Bird Communities in a Changing World: The Role of Interspecific Competition
Abstract
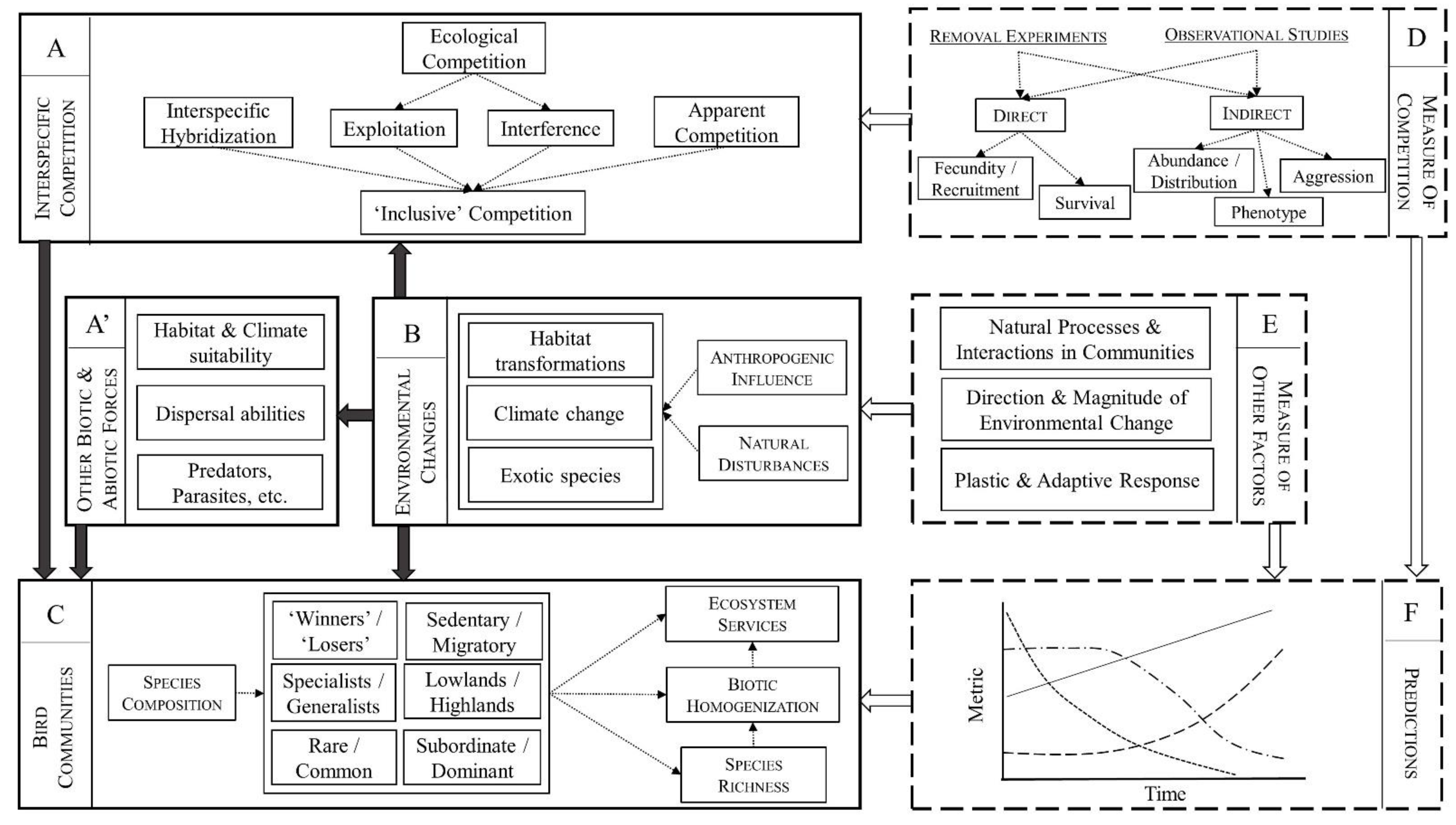
1. Introduction
2. Materials and Methods
3. Results
3.1. Prediction 1: Interspecific Competition May Hinder Species’ Ability to Shift Their Distribution towards Higher Latitude or Elevation in Response to Climate Warming
