Seed Germination and Seedling Growth of Robinia pseudoacacia Depending on the Origin of Different Geographic Provenances
Abstract
1. Introduction
2. Materials and Methods
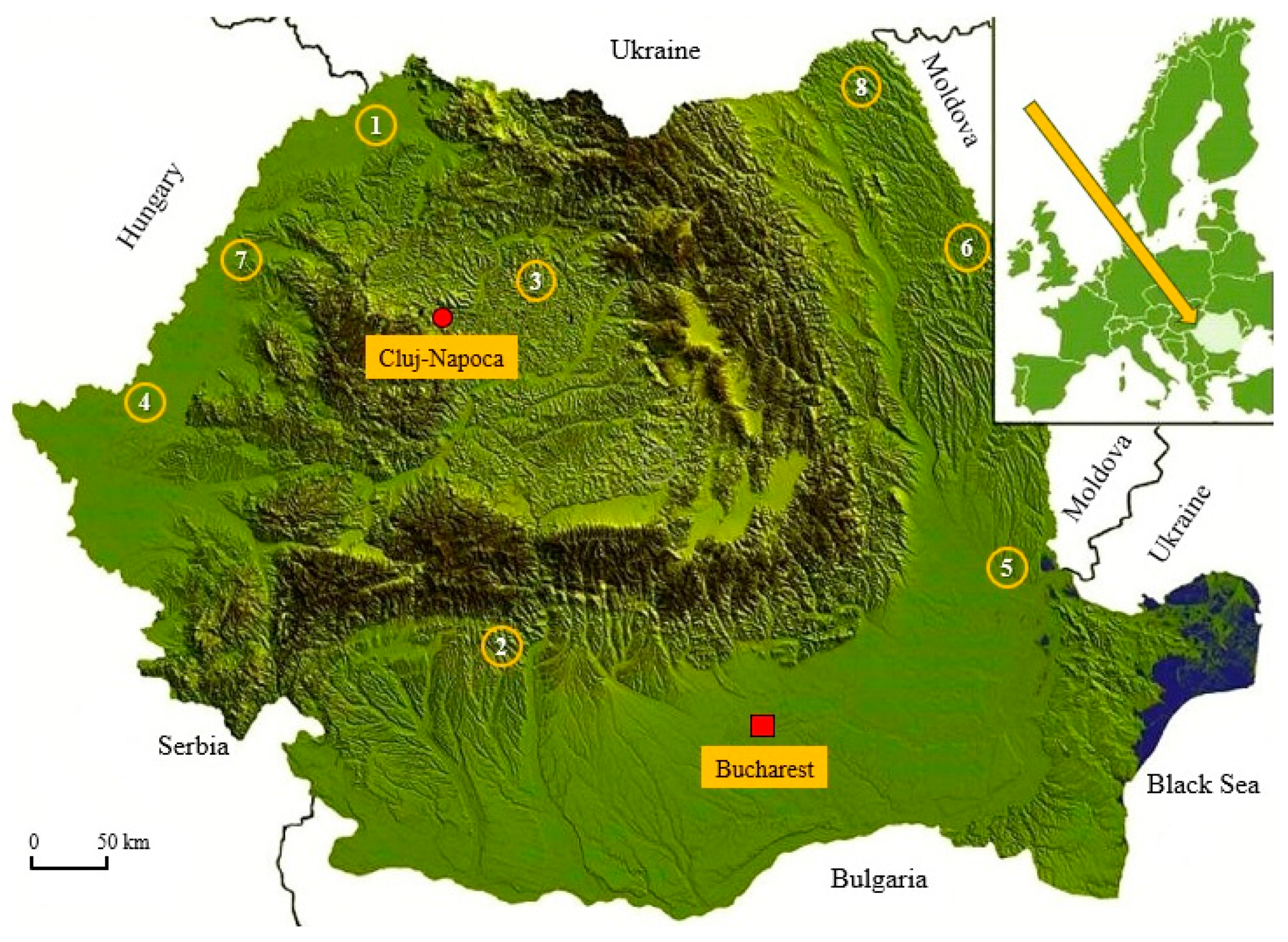
2.1. Biological Material
2.2. Experimental Procedures Performed in Order to Investigate the Germination Rate
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rédei, K.; Keserű, Z.; Rásó, J. Early evaluation of micropropagated black locust (Robinia pseudoacacia L.) clones in Hungary. For. Sci. Pract. 2013, 15, 81–84. [Google Scholar] [CrossRef]
- Jastrzębowski, S.; Ukalska, J.; Kantorowicz, W.; Klisz, M.; Wojda, T.; Sułkowska, M. Effects of thermal-time artificial scarification on the germination dynamics of black locust (Robinia pseudoacacia L.) seeds. Eur. J. For. Res. 2017, 136, 471–479. [Google Scholar] [CrossRef]
- Huntley, B. European vegetation history: Palaeovegetation maps from pollen data-13 000 yr BP to present. J. Quat. Sci. 1990, 5, 103–122. [Google Scholar] [CrossRef]
- Pepe, M.; Gratani, L.; Fabrini, G.; Varone, L. Seed germination traits of Ailanthus altissima, Phytolacca americana and Robinia pseudoacacia in response to different thermal and light requirements. Plant Species Biol. 2020, 35, 300–314. [Google Scholar] [CrossRef]
- Keresztesi, B. Black Locust: The Tree of Agriculture. Outlook Agric. 1988, 17, 77–85. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Carl, C.; Lehmann, J.R.K.; Landgraf, D.; Pretzsch, H. Robinia pseudoacacia L. in Short Rotation Coppice: Seed and Stump Shoot Reproduction as well as UAS-based Spreading Analysis. Forests 2019, 10, 235. [Google Scholar] [CrossRef]
- Lukasiewicz, M.; Kowalski, S.; Makarewicz, M. Antimicrobial an antioxidant activity of selected Polish herbhoneys. LWT 2015, 64, 547–553. [Google Scholar] [CrossRef]
- Quinkenstein, A.; Jochheim, H. Assessing the carbon sequestration potential of poplar and black locust short rotation coppices on mine reclamation sites in Eastern Germany—Model development and application. J. Environ. Manag. 2016, 168, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, V.-N.; Hernea, C.; Bakti, B.; Keserű, Z.; Antal, B.; Rédei, K. Black locust (Robinia pseudoacacia L.) as a multi-purpose tree species in Hungary and Romania: A review. J. For. Res. 2018, 29, 1449–1463. [Google Scholar] [CrossRef]
- Brus, R. Current Occurrence of Non-Native Tree Species in European Forest. In Proceedings of the Joint WG Meeting of COST Action 402 Non-Native Tree Species for European Forests Experiences, Risks and Opportunities (NNEXT), Lisbon, Portugal, 4 October 2016. [Google Scholar]
- Tateno, R.; Tokuchi, N.; Yamanaka, N.; Du, S.; Otsuki, K.; Shimamura, T.; Xue, Z.; Wang, S.; Hou, Q. Comparison of litterfall production and leaf litter decomposition between an exotic black locust plantation and an indigenous oak forest near Yan’an on the Loess Plateau, China. For. Ecol. Manag. 2007, 241, 84–90. [Google Scholar] [CrossRef]
