Comparative Approaches in Vertebrate Cartilage Histogenesis and Regulation: Insights from Lampreys and Hagfishes
Abstract
:1. Introduction
2. Overview of the Cartilaginous Skeleton of Lampreys and Hagfishes
2.1. History of Cyclostome Cartilage Research
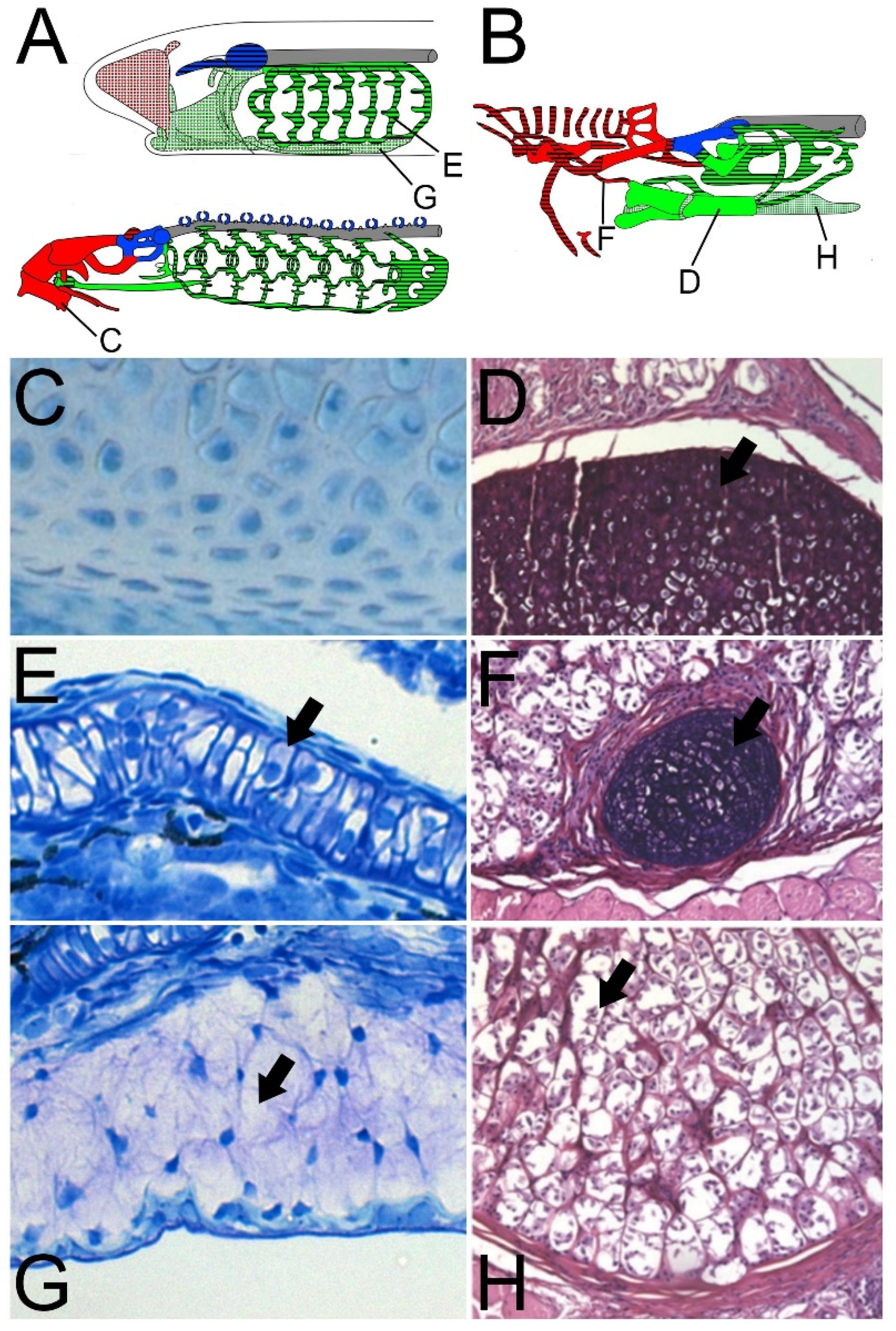
2.2. The “Hard” and “Soft” Cartilages of Cyclostomes
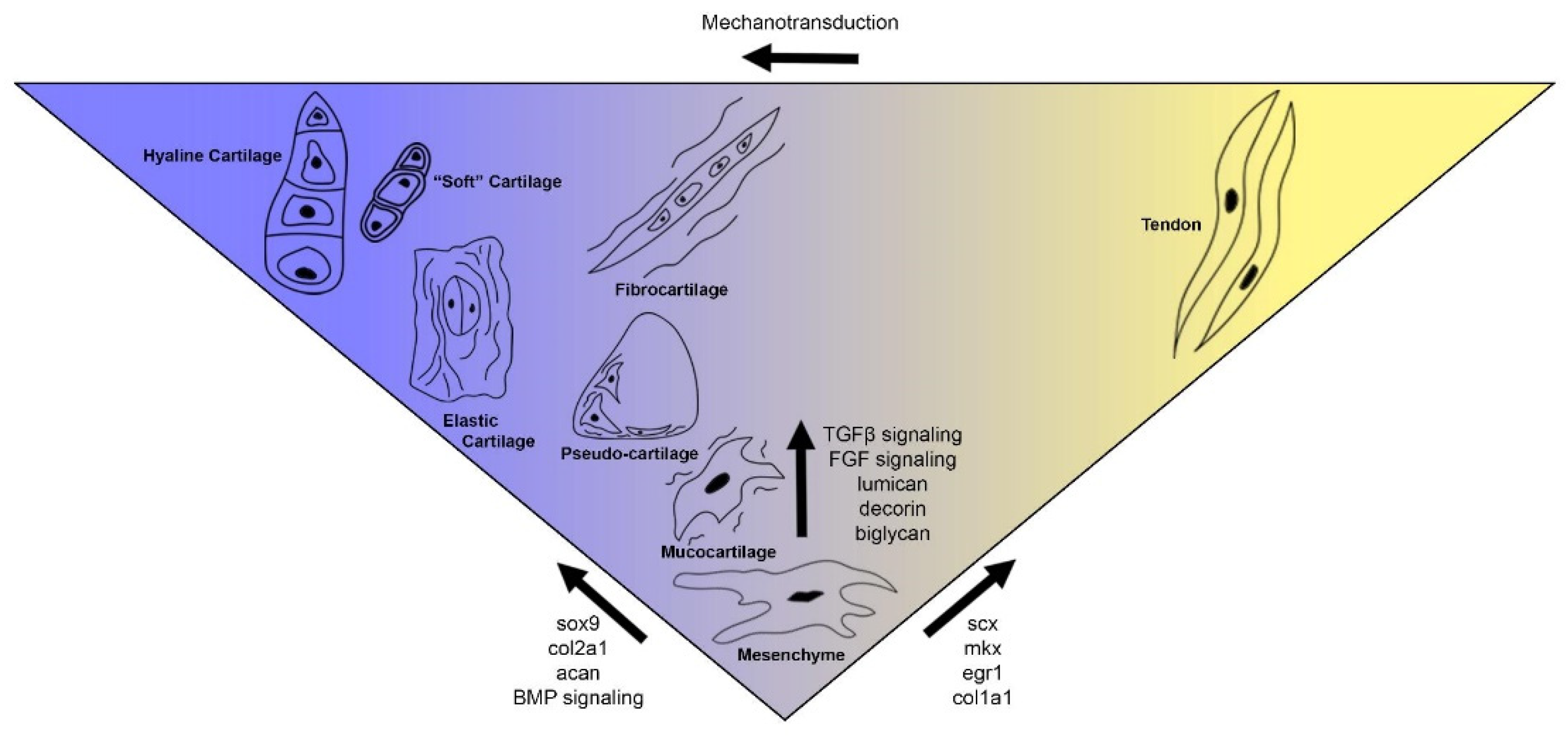
2.3. Cyclostome Cartilage-Like Tissues: Hagfish Pseudo-Cartilage and Lamprey Mucocartilage
2.4. Looking Forward: A Synthesis of Classic Cyclostome Histology
3. Overview of Gnathostome Cartilage Development
3.1. All Gnathostome Cartilages Develop Using a Conserved Gene Regulatory Network
3.2. Gnathostomes Have a Diversity of Cartilage Types
4. Current Understanding of Cyclostome Cartilage Development
4.1. Identification of Unique Proteins in Cyclostome Skeletons
4.2. Aspects of the Skeletal GRN of Hagfish Appear Similar to That in Gnathostomes
4.3. The Core Skeletal GRN of Lamprey Is Similar to That in Gnathostomes
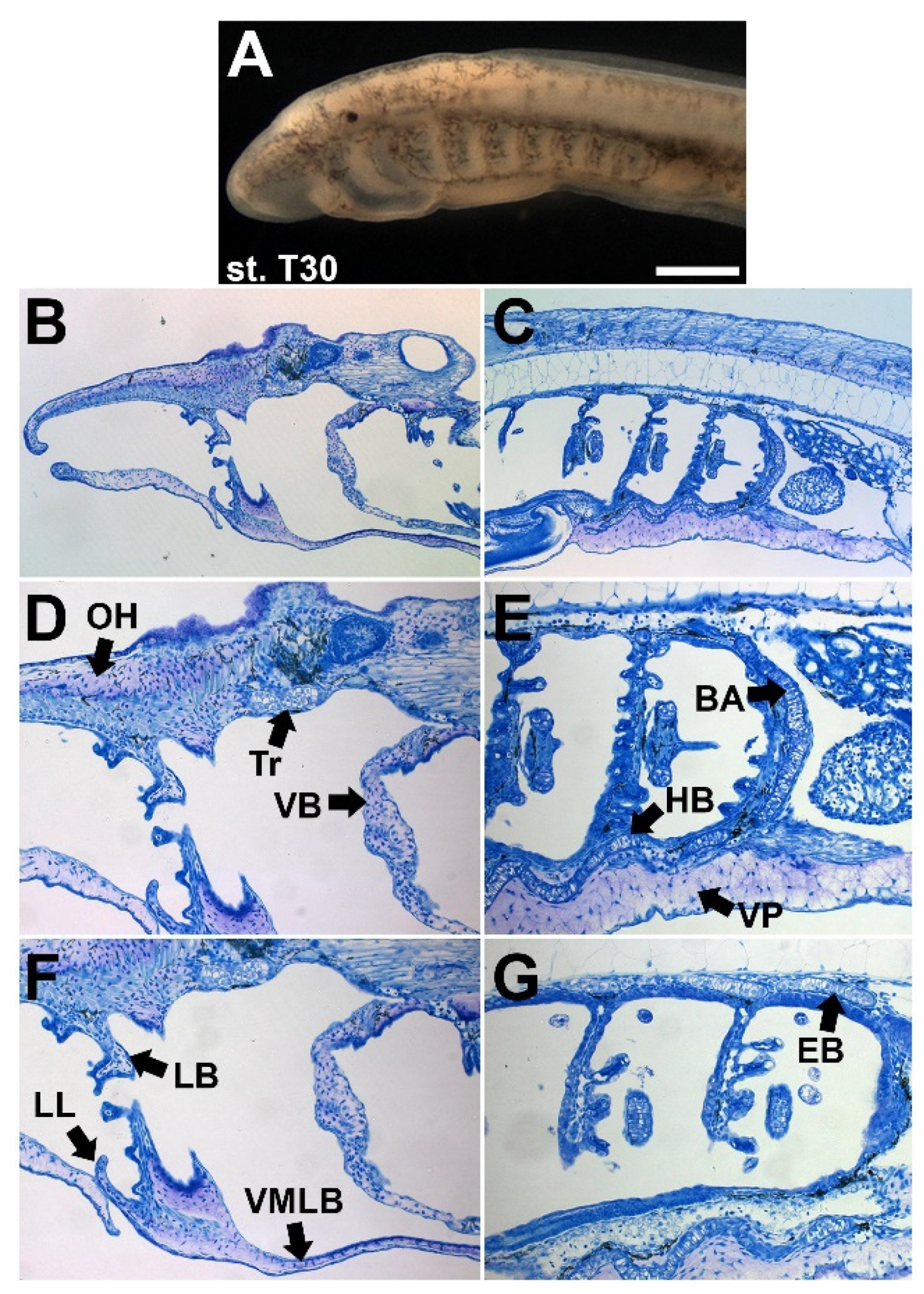
4.4. Larval Lampreys Have a Diversity of Cartilage Types
5. Future Areas of Interest in Cyclostome Cartilage Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Square, T.A. Evolution of the endothelin pathway drove neural crest cell diversification. Nature 2020, 585, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Martik, M.L. Evolution of the new head by gradual acquisition of neural crest regulatory circuits. Nature 2019, 574, 675–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuratani, S. Cephalic neural crest cells and the evolution of craniofacial structures in vertebrates: Morphological and embryological significance of the premandibular–mandibular boundary. Zoology 2005, 108, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Trainor, P.A. The development, patterning and evolution of neural crest cell differentiation into cartilage and bone. Bone 2020, 137, 115409. [Google Scholar] [CrossRef]
- York, J.R.; McCauley, D.W. The origin and evolution of vertebrate neural crest cells. Open Biol. 2020, 10, 190285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandzik, D. Evolution of the new vertebrate head by co-option of an ancient chordate skeletal tissue. Nature 2015, 518, 534–537. [Google Scholar] [CrossRef]
- Rychel, A.L. Evolution and development of the chordates: Collagen and pharyngeal cartilage. Mol. Biol. Evol. 2006, 23, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneto, S.; Wada, H. Regeneration of amphioxus oral cirri and its skeletal rods: Implications for the origin of the vertebrate skeleton. J. Exp. Zool. Part B Mol. Dev. Evol. 2011, 316, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, O.A. The genetic program for cartilage development has deep homology within Bilateria. Nature 2016, 533, 86–89. [Google Scholar] [CrossRef]
- Witten, P.; Huysseune, A.; Hall, B. A practical approach for the identification of the many cartilaginous tissues in teleost fish. J. Appl. Ichthyol. 2010, 26, 257–262. [Google Scholar] [CrossRef]
- Panopoulou, G.; Poustka, A.J. Timing and mechanism of ancient vertebrate genome duplications–the adventure of a hypothesis. Trends Genet. 2005, 21, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Holland, L.Z.; Daza, D.O. A new look at an old question: When did the second whole genome duplication occur in vertebrate evolution? Genom. Biol. 2018, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, Y.; Gu, J. Age distribution of human gene families shows significant roles of both large-and small-scale duplications in vertebrate evolution. Nat. Genet. 2002, 31, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Simakov, O. Deeply conserved synteny resolves early events in vertebrate evolution. Nat. Ecol. Evol. 2020, 4, 820–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.P.; Arora, J.; Isambert, H. Identification of ohnolog genes originating from whole genome duplication in early vertebrates, based on synteny comparison across multiple genomes. PLoS Comput. Biol. 2015, 11, e1004394. [Google Scholar] [CrossRef]
- Root, Z.D. Lamprey lecticans link new vertebrate genes to the origin and elaboration of vertebrate tissues. Dev. Biol. 2021, 476, 282–293. [Google Scholar] [CrossRef]
- Square, T. The origin and diversification of the developmental mechanisms that pattern the vertebrate head skeleton. Dev. Biol. 2017, 427, 219–229. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Crump, J.G. New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev. Biol. 2012, 371, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Yao, T. Development of lamprey mucocartilage and its dorsal–ventral patterning by endothelin signaling, with insight into vertebrate jaw evolution. J. Exp. Zool. Part B Mol. Dev. Evol. 2011, 316, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Cerny, R. Evidence for the prepattern/cooption model of vertebrate jaw evolution. Proc. Nat. Acad. Sci. USA 2010, 107, 17262–17267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.T. sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development. Development 2000, 127, 3815–3828. [Google Scholar] [CrossRef]