3.1.1. A Role for Interspecific Competition?
3.1.2. Can We Make Predictions?
3.2. Prediction 2: Human Activities Predictably Alter the Competitive Dynamics between Dominant and Subordinate Species
3.2.1. Overall Support for Prediction 2
3.2.2. A More Complex Picture
3.3. Prediction 3: Introduced Exotic Bird Species May Supplant Native Species
3.3.1. The Importance of Interspecific Hybridization
3.3.2. Introduced Birds: Not a Major Threat to Avian Diversity?
4. Discussion
4.1. Predictable ‘Winners’ and ‘Losers’
4.2. Implications for Ecosystem Functioning
4.3. Synthesis: Predictions Are Hampered by Multiple Sources of Uncertainty
4.3.1. What Role for Interspecific Competition?
4.3.2. Uncertainty Linked to Ecosystem Complexity and Future Changes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.R.; Collar, N.J. The future for Sundaic lowland forest birds: Long-term effects of commercial logging and fragmentation. Forktail 2002, 18, 127–146. [Google Scholar]
- Davis, A.J.; Jenkinson, L.S.; Lawton, J.H.; Shorrocks, B.; Wood, S. Making mistakes when predicting shifts in species range in response to global warming. Nature 1998, 391, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.E.; Urban, M.C.; Tewksbury, J.; Gilchrist, G.W.; Holt, R.D. A framework for community interactions under climate change. Trends Ecol. Evol. 2010, 25, 325–331. [Google Scholar] [CrossRef]
- Diamond, J.M. Distributional Ecology of New Guinea Birds: Recent ecological and biogeographical theories can be tested on the bird communities of New Guinea. Science 1973, 179, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.K.; Terborgh, J. Interspecific Aggression and Habitat Selection by Amazonian Birds. J. Anim. Ecol. 1995, 64, 1–11. [Google Scholar] [CrossRef]
- Jankowski, J.E.; Graham, C.H.; Parra, J.L.; Robinson, S.K.; Seddon, N.; Touchton, J.M.; Tobias, J.A. The role of competition in structuring tropical bird communities. Ornitol. Neotrop. 2012, 23, 97–106. [Google Scholar]
- Boyce, A.J.; E Martin, T.E. Interspecific aggression among parapatric and sympatric songbirds on a tropical elevational gradient. Behav. Ecol. 2019, 30, 541–547. [Google Scholar] [CrossRef]
- Freeman, B.G. Lower elevation animal species do not tend to be better competitors than their higher elevation relatives. Glob. Ecol. Biogeogr. 2019, 29, 171–181. [Google Scholar] [CrossRef]
- Price, T.D.; Kirkpatrick, M. Evolutionarily stable range limits set by interspecific competition. Proc. R. Soc. B: Boil. Sci. 2009, 276, 1429–1434. [Google Scholar] [CrossRef]
- Grant, P.R.; Grant, B.R. Evolution of Character Displacement in Darwin’s Finches. Science 2006, 313, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Mac Nally, R.; Bowen, M.; Howes, A.; McAlpine, C.; Maron, M. Despotic, high-impact species and the subcontinental scale control of avian assemblage structure. Ecology 2012, 93, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.E.; Cruz, A., Jr.; Grant, M.C.; Aid, C.S.; Strong, T.R. Field Experimental Evidence for Diffuse Competition Among Southwestern Riparian Birds. Am Nat. 1992, 140, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.R.; Martin, T.E. Ecological and fitness consequences of species coexistence: A removal experiment with wood warblers. Ecology 2001, 82, 189–206. [Google Scholar] [CrossRef]
- Quilodrán, C.; Austerlitz, F.; Currat, M.; Montoya-Burgos, J.I. Cryptic Biological Invasions: A General Model of Hybridization. Sci. Rep. 2018, 8, 2414. [Google Scholar] [CrossRef]
- Guillaumet, A.; Pons, J.M.; Godelle, B.; Crochet, P.-A. History of the Crested Lark in the Mediterranean region as revealed by mtDNA sequences and morphology. Mol. Phylogenet. Evol. 2006, 39, 645–656. [Google Scholar] [CrossRef]
- Guillaumet, A.; Ferdy, J.-B.; Desmarais, E.; Godelle, B.; Crochet, P.-A. Testing Bergmann’s rule in the presence of potentially confounding factors: A case study with three species of Galerida larks in Morocco. J. Biogeogr. 2007, 35, 579–591. [Google Scholar] [CrossRef]
- Guillaumet, A.; Gonin, J.; Prodon, R.; Crochet, P.-A. The geographic and seasonal dimensions of habitat use in Galerida larks: Implications for species coexistence and range limits. Ecography 2010, 33, 961–970. [Google Scholar] [CrossRef]
- Guillaumet, A.; Crochet, P.-A.; Pons, J.-M. Climate-driven diversification in two widespread Galerida larks. BMC Evol. Biol. 2008, 8, 32. [Google Scholar] [CrossRef]
- Guillaumet, A.; Leotard, G. Annoying neighbors: Multi-scale distribution determinants of two sympatric sibling species of birds. Curr. Zool. 2015, 61, 10–22. [Google Scholar] [CrossRef][Green Version]
- Laiolo, P. From inter-specific behavioural interactions to species distribution patterns along gradients of habitat heterogeneity. Oecologia 2012, 171, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 2007, 16, 743–753. [Google Scholar] [CrossRef]
- Engler, J.O.; Rödder, D.; Elle, O.; Hochkirch, A.; Secondi, J. Data from: Species distribution models contribute to determine the effect of climate and interspecific interactions in moving hybrid zones. J. Evol. Biol. 2013, 26, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.; Grey, M.J.; Catterall, C.P.; Major, R.E.; Oliver, D.L.; Clarke, M.F.; Loyn, R.H.; Mac Nally, R.; Davidson, I.; Thomson, J.R. Avifaunal disarray due to a single despotic species. Divers. Distrib. 2013, 19, 1468–1479. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.; Gemmell, N.; A Rand, T.; Ewers, R.M. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef]
- Walther, G.-R. Community and ecosystem responses to recent climate change. Philos. Trans. R. Soc. B: Biol. Sci. 2010, 365, 2019–2024. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Urban, M.C.; Tewksbury, J.J.; Sheldon, K.S. On a collision course: Competition and dispersal differences create no-analogue communities and cause extinctions during climate change. Proc. R. Soc. Lond. 2012, 279, 2072–2080. [Google Scholar] [CrossRef]
- Evans, T.; Jeschke, J.M.; Liu, C.; Redding, D.W.; Şekercioğlu, Ç.; Blackburn, T.M. What factors increase the vulnerability of native birds to the impacts of alien birds? Ecography 2021, 44, 727–739. [Google Scholar] [CrossRef]
- Soares, F.C.; Leal, A.I.; Palmeirim, J.M.; de Lima, R.F. Niche differences may reduce susceptibility to competition between native and non-native birds in oceanic islands. Divers. Distrib. 2021, 27, 1507–1518. [Google Scholar] [CrossRef]