- Grünewald, H.; Böhm, C.; Quinkenstein, A.; Grundmann, P.; Eberts, J.; Von Wühlisch, G. Robinia pseudoacacia L.: A Lesser Known Tree Species for Biomass Production. BioEnergy Res. 2009, 2, 123–133. [Google Scholar] [CrossRef]
- Balat, M. Bio-Oil Production from Pyrolysis of Black Locust (Robinia pseudoacacia) Wood. Energy Explor. Exploit. 2010, 28, 173–186. [Google Scholar] [CrossRef]
- González-García, S.; Gasol, C.M.; Moreira, M.T.; Gabarrell, X.; i Pons, J.R.; Feijoo, G. Environmental assessment of black locust (Robinia pseudoacacia L.)-based ethanol as potential transport fuel. Int. J. Life Cycle Assess. 2011, 16, 465–477. [Google Scholar] [CrossRef]
- Mally, R.; Ward, S.F.; Trombik, J.; Buszko, J.; Medzihorský, V.; Liebhold, A.M. Non-native plant drives the spatial dynamics of its herbivores: The case of black locust (Robinia pseudoacacia) in Europe. NeoBiota 2021, 69, 155–175. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological Flora of the British Isles: Robinia pseudoacacia . J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Wu, W.; Li, H.; Feng, H.; Si, B.; Chen, G.; Meng, T.; Li, Y.; Siddique, K.H. Precipitation dominates the transpiration of both the economic forest (Malus pumila) and ecological forest (Robinia pseudoacacia) on the Loess Plateau after about 15 years of water depletion in deep soil. Agric. For. Meteorol. 2020, 297, 108244. [Google Scholar] [CrossRef]
- Ussiri, D.A.N.; Lal, R.; Jacinthe, P.A. Soil Properties and Carbon Sequestration of Afforested Pastures in Reclaimed Minesoils of Ohio. Soil Sci. Soc. Am. J. 2006, 70, 1797–1806. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, X.; Cheng, J.; Yin, X. Effects of black locust (Robinia pseudoacacia) on soil properties in the loessial gully region of the Loess Plateau, China. Plant Soil 2010, 332, 207–217. [Google Scholar] [CrossRef]
- Wang, B.; Liu, G.; Xue, S. Effect of black locust (Robinia pseudoacacia) on soil chemical and microbiological properties in the eroded hilly area of China’s Loess Plateau. Environ. Earth Sci. 2011, 65, 597–607. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Chen, H.; Tang, M. Symbiosis of Arbuscular Mycorrhizal Fungi and Robinia pseudoacacia L. Improves Root Tensile Strength and Soil Aggregate Stability. PLoS ONE 2016, 11, e0153378. [Google Scholar] [CrossRef] [PubMed]
- Dalby, R. A honey of a tree: Black locust. Am. Bee J. 2004, 144, 382–384. [Google Scholar]
- Spyroglou, G.; Fotelli, M.; Nanos, N.; Radoglou, K. Assessing Black Locust Biomass Accumulation in Restoration Plantations. Forests 2021, 12, 1477. [Google Scholar] [CrossRef]
- Ciuvăț, A.L.; Abrudan, I.V.; Blujdea, V.; Marcu, C.; Dinu, C.; Enescu, C.; Nuță, I.S. Distribution and peculiarities of black locust in Romania. Rev. Silvic. Cinegetică 2013, 32, 76–85. [Google Scholar]
- Sitzia, T.; Cierjacks, A.; de Rigo, D.; Caudullo, G. Robinia pseudoacacia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 166–167. [Google Scholar]
