Species-Level Versus Community-Level Responses to Microhabitat Type and Diversity in an Experimental Plant Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Species
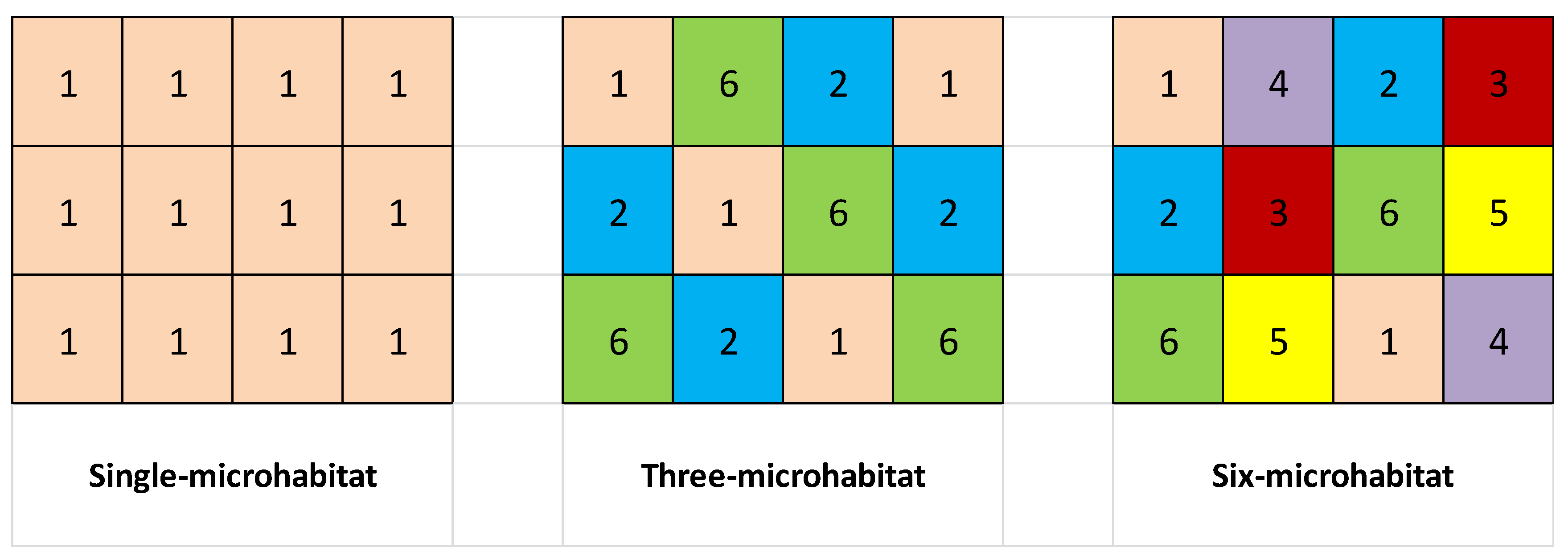
2.2. The Experiment
2.3. Harvest and Measurement
2.4. Data Analysis
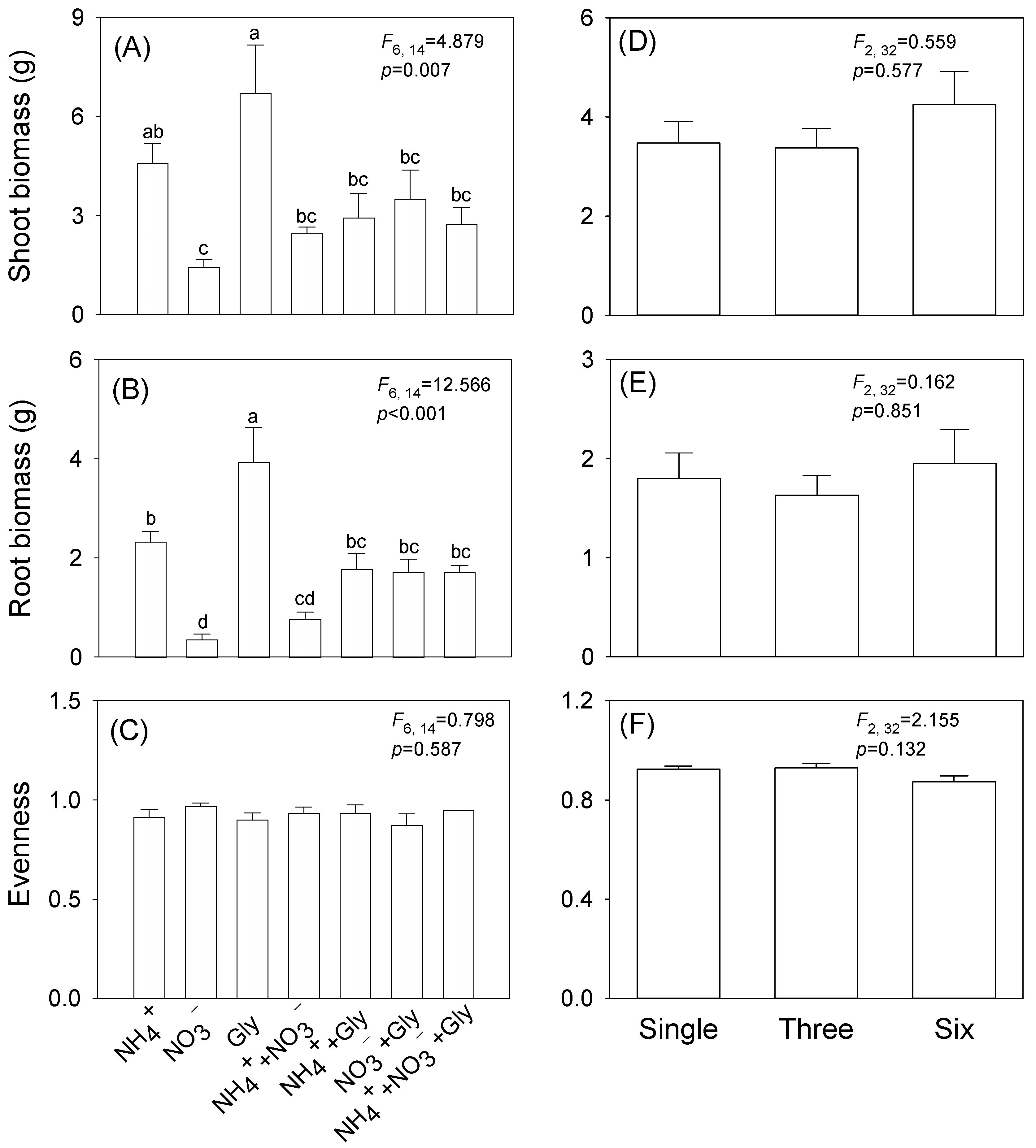
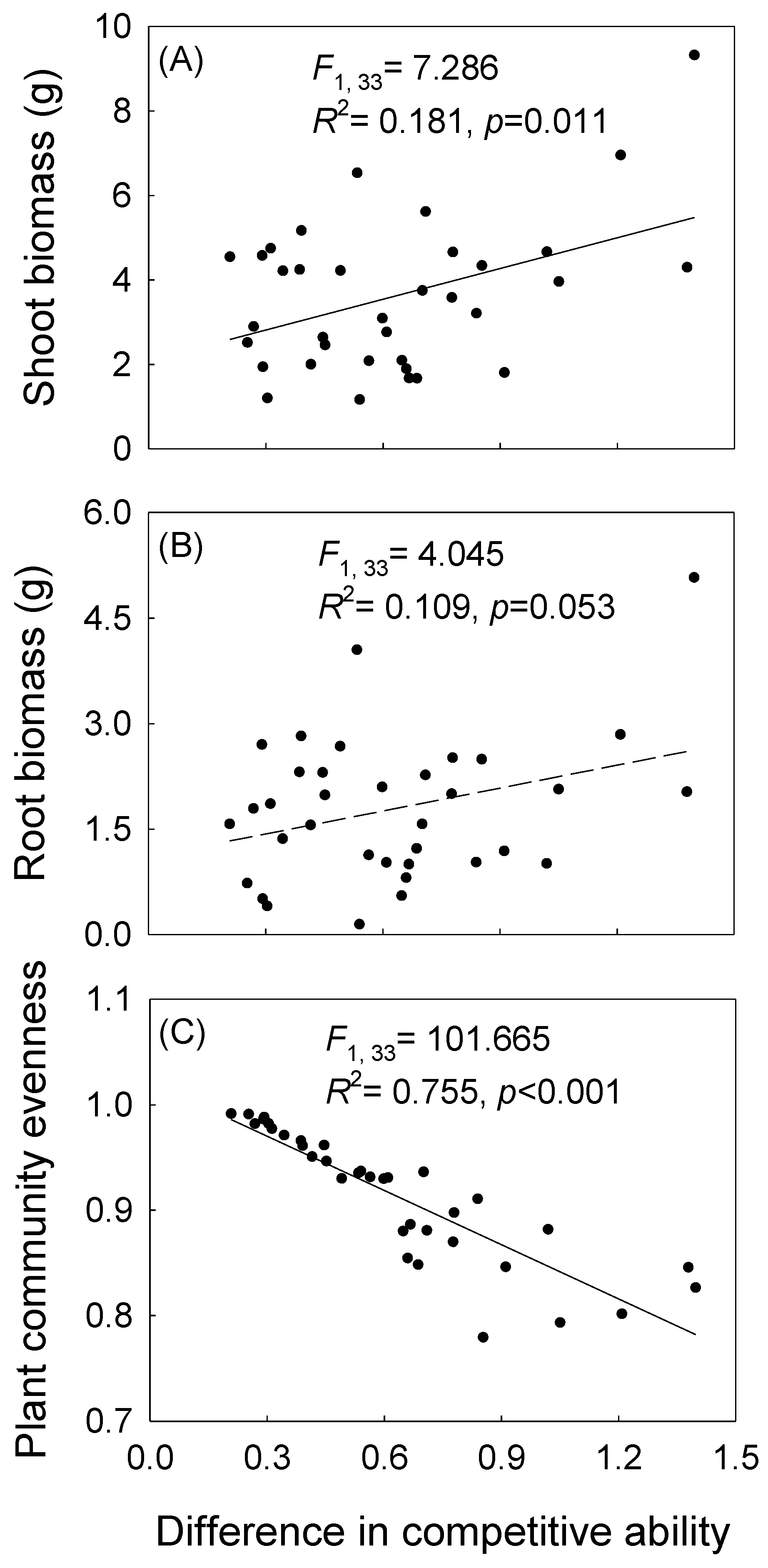
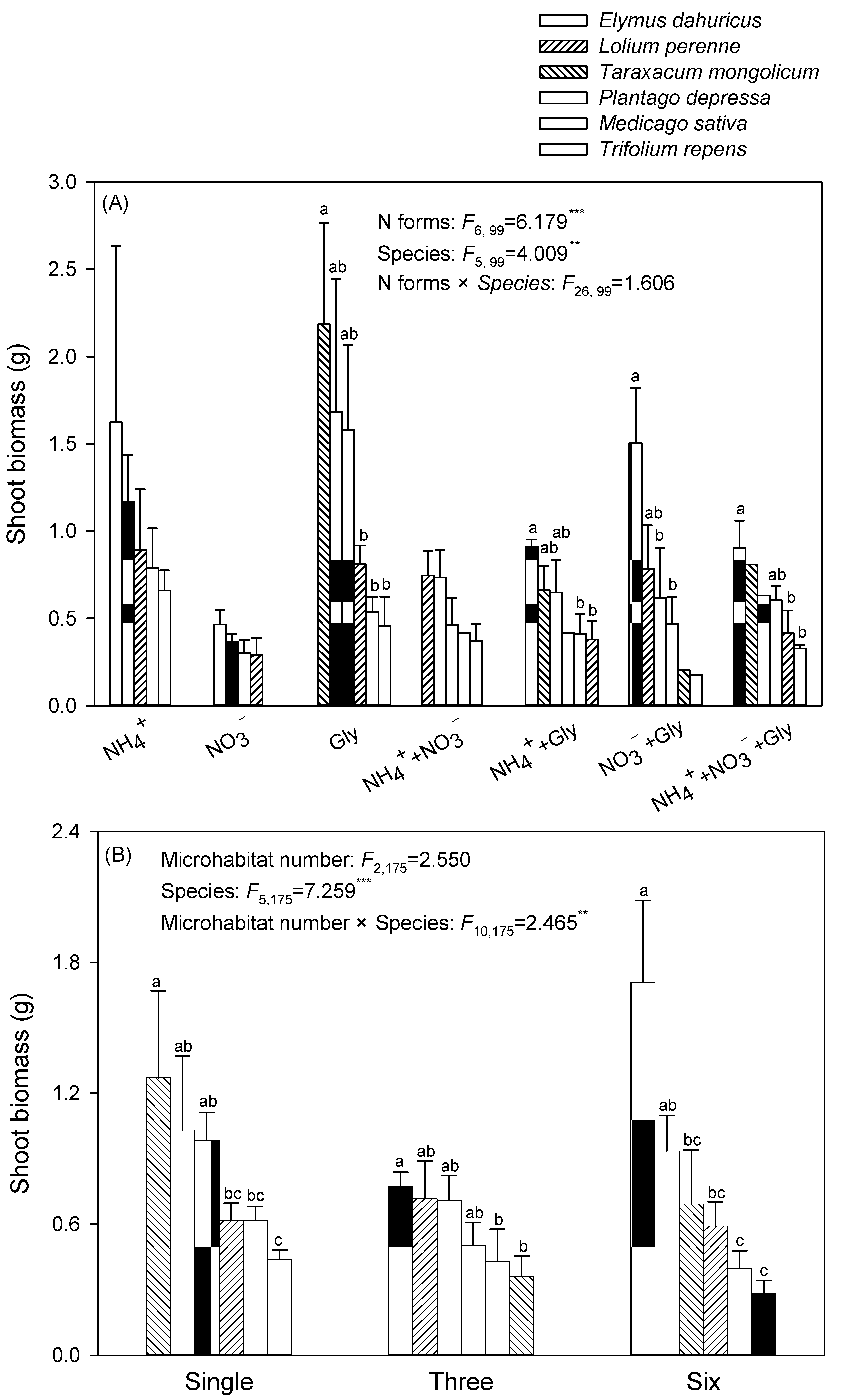
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tilman, P.; Pacala, S. The Maintenance of Species Richness in Plant Communities. In Species Diversity in Ecological Communities; Ricklefs, R.E., Schluter, D., Eds.; University of Chicago: Chicago, IL, USA, 1993; pp. 557–569. [Google Scholar]
- Shmida, A.; Wilson, M.V. Biological Determinants of Species Diversity. J. Biogeogr. 1985, 12, 1–20. [Google Scholar] [CrossRef]
- Silvertown, J. Plant Coexistence and the Niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Levine, J.M.; HilleRisLambers, J. The Importance of Niches for the Maintenance of Species Diversity. Nature 2009, 461, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.B. The Twelve Theories of Co-existence in Plant Communities: The Doubtful, the Important and the Unexplored. J. Veg. Sci. 2010, 22, 184–195. [Google Scholar] [CrossRef]
- Pausas, J.G. Species Richness Patterns in the Understorey of Pyrenean Pinus Sylvestris Forest. J. Veg. Sci. 1994, 5, 517–524. [Google Scholar] [CrossRef]
- Bakker, C.; Blair, J.M.; Knapp, A.K. Does Resource Availability, Resource Heterogeneity or Species Turnover Mediate Changes in Plant Species Richness in Grazed Grasslands? Oecologia 2003, 137, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Baer, S.G. Diversity Patterns from Sequentially Restored Grasslands Support the ‘Environmental Heterogeneity Hypothesis’. Oikos 2019, 128, 1116–1122. [Google Scholar] [CrossRef]