- Janvier, P. The dawn of the vertebrates: Characters versus common ascent in the rise of current vertebrate phylogenies. Palaeontology 1996, 39, 259–287. [Google Scholar]
- Donoghue, P.C.; Forey, P.L.; Aldridge, R.J. Conodont affinity and chordate phylogeny. Biol. Rev. 2000, 75, 191–251. [Google Scholar] [CrossRef] [PubMed]
- Stock, D.W.; Whitt, G.S. Evidence from 18S ribosomal RNA sequences that lampreys and hagfishes form a natural group. Science 1992, 257, 787–789. [Google Scholar] [CrossRef]
- Heimberg, A.M. microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc. Nat. Acad. Sci. USA 2010, 107, 19379–19383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuraku, S. Monophyly of lampreys and hagfishes supported by nuclear DNA–coded genes. J. Mol. Evol. 1999, 49, 729–735. [Google Scholar] [CrossRef]
- Mallatt, J.; Sullivan, J. 28S and 18S rDNA sequences support the monophyly of lampreys and hagfishes. Mol. Biol. Evol. 1998, 15, 1706–1718. [Google Scholar] [CrossRef] [Green Version]
- Yalden, D.W. Feeding mechanisms as evidence for cyclostome monophyly. Zool. J. Linnean Soc. 1985, 84, 291–300. [Google Scholar] [CrossRef]
- Miyashita, T. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological–molecular conflict in early vertebrate phylogeny. Proc. Nat. Acad. Sci. USA 2019, 116, 2146–2151. [Google Scholar]
- Kuraku, S.; Kuratani, S. Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zool. Sci. 2006, 23, 1053–1064. [Google Scholar] [CrossRef] [Green Version]
- Gess, R.W.; Coates, M.I.; Rubidge, B.S. A lamprey from the Devonian period of South Africa. Nature 2006, 443, 981–984. [Google Scholar] [CrossRef]
- Miyashita, T. Non-ammocoete larvae of Palaeozoic stem lampreys. Nature 2021, 591, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Bardack, D. First fossil hagfish (Myxinoidea): A record from the Pennsylvanian of Illinois. Science 1991, 254, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T. A Paleozoic stem hagfish Myxinikela siroka—revised anatomy and implications for evolution of the living jawless vertebrate lineages. Can. J. Zool. 2020, 98, 850–865. [Google Scholar] [CrossRef]
- Kuratani, S.; Oisi, Y.; Ota, K.G. Evolution of the vertebrate cranium: Viewed from hagfish developmental studies. Zool. Sci. 2016, 33, 229–238. [Google Scholar] [CrossRef]
- Oisi, Y. Development of the chondrocranium in hagfishes, with special reference to the early evolution of vertebrates. Zool. Sci. 2013, 30, 944–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuratani, S. Developmental and evolutionary significance of the mandibular arch and prechordal/premandibular cranium in vertebrates: Revising the heterotopy scenario of gnathostome jaw evolution. J. Anatom. 2013, 222, 41–55. [Google Scholar] [CrossRef]
- Ota, K.G. Late development of hagfish vertebral elements. J. Exp. Zool. Part B Mol. Dev. Evol. 2013, 320, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Ota, K.G. The origin of developmental mechanisms underlying vertebral elements: Implications from hagfish evo-devo. Zoology 2014, 117, 77–80. [Google Scholar] [CrossRef]
- Ota, K.G. Identification of vertebra-like elements and their possible differentiation from sclerotomes in the hagfish. Nat. Commun. 2011, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mallatt, J. The suspension feeding mechanism of the larval lamprey Petromyzon marinus. J. Zool. 1981, 194, 103–142. [Google Scholar] [CrossRef]
- Mallatt, J. Pumping rates and particle retention efficiencies of the larval lamprey, an unusual suspension feeder. Biol. Bull. 1982, 163, 197–210. [Google Scholar] [CrossRef]
- Rovainen, C.M. Feeding and breathing in lampreys. Brain Behav. Evol. 1996, 48, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Wright, G.M.; Youson, J. Transformation of mucocartilage to a definitive cartilage during metamorphosis in the sea lamprey, Petromyzon marinus. J. Morphol. 1987, 194, 1–21. [Google Scholar] [CrossRef]
- Müller, J. Vergleichende Anatomie der Myxinoiden; Königlichen Akademie der Wissenschaften: Berlin, Germany, 1834. [Google Scholar]
- Parker, W.K. X. On the Skeleton of the Marsipohranch Fishes.—Part II. Petromyzon; Philosophical Transactions of the Royal Society of London: London, UK, 1883; Volume 174, pp. 411–457. [Google Scholar]
- Parker, W.K. On the Skeleton of the Marsipobranch Fishes. Part I. The Myxinoids (Myxine, and Bdellostoma); Philosophical Transactions of the Royal Society of London: London, UK, 1883; Volume 174, pp. 373–409. [Google Scholar]
