Species Diversity of Gelidium from Southern Madagascar Evaluated by an Integrative Taxonomic Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results
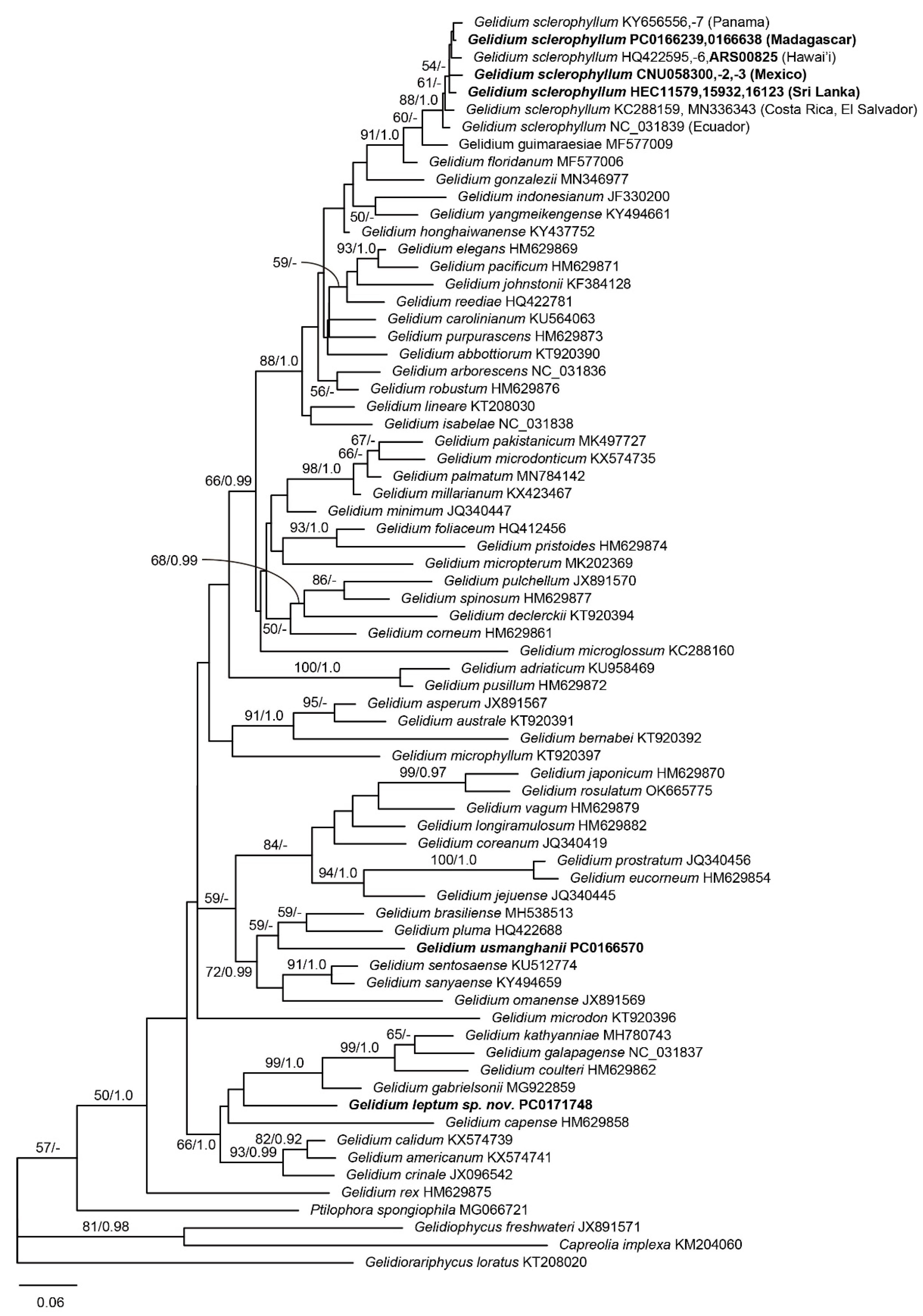
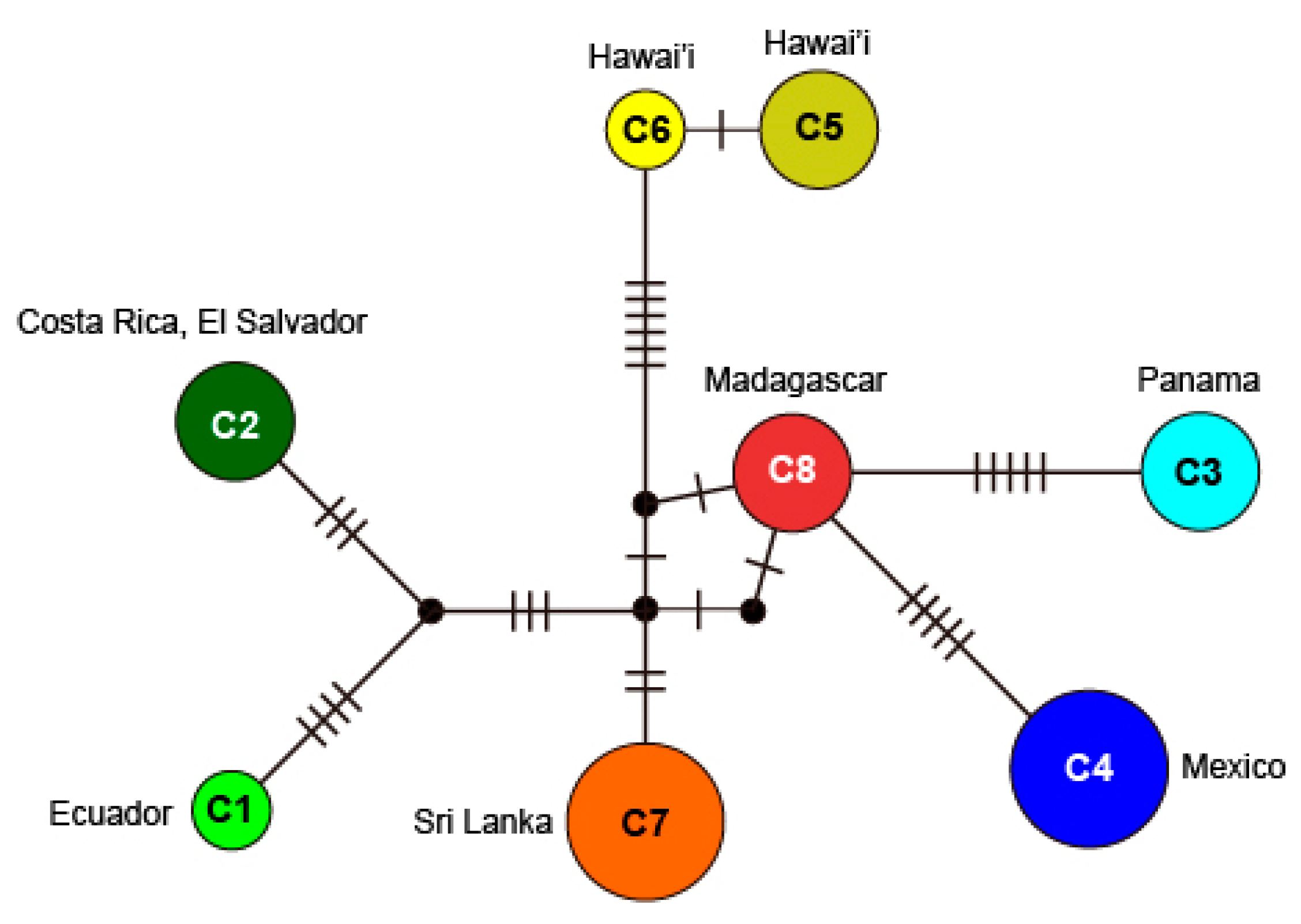