- Stevens, M.H.H.; Carson, W.P. Resource Quantity, Not Resource Heterogeneity, Maintains Plant Diversity. Ecol. Lett. 2002, 5, 420–426. [Google Scholar] [CrossRef]
- Gazol, A.; Tamme, R.; Price, J.N.; Hiiesalu, I.; Laanisto, L.; Paertel, M. A Negative Heterogeneity-diversity Relationship Found in Experimental Grassland Communities. Oecologia 2013, 173, 545–555. [Google Scholar] [CrossRef]
- Xue, W.; Bezemer, T.M.; Berendse, F. Soil Heterogeneity and Plant Species Diversity in Experimental Grassland Communities: Contrasting Effects of Soil Nutrients and pH at Different Spatial Scales. Plant Soil 2019, 442, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, D.K.; John, E.A.; Hutchings, M.J. Does Pattern of Soil Resource Heterogeneity Determine Plant Community Structure? An Experimental Investigation. J. Ecol. 2005, 93, 99–112. [Google Scholar] [CrossRef]
- Liu, Y.; De Boeck, H.J.; Li, Z.; Nijs, I. Unimodal Relationship Between Three-dimensional Soil Heterogeneity and Plant Species Diversity in Experimental Mesocosms. Plant Soil 2019, 436, 397–411. [Google Scholar] [CrossRef]
- Xue, W.; Huang, L.; Yu, F.-H. Increasing Soil Configurational Heterogeneity Promotes Plant Community Evenness Through Equalizing Differences in Competitive ability. Sci. Total Environ. 2021, 750, 142308. [Google Scholar] [CrossRef] [PubMed]
- Stover, H.J.; Henry, H.A.L. Legacy Effects of Soil Homogenization on Tallgrass Prairie Restoration: Toward Resolved Understanding of the Relationship Between Soil Heterogeneity and Plant Species Diversity. Restor. Ecol. 2020, 28, 93–103. [Google Scholar] [CrossRef]