- Cole, F.J. XXX.—A Monograph on the General Morphology of the Myxinoid Fishes, Based on a Study of Myxine. Part I. The Anatomy of the Skeleton; Earth and Environmental Science Transactions of The Royal Society of Edinburgh: Edinburgh, UK, 1906; Volume 41, pp. 749–788. [Google Scholar]
- Schaffer, J. Bemerkungen über die Histologie und Histogenese des Knorpels der Cyclostomen. Archiv. Mikroskopische Anatomie 1897, 50, 170–188. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A. Anatomie und Entwickelungsgeschichte von Petromyzon und Ammocoetes; Beitrage zur vergleichenden. Anatomie und Entwickelungsgeschichte der Wirbeltiere; Reimer Publishing: Berlin, Germany, 1879; pp. 85–92. [Google Scholar]
- Johnels, A.G. On the development and morphology of the skeleton of the head of Petromyzon. Acta Zool. 1948, 29, 139–279. [Google Scholar] [CrossRef]
- Miyashita, T. Comparative Analysis of the Anatomy of the Myxinoidea and the Ancestry of Early Vertebrate Lineages. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2012. [Google Scholar]
- Huxley, T.H. The nature of the craniofacial apparatus of Petromyzon. J. Anatomy Physiol. 1876, 10, 412. [Google Scholar]
- Wright, G.M.; Keeley, F.; Youson, J. Lamprin: A new vertebrate protein comprising the major structural protein of adult lamprey cartilage. Experientia 1983, 39, 495–497. [Google Scholar] [CrossRef]
- Benjamin, M.; Ralphs, J. Biology of fibrocartilage cells. Int. Rev. Cytol. 2004, 233, 1–46. [Google Scholar]
- Eames, B.F.; Medeiros, D.M.; Adameyko, I. On the Evolution of Skeletal Cells befire Abd after Neural Crest Evolving Neural Crest Cells; CRC Press: Boca Raton, NJ, USA, 2020. [Google Scholar]
- Zhang, P.; Jimenez, S.A.; Stokes, D.G. Regulation of human COL9A1 gene expression: Activation of the proximal promoter region by SOX9. J. Biol. Chem. 2003, 278, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Bridgewater, L.C. Adjacent DNA sequences modulate Sox9 transcriptional activation at paired Sox sites in three chondrocyte-specific enhancer elements. Nucl. Acids Res. 2003, 31, 1541–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, H. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojo, H.; Ohba, S. Insights into gene regulatory networks in chondrocytes. Int. J. Mol. Sci. 2019, 20, 6324. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Rossi, P.; De Crombrugghe, B. Transcriptional control of the mouse alpha 2 (I) collagen gene: Functional deletion analysis of the promoter and evidence for cell-specific expression. Mol. Cell. Biol. 1986, 6, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Thielen, N.G.; van der Kraan, P.M.; van Caam, A.P. TGFβ/BMP signaling pathway in cartilage homeostasis. Cells 2019, 8, 969. [Google Scholar]
- Hatakeyama, Y.; Tuan, R.S.; Shum, L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J. Cell. Biochem. 2004, 91, 1204–1217. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006, 2, e216. [Google Scholar] [CrossRef] [Green Version]
- Ray, A. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development 2015, 142, 1169–1179. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Li, T.F. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J. Bone Mineral Res. 2006, 21, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Lim, J. BMP–Smad4 signaling is required for precartilaginous mesenchymal condensation independent of Sox9 in the mouse. Developmental Biol. 2015, 400, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Dalcq, J. RUNX3, EGR1 and SOX9B form a regulatory cascade required to modulate BMP-signaling during cranial cartilage development in zebrafish. PLoS ONE 2012, 7, e50140. [Google Scholar] [CrossRef] [Green Version]
- Barlow, A.J. Expression of chick Barx-1 and its differential regulation by FGF-8 and BMP signaling in the maxillary primordia. Dev. Dynam. Off. Publ. Am. Assoc. Anatom. 1999, 214, 291–302. [Google Scholar]
- Tan, Z. Synergistic co-regulation and competition by a SOX9-GLI-FOXA phasic transcriptional network coordinate chondrocyte differentiation transitions. PLoS Genet. 2018, 14, e1007346. [Google Scholar] [CrossRef]
- Wuelling, M. Trps1, a regulator of chondrocyte proliferation and differentiation, interacts with the activator form of Gli3. Dev. Biol. 2009, 328, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Muraglia, A.; Cancedda, R.; Quarto, R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J. Cell Sci. 2000, 113, 1161–1166. [Google Scholar] [CrossRef]
