects, and medicine. Dev. Dyn. 2015, 244, 1357–1374. [Google Scholar] [CrossRef] [PubMed]
- Løvtrup, S. On von Baerian and Haeckelian Recapitulation. Syst. Zool. 1978, 27, 348–352. [Google Scholar] [CrossRef]
- De Beer, G.R. Embryos and Ancestors; Clarendon Press: Oxford, UK, 1940; 108p. [Google Scholar]
- Wood, J. On Human Muscular Variations and their Relation to Comparative Anatomy. J. Anat. Physiol. 1867, 1, 44–59. [Google Scholar]
- Wood, J., VII. On a group of varieties of the muscles of the human neck, shoulder, and chest, with their transi-tional forms and homologies in the mammalia. Philos. Trans. R. Soc. Lond. 1870, 83–116. [Google Scholar]
- Macalister, A. Additional observations on muscular anomalies in human anatomy (3rd series), with a cata-logue of the principal muscular variations hitherto published. Trans Roy Ir. Acad Sci. 1871, 25, 1–134. [Google Scholar]
- Smith, C.; Ziermann, J.; Diogo, R. Muscular and Skeletal Anomalies in Human Trisomy in an Evo-Devo Context Using 3-D Imaging and Anatomical Dissections, with Notes on Down Syndrome, Cyclopia and Medical Impli-cations. FASEB J. 2015, 29, 870–871. [Google Scholar] [CrossRef]
- Gould, S.J. Ontogeny and Phylogeny; The Belknap Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Gould, S.J. The Structure of Evolutionary Theory; Harvard University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Darwin, G. The Origin of Species by Natural Selection: Or the Preservation of Favored Races in the Struggle for Life, 1st ed.; Bantam Books: New York, NY, USA, 1859. [Google Scholar]
- Sanchez, S.; Dupret, V.; Tafforeau, P.; Trinajstic, K.M.; Ryll, B.; Gouttenoire, P.-J.; Wretman, L.; Zylberberg, L.; Peyrin, F.; Ahlberg, P.E. 3D Microstructural Architecture of Muscle Attachments in Extant and Fossil Vertebrates Revealed by Synchrotron Microtomography. PLoS ONE 2013, 8, e56992. [Google Scholar] [CrossRef] [PubMed]
- Woronowicz, K.C.; Gline, S.E.; Herfat, S.T.; Fields, A.J.; Schneider, R.A. Developmental Mechanisms Linking Form and Function During Jaw Evolution. bioRxiv 2018, 264556. [Google Scholar] [CrossRef]