- Lundholm, J.T. Plant Species Diversity and Environmental Heterogeneity: Spatial Scale and Competing Hypotheses. J. Veg. Sci. 2009, 20, 377–391. [Google Scholar] [CrossRef]
- Williams, B.M.; Houseman, G.R. Experimental Evidence that Soil Heterogeneity Enhances Plant Diversity During Community Assembly. J. Plant Ecol. 2014, 7, 461–469. [Google Scholar] [CrossRef]
- Baer, S.G.; Blair, J.M.; Collins, S.L. Environmental Heterogeneity Has a Weak Effect on Diversity During Community Assembly in Tallgrass Prairie. Ecol. Monogr. 2016, 86, 94–106. [Google Scholar] [CrossRef]
- Eilts, J.A.; Mittelbach, G.G.; Reynolds, H.L.; Gross, K.L. Resource Heterogeneity, Soil Fertility, and Species Diversity: Effects of Clonal Species on Plant Communities. Am. Nat. 2011, 177, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; McNaughton, S.J.; Ritchie, M.E. Scale-dependent Relationships Between the Spatial Distribution of a Limiting Resource and Plant Species Diversity in an African Grassland Ecosystem. Oecologia 2004, 139, 277–287. [Google Scholar] [CrossRef]
- Schimel, J.P.; Chapin, F.S. Tundra Plant Uptake of Amino Acid and NH4+ Nitrogen in Situ: Plants Complete Well for Amino Acid N. Ecology 1996, 77, 2142–2147. [Google Scholar] [CrossRef]
- Ohlund, J.; Nasholm, T. Growth of Conifer Seedlings on Organic and Inorganic Nitrogen Sources. Tree Physiol. 2001, 21, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.N.; Prescott, C.E. Organic and Inorganic Nitrogen Nutrition of Western Red Cedar, Western Hemlock and Salal in Mineral N-limited Cedar-hemlock Forests. Oecologia 2004, 141, 468–476. [Google Scholar] [CrossRef]
- Willey, N.; Tang, S. Some Effects of Nitrogen Nutrition on Caesium Uptake and Translocation by Species in the Poaceae, Asteraceae and Caryophyllidae. Environ. Exp. Bot. 2006, 58, 114–122. [Google Scholar] [CrossRef]