- Tamamura, Y. Developmental regulation of Wnt/β-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J. Biol. Chem. 2005, 280, 19185–19195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, H. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 2004, 18, 1072–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.-H.; Kim, S.J.; Kin, S.H.; Oh, C.D.; Hwang, S.G.; Chun, C.H.; Oh, S.H.; Seong, J.K.; Huh, T.L.; Chun, J.S. Regulation of the chondrocyte phenotype by β-catenin. J. Dev. 2002, 129, 5541–5550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eames, B.F.; De La Fuente, L.; Helms, J.A. Molecular ontogeny of the skeleton. Birth Defects Res. Part C Embryo Today Rev. 2003, 69, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.S.; Koyama, E.; Pacifici, M. Articular cartilage: Structural and developmental intricacies and questions. Curr. Osteoporosis Rep. 2015, 13, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Grogan, S.P. Zone-specific gene expression patterns in articular cartilage. Arthritis Rheumat. 2013, 65, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnoli, A. TGF-β signaling is essential for joint morphogenesis. J. Cell Biol. 2007, 177, 1105–1117. [Google Scholar] [CrossRef] [Green Version]
- Longobardi, L.; Li, T.; Myers, T.J.; O’Rear, L.; Ozkan, H.; Li, Y.; Contaldo, C.; Spagnoli, A. TGF-β Type II Receptor/MCP-5 Axis: At the Crossroad between Joint and Growth Plate Development. Dev. Cell 2012, 23, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Longobardi, L.; Myers, T.J.; Temple, J.D.; Chandler, R.L.; Özkan, H.; Contaldo, C.; Spagnoli, A. Joint TGF-β Type II Receptor-Expressing Cells: Ontogeny and Characterization as Joint Progenitors. Stem Cells Dev. 2013, 22, 1342–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, H.C.; Hodges, P.T.; Aguilera, X.M.; Missana, L.; Moylan, P.E. Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J. Histochem. Cytochem. 2000, 48, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Gelse, K.; Ekici, A.; Cipa, F.; Swoboda, B.; Carl, H.; Olk, A.; Hennig, F.; Klinger, P. Molecular differentiation between osteophytic and articular cartilage—Clues for a transient and permanent chondrocyte phenotype. Osteoarthr. Cartil. 2012, 20, 162–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leijten, J.C.H. Gremlin 1, frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheumat. 2012, 64, 3302–3312. [Google Scholar] [CrossRef] [Green Version]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Maenohara, Y.; Chijimatsu, R.; Tachibana, N.; Uehara, K.; Xuan, F.; Mori, D.; Murahashi, Y.; Nakamoto, H.; Oichi, T.; Chang, S.H.; et al. Lubricin Contributes to Homeostasis of Articular Cartilage by Modulating Differentiation of Superficial Zone Cells. J. Bone Miner. Res. 2020, 36, 792–802. [Google Scholar] [CrossRef]
- Flowers, S.; Zieba, A.; Örnros, J.; Jin, C.; Rolfson, O.; Björkman, L.I.; Eisler, T.; Kalamajski, S.; Kamali-Moghaddam, M.; Karlsson, N.G. Lubricin binds cartilage proteins, cartilage oligomeric matrix protein, fibronectin and collagen II at the cartilage surface. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Jones, A.R.; Gleghorn, J.; Hughes, C.; Fitz, L.J.; Zollner, R.; Wainwright, S.D.; Caterson, B.; Morris, E.A.; Bonassar, L.J.; Flannery, C.R. Binding and localization of recombinant lubricin to articular cartilage surfaces. J. Orthop. Res. 2007, 25, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Askary, A.; Smeeton, J.; Paul, S.; Schindler, S.; Braasch, I.; Ellis, N.A.; Postlethwait, J.; Miller, C.T.; Crump, J.G. Ancient origin of lubricated joints in bony vertebrates. eLife 2016, 5, e16415. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Q.; Nagano, K.; Moriishi, T.; Miyazaki, T.; Komori, H.; Ito, K.; Von Der Mark, K.; Sakane, C.; Kaneko, H.; et al. Runx2 is essential for the transdifferentiation of chondrocytes into osteoblasts. PLoS Genet. 2020, 16, e1009169. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Yue, S.; Lee, A.; Kikani, B.; Temnycky, A.; Barnes, K.M.; Baron, J. Persistent Sox9 expression in hypertrophic chondrocytes suppresses transdifferentiation into osteoblasts. Bone 2019, 125, 169–177. [Google Scholar] [CrossRef]
- Aghajanian, P.; Mohan, S. The art of building bone: Emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Picos, P.; Eames, B.F. On the evolutionary relationship between chondrocytes and osteoblasts. Front. Genet. 2015, 6, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, G.M.; Keeley, F.W.; Youson, J.H.; Babineau, D.L. Cartilage in the atlantic hagfish, Myxine glutinosa. Am. J. Anat. 1984, 169, 407–424. [Google Scholar] [CrossRef]
- Wright, G.M.; Youson, J.H. Ultrastructure of cartilage from young adult sea lamprey, Petromyzon marinus L: A new type of vertebrate cartilage. Am. J. Anat. 1983, 167, 59–70. [Google Scholar] [CrossRef]
- Debiais-Thibaud, M.; Simion, P.; Ventéo, S.; Muñoz, D.; Marcellini, S.; Mazan, S.; Haitina, T. Skeletal Mineralization in Association with Type X Collagen Expression Is an Ancestral Feature for Jawed Vertebrates. Mol. Biol. Evol. 2019, 36, 2265–2276. [Google Scholar] [CrossRef] [Green Version]
- Seidel, R.; Blumer, M.; Chaumel, J.; Amini, S.; Dean, M.N. Endoskeletal mineralization in chimaera and a comparative guide to tessellated cartilage in chondrichthyan fishes (sharks, rays and chimaera). J. R. Soc. Interface 2020, 17, 20200474. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M. The cranial cartilages of teleosts and their classification. J. Anat. 1990, 169, 153–172. [Google Scholar]