3.1. Molecular Phylogeny
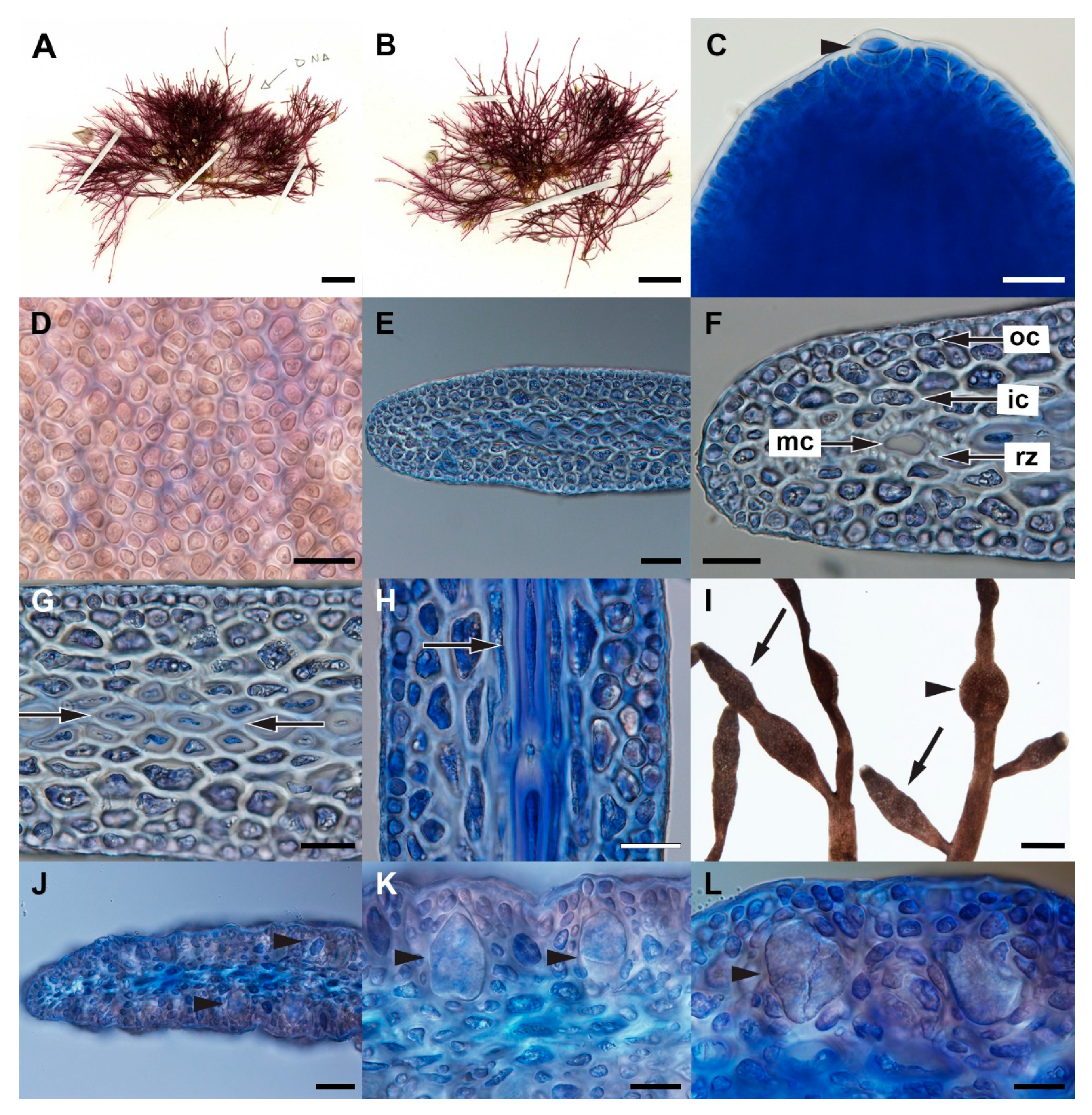
3.2. Gelidium leptum G.H.Boo, L.Le Gall, and H.S.Yoon, sp. nov.
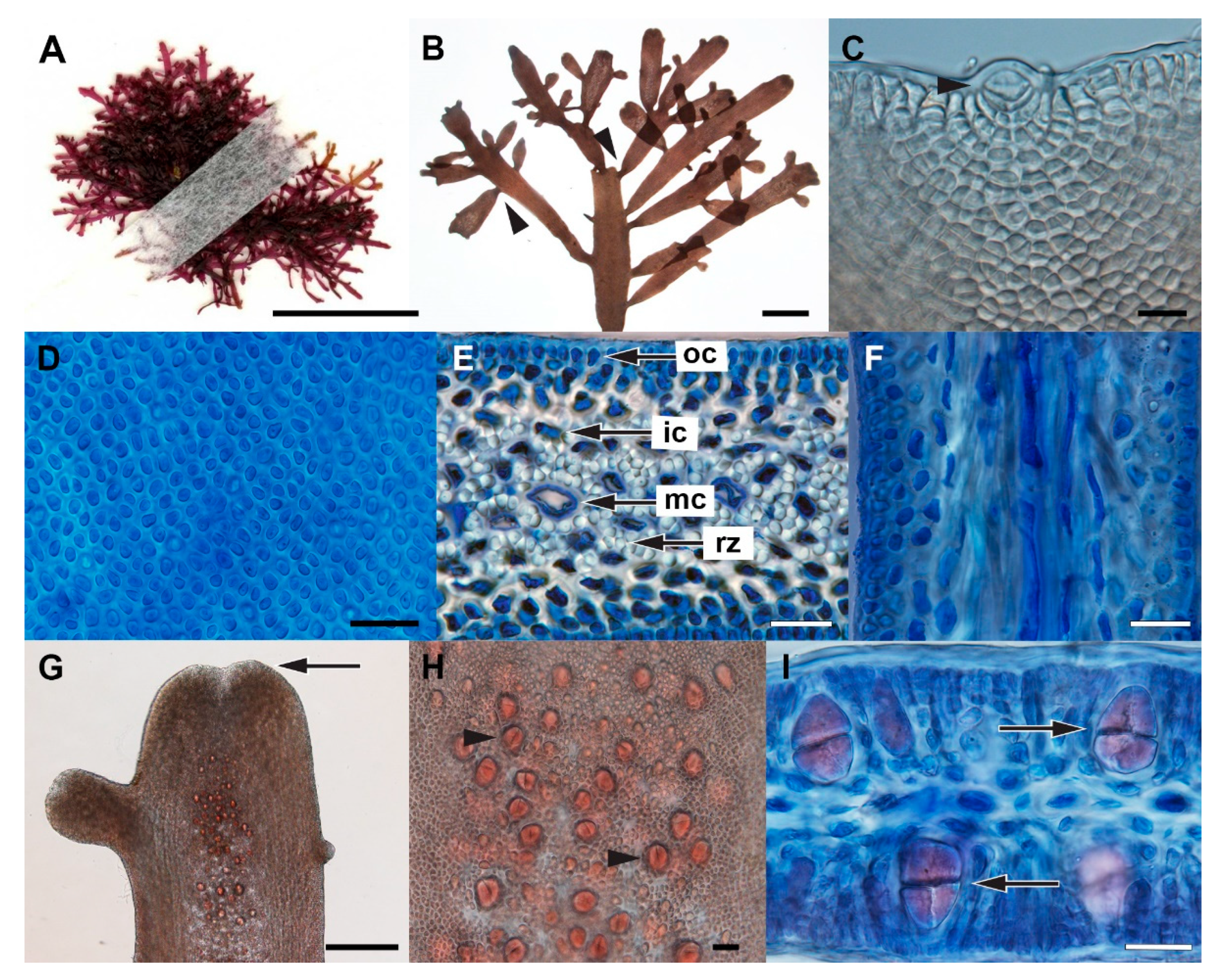
3.3. Gelidium sclerophyllum W.R.Taylor
3.4. Gelidium usmanghanii Afaq-Husain and Shameel
4. Discussion
4.1. Taxonomic Implication
4.2. Biogeographic Implication
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: http://www.algaebase.org (accessed on 5 March 2022).