- Marvier, M.; Kareiva, P.; Neubert, M.G. Habitat destruction, fragmentation, and disturbance promote invasion by habitat generalists in a multispecies metapopulation. Risk Anal. 2004, 24, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2010, 9, 222–228. [Google Scholar] [CrossRef]
- Büchi, L.; Vuilleumier, S. Coexistence of Specialist and Generalist Species Is Shaped by Dispersal and Environmental Factors. Am. Nat. 2014, 183, 612–624. [Google Scholar] [CrossRef]
- Berthold, P.; Fiedler, W.; Schlenker, R.; Querner, U. 25-year study of the population development of Central European songbirds: A general decline, most evident in long-distance migrants. Naturwissenschaften 1998, 85, 350–353. [Google Scholar] [CrossRef]
- Ahola, M.P.; Laaksonen, T.; Eeva, T.; Lehikoinen, E. Climate change can alter competitive relationships between resident and migratory birds. J. Anim. Ecol. 2007, 76, 1045–1052. [Google Scholar] [CrossRef]
- Samplonius, J.M.; Bartošová, L.; Burgess, M.D.; Bushuev, A.V.; Eeva, T.; Ivankina, E.V.; Kerimov, A.B.; Krams, I.; Laaksonen, T.; Mägi, M.; et al. Phenological sensitivity to climate change is higher in resident than in migrant bird populations among European cavity breeders. Glob. Change Biol. 2018, 24, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Samplonius, J.M.; Both, C. Climate Change May Affect Fatal Competition between Two Bird Species. Curr. Biol. 2019, 29, 327–331. [Google Scholar] [CrossRef]
- Wittwer, T.; O’Hara, R.B.; Caplat, P.; Hickler, T.; Smith, H.G. Long-term population dynamics of a migrant bird suggests interaction of climate change and competition with resident species. Oikos 2015, 124, 1151–1159. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Freeman, B.G.; Class Freeman, A.M. Rapid upslope shifts in New Guinean birds illustrate strong distributional responses of tropical montane species to global warming. Proc. Natl. Acad. Sci. USA 2014, 111, 4490–4494. [Google Scholar] [CrossRef]
- Bocedi, G.; Atkins, K.E.; Liao, J.; Henry, R.C.; Travis, J.M.; Hellmann, J.J. Effects of local adaptation and interspecific competition on species’ responses to climate change. Ann. NY Acad. Sci. 2013, 1297, 83–97. [Google Scholar] [CrossRef]
- Freeman, B.G.; Montgomery, G. Interspecific aggression by the Swainson’s Thrush (Catharus ustulatus) may limit the distribution of the threatened Bicknell’s Thrush (Catharus bicknelli) in the Adirondack Mountains. Ornithol. Appl. 2016, 118, 169–178. [Google Scholar] [CrossRef]
- Touchton, J.M.; Smith, J.N.M. Species loss, delayed numerical responses, and functional compensation in an antbird guild. Ecology 2011, 92, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Harvey, K.J.; French, K. Threats from introduced birds to native birds. Emu-Austral Ornithol. 2014, 114, 1–12. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Thompson, F.R., III. Poleward shifts in winter ranges of North American birds. Ecology 2007, 88, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Tingley, M.W.; Monahan, W.B.; Beissinger, S.R.; Moritz, C. Birds track their Grinnellian niche through a century of climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 19637. [Google Scholar] [CrossRef]
- Freeman, B.G.; Lee-Yaw, J.A.; Sunday, J.M.; Hargreaves, A.L. Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Global. Ecol. Biogeogr. 2018, 27, 1268–1276. [Google Scholar] [CrossRef]
- Freeman, B.G.; Scholer, M.N.; Ruiz-Gutierrez, V.; Fitzpatrick, J.W. Climate change causes upslope shifts and mountaintop extirpations in a tropical bird community. Proc. Natl. Acad. Sci. USA 2018, 115, 11982–11987. [Google Scholar] [CrossRef]
- Tingley, M.W.; Koo, M.; Moritz, C.; Rush, A.C.; Beissinger, S.R. The push and pull of climate change causes heterogeneous shifts in avian elevational ranges. Glob. Change Biol. 2012, 18, 3279–3290. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Durant, J.M.; Fowler, M.S.; Matthysen, E.; Adriaensen, F.; Jonzén, N.; Chan, K.-S.; Liu, H.; De Laet, J.; Sheldon, B.C.; et al. Testing for effects of climate change on competitive relationships and coexistence between two bird species. Proc. R. Soc. B Boil. Sci. 2015, 282, 20141958. [Google Scholar] [CrossRef]
- Round, P.D.; Gale, G.A. Changes in the Status of Lophura Pheasants in Khao Yai National Park, Thailand: A Response to Warming Climate? Biotropica 2007, 40, 225–230. [Google Scholar] [CrossRef]
- Taylor, S.A.; White, T.A.; Hochachka, W.M.; Ferretti, V.; Curry, R.L.; Lovette, I. Climate-Mediated Movement of an Avian Hybrid Zone. Curr. Biol. 2014, 24, 671–676. [Google Scholar] [CrossRef]
- Gee, J.M. Gene flow across a climatic barrier between hybridizing avian species, California and Gambel’s quail (Callipepla californica and C. gambelii). Evolution 2004, 58, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- DuBay, S.G.; Witt, C.C. Differential high-altitude adaptation and restricted gene flow across a mid-elevation hybrid zone in Andean tit-tyrant flycatchers. Mol. Ecol. 2014, 23, 3551–3565. [Google Scholar] [CrossRef]
- Vaughn, J.C.; Voelker, G.; Heatley, J.J. Glucose Concentrations in Closely Related Titmice (Baeolophus) Species Linked to Regional Habitat Differences Across an Avian Hybrid Zone. Open Ornithol. J. 2020, 13, 10–23. [Google Scholar] [CrossRef]
- Swenson, N.G. Gis-based niche models reveal unifying climatic mechanisms that maintain the location of avian hybrid zones in a North American suture zone. J. Evol. Biol. 2006, 19, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Carling, M.D.; Thomassen, H.A. The Role of Environmental Heterogeneity in Maintaining Reproductive Isolation between Hybridizing Passerina (Aves: Cardinalidae) Buntings. Int. J. Ecol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Buggs, R. Empirical study of hybrid zone movement. Heredity 2007, 99, 301–312. [Google Scholar] [CrossRef]
- McQuillan, M.A.; Rice, A.M. Differential effects of climate and species interactions on range limits at a hybrid zone: Potential direct and indirect impacts of climate change. Ecol. Evol. 2015, 5, 5120–5137. [Google Scholar] [CrossRef] [PubMed]
- Billerman, S.M.; Murphy, M.A.; Carling, M.D. Changing climate mediates sapsucker (Aves: Sphyrapicus) hybrid zone movement. Ecol. Evol. 2016, 6, 7976–7990. [Google Scholar] [CrossRef]