- Nicolescu, V.-N.; Rédei, K.; Mason, W.L.; Vor, T.; Pöetzelsberger, E.; Bastien, J.-C.; Brus, R.; Benčať, T.; Đodan, M.; Cvjetkovic, B.; et al. Ecology, growth and management of black locust (Robinia pseudoacacia L.), a non-native species integrated into European forests. J. For. Res. 2020, 31, 1081–1101. [Google Scholar] [CrossRef]
- Matei, I.; Pacurar, I.; Roșca, S.; Bilașco, Ș.; Sestras, P.; Rusu, T.; Jude, E.T.; Tăut, F.D. Land Use Favourability Assessment Based on Soil Characteristics and Anthropic Pollution. Case Study Somesul Mic Valley Corridor, Romania. Agronomy 2020, 10, 1245. [Google Scholar] [CrossRef]
- Curovic, M.; Spalevic, V.; Sestras, P.; Motta, R.; Dan, C.; Garbarino, M.; Vitali, A.; Urbinati, C. Structural and ecological characteristics of mixed broadleaved old-growth forest (Biogradska Gora-Montenegro). Turk. J. Agric. For. 2020, 44, 428–438. [Google Scholar] [CrossRef]
- Kraszkiewicz, A. Productivity of Black Locust (Robinia pseudoacacia L.) Grown on a Varying Habitats in Southeastern Poland. Forests 2021, 12, 470. [Google Scholar] [CrossRef]
- Deneau, K.A. The Effects of Black Locust (Robinia pseudoacacia L.) on Understory Vegetation and Soils in a Northern Hardwood Forest. Master’s Thesis, Michigan Technological University, Houghton, MI, USA, 2013. [Google Scholar]
- Annighöfer, P.; Mölder, I.; Zerbe, S.; Kawaletz, H.; Terwei, A.; Ammer, C. Biomass functions for the two alien tree species Prunus serotina Ehrh. and Robinia pseudoacacia L. in floodplain forests of Northern Italy. Eur. J. For. Res. 2012, 131, 1619–1635. [Google Scholar] [CrossRef]
- Wei, X.; Liang, W. Multifactor relationships between stand structure and soil and water conservation functions of Robinia pseudoacacia L. in the Loess Region. PLoS ONE 2019, 14, e0219499. [Google Scholar] [CrossRef]
- Allam, A.; Borsali, A.H.; Kefifa, A.; Zouidi, M.; DA Silva, A.M.F.; Rébufa, C. Impact of water erosion on the properties of forest soils. Not. Sci. Biol. 2021, 13, 10921. [Google Scholar] [CrossRef]
- Sestras, P.; Bondrea, M.V.; Cetean, H.; Sălăgean, T.; Bilaşco, Ş.; Naș, S.; Spalevic, V.; Fountas, S.; Cîmpeanu, S.M. Ameliorative, Ecological and Landscape Roles of Făget Forest, Cluj-Napoca, Romania, and Possibilities of Avoiding Risks Based on GIS Landslide Susceptibility Map. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 46, 292–300. [Google Scholar] [CrossRef]
- Hou, G.; Bi, H.; Wei, X.; Wang, N.; Cui, Y.; Zhao, D.; Ma, X.; Wang, S. Optimal configuration of stand structures in a low-efficiency Robinia pseudoacacia forest based on a comprehensive index of soil and water conservation ecological benefits. Ecol. Indic. 2020, 114, 106308. [Google Scholar] [CrossRef]
- Ciuvat, A.L.; Abrudan, I.V.; Blujdea, V.; Dutca, I.; Nuta, I.S.; Edu, E. Biomass Equations and Carbon Content of Young Black Locust (Robinia pseudoacacia L.) Trees from Plantations and Coppices on Sandy Soils in South-Western Romanian Plain. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 590–592. [Google Scholar] [CrossRef][Green Version]
- Olson, D.F.J.; Karrfalt, R.P. The Woody Plant Seed Manual; Robinia, L.L., Bonner, F., Karrfalt, R.P., Eds.; Agriculture Handbook; USDA Forest Service: Washington, DC, USA, 2008; Volume 727. [Google Scholar]
