Abstract
Significant changes in the environment have the potential to affect bird species abundance and distribution, both directly, through a modification of the landscape, habitats, and climate, and indirectly, through a modification of biotic interactions such as competitive interactions. Predicting and mitigating the consequences of global change thus requires not only a sound understanding of the role played by biotic interactions in current ecosystems, but also the recognition and study of the complex and intricate effects that result from the perturbation of these ecosystems. In this review, we emphasize the role of interspecific competition in bird communities by focusing on three main predictions derived from theoretical and empirical considerations. We provide numerous examples of population decline and displacement that appeared to be, at least in part, driven by competition, and were amplified by environmental changes associated with human activities. Beyond a shift in relative species abundance, we show that interspecific competition may have a negative impact on species richness, ecosystem services, and endangered species. Despite these findings, we argue that, in general, the role played by interspecific competition in current communities remains poorly understood due to methodological issues and the complexity of natural communities. Predicting the consequences of global change in these communities is further complicated by uncertainty regarding future environmental conditions and the speed and efficacy of plastic and evolutionary responses to fast-changing environments. Possible directions of future research are highlighted.
1. Introduction
Major changes in the environment, including habitat loss, landscape modification, direct anthropogenic mortality, and climate warming, can all have a strong impact on bird species abundance and distribution. For instance, nearly 3 billion birds have been lost in North America in less than half a century [1] and at least 32–50% of the species of lowland Sundaic forests have responded negatively to forest logging and/or fragmentation [2]. In addition, such major environmental changes can also affect the biotic interactions which influence avian distribution and abundance [3,4]. The idea that interspecific competition plays a prominent role in determining bird community structure and abundance has a long tradition in the literature (e.g., [5,6]) and yet, its relative importance remains a hotly debated issue to this day (e.g., [7,8,9]). Theoretical models point to the ability of competition to set evolutionarily stable range limits between species (e.g., [10]), but the actual mechanisms by which competition operates can be complex. Exploitation competition is particularly prejudicial during periods of resource scarcity (e.g., [11]) and interference competition may be best exemplified by the despotic aggressiveness of the Australian Noisy Miner Manorina melanocephala [12]. Less well-known mechanisms may include diffuse (communitywide) competition (e.g., [13]), apparent competition that can result from density-dependent functional shifts in nest predator behavior [14], as well as hybridization and genetic dilution that can lead to population declines even in the absence of exploitation or interference competition [15].
Galerida larks provide a notable example of the impact of historic (pre-dating the Industrial Revolution) climate and habitat changes on interspecific interactions, offering insights into the possible consequences of the fast-paced contemporaneous global changes on modern communities. In response to both natural climatic fluctuations and anthropogenic modification of the landscape, range expansion was detected in multiple taxa, leading to multiple secondary range contacts between species and incipient species [16,17]. In recently diverged taxa, abutting ranges and limited sympatry may have resulted from weak ecological differentiation and, possibly, hybridization [18]. Sympatric coexistence between more ancient lineages was permitted by morphological shifts in sympatry, possibly including character displacement [19], as well as habitat shifts as demonstrated by ecological release in space (in allopatry: [20]) and time (seasonally, during periods of high resource abundance: [18]). However, despite such evolutionary and ecological adjustments reducing the impact of interspecific competition, negative consequences of competition can still be observed today in the form of reduced abundance and truncated geographic distribution [20,21].
Biotic interactions may thus modulate species response to environmental changes and affect community-level responses including species composition, species richness, and functional biodiversity (e.g., [3,22,23,24]). Predicting and mitigating the consequences of global change thus requires not only a sound understanding of the role played by biotic interactions in current ecosystems (e.g., [3,4]), but also the recognition and study of the complex and intricate effects that result from the perturbation of these ecosystems [25,26]. A key aspect for possible intervention is the presumption that the competitive dynamics among species belonging to various ecological groups are affected in a non-random fashion, resulting in predictable ‘winners’ and ‘losers’ [27]. For instance, competition may alter species’ abilities to track their climatic niche, resulting in greater extinction risks for species with low dispersal ability [28]. Native species from islands and other species-poor communities, characterized by lower levels of interspecific competition, may be less resistant to competition by alien invaders than native species from species-rich communities ([29]; but see also [30]). Generalists and specialists may have characteristics that predispose them to being ‘winners’ and ‘losers’ of global change, respectively. The ‘winners’ tend to tolerate human disturbance and share traits such as high fecundity and broad diets, while ‘losers’ tend to be habitat specialists which normally outcompete generalists in species-rich metacommunities but are sensitive to the alteration of the landscape [27,31,32,33] Resident species may also gain a competitive edge against migratory species due to increased winter survival in warmer climate coupled with a greater capacity to track between-year temperature variation, and thus phenological optima through plasticity; in turn, higher population densities of resident species may lead to more intense competition for food or breeding sites [34,35,36]. A great example is provided by the competition between the migratory Pied Flycatcher Ficedula hypoleuca and the resident Blue Tit Cyanistes caeruleus and Great Tit Parus major in Europe. In a Dutch population, higher temperatures in December were positively related to beech crop, a predictor of Great Tit winter survival and Great Tit occupation in spring [37]. As a result, Pied Flycatchers competing with Great Tits for nest cavities were more likely to be killed by Great Tits in synchronous years (when flycatcher arrival coincided with peak egg-laying for the tits) and high-tit-density years [37]. Long-term time-series from Sweden suggest that the Blue Tit and Great Tit, which were favored by warmer spring temperatures, had a substantial negative effect on the Pied Flycatcher, which could have contributed to the eventual local extirpation of the Pied Flycatcher [38].