- Jankowski, J.E.; Robinson, S.K.; Levey, D.J. Squeezed at the top: Interspecific aggression may constrain elevational ranges in tropical birds. Ecology 2010, 91, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Surya, G.S.; Keitt, T.H. Altitudinal limits of Eastern Himalayan birds are created by competition past and present. PLoS ONE 2019, 14, e0217549. [Google Scholar] [CrossRef] [PubMed]
- Schumm, M.; White, A.E.; Supriya, K.; Price, T.D. Ecological Limits as the Driver of Bird Species Richness Patterns along the East Himalayan Elevational Gradient. Am. Nat. 2020, 195, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Elsen, P.R.; Tingley, M.W.; Kalyanaraman, R.; Ramesh, K.; Wilcove, D.S. The role of competition, ecotones, and temperature in the elevational distribution of Himalayan birds. Ecology 2017, 98, 337–348. [Google Scholar] [CrossRef]
- Boyce, A.J.; Shakya, S.; Sheldon, F.H.; Moyle, R.G.; Martin, T.E. Biotic interactions are the dominant drivers of phylogenetic and functional structure in bird communities along a tropical elevational gradient. Auk 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Freeman, B.G.; Class Freeman, A.M.; Hochachka, W.M. Asymmetric interspecific aggression in New Guinean songbirds that replace one another along an elevational gradient. Ibis 2016, 158, 726–737. [Google Scholar] [CrossRef]
- Freeman, B.G.; Tobias, J.A.; Schluter, D. Behavior influences range limits and patterns of coexistence across an elevational gradient in tropical birds. Ecography 2019, 42, 1832–1840. [Google Scholar] [CrossRef]
- Burner, R.C.; Boyce, A.J.; Bernasconi, D.; Styring, A.R.; Shakya, S.; Boer, C.; Rahman, M.A.; Martin, T.E.; Sheldon, F.H. Biotic interactions help explain variation in elevational range limits of birds among Bornean mountains. J. Biogeogr. 2020, 47, 760–771. [Google Scholar] [CrossRef]
- Freeman, B.G.; Strimas-Mackey, M.; Miller, E.T. Interspecific competition limits bird species’ ranges in tropical mountains. Science 2022, 377, 416–420. [Google Scholar] [CrossRef]
- Prodon, R.; Thibault, J.; Dejaifve, P. Expansion vs. compression of bird altitudinal ranges on a Mediterranean island. Ecology 2002, 83, 1294–1306. [Google Scholar] [CrossRef]
- Barve, S.; Dhondt, A.A. Elevational replacement of two Himalayan titmice: Interspecific competition or habitat preference? J. Avian Biol. 2017, 48, 1189–1194. [Google Scholar] [CrossRef]
- Freeman, B.G. Strong asymmetric interspecific aggression between two sympatric New Guinean robins. Ibis 2015, 158, 75–81. [Google Scholar] [CrossRef]
- Jones, S.E.I.; Tobias, J.A.; Freeman, R.; Portugal, S.J. Weak asymmetric interspecific aggression and divergent habitat preferences at an elevational contact zone between tropical songbirds. Ibis 2020, 162, 814–826. [Google Scholar] [CrossRef]
- Londoño, G.A.; Chappell, M.A.; Jankowski, J.E.; Robinson, S.K. Do thermoregulatory costs limit altitude distributions of Andean forest birds? Funct. Ecol. 2017, 31, 204–215. [Google Scholar] [CrossRef]
- Neate-Clegg, M.H.C.; Jones, S.E.I.; Tobias, J.A.; Newmark, W.D.; Şekercioǧlu, H. Ecological Correlates of Elevational Range Shifts in Tropical Birds. Front. Ecol. Evol. 2021, 9, 215. [Google Scholar] [CrossRef]
- Jankowski, J.E.; Londoño, G.A.; Robinson, S.K.; Chappell, M.A. Exploring the role of physiology and biotic interactions in determining elevational ranges of tropical animals. Ecography 2013, 36, 1–12. [Google Scholar] [CrossRef]
- Cimino, M.A.; Moline, M.A.; Fraser, W.R.; Patterson-Fraser, D.L.; Oliver, M.J. Climate-driven sympatry may not lead to foraging competition between congeneric top-predators. Sci. Rep. 2016, 6, srep18820. [Google Scholar] [CrossRef]
- Zonana, D.M. Mating On The Margins: The Impacts of Social Network Structure and Climate On Gene Flow In A Hybrid Zone Between California (Callipepla Californica) and Gambel’s Quail (Callipepla Gambelii). Ph.D. Thesis, University of Colorado, Boulder, CO, USA, 2019. [Google Scholar]
- Walsh, J.; Olsen, B.J.; Ruskin, K.J.; Shriver, W.G.; O’Brien, K.M.; Kovach, A.I. Extrinsic and intrinsic factors influence fitness in an avian hybrid zone. Biol. J. Linn. Soc. 2016, 119, 890–903. [Google Scholar] [CrossRef]
- Piper, S.D.; Catterall, C.P. A particular case and a general pattern: Hyperaggressive behaviour by one species may mediate avifaunal decreases in fragmented Australian forests. Oikos 2003, 101, 602–614. [Google Scholar] [CrossRef]
- Sætre, G.; Post, E.; Král, M. Can environmental fluctuation prevent competitive exclusion in sympatric flycatchers? Proc. R. Soc. Lond. 1999, 266, 1247–1251. [Google Scholar] [CrossRef]
- Vallin, N.; Rice, A.; Arntsen, H.; Kulma, K.; Qvarnstrom, A. Combined effects of interspecific competition and hybridization impede local coexistence of Ficedula flycatchers. Evol. Ecol. 2011, 26, 927–942. [Google Scholar] [CrossRef]
- Duckworth, R.A.; Badyaev, A.V. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl. Acad. Sci. USA 2007, 104, 15017–15022. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.J. Changes in the distribution and abundance of spotted owls during the past century. Stud. Avian Biol. 1994, 15, 293–300. [Google Scholar]
- Yackulic, C.B.; Bailey, L.L.; Dugger, K.M.; Davis, R.J.; Franklin, A.B.; Forsman, E.D.; Ackers, S.H.; Andrews, L.S.; Diller, L.V.; Gremel, S.A.; et al. The past and future roles of competition and habitat in the range-wide occupancy dynamics of Northern Spotted Owls. Ecol. Appl. 2019, 29, e01861. [Google Scholar] [CrossRef] [PubMed]
- Livezey, K.B. Range Expansion of Barred Owls, Part II: Facilitating Ecological Changes. Am. Midl. Nat. 2009, 161, 323–349. [Google Scholar] [CrossRef]
- Crozier, M.L.; Seamans, M.E.; Gutierrez, R.J.; Loschl, P.J.; Horn, R.B.; Sovern, S.G.; Forsman, E.D. Does the presence of barred owls suppress the calling behavior of spotted owls? Condor 2006, 108, 760–769. [Google Scholar] [CrossRef]
- Wiens, J.D.; Anthony, R.G.; Forsman, E.D. Competitive interactions and resource partitioning between northern spotted owls and barred owls in western Oregon. Wildl. Monogr. 2014, 185, 1–50. [Google Scholar] [CrossRef]
- Wood, C.M.; Kryshak, N.; Gustafson, M.; Hofstadter, D.F.; Hobart, B.K.; Whitmore, S.A.; Dotters, B.P.; Roberts, K.N.; Keane, J.J.; Sawyer, S.C.; et al. Density dependence influences competition and hybridization at an invasion front. Divers. Distrib. 2021, 27, 901–912. [Google Scholar] [CrossRef]
- Dugger, K.M.; Forsman, E.D.; Franklin, A.B.; Davis, R.J.; White, G.C.; Schwarz, C.J.; Burnham, K.P.; Nichols, J.D.; Hines, J.E.; Yackulic, C.B.; et al. The effects of habitat, climate, and Barred Owls on long-term demography of Northern Spotted Owls. Ornithol. Appl. 2015, 118, 57–116. [Google Scholar] [CrossRef]