- Metcalfe, R.J.; Nault, J.; Hawkins, B.J. Adaptations to Nitrogen form: Comparing Inorganic Nitrogen and Amino Acid Availability and Uptake by Four Temperate Forest Plants. Can. J. For. Res. 2011, 41, 1626–1637. [Google Scholar] [CrossRef]
- Yan, L.; Xu, X.; Xia, J. Different Impacts of External Ammonium and Nitrate Addition on Plant Growth in Terrestrial Ecosystems: A Meta-analysis. Sci. Total Environ. 2019, 686, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Ashton, I.W.; Miller, A.E.; Bowman, W.D.; Suding, K.N. Niche Complementarity Due to Plasticity in Resource Use: Plant Partitioning of Chemical N Forms. Ecology 2010, 91, 3252–3260. [Google Scholar] [CrossRef]
- Bowman, W.D.; Theodose, T.A.; Schardt, J.C.; Conant, R.T. Constraints of Nutrient Availability on Primary Production in Two Alpine Tundra Communities. Ecology 1993, 74, 2085–2097. [Google Scholar] [CrossRef]
- Jumpponen, A.; Hogberg, P.; Huss-Danell, K.; Mulder, C.P.H. Interspecific and Spatial Differences in Nitrogen Uptake in Monocultures and Two-species Mixtures in North European Grasslands. Funct. Ecol. 2002, 16, 454–461. [Google Scholar] [CrossRef]
- Spehn, E.M.; Scherer-Lorenzen, M.; Schmid, B.; Hector, A.; Caldeira, M.C.; Dimitrakopoulos, P.G.; Finn, J.A.; Jumpponen, A.; O’Donnovan, G.; Pereira, J.S.; et al. The Role of Legumes as a Component of Biodiversity in a Cross-European Study of Grassland Biomass Nitrogen. Oikos 2002, 98, 205–218. [Google Scholar] [CrossRef]
- Lambers, H.; Scheurwater, I.; Mata, C.; Nagel, O.W. Root Respiration of Fast- and Slow-growing Plants, as Dependent on Genotype and Nitrogen Supply: A Major Clue to the Functioning of Slow-growing Plants. Proc. Inherent Var. Plant Growth Physiol. Mech. Ecol. Conseq. 1998, 139–157. [Google Scholar]
