Phytoplankton Biodiversity in Two Tropical, High Mountain Lakes in Central Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
- (a)
- Lake area. The area–species richness relationship is well-known [1,2]. Lake area is closely related to habitat diversity and then higher species richness [3]. The surface of Lake El Sol is almost eight times larger than Lake La Luna [23]. Additionally, Lake El Sol is deeper and holds a larger water volume than Lake La Luna.
- (b)
- Macrophytes. The presence and development of macrophytes are important in influencing phytoplankton biodiversity [85]. Lake El Sol displays an extended vegetated area (e.g., Chara) on the shallow littoral, while Lake La Luna has no macrophytes. While macrophytes compete with phytoplankton and reduce its biodiversity [86], macrophytes also provide microhabitats that harbor benthic diatoms that eventually incorporate into the water column, augmenting the taxonomic richness found in phytoplankton. To date, empirical evidence shows that the number of habitats in a region is almost always positively correlated with the number of species inhabiting it [3]. In fact, habitat diversity is often a better predictor of species richness than area [87,88].
- (c)
- Trophic status. An inverse relationship has been found between trophic status and phytoplankton biodiversity [4,5,6]. However, this relationship is expressed when comparing oligo- versus eutrophic lakes. The trophic (chlorophyll and nutrient concentrations) difference between Lakes El Sol and La Luna is marginal since the former is oligotrophic and the latter ultraoligotrophic. Phytoplankton in both lakes are restricted by the low nutrient concentration.
- (d)
- (e)
- UV radiation. UVR at HML is higher than at sea level, and often HML are transparent (oligotrophic) and with scarce UVR attenuation substances (e.g., chromophoric dissolved organic matter) negatively impacting phytoplankton biodiversity [7,8]. The light attenuation coefficient (Kd) is lower in Lake La Luna (0.21 ± 0.06 m−1) than in Lake El Sol [0.37 ± 0.07 m−1], mirroring the higher turbidity of Lake El Sol compared to Lake La Luna. Consequently, UVR reaches all of the water column, down to the lake’s bottom in Lake La Luna, while in Lake El Sol, UVR is attenuated in the top 25% of the water column. This translated into an inexistent depth refuge (UVR safe zone 0% of the water column) in Lake La Luna, while larger (UVR safe zone 75% of the water column) in Lake El Sol [89].
- (f)
- Exotic fish species introduction. During the 1950´s there was a successful rainbow trout introduction in Lake El Sol that failed in Lake La Luna [31]. Fish introduction is associated with biodiversity loss in shallow lakes [11,12,90,91]. However, phytoplankton biodiversity in fish-stocked Lake El Sol was higher than in fishless Lake La Luna. In addition to promoting turbidity, rainbow trout also change the food webs, generate trophic cascade effects, and change the plankton functional groups with Cyanobacteria as a dominant group in fish-stocked lakes [92,93,94]. However, phytoplankton biodiversity in fish-stocked Lake El Sol was higher than in fishless Lake La Luna.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Søndergaad, M.; Jeepesen, E.; Jensen, J.P. Pond or lake: Does it make any difference? Arch. Hydrobiol. 2005, 162, 143–165. [Google Scholar] [CrossRef]
- Stomp, M.; Huisman, J.; Mittelbach, G.G.; Litchman, E.; Klausmeier, C.A. Large-scale biodiversity patterns in freshwaters phytoplankton. Ecology 2011, 92, 2096–2107. [Google Scholar] [CrossRef] [PubMed]
- Triantis, K.A.; Mylonas, M.; Weiser, M.D.; Lika, K.; Vardinoyannis, K. Species richness, environmental heterogeneity and area: A case study based on land snails in Skyros Archipelago (Aegean Sea, Greece). J. Biogeogr. 2005, 32, 1727–1735. [Google Scholar] [CrossRef]
- Baho, D.L.; Drakare, S.; Johnson, R.K.; Allen, C.R.; Angeler, D.G. Is the impact of eutrophication on phytoplankton diversity dependent on lake volume/ecosystem size? J. Limnol. 2017, 76, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Brahney, J.; Mahowald, N.; Ward, D.S.; Ballantyne, A.P.; Neff, J.C. Is atmospheric phosphorus pollution altering global alpine Lake stoichiometry? Glob. Biochem. Cycles 2015, 29, 1369–1383. [Google Scholar] [CrossRef] [Green Version]
- Kissman, C.E.H.; Willams, C.E.; Rose, K.C.; Saros, J.E. Response of phytoplankton in an alpine lake to inputs of dissolved organic matter through nutrient enrichment and trophic forcing. Limnol. Oceanogr. 2013, 58, 867–880. [Google Scholar] [CrossRef] [Green Version]
- Sommaruga, R.; Gunter, A. Seasonality in UV transparency of an alpine lake is associated to changes in phytoplankton biomass. Aquat. Sci. 2006, 68, 129–141. [Google Scholar] [CrossRef]
- Korbee, N.; Carrillo, P.; Mata, M.T.; Rosillo, S.; Medina-Sánchez, J.M.; Figueroa, F.L. Effect of ultraviolet radiation and nutrients on structure-function of phytoplankton in a high mountain lake. Photochem. Photobiol. Sci. 2012, 11, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Tolotti, M. Phytoplankton and littoral epilithic diatoms in high mountain lakes of the Adamello-Brenta Regional Park (Trentino, Italy) and their relation to trophic status and acidification risk. J. Limnol. 2001, 60, 171–188. [Google Scholar] [CrossRef]