- Wiens, J.D.; Dugger, K.M.; Higley, J.M.; Lesmeister, D.B.; Franklin, A.B.; Hamm, K.A.; White, G.C.; Dilione, K.E.; Simon, D.C.; Bown, R.R.; et al. Invader removal triggers competitive release in a threatened avian predator. Proc. Natl. Acad. Sci. USA 2021, 118, e2102859118. [Google Scholar] [CrossRef]
- Dugger, K.M.; Anthony, R.G.; Andrews, L.S. Transient dynamics of invasive competition: Barred owls, spotted owls, habitat, and the demons of competition present. Ecol. Appl. 2011, 21, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Van Lanen, N.J.; Franklin, A.B.; Huyvaert, K.P.; Reiser, R.F.; Carlson, P.C. Who hits and hoots at whom? Potential for interference competition between barred and northern spotted owls. Biol. Conserv. 2011, 144, 2194–2201. [Google Scholar] [CrossRef]
- Hamer, T.E.; Forsman, E.D.; Glenn, E.M. Home Range Attributes and Habitat Selection of Barred Owls and Spotted Owls in an Area of Sympatry. Condor 2007, 109, 750–768. [Google Scholar] [CrossRef]
- Long, L.L.; Wolfe, J.D. Review of the effects of barred owls on spotted owls. J. Wildl. Manag. 2019, 83, 1281–1296. [Google Scholar] [CrossRef]
- Walsh, J.; Shriver, W.G.; Olsen, B.J.; Kovach, A.I. Differential introgression and the maintenance of species boundaries in an advanced generation avian hybrid zone. BMC Evol. Biol. 2016, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Shriver, W.G.; Correll, M.D.; Olsen, B.J.; Elphick, C.S.; Hodgman, T.P.; Rowe, R.J.; O’Brien, K.M.; Kovach, A.I. Temporal shifts in the saltmarsh–Nelson’s sparrow hybrid zone revealed by replicated demographic and genetic surveys. Conserv. Genet. 2017, 18, 453–466. [Google Scholar] [CrossRef]
- Haynes, T.B.; Schmutz, J.A.; Lindberg, M.S.; Wright, K.G.; Uher-Koch, B.D.; Rosenberger, A.E. Occupancy of yellow-billed and pacific loons: Evidence for interspecific competition and Habitat mediated co-occurrence. J. Avian Biol. 2014, 45, 296–304. [Google Scholar] [CrossRef]
- Guay, P.-J.; Taysom, A.; Robinson, R.; Tracey, J.P. Hybridization between the Mallard and native dabbling ducks: Causes, consequences and management. Pac. Conserv. Biol. 2014, 20, 41–47. [Google Scholar] [CrossRef]
- Yamashina, Y. Notes on the Marianas mallard. Pac. Sci. 1948, 2, 121–124. [Google Scholar]
- Fowler, A.C.; Eadie, J.M.; Engilis, A., Jr. Identification of endangered Hawaiian ducks (Anas wyvilliana), introduced North American mallards (A. platyrhynchos) and their hybrids using multilocus genotypes. Conserv. Genet. 2008, 10, 1747–1758. [Google Scholar] [CrossRef]
- Mank, J.E.; Carlson, J.E.; Brittingham, M.C. A Century of Hybridization: Decreasing Genetic Distance Between American Black Ducks and Mallards. Conserv. Genet. 2004, 5, 395–403. [Google Scholar] [CrossRef]
- Thibault, M.; Vidal, E.; Potter, M.A.; Sanchez, T.; Brescia, F. The invasive Red-vented bulbul (Pycnonotus cafer) outcompetes native birds in a tropical biodiversity hotspot. PLoS ONE. 2018, 13, e0192249. [Google Scholar] [CrossRef] [PubMed]
- Shochat, E.; Lerman, S.B.; Anderies, J.M.; Warren, P.S.; Faeth, S.H.; Nilon, C.H. Invasion, Competition, and Biodiversity Loss in Urban Ecosystems. BioScience 2010, 60, 199–208. [Google Scholar] [CrossRef]
- Hopkins, J.M.; Edwards, W.; Laguna, J.M.; Schwarzkopf, L. An endangered bird calls less when invasive birds are calling. J. Avian Biol. 2020, 52, 1–9. [Google Scholar] [CrossRef]
- Colléony, A.; Shwartz, A. When the winners are the losers: Invasive alien bird species outcompete the native winners in the biotic homogenization process. Biol. Conserv. 2019, 241, 108314. [Google Scholar] [CrossRef]
- Strubbe, D.; Matthysen, E. Invasive ring-necked parakeets Psittacula krameri in Belgium: Habitat selection and impact on native birds. Ecography 2007, 30, 578–588. [Google Scholar] [CrossRef]
- Strubbe, D.; Matthysen, E. Experimental evidence for nest-site competition between invasive ring-necked parakeets (Psittacula krameri) and native nuthatches (Sitta europaea). Biol. Conserv. 2009, 142, 1588–1594. [Google Scholar] [CrossRef]
- Komdeur, J. Breeding of the Seychelles Magpie Robin Copsychus sechellarum and implications for its conservation. Ibis 1996, 138, 485–498. [Google Scholar] [CrossRef]
- Peck, H.L.; Pringle, H.E.; Marshall, H.H.; Owens, I.P.F.; Lord, A.M. Experimental evidence of impacts of an invasive parakeet on foraging behavior of native birds. Behav. Ecol. 2014, 25, 582–590. [Google Scholar] [CrossRef]
- Roach, M. Puerto Rican Vireo (Vireo latimeri), version 1.0. In Birds of the World; Schulenberg, T.S., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Bonter, D.N.; Zuckerberg, B.; Dickinson, J.L. Invasive birds in a novel landscape: Habitat associations and effects on established species. Ecography 2009, 33, 494–502. [Google Scholar] [CrossRef]
- Sol, D.; Bartomeus, I.; Griffin, A.S. The paradox of invasion in birds: Competitive superiority or ecological opportunism? Oecologia 2012, 169, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Vall-llosera, M.; Llimona, F.; de Cáceres, M.; Sales, S.; Sol, D. Competition, niche opportunities and the successful invasion of natural habitats. Biol. Invasions 2016, 18, 3535–3546. [Google Scholar] [CrossRef]
- Evans, T.; Kumschick, S.; Blackburn, T.M. Application of the Environmental Impact Classification for Alien Taxa (EICAT) to a global assessment of alien bird impacts. Divers. Distrib. 2016, 22, 919–931. [Google Scholar] [CrossRef]
- Freed, L.A.; Cann, R.L.; Bodner, G.R. Incipient extinction of a major population of the Hawaii akepa owing to introduced species. Evol. Ecol. Res. 2008, 10, 931–965. [Google Scholar]
- Freed, L.A.; Cann, R.L. Negative Effects of an Introduced Bird Species on Growth and Survival in a Native Bird Community. Curr. Biol. 2009, 19, 1736–1740. [Google Scholar] [CrossRef]
- Camp, R.J.; Pratt, T.K.; Gorresen, P.M.; Woodworth, B.L.; Jeffrey, J.J. Hawaiian forest bird trends: Using log-linear models to assess long-term trends is supported by model diagnostics and assumptions (reply to Freed and Cann 2013). Condor 2014, 116, 97–101. [Google Scholar] [CrossRef]
- Department of Climate Change, Energy, the Environment and Water of the Australian Government. Available online: https://www.dcceew.gov.au/environment/biodiversity/threatened/recovery-plans/buff-banded-rail-cocos-keeling-islands-gallirallus-philippensis-andrewsi-2006 (accessed on 7 October 2022).