- Botelho, J.F.; Smith-Paredes, D.; Soto-Acuña, S.; Mpodozis, J.; Palma, V.; Vargas, A.O. Skeletal plasticity in response to embryonic muscular activity underlies the development and evolution of the perching digit of birds. Sci. Rep. 2015, 5, 9840. [Google Scholar] [CrossRef]


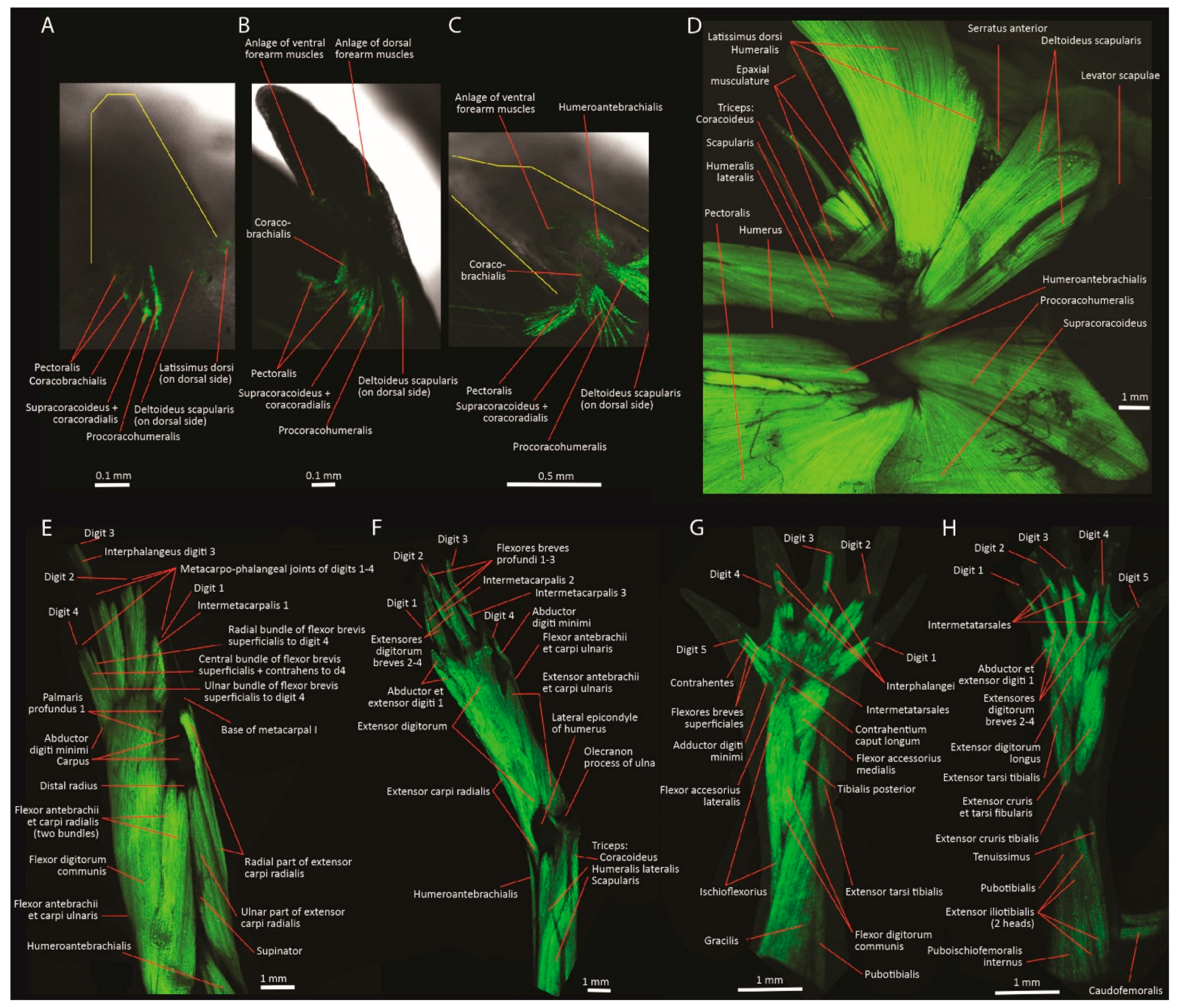
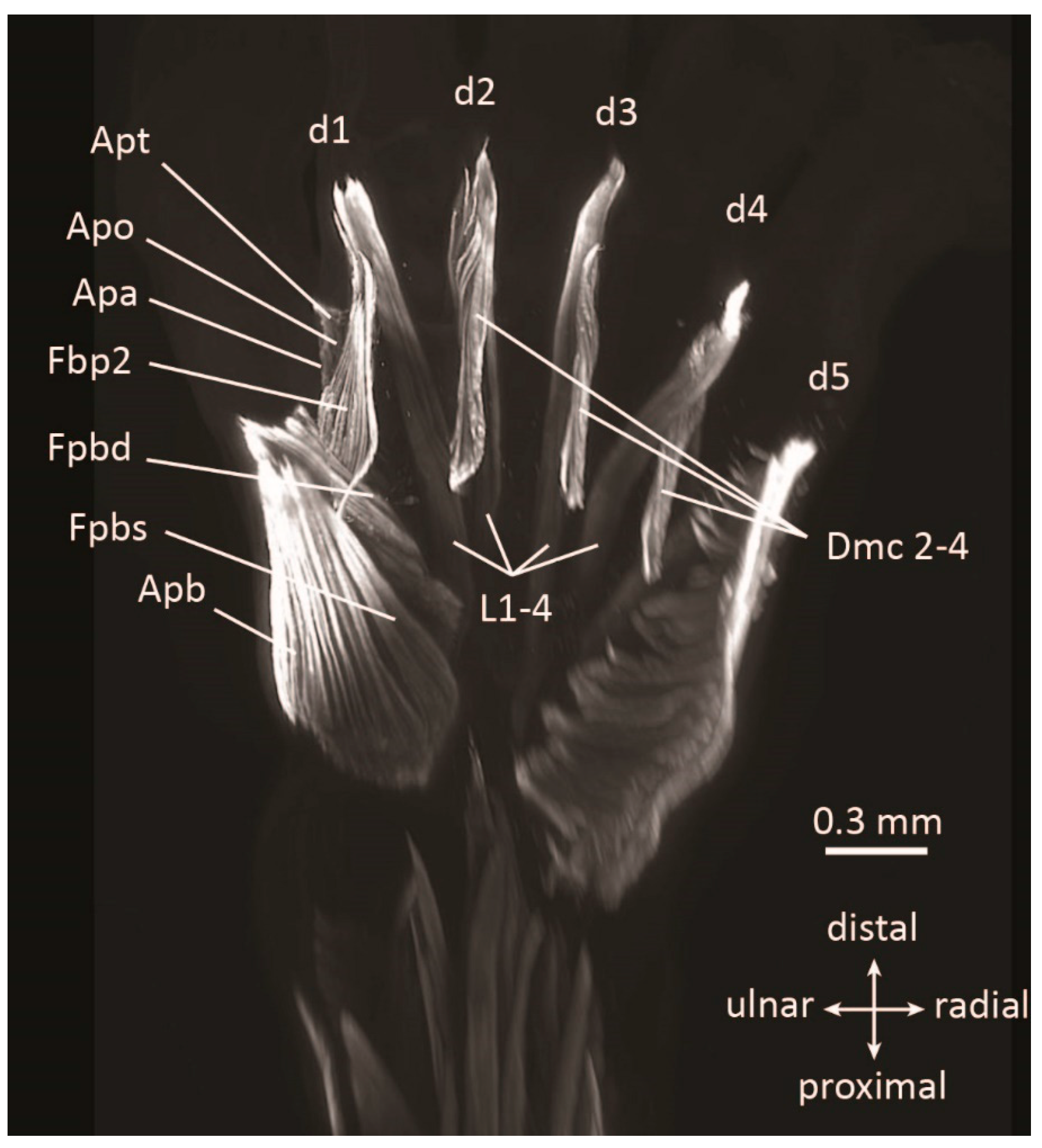
| Embryonic Stage | Clade | Forelimb Muscles | Hindlimb Muscles |
|---|---|---|---|
| CR10.5 mm | Tetrapoda | Serratus anterior | |
| Levator scapulae | |||
| Latissimus dorsi | |||
| Coracobrachialis | |||
| Triceps brachii | |||
| Amniota | Brachialis | Sartorius | |
| Biceps brachii | |||
| Mammalia | Pectoralis major | Gluteus maximus | |
| Pectoralis minor | Quadratus femoris | ||
| Infraspinatus | |||
| Supraspinatus | |||
| Subscapularis | |||
| Teres major | |||
| CR12–14 mm | Tetrapoda | Extensor digitorum | Gracilis |
| Pronator quadratus | Tibialis posterior | ||
| Supinator | |||
| Contrahentes | |||
| Amniota | Flexor carpi ulnaris | Tibialis anterior | |
| Flexor carpi radialis | Fibularis longus | ||
| Pronator teres | Fibularis brevis | ||
| Lumbricales | Flexor digitorum longus | ||
| Popliteus | |||
| Mammalia | Extensor carpi ulnaris | Gluteus medius | |
| Brachioradialis | Psoas minor | ||
| Anconeus | Iliopsoas | ||
| Extensor indicis | Pectineus | ||
| Obturator externus | |||
| Adductor brevis | |||
| Adductor longus | |||
| Semimembranosus | |||
| Semitendinosus | |||
| Extensor hallucis longus | |||
| Theria | Subclavius | Gluteus minimus | |
| Flexor digitorum superficialis | |||
| Palmaris longus | Piriformis | ||
| Euarchontoglires | Rhomboideus major | Tensor fasciae latae | |
| Rhomboideus minor | Soleus | ||
| Homo | Flexor hallucis longus | ||
| CR15–17 mm | Tetrapoda | Flexores breves profundi | Intermetatarsales |
| Intermetacarpales | Flexores breves profundi | ||
| Abd. dig. minimi | Abductor digiti minimi | ||