- Womersley, H.B.S.; Guiry, M.D. Order Gelidiales Kylin 1923:132. In The Marine Benthic Flora of Southern Australia. Part IIIA; Womersley, H.B.S., Ed.; Australian Biological Resources Study: Adelaide, Australia, 1994; pp. 118–142. [Google Scholar]
- Freshwater, D.W.; Fredericq, S.; Hommersand, M.H. A molecular phylogeny of the Gelidiales (Rhodophyta) based on analysis of plastid rbcL nucleotide sequences. J. Phycol. 1995, 31, 616–632. [Google Scholar] [CrossRef]
- Boo, G.H.; Kim, K.M.; Nelson, W.A.; Riosmena-Rodriguez, R.; Yoon, K.J.; Boo, S.M. Phylogenetic relationships and distribution of the selected species of the agarophyte genus Gelidium (Gelidiales, Rhodophyta). J. Appl. Phycol. 2014, 26, 1243–1251. [Google Scholar] [CrossRef]
- Iha, C.; Milstein, D.; Guimarães, S.M.P.B.; Freshwater, D.W.; Oliveira, M.C. DNA barcoding reveals high diversity in the Gelidiales of the Brazilian southeast coast. Bot. Mar. 2015, 58, 295–305. [Google Scholar] [CrossRef]
- Callaway, E. Lab staple agar hit by seaweed shortage. Nature 2015, 528, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Lamouroux, J.V. Essai sur les genres de la famille des thalassiophytes non articulées. Ann. Mus. Hist. Nat. 1813, 20, 21–47, 115–139, 267–293. [Google Scholar]
- Schmitz, F. Systematische Übersicht der bisher bekannten Gattungen der Florideen. Flora 1889, 72, 435–456. [Google Scholar]
- Fan, K.C. Morphological studies of the Gelidiales. Univ. Calif. Publ. Bot. 1961, 32, 315–368. [Google Scholar]
- Millar, A.J.K.; Freshwater, D.W. Morphology and molecular phylogeny of the marine algal order Gelidiales (Rhodophyta) from New South Wales, including Lord Howe and Norfolk Island. Aust. Syst. Bot. 2005, 18, 215–263. [Google Scholar] [CrossRef]
- Boo, G.H.; Park, J.K.; Han, K.S.; Yoon, H.S. Gelidium rosulatum (Gelidiales, Rhodophyta), a new species of subtidal marine algae from Korea. Phycologia 2022, 61, 332–340. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Rueness, J. Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia 1994, 33, 187–194. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Tudor, K.; O’Shaughnessy, K.; Wysor, B. DNA barcoding in the red algal order Gelidiales: Comparison of COI with rbcL and verification of the “barcoding gap”. Cryptogam. Algol. 2010, 31, 435–449. [Google Scholar]
- Kim, K.M.; Hwang, I.K.; Yoon, H.S.; Boo, S.M. Four novel Gelidium species (Gelidiales, Rhodophyta) discovered in Korea: G. coreanum, G. jejuense, G. minimum and G. prostratum. Phycologia 2012, 51, 461–474. [Google Scholar] [CrossRef]
- Perrone, C.; Bottalico, A.; Boo, G.H.; Boo, S.M.; Miller, K.A.; Freshwater, D.W. Gelidium adriaticum sp. nov. and Gelidium carolinianum sp. nov. (Gelidiales, Rhodophyta) from the Mediterranean Sea. Phycologia 2019, 58, 359–373. [Google Scholar] [CrossRef]
- Boo, G.H.; Le Gall, L.; Miller, K.A.; Freshwater, D.W.; Wernberg, T.; Terada, R.; Yoon, K.J.; Boo, S.M. A novel phylogeny of the Gelidiales (Rhodophyta) based on five genes including the nuclear CesA, with descriptions of Orthogonacladia gen. nov. and Orthogonacladiaceae fam. nov. Mol. Phylogenet. Evol. 2016, 101, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.C.; Freshwater, D.W. Molecular systematics of the Gelidiales: Inferences from separate and combined analyses of plastid rbcL and nuclear SSU gene sequences. Eur. J. Phycol. 1997, 32, 343–352. [Google Scholar] [CrossRef]
- Patwary, M.U.; Sensen, C.W.; MacKay, R.M.; van der Meer, J.P. Nucleotide sequences of small-subunit and internal transcribed spacer regions of nuclear rRNA genes support the autonomy of some genera of the Gelidiales (Rhodophyta). J. Phycol. 1998, 34, 299–305. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Fredericq, S.; Bailey, J.C. Characteristics and utility of nuclear-encoded large-subunit ribosomal gene sequences in phylogenetic studies of red algae. Phycol. Res. 1999, 47, 33–38. [Google Scholar] [CrossRef]
- Steen, F.; Vieira, C.; Leliaert, F.; Payri, E.C.; De Clerck, O. Biogeographic affinities of Dictyotales from Madagascar: A phylogenetic approach. Cryptogam. Algol. 2015, 36, 129–141. [Google Scholar] [CrossRef]
- Verbruggen, H.; Costa, J.E. Molecular survey of Codium species diversity in southern Madagascar. Cryptogam. Algol. 2015, 36, 171–187. [Google Scholar] [CrossRef]
- Vieira, C.; N’Yeurt, A.D.; Rasoamanendrinka, F.; D’Hondt, S.; Thi Tran, L.-A.; Spiegel, D.V.; Kawai, H.; De Clerck, O. Marine macroalgal biodiversity of northern Madagascar: Morpho-genetic systematics and implications of anthropic impacts for conservation. Biodivers. Conserv. 2021, 30, 1501–1546. [Google Scholar] [CrossRef]