- Whelan, C.J.; Şekercioğlu, H.; Wenny, D.G. Why birds matter: From economic ornithology to ecosystem services. J. Ornithol. 2015, 156, 227–238. [Google Scholar] [CrossRef]
- Inger, R.; Gregory, R.; Duffy, J.; Stott, I.; Voříšek, P.; Gaston, K.J. Common European birds are declining rapidly while less abundant species’ numbers are rising. Ecol. Lett. 2014, 18, 28–36. [Google Scholar] [CrossRef]
- García, D.; Martínez, D. Species richness matters for the quality of ecosystem services: A test using seed dispersal by frugivorous birds. Proc. R. Soc. B Boil. Sci. 2012, 279, 3106–3113. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jetz, W. The effect of range changes on the functional turnover, structure and diversity of bird assemblages under future climate scenarios. Glob. Change Biol. 2015, 21, 2917–2928. [Google Scholar] [CrossRef]
- Fricke, E.C.; Tewksbury, J.J.; Rogers, H.S. Defaunation leads to interaction deficits, not interaction compensation, in an island seed dispersal network. Glob. Change Biol. 2017, 24, e190–e200. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, G.; Wang, N.; Feng, G.; Yang, F.; Svenning, J.-C.; Yang, J. Taxonomic, phylogenetic and functional homogenization of bird communities due to land use change. Biol. Conserv. 2019, 236, 37–43. [Google Scholar] [CrossRef]
- Marcacci, G.; Westphal, C.; Wenzel, A.; Raj, V.; Nölke, N.; Tscharntke, T.; Grass, I. Taxonomic and functional homogenization of farmland birds along an urbanization gradient in a tropical megacity. Glob. Change Biol. 2021, 27, 4980–4994. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. Why do introduced species appear to devastate islands more than mainland areas? Pac. Sci. 1995, 49, 87–97. [Google Scholar]
- Soares, F.C.; Panisi, M.; Sampaio, H.; Soares, E.; Santana, A.; Buchanan, G.; Leal, A.I.; Palmeirim, J.M.; Lima, R. Land-use intensification promotes non-native species in a tropical island bird assemblage. Anim. Conserv. 2020, 23, 573–584. [Google Scholar] [CrossRef]
- Le Louarn, M.; Couillens, B.; Deschamps-Cottin, M.; Clergeau, P. Interference competition between an invasive parakeet and native bird species at feeding sites. J. Ethol. 2016, 34, 291–298. [Google Scholar] [CrossRef]
- Higgins, P.J.; Christidis, L.; Ford, H. Noisy Miner (Manorina melanocephala), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Crates, R.; Terauds, A.; Rayner, L.; Stojanovic, D.; Heinsohn, R.; Wilkie, C.; Webb, M. Spatially and temporally targeted suppression of despotic noisy miners has conservation benefits for highly mobile and threatened woodland birds. Biol. Conserv. 2018, 227, 343–351. [Google Scholar] [CrossRef]
- Val, J.; Eldridge, D.J.; Travers, S.K.; Oliver, I. Livestock grazing reinforces the competitive exclusion of small-bodied birds by large aggressive birds. J. Appl. Ecol. 2017, 55, 1919–1929. [Google Scholar] [CrossRef]
- Robertson, O.J.; McAlpine, C.; House, A.; Maron, M. Influence of Interspecific Competition and Landscape Structure on Spatial Homogenization of Avian Assemblages. PLoS ONE 2013, 8, e65299. [Google Scholar] [CrossRef]
- Thomson, J.R.; Maron, M.; Grey, M.J.; Catterall, C.P.; Major, R.E.; Oliver, D.L.; Clarke, M.F.; Loyn, R.H.; Davidson, I.; Ingwersen, D.; et al. Avifaunal disarray: Quantifying models of the occurrence and ecological effects of a despotic bird species. Divers. Distrib. 2015, 21, 451–464. [Google Scholar] [CrossRef]
- Grey, M.J.; Clarke, M.F.; Loyn, R.H. Initial changes in the avian communities of remnant eucalypt woodlands following a reduction in the abundance of noisy miners, Manorina melanocephala. Wildl. Res. 1997, 24, 631–648. [Google Scholar] [CrossRef]
- Grey, M.J.; Clarke, M.F.; Loyn, R.H. Influence of the Noisy Miner Manorina melanocephala on avian diversity and abundance in remnant Grey Box woodland. Pac. Conserv. Biol. 1998, 4, 55–69. [Google Scholar] [CrossRef]
- Debus, S.J.S. The effect of Noisy Miners on small bush birds: An unofficial cull and its outcome. Pac. Conserv. Biol. 2008, 14, 185–190. [Google Scholar] [CrossRef][Green Version]
- Mortelliti, A.; Ikin, K.; Tulloch, A.I.T.; Cunningham, R.; Stein, J.; Michael, D.; Lindenmayer, D.B. Surviving with a resident despot: Do revegetated patches act as refuges from the effects of the noisy miner (Manorina melanocephala) in a highly fragmented landscape? Divers. Distrib. 2016, 22, 770–782. [Google Scholar] [CrossRef]
- Bennett, J.M.; Clarke, R.H.; Thomson, J.R.; Mac Nally, R. Variation in abundance of nectarivorous birds: Does a competitive despot interfere with flower tracking? J. Anim. Ecol. 2014, 83, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Howes, A.; Mac Nally, R.; Loyn, R.; Kath, J.; Bowen, M.; McAlpine, C. Foraging guild perturbations and ecological homogenization driven by a despotic native bird species. Ibis 2014, 156, 341–354. [Google Scholar] [CrossRef]
- Bennett, J.M.; Clarke, R.H.; Horrocks, G.F.B.; Thomson, J.R.; Mac Nally, R. Climate drying amplifies the effects of land-use change and interspecific interactions on birds. Landsc. Ecol. 2015, 30, 2031–2043. [Google Scholar] [CrossRef]
- Howes, A.L.; Maron, M. Interspecific competition and conservation management of continuous subtropical woodlands. Wildl. Res. 2009, 36, 617–626. [Google Scholar] [CrossRef]
- Bennett, J.M.; Clarke, R.H.; Thomson, J.R.; Mac Nally, R. Fragmentation, vegetation change and irruptive competitors affect recruitment of woodland birds. Ecography 2015, 38, 163–171. [Google Scholar] [CrossRef]
- Bregman, T.P.; Lees, A.C.; Seddon, N.; Macgregor, H.E.A.; Darski, B.; Aleixo, A.; Bonsall, M.B.; Tobias, J.A. Species interactions regulate the collapse of biodiversity and ecosystem function in tropical forest fragments. Ecology 2015, 96, 2692–2704. [Google Scholar] [CrossRef]