- DeGomez, T.; Wagner, M.R. Culture and Use of Black Locust. HortTechnology 2001, 11, 279–288. [Google Scholar] [CrossRef]
- Rédei, K.; Csiha, I.; Keserű, Z.; Gál, J. Influence of Regeneration Method on the Yield and Stem Quality of Black Locust (Robinia pseudoacacia L.) Stands: A Case Study. Acta Silv. Lignaria Hung. 2012, 8, 103–112. [Google Scholar] [CrossRef]
- Roman, A.M.; Morar, I.M.; Truța, A.M.; Dan, C.; Sestraș, A.F.; Holonec, L.; Ioras, F.; Sestras, R.E. Trees, seeds and seedlings analyses in the process of obtaining a quality planting material for black locust (Robinia pseudoacacia L.). Not. Sci. Biol. 2020, 12, 940–958. [Google Scholar] [CrossRef]
- Thapliyal, M.; Kaliyathan, N.N.; Rathore, K. Seed germination response of Indian wild pear (Pyrus pashia) to gibberellic acid treatment and cold storage. Not. Sci. Biol. 2021, 13, 11044. [Google Scholar] [CrossRef]
- Lewak, S. Metabolic control of embryonic dormancy in apple seed: Seven decades of research. Acta Physiol. Plant. 2011, 33, 1–24. [Google Scholar] [CrossRef]
- Borkowska, L. Patterns of seedling recruitment in experimental gaps on mosaic vegetation of abandoned meadows. Acta Soc. Bot. Pol. 2011, 73, 343–350. [Google Scholar] [CrossRef][Green Version]
- Basbag, M.; Aydin, A.; Ayzit, D. The Effect of Different Temperatures and Durations on the Dormancy Breaking of Black Locust (Robinia pseudoacacia L.) and Honey Locust (Gleditsia triacanthos L.) Seeds. Not. Sci. Biol. 2010, 2, 125–128. [Google Scholar] [CrossRef]
- Mondoni, A.; Tazzari, E.; Zubani, L.; Orsenigo, S.; Rossi, G. Percussion as an effective seed treatment for herbaceous legumes (Fabaceae): Implications for habitat restoration and agriculture. Seed Sci. Technol. 2013, 41, 175–187. [Google Scholar] [CrossRef]
- Zencirkiran, M.; Tümsavaş, Z.; Ünal, H. The Effects of Different Acid Treatment and Stratification Duration on Germination of Cercis siliquastrum L. Seeds. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 159–163. [Google Scholar] [CrossRef]
- Mirzaei, M.; Moghadam, A.R.L.; Ardebili, Z.O. The induction of seed germination using sulfuric acid, gibberellic acid and hot water in Robinia pseudoacacia L. Int. Res. J. Appl. Basic Sci. 2013, 4, 96–98. [Google Scholar]
- Pârnuţă, G.S.E.; Budeanu, M.; Scărlătescu, V.; Marica, F.M.; Lalu, I.; Curtu, A.L. Catalogul Naţional al Resurselor Genetice 462 Forestiere [National Catalogue of Forest Genetic Resources]; Editura Silvică: Bucharest, Romania, 2011. (In Romanian) [Google Scholar]
- De Micco, V.; Aronne, G. Morpho-Anatomical Traits for Plant Adaptation to Drought. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 37–61. [Google Scholar]
- Belokopytova, L.; Zhirnova, D.; Kostyakova, T.; Babushkina, E. Dynamics of moisture regime and its reconstruction from a tree-ring width chronology of Pinus sylvestris in the downstream basin of the Selenga River, Russia. J. Arid. Land 2018, 10, 877–891. [Google Scholar] [CrossRef]