- Enochson, L.; Stenberg, J.; Brittberg, M.; Lindahl, A. GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthr. Cartil. 2014, 22, 566–577. [Google Scholar] [CrossRef] [Green Version]
- Inada, M.; Wang, Y.; Byrne, M.H.; Rahman, M.U.; Miyaura, C.; López-Otín, C.; Krane, S.M. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 2004, 101, 17192–17197. [Google Scholar] [CrossRef] [Green Version]
- Fosang, A.; Last, K.; Knauper, V.; Murphy, G.; Neame, P.J. Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett. 1996, 380, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Flannery, C.R.; Little, C.; Hughes, C.; Caterson, B. Expression of ADAMTS Homologues in Articular Cartilage. Biochem. Biophys. Res. Commun. 1999, 260, 318–322. [Google Scholar] [CrossRef]
- Collins-Racie, L.A.; Flannery, C.R.; Zeng, W.; Corcoran, C.; Annis-Freeman, B.; Agostino, M.J.; Arai, M.; DiBlasio-Smith, E.; Dorner, A.J.; Georgiadis, K.E.; et al. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol. 2004, 23, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Grogan, E.; Lund, R.; Greenfest-Allen, E. The Origin and Relationships of Early Chondrichthyans. Biol. Sharks Relat. 2012, 2, 3–29. [Google Scholar] [CrossRef]
- Keith, D.A.; Paz, A.; Gallop, P.M.; Glimcher, M.J. Histologic and biochemical identification and characterization of an elastin in cartilage. J. Histochem. Cytochem. 1977, 25, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Wu, J.-P.; Chen, H.H.; Kirk, T.; Xu, J. Elastin fibers display a versatile microfibril network in articular cartilage depending on the mechanical microenvironments. J. Orthop. Res. 2013, 31, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Field, J.; Rodger, G.; Hunter, J.; Serafini-Fracassini, A.; Spina, M. Isolation of elastin from bovine auricular cartilage. Arch. Biochem. Biophys. 1978, 191, 705–713. [Google Scholar] [CrossRef]
- Hellingman, C.A.; Verwiel, E.T.P.; Slagt, I.; Koevoet, W.; Poublon, R.M.L.; Nolst-Trenité, G.J.; De Jong, R.J.B.; Jahr, H.; Van Osch, G.J.V.M. Differences in Cartilage-Forming Capacity of Expanded Human Chondrocytes from Ear and Nose and Their Gene Expression Profiles. Cell Transplant. 2011, 20, 925–940. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Wang, W.; Liu, Z.; Hua, X.; Fu, S.; Dong, F.; Li, A.; Liu, Z.; Wang, P.; Dai, L.; et al. Fibrochondrogenic differentiation potential of tendon-derived stem/progenitor cells from human patellar tendon. J. Orthop. Transl. 2019, 22, 101–108. [Google Scholar] [CrossRef]
- Ruscitto, A.; Scarpa, V.; Morel, M.; Pylawka, S.; Shawber, C.; Embree, M. Notch Regulates Fibrocartilage Stem Cell Fate and Is Upregulated in Inflammatory TMJ Arthritis. J. Dent. Res. 2020, 99, 1174–1181. [Google Scholar] [CrossRef]
- Dyment, N.A.; Breidenbach, A.P.; Schwartz, A.G.; Russell, R.P.; Aschbacher-Smith, L.; Liu, H.; Hagiwara, Y.; Jiang, R.; Thomopoulos, S.; Butler, D.L.; et al. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev. Biol. 2015, 405, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, M.; Ralphs, J. Extracellular matrix of connective tissues in the heads of teleosts. J. Anat. 1991, 179, 137–148. [Google Scholar]
- Benjamin, M. Mucochondroid (mucous connective) tissues in the heads of teleosts. Anat. Embryol. 1988, 178, 461–474. [Google Scholar] [CrossRef]
- Duncan, W.P.; Da Costa, O.T.F.; Sakuragui, M.M.; Fernandes, M.N. Functional Morphology of the Gill in Amazonian Freshwater Stingrays (Chondrichthyes: Potamotrygonidae): Implications for Adaptation to Freshwater. Physiol. Biochem. Zool. 2010, 83, 19–32. [Google Scholar] [CrossRef]
- Egerbacher, M.; Böck, P. Myxoid Tissue: Its Morphology, Histochemistry, and Relationship with Other Supporting Tissues. Arch. Histol. Cytol. 1997, 60, 121–131. [Google Scholar] [CrossRef]
- Robson, P.; Wright, G.; Sitarz, E.; Maiti, A.; Rawat, M.; Youson, J.; Keeley, F. Characterization of lamprin, an unusual matrix protein from lamprey cartilage. Implications for evolution, structure, and assembly of elastin and other fibrillar proteins. J. Biol. Chem. 1993, 268, 1440–1447. [Google Scholar] [CrossRef]
- Bochicchio, B.; Pepe, A.; Tamburro, A. On (GGLGY) synthetic repeating sequences of lamprin and analogous sequences. Matrix Biol. 2001, 20, 243–250. [Google Scholar] [CrossRef]
- McBurney, K.; Keeley, F.; Kibenge, F.; Wright, G. Spatial and temporal distribution of lamprin mRNA during chondrogenesis of trabecular cartilage in the sea lamprey. Anat. Embryol. 1996, 193, 419–426. [Google Scholar] [CrossRef]
- Yokoyama, H.; Morino, Y.; Wada, H. Identification of a unique lamprey gene with tandemly repeated sequences and pharyngeal chondrocyte-specific expression. Gene 2019, 701, 9–14. [Google Scholar] [CrossRef]