- Ulrich, W.; Lens, L.; Tobias, J.; Habel, J.C. Contrasting Patterns of Species Richness and Functional Diversity in Bird Communities of East African Cloud Forest Fragments. PLoS ONE 2016, 11, e0163338. [Google Scholar] [CrossRef] [PubMed]
- Devictor, V.; Julliard, R.; Clavel, J.; Jiguet, F.; Lee, A.; Couvet, D. Functional biotic homogenization of bird communities in disturbed landscapes. Glob. Ecol. Biogeogr. 2007, 17, 252–261. [Google Scholar] [CrossRef]
- Julliard, R.; Clavel, J.; Devictor, V.; Jiguet, F.; Couvet, D. Spatial segregation of specialists and generalists in bird communities. Ecol. Lett. 2006, 9, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Richmond, C.E.; Breitburg, D.L.; Rose, K.A. The role of environmental generalist species in ecosystem function. Ecol. Model. 2005, 188, 279–295. [Google Scholar] [CrossRef]
- Drury, J.P.; Cowen, M.C.; Grether, G.F. Competition and hybridization drive interspecific territoriality in birds. Proc. Natl. Acad. Sci. USA 2020, 117, 12923–12930. [Google Scholar] [CrossRef]
- Lipshutz, S.E. Interspecific competition, hybridization, and reproductive isolation in secondary contact: Missing perspectives on males and females. Curr. Zool. 2017, 64, 75–88. [Google Scholar] [CrossRef]
- Harr, B.; Price, T. Climate change: A hybrid zone moves north. Curr. Biol. 2014, 24, R230–R232. [Google Scholar] [CrossRef]
- Grether, G.F.; Peiman, K.S.; Tobias, J.; Robinson, B. Causes and Consequences of Behavioral Interference between Species. Trends Ecol. Evol. 2017, 32, 760–772. [Google Scholar] [CrossRef]
- Gómez-Llano, M.; Germain, R.M.; Kyogoku, D.; McPeek, M.A.; Siepielski, A.M. When Ecology Fails: How Reproductive Interactions Promote Species Coexistence. Trends Ecol. Evol. 2021, 36, 610–622. [Google Scholar] [CrossRef]
- Irwin, D.; Schluter, D. Hybridization and the Coexistence of Species. Am. Nat. 2022, 200, E93–E109. [Google Scholar] [CrossRef]
- Todesco, M.; Pascual, M.A.; Owens, G.L.; Ostevik, K.L.; Moyers, B.T.; Hübner, S.; Heredia, S.M.; Hahn, M.A.; Caseys, C.; Bock, D.G.; et al. Hybridization and extinction. Evol. Appl. 2016, 9, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Confer, J.L.; Hartman, P.; Roth, A. Golden-winged Warbler (Vermivora chrysoptera), version 1.0. In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Godsoe, W.; Holland, N.J.; Cosner, C.; Kendall, B.E.; Brett, A.; Jankowski, J.; Holt, R.D. Interspecific interactions and range limits: Contrasts among interaction types. Theor. Ecol. 2016, 10, 167–179. [Google Scholar] [CrossRef]
- de la Torre Cerro, R.; Holloway, P. A review of the methods for studying biotic interactions in phenological analyses. Methods Ecol. Evol. 2021, 12, 227–244. [Google Scholar] [CrossRef]
- Flint, P.L.; Mallek, E.J.; King, R.J.; Schmutz, J.A.; Bollinger, K.S.; Derksen, D.V. Changes in abundance and spatial distribution of geese molting near Teshekpuk Lake, Alaska: Interspecific competition or ecological change? Polar Biol. 2007, 31, 549–556. [Google Scholar] [CrossRef]
- Burnham, K.K.; Burnham, J.L.; Konkel, B.W. Status of Peregrine falcon and Gyrfalcon populations in Northwest Greenland. In Proceedings of the North Water Polynya Conference, Copenhagen, Denmark, 22–24 November 2017; pp. 101–103. [Google Scholar]
- Carrete, M.; Lambertucci, S.A.; Speziale, K.; Ceballos, O.; Travaini, A.; Delibes, M.; Hiraldo, F.; Donázar, J.A. Winners and losers in Human-made habitats: Interspecific competition outcomes in two Neotropical vultures. Anim. Conserv. 2010, 13, 390–398. [Google Scholar] [CrossRef]
- Drury, J.; Clavel, J.; Manceau, M.; Morlon, H. Data from: Estimating the effect of competition on trait evolution using maximum likelihood inference. Syst. Biol. 2016, 65, 700–710. [Google Scholar] [CrossRef]
- Quintero, I.; Landis, M.J. Interdependent Phenotypic and Biogeographic Evolution Driven by Biotic Interactions. Syst. Biol. 2019, 69, 739–755. [Google Scholar] [CrossRef]
- Davitt, G.; Maute, K.; Major, R.E.; McDonald, P.G.; Maron, M. Short-term response of a declining woodland bird assemblage to the removal of a despotic competitor. Ecol. Evol. 2018, 8, 4771–4780. [Google Scholar] [CrossRef]
- Powell, L.L.; Ames, E.M.; Wright, J.R.; Matthiopoulos, J.; Marra, P.P. Interspecific competition between resident and wintering birds: Experimental evidence and consequences of coexistence. Ecology 2020, 102, e03208. [Google Scholar] [CrossRef]
- Cadena, D.C.; Loiselle, B.A. Limits to elevational distributions in two species of emberizine finches: Disentangling the role of interspecific competition, autoecology, and geographic variation in the environment. Ecography 2007, 30, 491–504. [Google Scholar] [CrossRef]
- Reif, J.; Reifová, R.; Skoracka, A.; Kuczyński, L. Competition-driven niche segregation on a landscape scale: Evidence for escaping from syntopy towards allotopy in two coexisting sibling passerine species. J. Anim. Ecol. 2018, 87, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D.; Kelling, S. eBird: A citizen-based bird observation network in the biological sciences. Biol. Conserv. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Donahue, M.J. How phenological tracking shapes species and communities in non-stationary environments. Biol. Rev. 2021, 96, 2810–2827. [Google Scholar] [CrossRef] [PubMed]
- Willis, E.O. The behavior of ocellated antbirds. Smithson. Contrib. Zool. 1973, 144, 1–57. [Google Scholar] [CrossRef]
- Altshuler, D.L. Flight performance and competitive displacement of hummingbirds across elevational gradients. Am. Nat. 2006, 167, 216–229. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Hawkins, S.J.; Southward, A.J.; Burrows, M.T. Modeling the Response of Populations of Competing Species to Climate Change. Ecology 2008, 89, 3138–3149. [Google Scholar] [CrossRef]