- Boo, G.H.; Leliaert, F.; Le Gall, L.; Coppejans, E.; De Clerck, O.; Nguyen, V.T.; Miller, K.A.; Yoon, H.S. Ancient Tethyan vicariance and long-distance dispersal drive global diversification and cryptic speciation in the red seaweed Pterocladiella. Front. Plant Sci. 2022, 13, 849476. [Google Scholar] [CrossRef] [PubMed]
- Andriamampandry, A.V. Beckerella pterocladioides sp. nov. et Gelidium madagascariense sp. nov. deux espèces de Gelidiales-rhodophytes de Fort-Dauphin (Madagascar). Cryptogam. Algol. 1988, 9, 243–259. [Google Scholar]
- Silva, P.C.; Basson, P.W.; Moe, R.L. Catalogue of the benthic marine algae of the Indian Ocean. Univ. Calif. Publ. Bot. 1996, 27, 1–1259. [Google Scholar]
- Cormaci, M.; Furnari, G.; Alongi, G. Benthic marine flora of the Mediterranean Sea: Rhodophyta—Rhodymeniophycidae I. Acrosymphytales, Bonnemaisoniales, Gelidiales, Gigartinales, Gracilariales. Boll. Accad. Gioenia Nat. Sci. 2020, 53, FP11–FP346. [Google Scholar] [CrossRef]
- Mollion, J. The seaweed resources of Madagascar. Bot. Mar. 2020, 63, 97–104. [Google Scholar] [CrossRef]
- Boo, G.H.; Le Gall, L.; Rousseau, F.; De Reviers, B.; Coppejans, E.; Anderson, R.; Boo, S.M. Phylogenetic relationships and morphology of Gelidiella (Gelidiales, Rhodophyta) from Madagascar with a description of Gelidiella incrassata sp. nov. Cryptogam. Algol. 2015, 36, 219–237. [Google Scholar] [CrossRef]
- Manghisi, A.; Morabito, M.; Boo, G.H.; Boo, S.M.; Bonillo, C.; De Clerck, O.; Le Gall, L. Two novel species of Yonagunia (Halymeniales, Rhodophyta) were uncovered in the South of Madagascar during the Atimo-Vatae expediction. Cryptogam. Algol. 2015, 36, 199–217. [Google Scholar] [CrossRef]
- Boo, G.H.; Le Gall, L.; Hwang, I.K.; Boo, S.M. Pterocladiella feldmannii sp. nov. and P. hamelii sp. nov. (Gelidiales, Rhodophyta), two new species uncovered in Madagascar during the Atimo Vatae Expedition. Cryptogam. Algol. 2016, 37, 179–198. [Google Scholar] [CrossRef]
- Boo, G.H.; Nguyen, T.V.; Kim, J.Y.; Le Gall, L.; Rico, J.M.; Bottalico, A.; Boo, S.M. A revised classification of the Gelidiellaceae (Rhodophyta) with descriptions of three new genera: Huismaniella, Millerella and Perronella. Taxon 2016, 65, 965–979. [Google Scholar] [CrossRef]
- Boo, G.H.; Le Gall, L.; Hwang, I.K.; Miller, K.A.; Boo, S.M. Phylogenetic relationships and biogeography of Ptilophora (Gelidiales, Rhodophyta) with descriptions of P. aureolusa, P. malagasya, and P. spongiophila from Madagascar. J. Phycol. 2018, 54, 249–263. [Google Scholar] [CrossRef]
- Boo, G.H.; Zubia, M.; Hughey, J.R.; Sherwood, A.R.; Fujii, M.T.; Boo, S.M.; Miller, K.A. Complete mitochondrial genomes reveal population-revel patterns in the widespread red alga Gelidiella fanii (Gelidiales, Rhodophyta). Front. Mar. Sci. 2020, 7, 583957. [Google Scholar] [CrossRef]
- Lin, S.-M.; Fredericq, S.; Hommersand, M.H. Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on large subunit rDNA and rbcL sequences, including the Phycodryoideae, subfam. nov. J. Phycol. 2001, 37, 881–889. [Google Scholar] [CrossRef]
- Gavio, B.; Fredericq, S. Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora. Eur. J. Phycol. 2002, 37, 349–359. [Google Scholar] [CrossRef]
- Geraldino, P.J.L.; Yang, E.C.; Boo, S.M. Morphology and molecular phylogeny of Hypnea flexicaulis (Gigartinales, Rhodophyta) from Korea. Algae 2006, 21, 417–423. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Iha, C.; Freshwater, D.W.; Guimarães, S.M.P.B.; Oliveira, M.C. Gelidiorariphycus gen. nov. (Gelidiales, Rhodophyta): A rare genus found in the Americas. Phycologia 2022, 61, 473–483. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Grusz, A.L.; Freshwater, D.W. Studies of Costa Rican Gelidiales (Florideophyceae). II. Two Pacific taxa including Gelidium microglossum, n. sp. Pac. Sci. 2014, 68, 97–110. [Google Scholar] [CrossRef]
- Taylor, W.R. Pacific marine algae of the Allan Hancock expeditions to the Galapagos Islands. Allan Hancock Pac. Exped. 1945, 12, 1–528. [Google Scholar]
- Boo, G.H.; Hughey, J.R.; Miller, K.A.; Boo, S.M. Mitogenomes from type specimens, a genotyping tool for morphologically simple species: Ten genomes of agar-producing red algae. Sci. Rep. 2016, 6, 35337. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-González, N.; Ponce-Márquez, M.A.E.; Fernández-García, C.; Rodríguez, D. Gelidium gonzalezii sp. nov. (Gelidiales, Rhodophyta) from the Mexican tropical Pacific based on molecular and morphological evidence. Phytotaxa 2020, 459, 124–138. [Google Scholar] [CrossRef]