- Konnerup, D.; Brix, H. Nitrogen Nutrition of Canna Indica: Effects of Ammonium Versus Nitrate on Growth, Biomass Allocation, Photosynthesis, Nitrate Reductase Activity and N Uptake Rates. Aquat. Bot. 2010, 92, 142–148. [Google Scholar] [CrossRef]
- Owen, A.G.; Jones, D.L. Competition for Amino Acids Between Wheat Roots and Rhizosphere Microorganisms and the Role of Amino Acids in Plant N Acquisition. Soil Biol. Biochem. 2001, 33, 651–657. [Google Scholar] [CrossRef]
- Nasholm, T.; Huss-Danell, K.; Hogberg, P. Uptake of Organic Nitrogen in the Field by Four Agriculturally Important Plant Species. Ecology 2000, 81, 1155–1161. [Google Scholar] [CrossRef]
- Nasholm, T.; Persson, J. Plant Acquisition of Organic Nitrogen in Boreal Forests. Physiol. Plant. 2001, 111, 419–426. [Google Scholar] [CrossRef]
- Luo, S.; Schmid, B.; De Deyn, G.B.; Yu, S.X. Soil Microbes Promote Complementarity Effects Among Co-existing Trees Through Soil Nitrogen Partitioning. Funct. Ecol. 2018, 32, 1879–1889. [Google Scholar] [CrossRef]
- Puri, G.; Ashman, M.R. Microbial Immobilization of N-15-labelled Ammonium and Nitrate in a Temperate Woodland Soil. Soil Biol. Biochem. 1999, 31, 929–931. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Why Plants Bother: Root Proliferation Results in Increased Nitrogen Capture from an Organic Patch when Two Grasses Compete. Plant Cell Environ. 1999, 22, 811–820. [Google Scholar] [CrossRef]
- Hodge, A.; Stewart, J.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Root Proliferation, Soil Fauna and Plant Nitrogen Capture from Nutrient-rich Patches in Soil. New Phytol. 1998, 139, 479–494. [Google Scholar] [CrossRef]