- Triadó-Margarit, X.; Casamayor, E. Genetic diversity of planktonic eukaryotes in high mountain lakes (Central Pyrenees, Spain). Environ. Microbiol. 2012, 14, 2445–2456. [Google Scholar] [CrossRef]
- Tiberti, R.; Hardenberg, A.; Bogliani, G. Ecological impact of introduced fish in high altitude lakes: A case of study from European Alps. Hidrobiologia 2013, 724, 1–19. [Google Scholar] [CrossRef]
- Cantonati, M.; Zorza, R.; Bertoli, M.; Pastorino, P.; Salvi, G.; Platania, G.; Prearo, M.; Pizzul, E. Recent and subfossil assemblages as indicator of environmental change (including fish introduction in a high-mountain lake). Ecol. Indic. 2021, 125, 107603. [Google Scholar] [CrossRef]
- Catalan, J.; Camarero, L.; Felip, M.; Pla, S.; Ventura, M.; Buchaca, T.; Bartumeus, F.; Mendoza, G.D.; Miró, A.; Casamayor, E.O.; et al. High mountain lakes: Extreme habitats and witnesses of environmental changes. Limnetica 2006, 25, 551–584. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M. High-Mountain Lakes, Indicators of Global Change: Ecological characterization and environmental pressures. Diversity 2020, 12, 260. [Google Scholar] [CrossRef]
- Löffler, H. Aspects of the history and evolution of alpine lakes in Austria. Hydrobiologia 1983, 100, 143–152. [Google Scholar] [CrossRef]
- Ortiz-Álvarez, R.; Triadó-Margarit, X.; Camareno, L.; Casamayor, E.O.; Catalan, J. High planktonic diversity in mountain lakes contains similar contributions of autotrophic, heterotrophic and parasitic eukaryotic life forms. Sci. Rep. 2018, 8, 4457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastorino, P.; Elia, A.C.; Caldaroni, B.; Menconi, V.; Abete, M.C.; Brizia, P.; Bertoli, M.; Zaccaroni, A.; Gabriele, M.; Dörr, A.J.M.; et al. Oxidative stress ecology in brook trout (Salvelinus fontinalis) from a high-mountain lake (Cottian Alps). Sci. Total Environ. 2020, 715, 136946. [Google Scholar] [CrossRef] [PubMed]
- Catalan, J.; Donato Rondón, J.C. Perspectives for an integrated understanding of tropical and temperate high-mountain lakes. J. Limnol. 2016, 75, 215–234. [Google Scholar] [CrossRef] [Green Version]
- Casallas, J.; Gunkel, G. Algunos aspectos limnológicos de un lago altoandino, el Lago San Pablo, Ecuador. Limnetica 2001, 20, 29–46. [Google Scholar] [CrossRef]
- Llorente-Bousquets, J.; Ocegueda, S. Estado del conocimiento de la biota. In Capital Natural de México, Vol. 1. Conocimiento Actual de la Biodiversidad; Conabio, Ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Ciudad de México, Mexico, 2008; pp. 283–322. [Google Scholar]
- Lewis, W.M., Jr. Tropical limnology. Annu. Rev. Ecol. Evol. Syst. 1987, 18, 159–184. [Google Scholar] [CrossRef]
- Armienta, M.A.; de la Cruz-Reina, S.; Macias, J.L. Chemical characteristics of the crater lakes of Popocatepetl, El Chichón and Nevado de Toluca volcanoes. J. Volcanol. Geotherm. Res. 2000, 97, 105–125. [Google Scholar] [CrossRef]
- Alcocer, J.; Oseguera, L.A.; Escobar, E.; Peralta, L.; Lugo, A. Phytoplankton biomass and water chemistry in two high mountain tropical lakes in Central Mexico. Arct. Antarct. Alp. Res. 2004, 36, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Elías-Gutiérrez, M.; Ciros-Pérez, J.; Gutiérrez-Aguirre, M.; Cervantes-Martínez, A. A checklist of the littoral cladocerans from Mexico, with descriptions of five taxa recently recorded from the Neovolcanic Province. Hydrobiologia 1997, 360, 63–73. [Google Scholar] [CrossRef]
- Sarma, S.S.; Elías-Gutiérrez, M.; Serranía Soto, C. Rotifers from high altitude crater-lakes at Nevado de Toluca volcano, México. Hidrobiológica 1997, 6, 33–38. [Google Scholar]
- Dimas-Flores, N.; Alcocer, J.; Ciros-Pérez, J. The structure of the zooplankton assemblages from two neighboring tropical high mountain lakes. J. Fresh Water Ecol. 2008, 23, 21–31. [Google Scholar] [CrossRef]
- Oseguera, L.A.; Alcocer, J.; Escobar, E. Macroinvertebrados bentónicos de dos lagos tropicales de alta montaña en el volcán Nevado de Toluca, en el centro de México. Hidrobiológica 2016, 26, 419–432. [Google Scholar]
- Löffler, H. Contribution to the limnology of high mountain lakes in Central America. Int. Rev. Gesamten Hydrobiol. 1972, 57, 397–408. [Google Scholar] [CrossRef]
- Caballero-Miranda, M. The diatom flora of two acid lakes in central Mexico. Diatom Res. 1996, 11, 227–240. [Google Scholar] [CrossRef]
- Banderas-Tarabay, A.G. Phycoflora of the tropical high mountain lake El Sol, Central Mexico, and some biogeographical relationships. Hydrobiologia 1997, 354, 17–40. [Google Scholar] [CrossRef]
- Cuna, E.; Zawisza, E.; Caballero, M.; Ruiz-Fernández, A.C.; Lozano-García, M.S.; Alcocer, J. Environmental impact of the Little Ice Age cooling in Central Mexico: The record from a tropical alpine lake. J. Paleolimnol. 2014, 51, 1–14. [Google Scholar] [CrossRef]
- SMN-CONAGUA. Servicio Meteorológico Nacional, Comisión Nacional del Agua. 2017. Available online: http://smn1.conagua.gob.mx/emas (accessed on 6 January 2022).