| Contrahentes | |||
| Amniota | Abd. pollicis brevis | Dorsometatarsales | |
| Dorsometacarpales | |||
| Mammalia | Dorsoepitrochlearis | Plantaris | |
| Flexor hallucis brevis | |||
| Flexor digitorum brevis | |||
| Theria | Flexor pollicis brevis | ||
| Euarchontoglires | Gastrocnemius | ||
| Homo | Opponens pollicis | ||
| CR18–20 mm | Tetrapoda | Extensor digitorum longus | |
| Amniota | Abductor pollicis longus | ||
| Mammalia | Extensor pollicis longus | Adductor magnus | |
| Extensor digiti minimi | Rectus femoris | ||
| Teres minor | Vastus lateralis | ||
| Vastus medialis | |||
| Theria | Flexor digitorum profundus | ||
| Flexor digiti minimi brevis | |||
| Adductor pollicis | |||
| Palmaris brevis | |||
| Euarchontoglires | Opponens digiti minimi | ||
| Homo | Extensor pollicis brevis | Fibularis tertius | |
| Deltoideus Flexor pollicis longus | Adductor hallucis | ||
| CR25–36mm | Amniota | Epitrochleoanconeus | |
| Mammalia | Flexor digiti minimi brevis | ||
| Theria | Adductor pollicis accessorius | Gemellus interior | |
| Extensor carpi radialis longus | Gemellus superior | ||
| Extensor carpi radialis brevis | Obturator internus | ||
| Homo | Interossei dorsales | ||
| Interossei plantares | |||
| Adductor hallucis accessorius | |||
| CR51 mm | Homo | Interossei dorsalis | |
| Interossei palmaris |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnar, J.L.; Diogo, R. Evolution, Homology, and Development of Tetrapod Limb Muscles. Diversity 2021, 13, 393. https://doi.org/10.3390/d13080393
Molnar JL, Diogo R. Evolution, Homology, and Development of Tetrapod Limb Muscles. Diversity. 2021; 13(8):393. https://doi.org/10.3390/d13080393
Chicago/Turabian StyleMolnar, Julia L., and Rui Diogo. 2021. "Evolution, Homology, and Development of Tetrapod Limb Muscles" Diversity 13, no. 8: 393. https://doi.org/10.3390/d13080393
APA StyleMolnar, J. L., & Diogo, R. (2021). Evolution, Homology, and Development of Tetrapod Limb Muscles. Diversity, 13(8), 393. https://doi.org/10.3390/d13080393