- Yang, B.; Peng, C.; Harrison, S.P.; Wei, H.; Wang, H.; Zhu, Q.; Wang, M. Allocation Mechanisms of Non-Structural Carbohydrates of Robinia pseudoacacia L. Seedlings in Response to Drought and Waterlogging. Forests 2018, 9, 754. [Google Scholar] [CrossRef]
- Giuliani, C.; Lazzaro, L.; Calamassi, R.; Fico, G.; Foggi, B.; Lippi, M.M. Induced water stress affects seed germination response and root anatomy in Robinia pseudoacacia (Fabaceae). Trees 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Sestras, A.F. Biostatistica si Tehnica Experimentala Forestiera: Manual Didactic; Editura Academic Press: Cluj-Napoca, Romania, 2018. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4–9. [Google Scholar]
- Sestras, A.F. Modele Statistice Aplicate în Cercetarea Horticola; Editura Risoprint: Cluj-Napoca, Romania, 2019. [Google Scholar]
- Rajora, O.P.; Mosseler, A. Challenges and opportunities for conservation of forest genetic resources. Euphytica 2001, 118, 197–212. [Google Scholar] [CrossRef]
- Tozer, M.G.; Ooi, M. Humidity-regulated dormancy onset in the Fabaceae: A conceptual model and its ecological implications for the Australian wattle Acacia saligna. Ann. Bot. 2014, 114, 579–590. [Google Scholar] [CrossRef]
- Safdar, M.E.; Wang, X.; Abbas, M.; Ozaslan, C.; Asif, M.; Adnan, M.; Zuan, A.T.K.; Wang, W.; Gasparovic, K.; Nasif, O.; et al. The impact of aqueous and N-hexane extracts of three Fabaceae species on seed germination and seedling growth of some broadleaved weed species. PLoS ONE 2021, 16, e0258920. [Google Scholar] [CrossRef] [PubMed]
- Kheloufi, A.; Mansouri, L.; Aziz, N.; Sahnoune, M.; Boukemiche, S.; Ababsa, B. Breaking seed coat dormancy of six tree species. Reforesta 2018, 5, 4–14. [Google Scholar] [CrossRef]
- Pedrol, N.; Puig, C.G.; López-Nogueira, A.; Pardo-Muras, M.; González, L.; Alonso, P.S. Optimal and synchronized germination of Robinia pseudoacacia, Acacia dealbata and other woody Fabaceae using a handheld rotary tool: Concomitant reduction of physical and physiological seed dormancy. J. For. Res. 2017, 29, 283–290. [Google Scholar] [CrossRef]
- Kolbek, J.V.M.; Vetvicka, V. From history of Central European Robinia growths and its communities. Zprávy České Bot. Společnosti 2004, 39, 287–298. [Google Scholar]
- Masaka, K.; Yamada, K. Variation in germination character of Robinia pseudoacacia L. (Leguminosae) seeds at individual tree level. J. For. Res. 2009, 14, 167–177. [Google Scholar] [CrossRef]
- Zoghi, Z.; Azadfar, D.; Kooch, Y. The Effect of Different Treatments on Seeds Dormancy Breaking and Germination of Caspian Locust (Gleditschia caspica) Tree. Ann. Biol. Res. 2011, 2, 400–406. [Google Scholar]
- Staska, B.; Essl, F.; Samimi, C. Density and age of invasive Robinia pseudoacacia modulate its impact on floodplain forests. Basic Appl. Ecol. 2014, 15, 551–558. [Google Scholar] [CrossRef]
- Rédei, K.; Csiha, I.; Keserű, Z.; Végh Ágnes, K.; Gyori, J. The Silviculture of Black Locust (Robinia pseudoacacia L.) in Hungary: A Review. South-East Eur. For. 2011, 2, 101–107. [Google Scholar] [CrossRef]