- Alcocer, J.; Roberson, J.; Oseguera, L.A.; Lewis, M.W., Jr. Rhythmic episodes of heating and cooling control thermal stratification of two tropical high mountain lakes. Aquat. Sci. 2020, 82, 58. [Google Scholar] [CrossRef]
- Batarbe, R.W. Diatom analysis. In Handbook of Holocene Palaeoecology and Palaeohydrology; Berlung, E.B., Ed.; John Wiley & Sons: Chichester, UK, 1986; pp. 423–448. [Google Scholar]
- Comas, A. Las Chlorococcales Dulceacuícolas de Cuba. Biblioteca Phicologica Band 99; J. Cramer: Berlin, Germany; Stuttgart, Germany, 1996. [Google Scholar]
- Desikachary, T.V. Cyanophyta; I.C.A.R.: New Delhi, India, 1959. [Google Scholar]
- Dillar, G.E. Freshwater Algae of the Southeastern United States, Part 1; J. Cramer: Berlin, Germany; Stuttgart, Germany, 1989. [Google Scholar]
- Dillar, G.E. Freshwater Algae of the Southeastern United States, Part 3 (Section 2); J. Cramer: Berlin, Germany; Stuttgart, Germany, 1990. [Google Scholar]
- Dillar, G.E. Freshwater Algae of the Southeastern United States, Part 4 (Section 4); J. Cramer: Berlin, Germany; Stuttgart, Germany, 1991. [Google Scholar]
- Dillar, G.E. Freshwater Algae of the Southeastern United States, Part 6 (Section 4); J. Cramer: Berlin, Germany; Stuttgart, Germany, 1993. [Google Scholar]
- Ettl, H.; Gärtner, G. Suβwasserflora von Mitteleuropa. Chlorophyta II. Band 10: Tetrasporales, Chlorococcales, Gloeodendrales; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1988. [Google Scholar]
- Huber-Pestalozzi, G. (Grünalgen) Ordnung: Volvocales; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1961. [Google Scholar]
- Huber-Pestalozzi, G. Das Phytoplankton des Süβwassers Systematik und Biologic. 2 Teil Chrysophyceen. Farblose Flagellaten Heterokonten; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1962. [Google Scholar]
- Huber-Pestalozzi, G. Das Phytoplankton des Süβwassers Systematik und Biologic. 3 Teil Chryptophyceae, Chloromonadaceae, Dinophyceae; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1968. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales; Gustav Fischer: Jena, Germany; Stuttgart, Germany; Lübeck, Germany; Ulm, Germany, 1999. [Google Scholar]
- Komárek, J.; Fott, B. Chlorophyceae. (Güelgen). Ordnurg: Chlorococccales. 7/1. 16. Das Phytoplankton des Sübwassers, Systematick und Biologie. Die Binnengëwasser; Huber-Pestalozzi, G., Ed.; E. Schweizerbart’sche Stuttgart Verlagsbuchhandlung: Stuttgart, Germany, 1983. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süβwasser Flora von Mitteleuropa. Band 2/1: Bacillariophyceae. 2. Teil: Naviculaceae; Gustav Fisher: Jena, Germany, 1986. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süβwasser Flora von Mitteleuropa. Band 2/2: Baccillariophyceae (Epithemiaceae, Surirellaceae); Gustav Fisher: Jena, Germany, 1988. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süβwasser Flora von Mitteleuropa. Band 2/2: Baccillariophyceae (Centrales, Fragilariaceae, Eunotiacea); Gustav Fisher: Jena, Germany, 1991. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süβwasser Flora von Mitteleuropa. Band 2/4: Baccillariophyceae (Achnanthes, Kristiche Ergannzunge zu Navicula (Lineolatae) und Gomphonema); Gustav Fisher: Jena, Germany, 1991. [Google Scholar]
- Philipose, M.D. Chlorococcales; Indian Council of Agricultural Research: New Delhi, India, 1967. [Google Scholar]
- Popovský, J.; Pfiester, L.A. Subeasserflora von Mitteleuropa, Band 6, Dinophyceae (Dinoflagellida); Gustav Fischer Verlag: Jena, Germany; Stuttgart, Germany, 1990. [Google Scholar]
- Prescott, G.W.; Croasdale, H.T.; Vinyard, W.C. A Synopsis of North American Desmids. Part II, Section I; University of Nebraska Press: Lincoln, NE, USA, 1975. [Google Scholar]
- Prescott, G.W.; Croasdale, H.T.; Vinyard, W.C. A Synopsis of North American Desmids. Part II, Section 2; University of Nebraska Press: Lincoln, NE, USA, 1977. [Google Scholar]