- Wei, G.W.; van Kleunen, M. Soil Heterogeneity Tends to Promote the Growth of Naturalized Aliens when Competing with Native Plant Communities. J. Ecol. 2022, 110, 1161–1173. [Google Scholar] [CrossRef]
- Liang, J.F.; Yuan, W.Y.; Gao, J.Q.; Roiloa, S.R.; Song, M.H.; Zhang, X.Y.; Yu, F.H. Soil Resource Heterogeneity Competitively Favors an Invasive Clonal Plant Over a Native One. Oecologia 2020, 193, 155–165. [Google Scholar] [CrossRef]
- Hendriks, M.; Ravenek, J.M.; Smit-Tiekstra, A.E.; van der Paauw, J.W.; de Caluwe, H.; van der Putten, W.H.; de Kroon, H.; Mommer, L. Spatial Heterogeneity of Plant-soil Feedback Affects Root Interactions and Interspecific Competition. New Phytol. 2015, 207, 830–840. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Shu, M.; Mou, P.; Weiner, J. Fine Root Responses to Temporal Nutrient Heterogeneity and Competition in Seedlings of Two Tree Species with Different Rooting Strategies. Ecol. Evol. 2018, 8, 3367–3375. [Google Scholar] [CrossRef]
- Song, M.H.; Yu, F.H. Reduced Compensatory Effects Explain the Nitrogen-mediated Reduction in Stability of an Alpine Meadow on the Tibetan Plateau. New Phytol. 2015, 207, 70–77. [Google Scholar] [CrossRef]
- Huangfu, C.H.; Li, H.Y.; Chen, X.W.; Liu, H.M.; Wang, H.; Yang, D.L. Response of an Invasive Plant, Flaveria Bidentis, to Nitrogen Addition: A Test of form-Preference Uptake. Biol. Invasions 2016, 18, 3365–3380. [Google Scholar] [CrossRef]
- Grassein, F.; Lemauviel-Lavenant, S.; Lavorel, S.; Bahn, M.; Bardgett, R.D.; Desclos-Theveniau, M.; Laine, P. Relationships Between Functional Traits and Inorganic Nitrogen Acquisition Among Eight Contrasting European Grass Species. Ann. Bot. 2015, 115, 107–115. [Google Scholar] [CrossRef]