- Kleinbauer, I.; Dullinger, S.; Peterseil, J.; Essl, F. Climate change might drive the invasive tree Robinia pseudacacia into nature reserves and endangered habitats. Biol. Conserv. 2010, 143, 382–390. [Google Scholar] [CrossRef]
- Jin, T.T.; Fu, B.J.; Liu, G.H.; Wang, Z. Hydrologic feasibility of artificial forestation in the semi-arid Loess Plateau of China. Hydrol. Earth Syst. Sci. 2011, 15, 2519–2530. [Google Scholar] [CrossRef]
- Bouteiller, X.P.; Porté, A.J.; Mariette, S.; Monty, A. Using automated sanding to homogeneously break seed dormancy in black locust (Robinia pseudoacacia L., Fabaceae). Seed Sci. Res. 2017, 27, 243–250. [Google Scholar] [CrossRef]
- Bouteiller, X.P.; Barraquand, F.; Garnier-Géré, P.; Harmand, N.; Laizet, Y.; Raimbault, A.; Segura, R.; Lassois, L.; Monty, A.; Verdu, C.; et al. No evidence for genetic differentiation in juvenile traits between Belgian and French populations of the invasive tree Robinia pseudoacacia . Plant Ecol. Evol. 2018, 151, 5–17. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Freese, D. Black locust (Robinia pseudoacacia L.) ecophysiological and morphological adaptations to drought and their consequence on biomass production and water-use efficiency. N. Z. J. For. Sci. 2014, 44, 29. [Google Scholar] [CrossRef]





| Provenance-Population | County | Administrative Location 1 | Latitude/ Longitude | Average Yearly Temperature (°C) | Average Annual Precipitation (mm) | Altitude (m asl) |
|---|---|---|---|---|---|---|
| Foieni | Satu Mare | OS Carei, UPIII, u.a.57L, 58A | 47°42′ N/22°24′ E | 10.9 | 619.2 | 130–130 |
| Budești | Vâlcea | RNP Romsilva, OS Stoiceni UPII, u.a.5L, 5M | 45°03′ N/24°26′ E | 8.2 | 813.1 | 300–350 |
| Pădurea Cetății | Bistrița Năsăud | RNP Romsilva, OSE Lechința UP.7, u.a.80C | 47°00′ N/24°20′ E | 6.3 | 802.9 | 380–400 |
| Moneasa | Arad | Private Orchard Bărzani (Ruben Budău) | 46°19′ N/21°40′ E | 11.8 | 575.7 | 110–150 |
| Drăgănești | Galați | RNP Romsilva, OS Tecuci UP.VI, u.a.49B | 45°46′ N/27°30′ E | 11.6 | 480.1 | 45–45 |
| Borșa | Iași | RNP Romsilva, OS Iași UP. III, u.a.105E | 47°25′ N/27°20′ E | 10.5 | 540.9 | 60–160 |
| Curtuișeni | Bihor | RNP Romsilva, OS Săcuieni UP. IV, u.a.45C | 47°32′ N/22°09′ E | 10.7 | 617.6 | 140–140 |
| Liveni | Botoșani | RNP Romsilva, OS Darabani UP. III, u.a.26D | 48°02′ N/27°01′ E | 10.1 | 526.6 | 200–200 |
| Provenance | Seeds Length (mm) | Seeds Width (mm) | Seeds Weight (g) | |||
|---|---|---|---|---|---|---|
| Mean ± SEM | CV% | Mean ± SEM | CV% | Mean ± SEM | CV% | |
| Satu-Mare | 3.13 ab ± 0.27 | 15.0 | 1.14 a ± 0.04 | 6.1 | 0.031 a ± 0.002 | 10.2 |
| Vâlcea | 3.00 ab ± 0.06 | 3.2 | 0.63 cd ± 0.05 | 14.6 | 0.022 bc ± 0.001 | 16.0 |
| Bistrița N. | 2.28 c ± 0.22 | 16.9 | 0.56 cd ± 0.19 | 5.7 | 0.017 d ± 0.001 | 4.5 |
| Arad | 2.77 bc ± 0.15 | 9.4 | 0.72 bc ± 0.10 | 22.9 | 0.024 b ± 0.001 | 7.7 |
| Galați | 2.36 c ± 0.10 | 7.3 | 0.40 d ± 0.02 | 8.3 | 0.019 cd ± 0.000 | 3.1 |