- Prescott, G.W.; Croasdale, H.T.; Vinyard, W.C.; Bicudo, C.E. A Synopsis of North American Desmids. Part II, Section 3; University of Nebraska Press: Lincoln, NE, USA, 1981. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2019; Available online: http://www.algaebase.org (accessed on 21 July 2019).
- Wetzel, R.G.; Likens, G.E. Limnological Analysis; Springer: New York, NY, USA, 2000. [Google Scholar]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, G.E. A Treatise on Limnology. Volume II. Introduction to Lake Biology and Limnoplankton; Wiley: New York, NY, USA, 1967. [Google Scholar]
- Margulis, L.; Chapman, M.J. Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth; Academic Press/Elsevier: Amsterdam, The Netherlands, 2009; 566p, ISBN 978-0123736215. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; W.H. Freeman: San Francisco, CA, USA, 1981; 859p. [Google Scholar]
- Pedroche, F.F.; Dreckmann, K.M.; Sentíes, G.A.; Margain-Hernández, R. Diversidad algal en México. Rev. Soc. Mex. Hist. Nat. 1993, XLIV, 69–92. [Google Scholar]
- Oliva-Martínez, M.G.; Godínez-Ortega, J.L.; Zuñiga-Ramos, C.A. Biodiversidad del fitoplancton de aguas continentales en México. Rev. Mex. Biodivers. 2014, 85, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Vilaclara, G.; Cuna, E.; Zeeb, B. Subfossil chrysophyte cyst morphotypes from two tropical, high-mountain lakes in Nevado de Toluca volcano, Central Mexico. Nova Hedwig. 2005, 128, 309–335. [Google Scholar]
- Reynolds, C. The concept of ecological succession applied to seasonal periodicity of freshwater phytoplankton. Verh. Des Int. Ver. Limnol. 1988, 23, 683–691. [Google Scholar] [CrossRef]
- Garduño-Solórzano, G.; Martínez-García, M.; Scotta Hentschke, G.; Lopes, G.; Castelo Branco, R.; Oliveira Vasconcelos, V.M.; Campos, J.E.; López-Cano, R.; Quintanar-Zúñiga, R.E. The phylogenetic placement of Temnogametum (Zygnemataceae) and description of Temnogametum iztacalense sp. nov., from a tropical high mountain lake in Mexico. Eur. J. Phycol. 2021, 56, 159–173. [Google Scholar] [CrossRef]
- Cervantes-Martínez, A.; Gutiérrez-Aguirre, M.; Elías-Gutiérrez, M. Description of Iliocryptus nevadensis (Branchiopoda, Anomopoda), a new species from high altitude crater lake in the volcano Nevado de Toluca, Mexico. Crustaceana 2000, 354, 311–321. [Google Scholar]
- Sinev, A.Y.; Zawisza, E. Comments on cladocerans of crater lakes of the Nevado de Toluca Volcano (Central Mexico), with the description of a new species, Alona manueli sp. nov. Zootaxa 2013, 3647, 390–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, W.M., Jr. Global primary production of lakes: 19th Baldi Memorial Lecture. Inland Waters 2011, 1, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Garduño Solórzano, G.; Oliva Martínez, M.G.; Ortega, M. Algas. In La Diversidad Biológica del Estado de México. Estudio de Estado; Ceballos, G., List, R., Garduno, G., López Cano, R., Muñozcano Quintanar, M.J., Collado, E., San Román, J.E., Eds.; Colección Mayor, Gobierno del Estado de Mexico, CONABIO: Mexico City, Mexico, 2009; pp. 153–161. [Google Scholar]
- Godínez-Ortega, J.L.; Oliva-Martínez, M.G.; Escobar-Oliva, M.A.; Mendoza-Garfias, M.B. Diversidad algal del Parque Nacional Lagunas de Zempoala, México, excepto diatomeas. Hidrobiológica 2017, 27, 45–58. [Google Scholar] [CrossRef]
- León López, N.; Rivera Rondón, C.A.; Zapata, A.; Jiménez, J.; Villamil, W.; Arenas, G.; Rincón, C.; Sánchez, T. Factors controlling phytoplankton in tropical high-mountain drinking-water reservoirs. Limnetica 2012, 31, 305–322. [Google Scholar] [CrossRef]
- Krupa, E.G.; Barinova, S.M.; Romanova, S.M.; Malybekov, A.B. Hydrobiological assessment of the high mountain Kolsay lakes (Kungey Alatau, Southeastern Kazakhstan) ecosystems in climatic gradient. Br. J. Environ. Clim. Chang. 2016, 6, 259–278. [Google Scholar] [CrossRef]