- Gaiad, S.; Rakocevic, M.; Reissmann, C.B. N Sources Affect Growth, Nutrient Content, and Net Photosynthesis In Maté (Ilex Paraguariensis St. Hil.). Braz. Arch. Biol. Technol. 2006, 49, 689–697. [Google Scholar] [CrossRef]
- Hutchings, M.J.; John, E.A.; Wijesinghe, D.K. Toward Understanding the Consequences of Soil Heterogeneity for Plant Populations and Communities. Ecology 2003, 84, 2322–2334. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, W.; He, D.; Xiang, Y.; Liu, J.; Huang, H.; Chen, M.; Tao, J. Soil Resource Availability Is Much More Important than Soil Resource Heterogeneity in Determining the Species Diversity and Abundance of Karst Plant Communities. Ecol. Evol. 2021, 11, 16680–16692. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Xu, Z.-W.; Xue, W.; Yu, F.-H. Species-Level Versus Community-Level Responses to Microhabitat Type and Diversity in an Experimental Plant Community. Diversity 2022, 14, 803. https://doi.org/10.3390/d14100803
Hu B, Xu Z-W, Xue W, Yu F-H. Species-Level Versus Community-Level Responses to Microhabitat Type and Diversity in an Experimental Plant Community. Diversity. 2022; 14(10):803. https://doi.org/10.3390/d14100803
Chicago/Turabian StyleHu, Bing, Zhu-Wen Xu, Wei Xue, and Fei-Hai Yu. 2022. "Species-Level Versus Community-Level Responses to Microhabitat Type and Diversity in an Experimental Plant Community" Diversity 14, no. 10: 803. https://doi.org/10.3390/d14100803
APA StyleHu, B., Xu, Z.-W., Xue, W., & Yu, F.-H. (2022). Species-Level Versus Community-Level Responses to Microhabitat Type and Diversity in an Experimental Plant Community. Diversity, 14(10), 803. https://doi.org/10.3390/d14100803