| Iași | 2.78 bc ± 0.13 | 8.4 | 0.89 b ± 0.10 | 19.2 | 0.031 a ± 0.000 | 1.1 |
| Bihor | 3.25 a ± 0.03 | 1.4 | 0.61 cd ± 0.09 | 24.1 | 0.028 a ± 0.002 | 11.3 |
| Botoșani | 2.71 bc ± 0.15 | 9.6 | 0.43 d ± 0.06 | 23.7 | 0.023 bc ± 0.000 | 11.5 |
| Provenance | Sulphuric Acid Treatment/Concentration | Mean Provenance ** | ||
|---|---|---|---|---|
| 50% | 70% | 90% | ||
| Satu-Mare | 5.5 gh * | 15.8 e–g | 40.0 ab | 20.4 CD |
| Vâlcea | 45.3 a | 21.5 d–f | 30.0 b–d | 32.3 A |
| Bistrița N. | 30.5 b–d | 25.0 c–e | 34.8 a–c | 30.1 AB |
| Arad | 39.8 ab | 10.3 f–h | 25.5 c–e | 25.2 A–C |
| Galați | 39.8 ab | 16.0 e–g | 20.5 d–f | 25.4 A–C |
| Iași | 40.8 ab | 16.3 e–g | 26.8 c–e | 27.9 A–C |
| Bihor | 0.0 h | 34.8 a–c | 0.0 h | 11.6 D |
| Botoșani | 45.0 a | 4.8 gh | 20.5 d–f | 23.4 CD |
| Mean treatment *** | 30.8 Z | 18.0 Y | 24.7 X | - |
| Provenance | Thermal Treatment/Temperatures | Mean Provenance | ||
| 18 °C | 45 °C, −20 °C | 60 °C, −20 °C | ||
| Satu-Mare | 20.5 ij | 49.8 b–d | 10.3 k | 26.8 EF |
| Vâlcea | 30.0 g–j | 37.0 d–h | 43.8 b–f | 36.9 CD |
| Bistrița N. | 40.3 c–g | 25.0 h–j | 31.0 f–j | 32.1 DE |
| Arad | 25.5 h–j | 25.5 h–j | 20.0 i–j | 23.7 F |
| Galați | 70.8 a | 20.8 ij | 49.8 b–d | 47.1 B |
| Iași | 51.8 bc | 46.8 b–e | 34.5 e–i | 44.3 BC |
| Bihor | 55.3 b | 75.0 a | 74.3 a | 68.2 A |
| Botoșani | 30.5 f–j | 50.0 b–d | 55.0 b | 45.2 BC |
| Mean treatment | 40.6 Z | 41.2 Z | 39.8 Z | - |
| Provenance | Sulphuric Acid Treatment/Concentration | Mean Provenance ** | ||
|---|---|---|---|---|
| 50% | 70% | 90% | ||
| Satu-Mare | 24.5 f–j * | 28.5 e–h | 31.5 c–f | 28.2 BC |
| Vâlcea | 10.8 l | 8.3 l | 9.3 l | 9.4 E |
| Bistrița N. | 20.3 h–k | 19.5 i–k | 21.5 g–k | 20.4 D |
| Arad | 45.0 a | 37.5 a–d | 40.5 ab | 41.0 A |
| Galați | 30.0 d–g | 24.0 f–k | 27.5 e–i | 27.2 C |
| Iași | 39.3 a–c | 29.8 d–g | 33.8 b–e | 34.3 B |
| Bihor | 0.0 m | 31.8 c–f | 0.0 m | 10.6 E |
| Botoșani | 22.0 g–k | 15.5 k–l | 18.8 jk | 18.8 D |
| Mean treatment *** | 24.0 x | 24.3 x | 22.8 x | - |
| Provenance | Thermal Treatment/Temperatures | Mean Provenance | ||
| 18 °C | 45 °C, −20 °C | 60 °C, −20 °C | ||
| Satu-Mare | 24.0 h–l | 31.0 e–g | 18.8 kl | 24.6 C |
| Vâlcea | 8.0 m | 8.6 m | 9.5 m | 8.7 F |
| Bistrița N. | 21.0 j–l | 17.6 l | 20.0 k–l | 19.5 E |
| Arad | 45.0 a | 42.5 ab | 38.5 bc | 42.0 A |
| Galați | 26.9 f–j | 18.7 kl | 25.3 g–k | 23.6 CD |
| Iași | 38.0 b–d | 36.0 c–e | 30.8 e–g | 34.9 A |
| Bihor | 28.5 f–i | 30.0 e–h | 32.1 d–f | 30.2 B |
| Botoșani | 17.5 l | 22.0 i–l | 23.8 h–l | 21.1 DE |
| Mean treatment | 26.1 X | 25.8 X | 24.8 X | - |
| Provenance | Sulphuric Acid Treatment/Concentration | Mean Provenance ** | ||
|---|---|---|---|---|
| 50% | 70% | 90% | ||
| Satu-Mare | 3.2 c–g * | 4.3 a–d | 5.2 a | 4.2 A |
| Vâlcea | 5.0 a | 3.7 b–f | 4.4 a–c | 4.3 A |
| Bistrița N. | 2.2 g | 2.5 fg | 3.1 d–g | 2.6 B |
| Arad | 5.3 a | 4.0 a–e | 4.8 ab | 4.7 A |
| Galați | 3.1 d–g | 2.2 g | 3.0 e–g | 2.7 B |
| Iași | 3.5 c–g | 2.3 g | 2.6 fg | 2.8 A |
| Bihor | 0.0 h | 3.3 c–g | 0.0 h | 1.1 C |