- Sherwood, A.R.; Kurihara, A.; Conklin, K.Y.; Sauvage, T.; Presting, G.G. The Hawaiian Rhodophyta biodiversity survey (2006–2010): A summary of principal findings. BMC Plant Biol. 2010, 10, 258. [Google Scholar] [CrossRef]
- Fernández-García, C.; Riosmena-Rodríguez, R.; Wysor, B.; Tejada, O.L.; Cortéz, J. Checklist of the Pacific marine macroalgae of Central America. Bot. Mar. 2011, 54, 53–73. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Idol, J.W.; Parham, S.L.; Fernández-García, C.; León, N.; Gabrielson, P.W.; Wysor, B. Molecular assisted identification reveals hidden red algae diversity from the Burica Peninsula, Pacific Panama. Diversity 2017, 9, 19. [Google Scholar] [CrossRef]
- Afaq-Husain, S.; Shameel, M. Morphology, anatomy and reproduction of the populations of a new alga, Gelidium usmanghanii (Gelidiales, Rhodophyta). Candollea 1996, 51, 433–443. [Google Scholar]
- Afaq-Husain, S.; Shameel, M. Further studies on Gelidium (Rhodophyta) from the coast of Pakistan. Pak. J. Bot. 1999, 31, 371–382. [Google Scholar]
- Freshwater, D.W.; Shahnaz, L. Phylogenetic relationships of Pakistan Gelidium (Gelidiales, Rhodophyta) species with recognition of Gelidium pakistanicum stat. nov. Bot. Mar. 2019, 62, 141–147. [Google Scholar] [CrossRef]
- Wynne, M.J.; Freshwater, D.W. Gelidium omanense sp. nov. (Gelidiaceae, Rhodophyta) from the Sultanate of Oman. Bot. Mar. 2004, 47, 64–72. [Google Scholar] [CrossRef]
- Norris, J.N. Marine Algae of the Northern Gulf of California II. Rhodophyta; Smithsonian Contributions to Botany, Number 96; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2014; p. 555. [Google Scholar]
- Lamouroux, J.V. Dissertations sur Plusieurs Espèces de Fucus, peu Connues ou Nouvelles Avec Leur Description en Latin et en Français; de l’Imprimerie de Raymond Nouvel & Chez Treuttel et Würtz: Paris, France, 1805; p. 83. [Google Scholar]
- Børgesen, F. Some marine algae from Mauritius, III. Rhodophyceae, Part 2: Gelidiales, Cryptonemiales, Gigartinales. Kongelige Danske Videnskabernes Sleskab, Biologiske Meddelelser 1943, 19, 1–85. [Google Scholar]
- Norris, R.E. A critique on the taxonomy of an important agarophyte, Gelidium amansii. Jpn. J. Phycol. 1990, 38, 35–42. [Google Scholar]
- Santelices, B. A reassessment of the taxonomic status of Gelidium amansii (Lamouroux) Lamouroux. In Taxonomy of Economic Seaweeds; Abbott, I.A., Ed.; California Sea Grant College Program: La Jolla, CA, USA, 1994; Volume 4, pp. 37–53. [Google Scholar]
- Stegenga, H.; Bolton, J.J.; Anderson, R.J. Seaweeds of the South African West Coast; Contributions from the Bolus Herbarium: Cape Town, South Africa, 1997; pp. 1–655. [Google Scholar]
- Kylin, H. Verzeichnis einiger Rhodophyceen von Südafrica. Lunds Universitets Årsskrift, Ny Följd 1938, 34, 1–26. [Google Scholar]
- Norris, R.E. The marine red algae of Natal, South Africa: Order Gelidiales (Rhodophyta). Mem. Bot. Surv. S. Afr. 1992, 61, 1–43. [Google Scholar]
- Iha, C.; O’haughnessy, K.A.; Guimarães, S.M.P.B.; Oliveira, M.C.; Freshwater, D.W. Taxonomic reappraisal of Gelidium coarctatum (Gelidiales, Rhodophyta) and Gelidium lineare sp. nov. from the tropical western Atlantic. Phycologia 2016, 55, 555–563. [Google Scholar] [CrossRef]
- Boo, G.H.; Hughey, J.R. Phylogenomics and multigene phylogenies decipher two new cryptic marine algae from California, Gelidium gabrielsonii and G. kathyanniae (Gelidiales, Rhodophyta). J. Phycol. 2019, 55, 160–172. [Google Scholar] [CrossRef]
- Boo, S.M.; Fredriksen, S.; Rueness, J.; Lee, I.K. Field and culture studies on the life history of Camylaephora crassa (Okamura) Nakamura (Ceramiaceae, Rhodophyta). Bot. Mar. 1991, 34, 437–445. [Google Scholar] [CrossRef]
- Shahnaz, L.; Shehnaz, H.; Haider, A. Fourier transform infrared (FT-IR) spectroscopic investigations of four agarophytes from Northern Arabian Sea. Bangladesh J. Bot. 2019, 48, 925–932. [Google Scholar] [CrossRef]
- Rodríguez, D.; López, N.; González-González, J. Gelidiales (Rhodophyta) en las costas del Pacífico mexicano con énfasis en las especies tropicales. In Monografías Ficológicas; Sentíes, A., Dreckmann, K., Eds.; Universidad Autónoma Metropolitana: Mexico City, Mexico, 2008; Volume 3, pp. 27–74. [Google Scholar]