- Simona, M.; Barbieri, A.; Veronesi, M.; Malusardi, S.; Straškrabová, V. Seasonal Dynamics of plankton in a mountain lake in the southern Alps (Laghetto Inferiore, Switzerland). J. Limnol. 1999, 58, 169–178. [Google Scholar] [CrossRef]
- Fott, J.; Blažo, M.; Stuchlík, E.; Strunecký, O. Phytoplankton in three Tatra Mountain lakes of different acidification status. J. Limnol. 1999, 58, 107–116. [Google Scholar] [CrossRef]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Polato, N.R.; Gill, B.A.; Shah, A.A.; Gray, M.M.; Casner, K.L.; Barthelet, A.; Messer, P.W.; Simmons, M.P.; Guayasamin, J.M.; Encalada, A.C.; et al. Narrow thermal tolerance and low dispersal drive higher speciation in tropical mountains. Proc. Natl. Acad. Sci. USA 2018, 115, 12471–12476. [Google Scholar] [CrossRef] [Green Version]
- Usinowicz, J.; Chang-Yang, C.; Chen, Y.; Clark, J.S.; Fletcher, C.; Garwood, N.C.; Hao, Z.; Johnstone, J.; Lin, Y.; Metz, M.R.; et al. Temporal coexistence mechanisms contribute to the latitudinal gradient in forest diversity. Nature 2017, 550, 105–108. [Google Scholar] [CrossRef]
- Novelo, E.; Tavera, R. Un panorama gráfico de las algas de agua dulce de México. Hidrobiológica 2011, 21, 333–341. [Google Scholar]
- Rivera, C.; Solano, D.; Zapata, A.; Donato, J. Phytoplankton diversity in a tropical high mountain lake. Verh. Int. Ver. Theor. Angew. Limnol. 2005, 29, 418–421. [Google Scholar] [CrossRef]
- Tolotti, M.; Thies, H.; Cantonati, M.; Hansen, C.; Thaler, B. Flagellate algae (Chrysophyceae, Dinophyceae, Cryptophyceae) en 48 high mountain lakes of the Northern and Southern slope of the Eastern Alps: Biodiversity, taxa distribution and their driving variables. Hydrobiologia 2001, 502, 331–348. [Google Scholar] [CrossRef]
- Unrein, F.; Massana, R.; Alonso-Saénz, L.; Gasol, J.M. Significant year-round of small mixotrophic flagellates on bacterioplankton in an oligotrophic coastal system. Limnol. Oceanogr. 2007, 52, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.S. What factors influence the species composition of phytoplankton in lakes of different trophic status. Hydrobiologia 1998, 369, 11–26. [Google Scholar] [CrossRef]
- Porcel, S.; Chaparro, G.; Marinone, M.C.; Saad, J.F.; Lancelotti, J.; Izaguirre, I. The role of environmental, geographical, morphometric and spatial variables on plankton communities in lakes of the arid Patagonian plateau. J. Plankton Res. 2020, 42, 173–187. [Google Scholar] [CrossRef]
- Kufel, L.; Kufel, I. Chara beds acting as nutrient sinks in shallow lakes—A review. Aquat. Bot. 2002, 72, 249–260. [Google Scholar] [CrossRef]
- Hortal, J.; Triantis, K.; Meiri, S.; Thebault, E.; Sfenthourakis, S. Island species richness increases with hábitat diversity. Am. Nat. 2009, 174, E205–E217. [Google Scholar] [CrossRef] [Green Version]
- Triantis, K.A.; Nogués-Bravo, D.; Hortal, J.; Borges, P.A.; Adsersen, H.; Fernández-Palacios, J.M.; Araujo, M.B.; Whittaker, R.J. Measurements of area and the (island) species area relationship: New direction for old pattern. Oikos 2008, 117, 1555–1559. [Google Scholar] [CrossRef] [Green Version]
- Alcocer, J.; Delgado, C.N.; Sommaruga, R. Photoprotective compounds in zooplankton of two adjacent tropical high mountain lakes with contrasting underwater light climate and fish occurrence. J. Plankton Res. 2020, 42, 105–118. [Google Scholar] [CrossRef]