- Robson, P.; Wright, G.M.; Keeley, F.W. Distinct non-collagen based cartilages comprising the endoskeleton of the Atlantic hagfish, Myxine glutinosa. Anat. Embryol. 2000, 202, 281–290. [Google Scholar] [CrossRef]
- Zhang, G.; Cohn, M.J. Hagfish and lancelet fibrillar collagens reveal that type II collagen-based cartilage evolved in stem vertebrates. Proc. Natl. Acad. Sci. USA 2006, 103, 16829–16833. [Google Scholar] [CrossRef] [Green Version]
- Ota, K.G.; Kuratani, S. Expression pattern of two collagen type 2 α1 genes in the Japanese inshore hagfish (Eptatretus burgeri) with special reference to the evolution of cartilaginous tissue. J. Exp. Zool. Part B Mol. Dev. Evol. 2009, 9999B, 157–165. [Google Scholar] [CrossRef]
- Lakiza, O.; Miller, S.; Bunce, A.; Lee, E.M.-J.; McCauley, D.W. SoxE gene duplication and development of the lamprey branchial skeleton: Insights into development and evolution of the neural crest. Dev. Biol. 2011, 359, 149–161. [Google Scholar] [CrossRef] [Green Version]
- McCauley, D.W.; Bronner-Fraser, M. Importance of SoxE in neural crest development and the evolution of the pharynx. Nature 2006, 441, 750–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Miyamoto, M.M.; Cohn, M.J. Lamprey type II collagen and Sox9 reveal an ancient origin of the vertebrate collagenous skeleton. Proc. Natl. Acad. Sci. USA 2006, 103, 3180–3185. [Google Scholar] [CrossRef] [Green Version]
- McCauley, D.W.; Bronner-Fraser, M. Conservation and divergence of BMP2/4 genes in the lamprey: Expression and phylogenetic analysis suggest a single ancestral vertebrate gene. Evol. Dev. 2004, 6, 411–422. [Google Scholar] [CrossRef]
- Jandzik, D.; Hawkins, M.; Cattell, M.V.; Cerny, R.; Square, T.; Medeiros, D. Roles for FGF in lamprey pharyngeal pouch formation and skeletogenesis highlight ancestral functions in the vertebrate head. Development 2014, 141, 629–638. [Google Scholar] [CrossRef] [Green Version]
- Cattell, M.; Lai, S.; Cerny, R.; Medeiros, D.M. A New Mechanistic Scenario for the Origin and Evolution of Vertebrate Cartilage. PLoS ONE 2011, 6, e22474. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, K.; Yao, T.; Kobayashi, M.; Kusakabe, R.; Kuratani, S.; Wada, H. Expression of Sox and fibrillar collagen genes in lamprey larval chondrogenesis with implications for the evolution of vertebrate cartilage. J. Exp. Zool. Part B Mol. Dev. Evol. 2008, 310, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Yutaka, T. Normal Stages of Development in the Lamprey, Lampetra reissued (Dybowski). Zool. Sci. 1988, 5, 109–118. [Google Scholar]
- Xia, J.; Kang, Z.; Xue, Y.; Ding, Y.; Gao, S.; Zhang, Y.; Lv, P.; Wang, X.; Ma, D.; Wang, L. A single-cell resolution developmental atlas of hematopoietic stem and progenitor cell expansion in zebrafish. Proc. Natl. Acad. Sci. USA 2021, 118, e2015748118. [Google Scholar] [CrossRef]
- Moreno-Ayala, R.; Junker, J.P. Single-cell genomics to study developmental cell fate decisions in zebrafish. Briefings Funct. Genom. 2021. [Google Scholar] [CrossRef]
- Square, T.; Romášek, M.; Jandzik, D.; Cattell, M.V.; Klymkowsky, M.; Medeiros, D.M. CRISPR/Cas9-mediated mutagenesis in the sea lamprey, Petromyzon marinus: A powerful tool for understanding ancestral gene functions in vertebrates. Development 2015, 142, 4180–4187. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 37–51. [Google Scholar] [CrossRef] [Green Version]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef] [Green Version]
- Long, F.; Zhang, X.M.; Karp, S.; Yang, Y.; McMahon, A.P. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 2001, 128, 5099–5108. [Google Scholar] [CrossRef]
- Kim, E.-J.; Cho, S.-W.; Shin, J.-O.; Lee, M.-J.; Kim, K.-S.; Jung, H.-S. Ihh and Runx2/Runx3 Signaling Interact to Coordinate Early Chondrogenesis: A Mouse Model. PLoS ONE 2013, 8, e55296. [Google Scholar] [CrossRef] [Green Version]
- Keller, B.; Yang, T.; Chen, Y.; Munivez, E.; Bertin, T.; Zabel, B.; Lee, B. Interaction of TGFβ and BMP Signaling Pathways during Chondrogenesis. PLoS ONE 2011, 6, e16421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, K.L. Expression of patched, prdm1 and engrailed in the lamprey somite reveals conserved responses to Hedgehog signaling. Evol. Dev. 2009, 11, 27–40. [Google Scholar] [CrossRef]
- Kano, S.; Xiao, J.-H.; Osório, J.; Ekker, M.; Hadzhiev, Y.; Müller, F.; Casane, D.; Magdelenat, G.; Rétaux, S. Two Lamprey Hedgehog Genes Share Non-Coding Regulatory Sequences and Expression Patterns with Gnathostome Hedgehogs. PLoS ONE 2010, 5, e13332. [Google Scholar] [CrossRef] [Green Version]
- Sugahara, F.; Aota, S.-I.; Kuraku, S.; Murakami, Y.; Takio-Ogawa, Y.; Hirano, S.; Kuratani, S. Involvement of Hedgehog and FGF signalling in the lamprey telencephalon: Evolution of regionalization and dorsoventral patterning of the vertebrate forebrain. Development 2011, 138, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Hernández, M.E.; Bustamante, M.; Galván-Hernández, C.I.; Chimal-Monroy, J. Irx1 and Irx2 Are Coordinately Expressed and Regulated by Retinoic Acid, TGFβ and FGF Signaling during Chick Hindlimb Development. PLoS ONE 2013, 8, e58549. [Google Scholar] [CrossRef] [Green Version]