- Coleman, M.A.; Reddy, M.; Nimbs, M.J.; Marchell, A.; Al-Ghassani, S.A.; Bolton, J.J.; Jupp, B.P.; de Clerck, O.; Leliaert, F.; Champion, C.; et al. Loss of a globally unique kelp forest from Oman. Sci. Rep. 2022, 12, 5020. [Google Scholar] [CrossRef]
- Tronchin, E.M.; Freshwater, D.W.; Bolton, J.J.; Anderson, R.J. A reassessment and reclassification of species in the genera Onikusa Akatsuka and Suhria J. Agardh ex Endlicher (Gelidiales, Rhodophyta) based on molecular and morphological data. Bot. Mar. 2002, 45, 548–558. [Google Scholar] [CrossRef]
- Tronchin, E.M.; Freshwater, D.W. Four Gelidiales (Rhodophyta) new to southern Africa, Aphanta pachyrrhiza gen. et sp. nov., Gelidium profundum sp. nov., Pterocladiella caerulescens and P. psammophila sp. nov. Phycologia 2007, 46, 325–348. [Google Scholar] [CrossRef]


| Species | Voucher Code | Collection Information | cox1 | rbcL |
|---|---|---|---|---|
| Gelidium leptum | PC0171748-1 (MAD1729-1) | Baie des Galions, Madagascar (25°08′48″ S, 46°45′00″ E); 15.v.2010; E.Coppejans | OP137177 | OP137188 |
| G. leptum | PC0171748-2 (MAD1729-2) | Baie des Galions, Madagascar; 15.v.2010; E.Coppejans | NA | NA |
| G. leptum | PC0171748-3 (MAD1729-3) | Baie des Galions, Madagascar; 15.v.2010; E.Coppejans | NA | NA |
| G. sclerophyllum | PC0166239 (MAD0272) | Plage monseigneur, Fort Dauphin, Madagascar (25°02′09″ S, 46°59′54″ E); 2.v.2010; F.Rousseau, R.Anderson, and J.Tsarahevitra | OP137178 | OP137189 |
| G. sclerophyllum | PC0166638 (MAD0671) | Flacourt, Madagascar (25°01′42″ S, 47°00′06″ E); 13.v.2010; F.Rousseau, R.Anderson, and J.Tsarahevitra | OP137179 | OP137190 |
| G. sclerophyllum | UC1884229 | near northern limit of Bahia San Francisco, Esmeraldas, Ecuador; 11.ii.1934; W.R.Taylor | NC_031839 | OP137191 |
| G. sclerophyllum | CNU058300 | Cabo San Lucas, La Paz, Baja California Sur, Mexico; 01.iii.2014 | OP137180 | OP137192 |
| G. sclerophyllum | CNU058302 | Cabo San Lucas, La Paz, Baja California Sur, Mexico; 01.iii.2014 | OP137181 | NA |
| G. sclerophyllum | CNU058303 | Cabo San Lucas, La Paz, Baja California Sur, Mexico; 01.iii.2014 | OP137182 | NA |
| G. sclerophyllum | HEC11579 | Weligama, Sri Lanka; 07.i.1997 | OP137183 | NA |
| G. sclerophyllum | HEC15932 | Polhena Beach, Matara, Sri Lanka; 16.viii.2006 | OP137184 | OP137193 |
| G. sclerophyllum | HEC16123 | Hikkaduwa, Sri Lanka; 02.i.2007 | OP137185 | NA |
| G. sclerophyllum | ARS00825 | West Beach, Ewa Beach, Oahu, Hawai’i, USA; 3.iii.1985 | OP137186 | NA |
| G. usmanghanii | PC0166570-2 (MAD0603-2) | Sud de la baie, Lokaro, Madagascar (24°57′00″ S, 47°06′30″ E); 12.v.2010; F.Rousseau, R.Anderson, and J.Tsarahevitra | OP137187 | OP137194 |
| G. usmanghanii | PC0166570-3 (MAD0603-3) | Sud de la baie, Lokaro, Madagascar; 12.v.2010; F.Rousseau, R.Anderson, and J.Tsarahevitra | NA | NA |
| Gelidium leptum G.H.Boo, L.Le Gall, and H.S.Yoon | Gelidium sclerophyllum W.R.Taylor | Gelidium usmanghanii Afaq-Husain and Shameel | Gelidium amansii (J.V.Lamouroux) J.V.Lamouroux | Gelidium applanatum Stegenga, Bolton, and R.J.Anderson | Gelidium arenarium Kylin | Gelidium minusculum (Weber-van Bosse) R.E. Norris | Gelidium reptans (Suhr) Kylin | |
|---|---|---|---|---|---|---|---|---|
| Type locality | Baie des Galions, southern Madagascar | at a depth of 5.4 m near the northeast side of Ensenada de San Francisco, Esmeraldas, Ecuador | Buleji, Karachi, Pakistan | Madagascar | at a depth of 15 m, Vulcan Rock off Hout Bay, Cape Peninsula, South Africa | Isipingo Beach, south of Durban, South Africa | Indonesia | Cape of Good Hope, South Africa |
| Habitat | intertidal | intertidal to subtidal, on crustose coralline algae, barnacles, attached mollusks | on margins and vertical side of protected rock or boulders | on the coast | Subtidal, on Pyura stolonifera | lower intertidal | Mvoti River region, north Durban | upper intertidal to shallow subtidal, on rocks, or on limpets or barnacles |
| Thallus size | up to 5.5 cm | up to 2.5 cm | up to 7 cm | about 15 cm | up to 0.5 cm | 3–6 cm | about 0.2 cm | 0.5–2 cm |
| Erect axes | thin, slender, and flattened | terete to compressed proximally, flattened distally | flattened, distorted | cylindrical to compressed | foliaceous, simple to cuneate | terete, slender, about 250 μm in diam. | simple, flattened lanceolate, entangled | flattened or partly terete, about 1 mm wide |
| Branches | alternate to irregular, distichous, thin and flattened | 2–3 orders of subopposite to alternate, constricted basally | irregular, cuneate basally, densely | alternate, mostly secondary, distichous | irregularly branched | irregular | irregular | irregular, with entire margin |