- Izaguirre, I.; Lancelotti, J.; Saad, J.F.; Porcel, S.; O’Farrell, I.; Marinone, M.C.; Roesler, I.; Dieguez, M.C. Influence of fish introduction and water level decrease on lakes of the arid Patagonian plateaus with importance for biodiversity conservation. Glob. Ecol. Conserv. 2018, 14, e00391. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M.; Bertoli, M.; Menconi, V.; Esposito, G.; Righetti, M.; Mugetti, D.; Pederiva, S.; Abete, M.C.; Pizzul, E. Assessment of biological and sanitary condition of alien fish from a high-mountain lake (Cottian Alps). Water 2020, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Izaguirre, I.; Saad, J.F. Phytoplankton from natural water bodies of Patagonian plateau. Adv. Limnol. 2014, 65, 309–319. [Google Scholar] [CrossRef]
- Lancelotti, J.L.; Marinone, M.C.; Roesler, I. Rainbow trout effects on zooplankton in the reproductive area of the critically endangered hooded grebe. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 27, 128–136. [Google Scholar] [CrossRef]
- Saad, J.F.; Porcel, S.; Lancelotti, J.; O’Farrel, I.; Izaguirre, I. Both lake regime and fish introduction shape autotrophic planktonic communities of lakes from the Patagonian plateau (Argentina). Hydrobiologia 2018, 831, 133–145. [Google Scholar] [CrossRef]

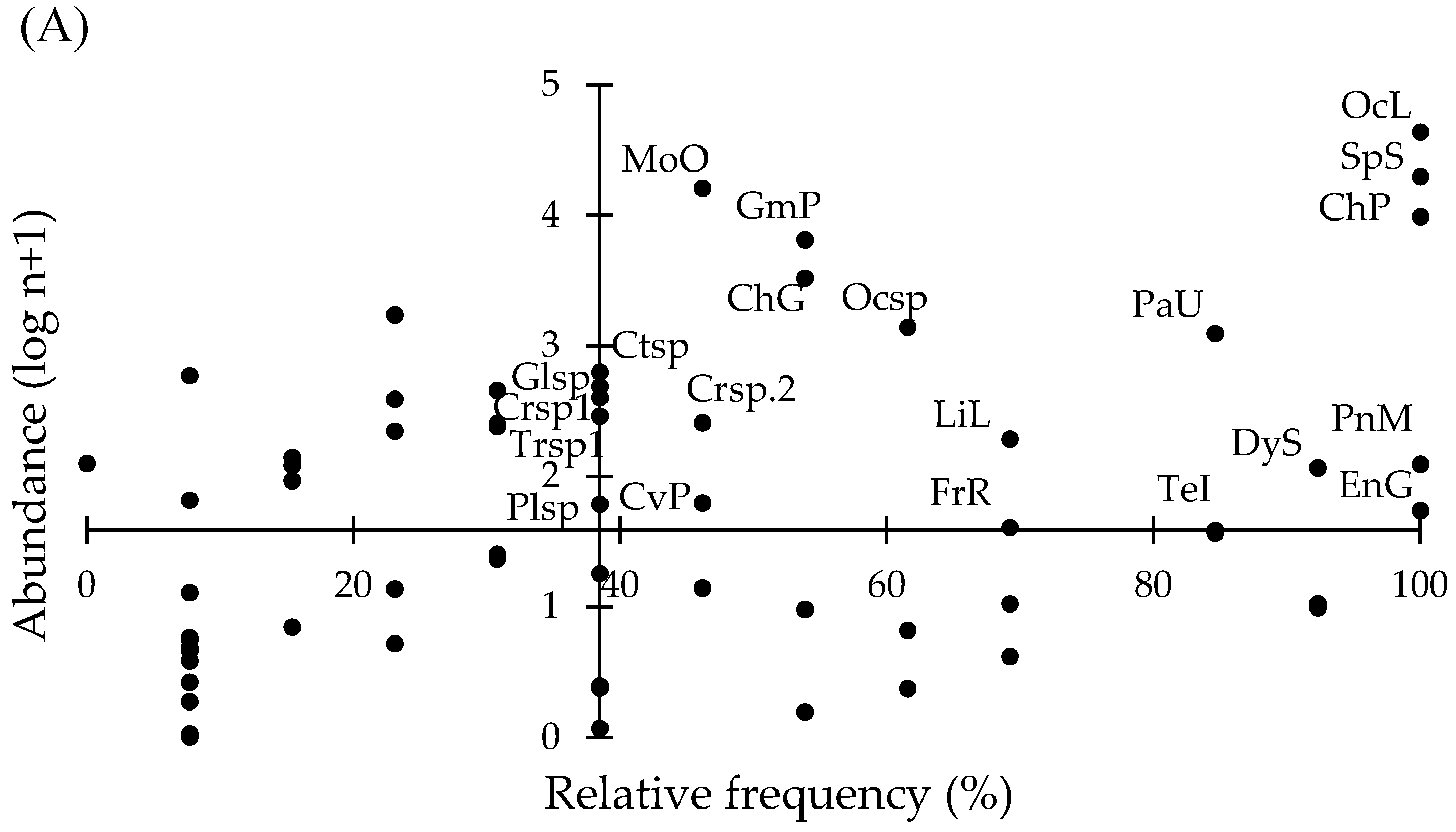
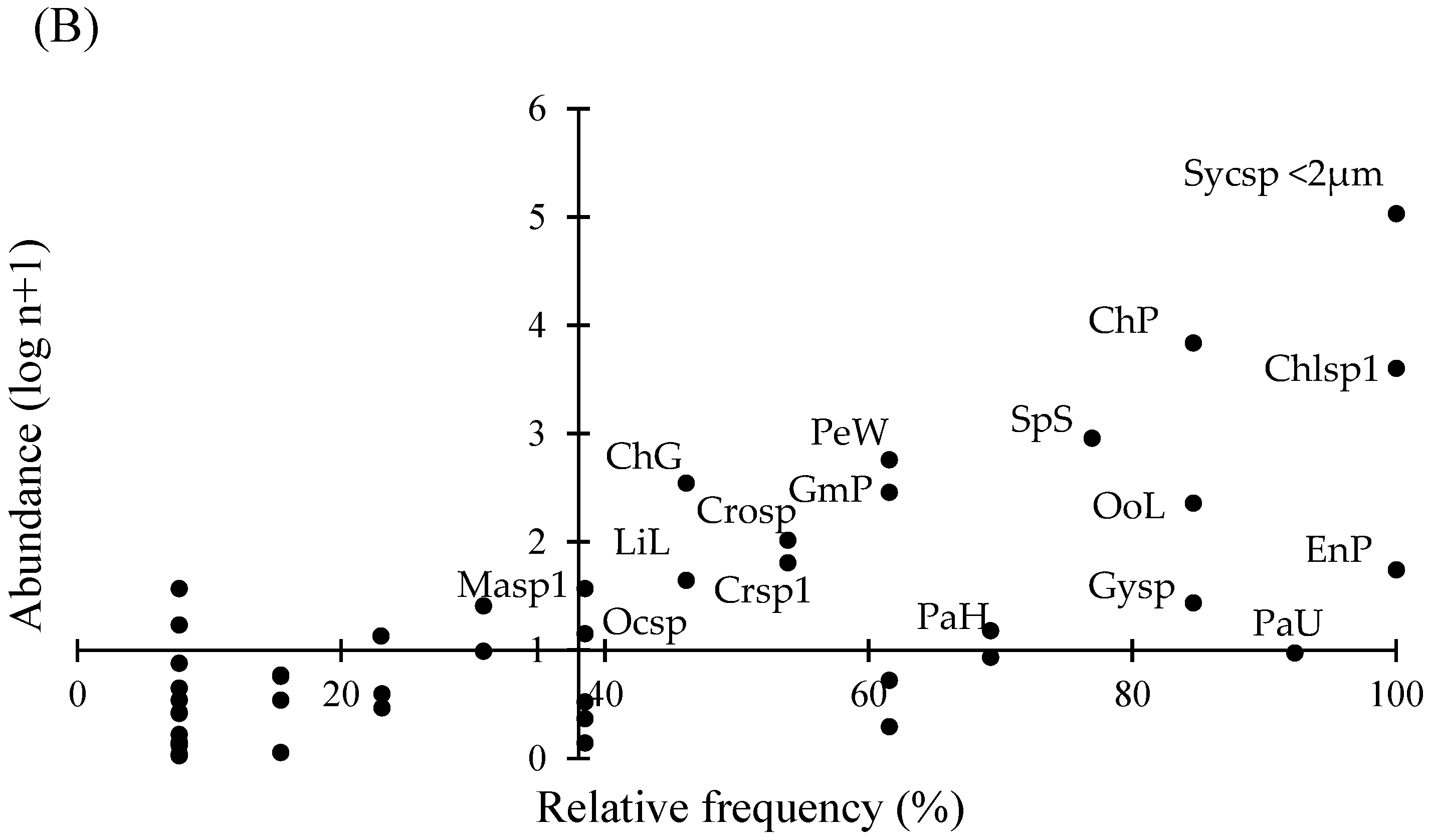
| Variable | Lake El Sol | Lake La Luna | ||||
|---|---|---|---|---|---|---|
| X ± s.d. | Min | Max | X ± s.d. | Min | MAX | |
| Temp (°C) | 9.2 ± 1.5 | 5.2 | 10.81 | 8.5 ± 1.7 | 5.7 | 10.9 |
| DO (mg L−1) | 6.6 ± 0.6 | 5.76 | 7.74 | 6.8 ± 0.8 | 5.9 | 8.6 |
| pH * | 5.5 ± 0.3 | 4.9 | 6.1 | 4.7 ± 0.3 | 4.5 | 5.6 |
| K25 (µS cm−1) * | 16 ± 1 | 15 | 18 | 14 ± 0.8 | 13 | 15 |
| %SPAR * | 10 ± 5 | 3 | 26 | 44 ± 12 | 17 | 53 |
| N-NO2 (µg L−1) * | 1.9 ± 0.4 | 0.56 | 4.3 | 0.5 ± 0.2 | 0.3 | 1.0 |
| N-NO3 (µg L−1) * | 66.0 ± 44.3 | 1.26 | 138 | 316.7 ± 28.0 | 278.7 | 362.6 |
| N-NH4 (µg L−1) | 38.1 ± 21.4 | 15.0 | 77.1 | 39.0 ± 20.7 | 15.0 | 77.1 |
| DIN (µg L−1) * | 106.7 ± 57.7 | 16.9 | 185 | 356.4 ± 23.6 | 299.3 | 368.7 |
| P-SRP (µg L−1) | 5.8 ± 3.6 | 1.1 | 11.1 | 4.0 ± 4.5 | 0.4 | 17.2 |
| Si-SRSi (µg L−1) * | 68.9 ± 56.6 | 23.5 | 205.6 | 17.3 ± 28.4 | 3.3 | 108.4 |
| DIN:P-SRP * | 37 ± 44 | 2 | 143 | 244 ± 256 | 21 | 832 |
| Chl-a (µg L−1) | 1.7 ± 1.6 | 0.2 | 5.1 | 0.6 ± 0.35 | 0.29 | 1.1 |
| Taxa | El Sol | La Luna |
|---|---|---|
| Phylum: Cyanobacteria | ||
| Class: Cyanophyceae | ||
| Order: Chroococcales | ||
| Limnococcus limneticus [Lemmerman] Komárkova, Jezbarová, Komárek & Zapomelová | X | X |
| Order: Nostocales | ||
| Anabaena cf. lapponica Borge | X | |
| Anabaena sp. | X | |
| Order: Oscillatoriales | ||
| Limnoraphis birgei [Smith] Komárek, Zapomelová, Smarda, Kopecký. Rejimánková, Woodhouse, Neilan & Komárková | X | |
| Oscillatoria sp. | X | X |
| Order: Synechococcales | ||
| Merismopedia sp. | X | |
| Synechocystis minuscula Woronichin | X | |
| Synechocystis sp. | X | X |
| Pseudoanabaena sp. | X | |
| Phylum: Ochrophyta | ||
| Class: Chrysophyceae | ||
| Order: Chromulinales | ||
| Chromulina sp. | X | X |
| Chrysococcus minutus [Fritsch] Nygaard | X | |
| Dinobryon cf. sociale [Ehrenberg] Ehrenberg | X | |
| Ochromonas sp. | X | X |
| Class: Synurophyceae | ||
| Order: Synurales | ||
| Mallomonas cf. acaroides Perty | X | |
| Mallomonas sp. 1 | X | X |
| Mallomonas sp. 2 | X | |
| Phylum: Bacillariophyta | ||
| Class: Coscinodiscophyceae | ||
| Order: Aulacoseirales | ||
| Aulacoseira nivaloides [Cambrun] English & Potatova | X | X |
| Aulacoseira cf. alpigena [Grunow] Krammer | X | X |
| Class: Mediophyceae | ||
| Order: Stephanodiscales | ||
| Cyclotella aff. quillensis Bailey | X | X |
| Cyclotella sp. | X | X |
| Class: Bacillaripophyceae | ||
| Order: Bacillariales | ||
| Nitzschia cf. acidoclinata Lange-Bertalot * | X | X |
| Nitzschia sp. * | X | X |
| Order: Cocconeidales | ||
| Achnanthidium minutissimum [Kützing] Czarnecki * | X | |
| Psammothidium helveticum [Hustedt] Bukhtiyarova & Round * | X | X |
| Psammothidium levanderi [Hustedt] Bukhtiyarova & Round * | X | X |
| Order: Cymbellales | ||
| Encyonema gracile Rabenhorst * | X | |
| Encyonema perpusillum [Cleve-Euler] Mann * | X | X |