- Ainley, D.G.; Hyrenbach, K.D. Top-down and bottom-up factors affecting seabird population trends in the California current system (1985–2006). Prog. Oceanogr. 2010, 84, 242–254. [Google Scholar] [CrossRef]
- Montoya, J.M.; Raffaelli, D. Climate change, biotic interactions and ecosystem services. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2013–2018. [Google Scholar] [CrossRef]
- Crawford, R.J.M.; Makhado, A.B.; Waller, L.J.; Whittington, P.A. Winners and losers—responses to recent environmental change by South African seabirds that compete with purse-seine fisheries for food. Ostrich 2014, 85, 111–117. [Google Scholar] [CrossRef]
- Yackulic, C.B. Competitive exclusion over broad spatial extents is a slow process: Evidence and implications for species distribution modeling. Ecography 2017, 40, 305–313. [Google Scholar] [CrossRef]
- Rodenhouse, N.L.; Matthews, S.N.; McFarland, K.P.; Lambert, J.D.; Iverson, L.R.; Prasad, A.; Sillett, T.S.; Holmes, R.T. Potential effects of climate change on birds of the Northeast. Mitig. Adapt. Strateg. Glob. Change 2007, 13, 517–540. [Google Scholar] [CrossRef]
- Matthews, S.N.; Iverson, L.R.; Prasad, A.M.; Peters, M.P. Changes in potential habitat of 147 North American breeding bird species in response to redistribution of trees and climate following predicted climate change. Ecography 2011, 34, 933–945. [Google Scholar] [CrossRef]
- Cruz, J.; Windels, S.K.; Thogmartin, W.E.; Crimmins, S.M.; Grim, L.H.; Larson, J.H.; Zuckerberg, B. Top-down effects of repatriating bald eagles hinder jointly recovering competitors. J. Anim. Ecol. 2019, 88, 1054–1065. [Google Scholar] [CrossRef]
- Auer, S.K.; Martin, T.E. Climate change has indirect effects on resource use and overlap among coexisting bird species with negative consequences for their reproductive success. Glob. Change Biol. 2012, 19, 411–419. [Google Scholar] [CrossRef]
- Remsen, J.V., Jr.; Graves, W.S. Distribution patterns of Buarremon brush-finches (Emberizinae) and interspecific competition in Andean birds. Auk 1995, 112, 224–236. [Google Scholar] [CrossRef]
- Schaefer, T.; Ledebur, G.; Beier, J.; Leisler, B. Reproductive responses of two related coexisting songbird species to environmental changes: Global warming, competition, and population sizes. J. Ornithol. 2006, 147, 47–56. [Google Scholar] [CrossRef]
- Furnas, B.J. Rapid and varied responses of songbirds to climate change in California coniferous forests. Biol. Conserv. 2020, 241, 108347. [Google Scholar] [CrossRef]
- Vallin, N.; Rice, A.M.; Bailey, R.I.; Husby, A.; Qvarnström, A. Positive feedback between ecological and reproductive character displacement in a young avian hybrid zone. Evolution 2012, 66, 1167–1179. [Google Scholar] [CrossRef]
- Staude, I.R.; Overbeck, G.E.; Fontana, C.S.; Bencke, G.A.; da Silva, T.W.; Mimet, A.; Pereira, H.M. Specialist Birds Replace Generalists in Grassland Remnants as Land Use Change Intensifies. Front. Ecol. Evol. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Charmantier, A.; Gienapp, P. Climate change and timing of avian breeding and migration: Evolutionary versus plastic changes. Evol. Appl. 2013, 7, 15–28. [Google Scholar] [CrossRef]
- Kopp, M.; Matuszewski, S. Rapid evolution of quantitative traits: Theoretical perspectives. Evol. Appl. 2013, 7, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Catullo, R.A.; Llewelyn, J.; Phillips, B.L.; Moritz, C.C. The Potential for Rapid Evolution under Anthropogenic Climate Change. Curr. Biol. 2019, 29, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Bonnet-Lebrun, A.-S.; Larsen, T.; Frederiksen, M.; Fox, D.; le Bouard, F.; Boutet, A.; Þórarinsson, Þ.L.; Kolbeinsson, Y.; Deville, T.; Ratcliffe, N. Effects of competitive pressure and habitat heterogeneity on niche partitioning between Arctic and boreal congeners. Sci. Rep. 2021, 11, 22133. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.B.; Barlow, C.R.; Bensusan, K.; Perez, C.; Willis, S.G. Phenological trends in the pre- and post-breeding migration of long-distance migratory birds. Glob. Change Biol. 2022, 28, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Bearhop, S.; Fiedler, W.; Furness, R.W.; Votier, S.C.; Waldron, S.; Newton, J.; Bowen, G.J.; Berthold, P.; Farnsworth, K. Assortative mating as a mechanism for rapid evolution of a migratory divide. Science 2005, 310, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Fiedler, W.; Wikelski, M.; Flack, A. “Closer-to-home” strategy benefits juvenile survival in a long-distance migratory bird. Ecol. Evol. 2019, 9, 8945–8952. [Google Scholar] [CrossRef] [PubMed]
- Rybinski, J.; Sirkiä, P.M.; McFarlane, S.E.; Vallin, N.; Wheatcroft, D.; Ålund, M.; Qvarnström, A. Competition-driven build-up of habitat isolation and selection favoring modified dispersal patterns in a young avian hybrid zone. Evolution 2016, 70, 2226–2238. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillaumet, A.; Russell, I.J. Bird Communities in a Changing World: The Role of Interspecific Competition. Diversity 2022, 14, 857. https://doi.org/10.3390/d14100857
Guillaumet A, Russell IJ. Bird Communities in a Changing World: The Role of Interspecific Competition. Diversity. 2022; 14(10):857. https://doi.org/10.3390/d14100857
Chicago/Turabian StyleGuillaumet, Alban, and Ivory Jordan Russell. 2022. "Bird Communities in a Changing World: The Role of Interspecific Competition" Diversity 14, no. 10: 857. https://doi.org/10.3390/d14100857
APA StyleGuillaumet, A., & Russell, I. J. (2022). Bird Communities in a Changing World: The Role of Interspecific Competition. Diversity, 14(10), 857. https://doi.org/10.3390/d14100857