| Ultimate branches | irregular branchlets | pinnate branchlets, clavate | foliose, without pinnate branchlets | simple branchlets | sometimes marginal proliferations, irregularly arising | simple to trichotomous | simple, flattened | simple, without pinnate branchlets |
| Apex | attenuated | obtuse to retuse | retuse or protruding | gradually attenuated | retuse | - | obtuse | roundish |
| Surface cortical cells | angular to rounded, arranged in pairs or randomly | globose to cuboid, arranged randomly | angular to rounded, arranged randomly | - | - | larger than surface cells of G. crinale | - | angular, arranged randomly |
| Cortical layers | 3–4 layers of angular to elongate cells | 4–5 layers of angular to elongate cells | 3–4 layers of rectangular cells | 5–7 layers of roundish or angular cells | 3–4 layers of rectangular cells | - | 3–4 layers of rectangular cells | 2–3 layers of small cells |
| Medulla | 3–4 layers of thick-walled cells transversally or filamentous cells longitudinally | 2–3 layers of elongate, thick-walled cells | 2–3 layers of elongate cells | 4–5 layers of thick-walled cells | 3–4 layers of irregularly shaped cells | - | 4–5 layers of various shaped cells with a row of round cell in the center | 4–5 layers of thick-walled filaments |
| Thick-walled cells | compactly arranged in inner cortex and medulla | rarely occurring in medulla from Costa Rican specimens | absent | compactly arranged in medulla | absent | absent | absent | loosely arranged in medulla |
| Rhizoidal filaments | rare at the margin of branches | abundant in both inner cortex and medulla | congested in medulla | abundant in inner cortex | rare in medulla | sparse in medulla | abundant in medulla | in medulla |
| Cystocarps | - | bilocular | bilocular, with 1-2 ostioles on opposite sides | - | bilocular, ostioles on opposite sides, subapically on the lobes | - | - | bilocular with ostioles on opposite side |
| Spermatangia | - | sori on ultimate branchlets | - | - | - | - | present in cultured plants | - |
| Tetrasporangial sori | elongate at the apices of branches, without sterile margin, roundish parasporangial cluster | with wide sterile margin | with wide sterile margin | apiculate, up to 1 mm broad, short stalks, not geniculate | - | on spatulate apices with sterile margin or on trichotomous apices | - | on slightly widened apices, with sterile margin |
| Tetrasporangia | irregularly arranged, 19–30 × 31–48 µm in size, decussately divided | irregularly arranged, 18–22 × 21–27 µm in size, cruciately or decussately divided | irregularly arranged, 25–31 × 34–41 µm in size, cruciately or decussately divided | - | - | irregularly arranged on sori, about 30 µm in diam. | - | V-shaped arrangement |
| DNA sequences | rbcL, cox1 from type specimen | rbcL, cox1, COI-5P, mitogenome from type specimen | rbcL from topotype material, cox1 and Madagascar | - | - | - | - | - |
| Distribution | southern Madagascar | southern Madagascar, Sri Lanka, Hawai’i, Mexico to Costa Rica | Oman, Pakistan, southern Madagascar | Madagascar | South Africa | Kenya, South Africa | Australia, South Africa, Mediterranean Sea, Indonesia | South Africa, Mozambique |
| References | The present study | The present study, [43,44,54] | The present study, [50,51,52] | [7,25,55,56,57,58] | [59] | [60,61] | [61] | [60,61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boo, G.H.; Le Gall, L.; Hwang, I.K.; Rousseau, F.; Yoon, H.S. Species Diversity of Gelidium from Southern Madagascar Evaluated by an Integrative Taxonomic Approach. Diversity 2022, 14, 826. https://doi.org/10.3390/d14100826
Boo GH, Le Gall L, Hwang IK, Rousseau F, Yoon HS. Species Diversity of Gelidium from Southern Madagascar Evaluated by an Integrative Taxonomic Approach. Diversity. 2022; 14(10):826. https://doi.org/10.3390/d14100826
Chicago/Turabian StyleBoo, Ga Hun, Line Le Gall, Il Ki Hwang, Florence Rousseau, and Hwan Su Yoon. 2022. "Species Diversity of Gelidium from Southern Madagascar Evaluated by an Integrative Taxonomic Approach" Diversity 14, no. 10: 826. https://doi.org/10.3390/d14100826
APA StyleBoo, G. H., Le Gall, L., Hwang, I. K., Rousseau, F., & Yoon, H. S. (2022). Species Diversity of Gelidium from Southern Madagascar Evaluated by an Integrative Taxonomic Approach. Diversity, 14(10), 826. https://doi.org/10.3390/d14100826