| Botoșani | 3.1 c–g | 2.3 g | 2.5 fg | 2.6 B |
| Mean treatment *** | 3.1 X | 3.1 X | 3.2 X | - |
| Provenance | Thermal Treatment/Temperatures | Mean Provenance | ||
| 18 °C | 45 °C, −20 °C | 60 °C, −20 °C | ||
| Satu-Mare | 4.2 a–e | 5.3 a | 3.7 b–g | 4.4 AB |
| Vâlcea | 3.1 c–g | 4.0 a–e | 4.3 a–d | 3.8 BC |
| Bistrița N. | 3.3 c–g | 2.8 e–g | 3.1 c–g | 3.0 CD |
| Arad | 5.3 a | 4.7 ab | 4.5 a–c | 4.8 A |
| Galați | 2.8 e–g | 2.3 g | 3.0 d–g | 2.7 D |
| Iași | 3.8 b–f | 3.0 d–g | 2.9 e–g | 3.2 CD |
| Bihor | 2.9 d–g | 3.0 d–g | 3.5 b–g | 3.1 CD |
| Botoșani | 2.5 f–g | 3.4 b–g | 3.5 b–g | 3.1 CD |
| Mean treatment | 3.5 X | 3.5 X | 3.7 X | - |
| Provenance | Sulphuric Acid Treatment/Concentration | Mean Provenance ** | ||
|---|---|---|---|---|
| 50% | 70% | 90% | ||
| Satu-Mare | 7 d–f * | 7 d–f | 11.9 a | 8.6 AB |
| Vâlcea | 9 b–d | 7 d–f | 7 d–f | 7.7 BC |
| Bistrița N. | 8 c–e | 6 ef | 10 a–c | 8.0 AB |
| Arad | 11 ab | 9.1 bd | 8 c–e | 9.4 A |
| Galați | 8 c–e | 5 f | 6 ef | 6.3 CD |
| Iași | 10 a–c | 7 d–f | 8 c–e | 8.3 AB |
| Bihor | 0 g | 11 ab | 0 g | 3.7 E |
| Botoșani | 7 d–f | 4.9 f | 6 ef | 6.0 D |
| Mean treatment *** | 7.5 X | 7.1 X | 7.1 X | - |
| Provenance | Thermal Treatment/Temperatures | Mean Provenance | ||
| 18 °C | 45 °C, −20 °C | 60 °C, −20 °C | ||
| Satu-Mare | 9 d–e | 12 a | 7 d–g | 9.3 AB |
| Vâlcea | 6 fg | 7 d–g | 7 d–g | 6.7 CD |
| Bistrița N. | 10 a–c | 6 fg | 7 d–g | 7.7 BC |
| Arad | 9.3 b–d | 9 d–e | 7 d–g | 8.4 B |
| Galați | 6.3 e–g | 5 g | 6 fg | 5.8 D |
| Iași | 9.8 a–d | 8.3 c–f | 6.3 e–g | 8.1 BC |
| Bihor | 10.0 a–c | 11.8 ab | 10.8 a–c | 10.8 A |
| Botoșani | 7.3 d–g | 8 c–f | 8.3 c–f | 7.8 BC |
| Mean treatment | 8.4 X | 8.4 X | 7.4 Y | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, A.M.; Truta, A.M.; Viman, O.; Morar, I.M.; Spalevic, V.; Dan, C.; Sestras, R.E.; Holonec, L.; Sestras, A.F. Seed Germination and Seedling Growth of Robinia pseudoacacia Depending on the Origin of Different Geographic Provenances. Diversity 2022, 14, 34. https://doi.org/10.3390/d14010034
Roman AM, Truta AM, Viman O, Morar IM, Spalevic V, Dan C, Sestras RE, Holonec L, Sestras AF. Seed Germination and Seedling Growth of Robinia pseudoacacia Depending on the Origin of Different Geographic Provenances. Diversity. 2022; 14(1):34. https://doi.org/10.3390/d14010034
Chicago/Turabian StyleRoman, Andrea M., Alina M. Truta, Oana Viman, Irina M. Morar, Velibor Spalevic, Catalina Dan, Radu E. Sestras, Liviu Holonec, and Adriana F. Sestras. 2022. "Seed Germination and Seedling Growth of Robinia pseudoacacia Depending on the Origin of Different Geographic Provenances" Diversity 14, no. 1: 34. https://doi.org/10.3390/d14010034
APA StyleRoman, A. M., Truta, A. M., Viman, O., Morar, I. M., Spalevic, V., Dan, C., Sestras, R. E., Holonec, L., & Sestras, A. F. (2022). Seed Germination and Seedling Growth of Robinia pseudoacacia Depending on the Origin of Different Geographic Provenances. Diversity, 14(1), 34. https://doi.org/10.3390/d14010034