| Gomphonema angustatum [Kützing] Rabenhorst * | X | X |
| Order: Fragilariales | ||
| Staurosira aff. venter [Ehrenberg] Cleve & Möller * | X | X |
| Staurosira pseudoconstruens [Marciniak] Lange-Bertalot * | X | X |
| Staurosirella pinnata [Ehrenberg] Williams & Round * | X | X |
| Order: Naviculales | ||
| Brachysira sp. * | X | X |
| Cavinula pseudocutiformis [Hustedt] Man & Stickle * | X | X |
| Frustulia rhomboides [Ehrenberg] De Toni * | X | X |
| Navicula NTA * | X | X |
| Navicula NTB * | X | |
| Pinnularia microstauron [Ehrenberg] Cleve * | X | X |
| Pinnularia cf. viridis [Nitzsch] Ehrenberg * | X | X |
| Pinnularia subcapitata W. Gregory * | X | X |
| Pinnularia sp. 1 * | X | |
| Pinnularia sp. 2 * | X | X |
| Sellaphora sp. * | X | |
| Stauroneis anceps Ehrenberg * | X | X |
| Order: Surirellales | ||
| Stenopterobia sp. 1 * | X | X |
| Stenopterobia sp. 2 * | X | X |
| Surirella cf. linearis Smith * | X | X |
| Surirella cf. angusta Kützing * | X | X |
| Surirella sp. * | X | X |
| Phylum: Haptophyta | ||
| Class: Coccolithophyceae | ||
| Order: Prymnesiales | ||
| Chrysochromulina aff. parva Lackey | X | X |
| Phylum: Cryptophyta | ||
| Class: Cryptophyceae | ||
| Order: Cryptomonadales | ||
| Chroomonas nordstedt Hansgirg | X | |
| Chroomonas sp. | X | X |
| Cryptomonas sp. 1 | X | X |
| Cryptomonas sp. 2 | X | X |
| Phylum: Miozoa | ||
| Class: Dinophyceae | ||
| Order: Gymnodiniales | ||
| Gymnodinum sp. | X | X |
| Order: Peridiniales | ||
| Parvodinium umbonatum [Stein] Carty | X | X |
| Peridinium cf. volzii Lemmermann | X | |
| Peridinium willei Huitfeldt-Kaas | X | X |
| Phylum Euglenozoa | ||
| Class: Euglenophyceae | ||
| Order: Euglenales | ||
| Euglena sp. | X | |
| Lepocinclis sp. | X | |
| Trachelomonas sp. 1 | X | |
| Trachelomonas sp. 2 | X | |
| Phylum: Chlorophyta | ||
| Class: Chlorophyceae | ||
| Order: Chlamydomonadales | ||
| Chlamydomonas sp. 1 | X | X |
| Chlamydomonas sp. 2 | X | X |
| Carteria sp. | X | X |
| Gemellicystis planctonica [Woronichin] Lund | X | X |
| Microglena sp. | X | |
| Palmella sp. | X | X |
| Sphaerocystis schroeteri Chodat | X | X |
| Order: Chlorellales | ||
| Oocystis lacustris Chodat | X | X |
| Oocystis sp. | X | X |
| Order: Oedogoniales | ||
| Oedogonium sp. | X | X |
| Order: Sphaeropleales | ||
| Ankistrodesmus sp. | X | |
| Coelastrum sp. | X | |
| Desmodesmus abundans [Kirchner] Hegewald | X | X |
| Desmodesmus spinosus [Chodat] Hegewa | X | |
| Gloeocystis sp. | X | |
| Monoraphidium minutum [Nägeli] Komárková-Legnerová | X | |
| Monoraphidium obtusum [Korshikov] Komárková-Legnerová | X | X |
| Tetradesmus obliquus [Turpin] Wynne | X | |
| Order:Trebouxiales | ||
| Botryococcus braunii Kützing | X | X |
| Phylum: Charophyta | ||
| Class: Charophyceae | ||
| Order: Charales | ||
| Nitella sp. | X | |
| Class: Coleochaetophyceae | ||
| Order: Chaetosphaeridiales | ||
| Chaetosphaeridium globosum [Nordstedt] Klebahn | X | X |
| Class: Conjugatophyceae | ||
| Order: Desmidiales | ||
| Cosmarium cf. exiguum Archer | X | X |
| Closterium lunula Ehrenberg & Hemprich ex Ralfs | X | X |
| Closterium sp. | X | X |
| Desmidium sp. | X | X |
| Euastrum cf. oblongum Ralfs | X | X |
| Micrasterias radiosa Ralfs | X | X |
| Staurastrum sp. | X | X |
| Order: Zygnematales | ||
| Temnogametum iztacalense Garduño & Martínez | X | X |
| Spirogyra sp. | X | |
| Zygnema sp. | X | X |
| Total | 92 | 63 |
| Lake El Sol | Lake La Luna |
|---|---|
| Chaetosphaeridium globosum [ChG] | |
| Chryptomonas sp. 1 [Crsp1] | |
| Chrysochromulina aff. parva [ChP] | |
| Gemellicystis planctonica [GmP] | |
| Limnococcus limneticus [LiL] | |
| Ochromonas sp [Ocsp] | |
| Oocystis lacustris [OcL] | |
| Parvodinium umbonatum [PaU] | |
| Sphaerocystis schroeteri [SpS] | |
| Chryptomonas sp. 2 [Crsp2] | Chlamydomonas sp. 1 [Chsp1] |