- Zülch, A.; Becker, M.-B.; Gruss, P. Expression pattern of Irx1 and Irx2 during mouse digit development. Mech. Dev. 2001, 106, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Askary, A.; Mork, L.; Paul, S.; He, X.; Izuhara, A.K.; Gopalakrishnan, S.; Ichida, J.K.; McMahon, A.P.; Dabizljevic, S.; Dale, R.; et al. Iroquois Proteins Promote Skeletal Joint Formation by Maintaining Chondrocytes in an Immature State. Dev. Cell 2015, 35, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Square, T.; Jandzik, D.; Cattell, M.; Coe, A.; Doherty, J.; Medeiros, D.M. A gene expression map of the larval Xenopus laevis head reveals developmental changes underlying the evolution of new skeletal elements. Dev. Biol. 2015, 397, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Nichols, J.T.; Pan, L.; Moens, C.; Kimmel, C.B. barx1 represses joints and promotes cartilage in the craniofacial skeleton. Development 2013, 140, 2765–2775. [Google Scholar] [CrossRef] [Green Version]
- Michikami, I.; Fukushi, T.; Honma, S.; Yoshioka, S.; Itoh, S.; Muragaki, Y.; Kurisu, K.; Ooshima, T.; Wakisaka, S.; Abe, M. Trps1 is necessary for normal temporomandibular joint development. Cell Tissue Res. 2012, 348, 131–140. [Google Scholar] [CrossRef]
- Itoh, S.; Kanno, S.; Gai, Z.; Suemoto, H.; Kawakatsu, M.; Tanishima, H.; Morimoto, Y.; Nishioka, K.; Hatamura, I.; Yoshida, M. Trps1 plays a pivotal role downstream of Gdf5 signaling in promoting chondrogenesis and apoptosis of ATDC5 cells. Genes Cells 2008, 13, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Li, Y.; Gui, C.; Ma, Y.; Ge, Y.; Dai, H.; Zhang, K.; Du, J.; Guo, Y.; Jiang, Y.; et al. Fibronectin Enhances Cartilage Repair by Activating Progenitor Cells Through Integrin α5β1 Receptor. Tissue Eng. Part A 2018, 24, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Burton-Wurster, N.; Lust, G. Fibronectin in cartilage. In Fibronectin in Health and Disease; CRC Press: Boca Raton, FL, USA, 2018; pp. 243–254. [Google Scholar]
- Sun, Y.; Wang, T.; Toh, W.S.; Pei, M. The role of laminins in cartilaginous tissues: From development to regeneration. Eur. Cells Mater. 2017, 34, 40–54. [Google Scholar] [CrossRef]
- Dürr, J.; Lammi, P.; Goodman, S.L.; Aigner, T.; Von Der Mark, K. Identification and Immunolocalization of Laminin in Cartilage. Exp. Cell Res. 1996, 222, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Quintarelli, G.; Starcher, B.C.; Vocaturo, A.; Di Gianfilippo, F.; Gotte, L.; Mecham, R.P. Fibrogenesis and Biosynthesis of Elastin in Cartilage. Connect. Tissue Res. 1979, 7, 1–19. [Google Scholar] [CrossRef]
- Silva Jr, W.A. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells 2003, 21, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.H.; Kim, B.; Choi, I.; Kim, H.; Choi, H.S.; Suh, K.; Bae, Y.C.; Jung, J.S. Characterization and Expression Analysis of Mesenchymal Stem Cells from Human Bone Marrow and Adipose Tissue. Cell. Physiol. Biochem. 2004, 14, 311–324. [Google Scholar] [CrossRef]
- Liu, T.M.; Martina, M.; Hutmacher, D.W.; Hui, J.H.P.; Lee, E.H.; Lim, B. Identification of common pathways mediating differentiation of bone marrow-and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 2007, 25, 750–760. [Google Scholar] [CrossRef]
- Covas, D.T.; Panepucci, R.; Fontes, A.M.; Silva, W.A.; Orellana, M.D.; Freitas, M.C.; Neder, L.; Santos, A.R.; Peres, L.C.; Jamur, M.C.; et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp. Hematol. 2008, 36, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Muneta, T.; Makino, H.; Nimura, A.; Mochizuki, T.; Ju, Y.-J.; Ezura, Y.; Umezawa, A.; Sekiya, I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J. Orthop. Res. 2009, 27, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Shenaq, D.S.; Rastegar, F.; Petkovic, D.; Zhang, B.-Q.; He, B.-C.; Chen, L.; Zuo, G.-W.; Luo, Q.; Shi, Q.; Wagner, E.R.; et al. Mesenchymal Progenitor Cells and Their Orthopedic Applications: Forging a Path towards Clinical Trials. Stem Cells Int. 2010, 2010, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipour, F.; Parham, A.; Mehrjerdi, H.K.; Dehghani, H. Equine Adipose-Derived Mesenchymal Stem Cells: Phenotype and Growth Characteristics, Gene Expression Profile and Differentiation Potentials. Cell J. 2015, 16, 456–465. [Google Scholar] [PubMed]
- Asou, Y. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. J. Orthop. Res. 2002, 20, 827–833. [Google Scholar] [CrossRef]