| Cavinula pseudocutiformis [CvP] | Chroomonas sp. [Crosp] |
| Dynobryon cf. sociale [DyS] | Encyonema perpusillum [EnP) |
| Encyonema gracile [EnG] | Gymnodinum sp. [Gysp] |
| Frustulia rhomboides [FrR] | Mallomonas sp. 1 [Masp1] |
| Gloeocystis sp. [Glsp] | Peridinium willei [PeW] |
| Monoraphidium obtusum [MoO] | Psammothidium helveticum [PaH] |
| Palmella sp. [Plsp] | Synechocystis sp. < 2 µm [Sycsp] |
| Carteria sp. [Ctsp] | |
| Pinnularia microstauron [PnM] | |
| Temnogametum iztacalense [TeI] | |
| Trachelomonas sp. 1 [Trsp1] | |
| Lake El Sol | La Luna | ||
|---|---|---|---|
| Abundance | % | Abundance | % |
| Oocystis lacustris | 58.2 | Synechocystis sp. | 89.1 |
| Sphaerocystis schroeteri | 17.9 | Chrysochromulina aff. parva | 5.8 |
| Monoraphidium obtusum | 8.7 | Chlamydomonas sp. 1 | 3.3 |
| Chrysochromulina aff. parva | 7.5 | ||
| Gemellicystis planctonica | 5.9 | ||
| Biomass | % | Biomass | % |
| Monoraphidium obtusum | 19.0 | Gymnodinum sp. | 17.5 |
| Sphaerocystis schroeteri | 14.5 | Parvodinium umbonatum | 16.2 |
| Oocystis lacustris | 10.6 | Synechocystis sp. | 14.8 |
| Encyonema perpusillum | 14.7 | ||
| Peridinium willei | 14.6 | ||
| Phytoplankton Phylum (Class) | Mex1 | Mex2 | Sol * | Luna * | ZL a* | SP * | CR * | KL | LI | AL b | TML |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 55 | 56 | 1 | 1 | 5 | 1 | 4 | 4 | 1 | 16 | 3 | |
| Cyanobacteria (Cyanophyceae) | 187 | 65 | 9 | 3 | 12 | 1 | 6 | 3 | 2 | 1 | 1 |
| 18% | 10% | 10% | 5% | 22% | 4% | 6% | 11% | 6% | 1% | 2% | |
| Ochrophyta (Chrysophyceae and Synurophyceae) | 37 | 10 | 7 | 3 | 2 | 0 | 2 | 0 | 4 | 16 | 11 |
| 4% | 1% | 8% | 5% | 4% | 0% | 2% | 0% | 11% | 20% | 27% | |
| Bacillariophyta (Coscinodiscophycea, Mediophyceae, and Bacillariophyceae) | 327 | 262 | 32 | 27 | - | 5 | 15 | 15 | 4 | 32 | 6 |
| 32% | 40% | 35% | 43% | - | 18% | 16% | 54% | 11% | 40% | 15% | |
| Haptophyta | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (Coccolitophyceae) | 0% | 0% | 1% | 2% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| Cryptophyta (Cryptophyceae) | 8 | 2 | 4 | 3 | 1 | 3 | 1 | 0 | 5 | 7 | 2 |
| 1% | (<1%) | 4% | 5% | 2% | 11% | 1% | 0% | 14% | 9% | 5% | |
| Miozoa | 28 | 6 | 4 | 3 | 2 | 2 | 3 | 0 | 5 | 7 | 6 |
| (Dinophyceae) | 3% | 1% | 4% | 5% | 4% | 7% | 3% | 0% | 14% | 9% | 15% |
| Euglenozoa (Euglenophyceae) | 95 | 13 | 4 | 0 | 7 | 2 | 9 | 2 | 0 | 0 | 1 |
| 9% | 2% | 4% | 0% | 13% | 7% | 9% | 7% | 0% | 0% | 2% | |
| Chorophyta (Chlorophyceae) | 233 | 307 | 19 | 12 | 21 | 13 | 37 | 7 | 8 | 6 | 10 |
| 23% | 46% | 21% | 19% | 38% | 48% | 38% | 25% | 23% | 7% | 25% | |
| Charophyta (Charophyceae, Coleochaetophyceae, and Conjugatophyceae) | 108 | _ | 12 | 11 | 8 | 1 | 23 | 1 | 6 | 10 | 3 |
| 10% | 13% | 17% | 15% | 4% | 24% | 4% | 17% | 12% | 7% | ||
| Total | 1025 | 668 | 92 | 63 | 55 | 27 | 96 | 28 | 35 | 79 | 40 |
| (Richness per lake) | (>200) | (1–40) | (6–49) | (6–15) |
| Surface Area | Macrophytes | Trophic Status | pH | UVR | Fish Introduction | ||
|---|---|---|---|---|---|---|---|
| Coverage | Microhabitats | ||||||
| Theoretical | + | − | + | − | + | − | − |
| Actual | ✔ | X | ✔ | X | ✔ | ✔ | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuna, E.; Alcocer, J.; Gaytán, M.; Caballero, M. Phytoplankton Biodiversity in Two Tropical, High Mountain Lakes in Central Mexico. Diversity 2022, 14, 42. https://doi.org/10.3390/d14010042
Cuna E, Alcocer J, Gaytán M, Caballero M. Phytoplankton Biodiversity in Two Tropical, High Mountain Lakes in Central Mexico. Diversity. 2022; 14(1):42. https://doi.org/10.3390/d14010042
Chicago/Turabian StyleCuna, Estela, Javier Alcocer, Martha Gaytán, and Margarita Caballero. 2022. "Phytoplankton Biodiversity in Two Tropical, High Mountain Lakes in Central Mexico" Diversity 14, no. 1: 42. https://doi.org/10.3390/d14010042