- Soeda, T.; Deng, J.M.; de Crombrugghe, B.; Behringer, R.R.; Nakamura, T.; Akiyama, H. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis 2010, 48, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Blitz, E.; Sharir, A.; Akiyama, H.; Zelzer, E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 2013, 140, 2680–2690. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.-L.; Ahmad, R.E.; Ahmad, T.S.; Merican, A.M.; Abbas, A.A.; Ng, W.M.; Kamarul, T. Effect of Growth Differentiation Factor 5 on the Proliferation and Tenogenic Differentiation Potential of Human Mesenchymal Stem Cells in vitro. Cells Tissues Organs 2012, 196, 325–338. [Google Scholar] [CrossRef]
- Tan, S.-L.; Ahmad, T.S.; Ng, W.-M.; Azlina, A.A.; Azhar, M.M.; Selvaratnam, L.; Kamarul, T. Identification of Pathways Mediating Growth Differentiation Factor5-Induced Tenogenic Differentiation in Human Bone Marrow Stromal Cells. PLoS ONE 2015, 10, e0140869. [Google Scholar] [CrossRef] [Green Version]
- Spaapen, F.; Akker, G.G.H.V.D.; Caron, M.M.J.; Prickaerts, P.; Rofel, C.; Dahlmans, V.E.H.; Surtel, D.A.M.; Paulis, Y.; Schweizer, F.; Welting, T.J.M.; et al. The Immediate Early Gene Product EGR1 and Polycomb Group Proteins Interact in Epigenetic Programming during Chondrogenesis. PLoS ONE 2013, 8, e58083. [Google Scholar] [CrossRef]
- Brent, A.E.; Braun, T.; Tabin, C.J. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 2005, 132, 515–528. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xu, J.; Jiang, R. Mkx -Deficient Mice Exhibit Hedgehog Signaling–Dependent Ectopic Ossification in the Achilles Tendons. J. Bone Miner. Res. 2018, 34, 557–569. [Google Scholar] [CrossRef]
- Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S.; et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 10538–10542. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Watson, S.S.; Lan, Y.; Keene, D.R.; Ovitt, C.E.; Liu, H.; Schweitzer, R.; Jiang, R. The Atypical Homeodomain Transcription Factor Mohawk Controls Tendon Morphogenesis. Mol. Cell. Biol. 2010, 30, 4797–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Chen, P.; Chen, L.; Zhou, Y.; Wang, A.; Zheng, Q.; Mitchell, C.A.; Leys, T.; Tuan, R.S.; Zheng, M.H. Reduction of mechanical loading in tendons induces heterotopic ossification and activation of the β-catenin signaling pathway. J. Orthop. Transl. 2021, 29, 42–50. [Google Scholar] [CrossRef]
- Subramanian, A.; Kanzaki, L.F.; Galloway, J.L.; Schilling, T.F. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. eLife 2018, 7, e38069. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J.; Bürgisser, G.M. Biomechanics of Tendons and Ligaments: Tissue Reconstruction and Regeneration; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Wang, Z.; Jing, Y.; Chen, D.; Ma, C.; Feng, J.Q. Tendon Cells Directly Form Bone Ridges via Multiple Cell Transdifferentiation: Tendon-Fibroblast-Bone Cells beyond Simply Connecting Bone And Muscles. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Gaspar, D.; Zeugolis, D. Macromolecular Crowding and Mechanical Stimulation for Tenogenic Phenotype Maintenance and Differentiation/Transdifferentiation. In Proceedings of the Orthopaedic Proceedings, Munich, Germany, 13–15 September 2017; The British Editorial Society of Bone & Joint Surgery: London, UK, 2018. [Google Scholar]
- de Mos, M. Intrinsic differentiation potential of adolescent human tendon tissue: An in-vitro cell differentiation study. BMC Muscul. Disord. 2007, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Eyal, S.; Rubin, S.; Krief, S.; Levin, L.; Zelzer, E. Common cellular origin and diverging developmental programs for different sesamoid bones. Development 2019, 146, dev.167452. [Google Scholar] [CrossRef] [Green Version]
- Eyal, S.; Blitz, E.; Shwartz, Y.; Akiyama, H.; Schweitzer, R.; Zelzer, E. On the development of the patella. Development 2015, 142, 1831–1839. [Google Scholar] [CrossRef] [Green Version]
- Screen, H.R.C.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon Functional Extracellular Matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Root, Z.D.; Gould, C.; Brewer, M.; Jandzik, D.; Medeiros, D.M. Comparative Approaches in Vertebrate Cartilage Histogenesis and Regulation: Insights from Lampreys and Hagfishes. Diversity 2021, 13, 435. https://doi.org/10.3390/d13090435
Root ZD, Gould C, Brewer M, Jandzik D, Medeiros DM. Comparative Approaches in Vertebrate Cartilage Histogenesis and Regulation: Insights from Lampreys and Hagfishes. Diversity. 2021; 13(9):435. https://doi.org/10.3390/d13090435
Chicago/Turabian StyleRoot, Zachary D., Claire Gould, Margaux Brewer, David Jandzik, and Daniel M. Medeiros. 2021. "Comparative Approaches in Vertebrate Cartilage Histogenesis and Regulation: Insights from Lampreys and Hagfishes" Diversity 13, no. 9: 435. https://doi.org/10.3390/d13090435
APA StyleRoot, Z. D., Gould, C., Brewer, M., Jandzik, D., & Medeiros, D. M. (2021). Comparative Approaches in Vertebrate Cartilage Histogenesis and Regulation: Insights from Lampreys and Hagfishes. Diversity, 13(9), 435. https://doi.org/10.3390/d13090435