2. Materials and Methods
In this review, we examine how interspecific competition may modulate the effect of global change on the composition and management of natural bird communities by focusing on three predictions derived from theoretical and empirical considerations. We provide examples taken from the literature that support or contradict the predictions, discuss the potential implications for the functioning of ecosystems in a changing world, and point out gaps in knowledge and other possible areas of future research. A literature search was performed using targeted Google Scholar searches conducted between March 2021 and March 2022 focusing on the different predictions (see below) as well as a number of well-documented cases studies we wished to emphasize (e.g., Barred Owl Strix varia vs. Spotted Owl Strix occidentalis, the impact of Noisy Miner on East Australian bird communities, etc.). To make sure we did not miss any important topic, we conducted a complementary generic search in Web of Science on 26 May 2022 using the following keywords: (“bird*” OR “avian”) AND (“global change” OR “climat* change” OR “climat* warming” OR “land-use change” OR “habitat change” OR “habitat transformation” OR “habitat destruction” OR “invasiv*” OR “alien” OR “exotic” OR “non-native”) AND (“interspecific competition” OR “compet*”). This search resulted in 1940 articles. Many of these articles were redundant with our previous searches or irrelevant, including studies focusing on different taxa and topics (e.g., bacteriological studies), so we restricted our examination to all 151 review articles as well as an additional subset of 166 articles that had the term “compet*” in their title.
Prediction 1. Interspecific competition may hinder species’ ability to shift their distribution towards higher latitude or elevation in response to climate warming.
Rationale—In response to climate warming, species tend to shift their distributions towards higher latitude or elevation (e.g., [39,40]). However, modelling suggests that interspecific competition has the potential to create novel combinations of species, slow range shifts, induce lags in climate tracking and be responsible for population decline and species extinction [28,41].
Prediction 2. Human activities predictably alter the competitive dynamics between dominant and subordinate species.
Rationale—When human activities promote the expansion of dominant competitors, subordinate competitors are expected to be negatively impacted (e.g., [42]). Conversely, when human activities cause the decline or loss of dominant competitors, relaxed competition may favor the expansion of subordinate competitors [43].
Prediction 3. Introduced exotic bird species may supplant native species.
Rationale—Invasion by introduced species is a global threat to biodiversity and is one of the main causes of animal extinction globally [44]. In addition to negative effects due to predation and disease, the introduction of exotic bird species can lead to the decline or extinction of native bird species via interspecific competition for food or nest sites and hybridization (extinction by introgression: [15]).
3. Results
3.1. Prediction 1: Interspecific Competition May Hinder Species’ Ability to Shift Their Distribution towards Higher Latitude or Elevation in Response to Climate Warming
Observations generally support the view that climate warming may affect species distribution and abundance via niche tracking, with species distributions shifting towards increased latitudes and elevations ([39,40,45,46,47,48]; but see also [49]). The fact that species tend to track their climatic niche may, in turn, disrupt interspecific coexistence. For instance, spring temperatures have affected the relative abundance of Blue Tits and Great Tits in Belgium [50]. In Thailand’s Khao Yai National Park, the relative abundance of two Lophura pheasants has changed markedly over a period of two decades, from a predominance of a high-elevation species, the Silver Pheasant L. nycthemera, to a predominance of a low-elevation species, the Siamese Fireback Lophura diardi [51]. In a Peruvian mountain, high-elevation species have declined in range size and abundance, and several previously common mountaintop species have disappeared, indicative of what has been called a climate-driven ‘escalator to extinction’ [48].
Tracking avian hybrid zone movements can provide further insights into the consequences of climate change on species interactions [52]. This is because competitive dominance as well as physiological adaptations to different climatic niches often play key roles in determining range boundaries in hybridizing species (e.g., [52,53,54,55]). As a result, the center position of hybrid zones is often determined by climatic factors such as temperature, a major driver of latitudinal and altitudinal range boundaries (e.g., [52,56]), and aridity, which combines the influence of temperature and precipitation, as evidenced in Callipepla quails [53], Galerida larks [18], Passerina buntings [57], and Baeolophus titmice [55], rather than being coincident with topographical barriers or habitat shift [18,52]. Data suggest that hybrid zone movements are commonplace [58], and climate change is invoked as a possible factor in about a third of moving avian hybrid zones [52]. For instance, the northward movement of the contact zone between Black-capped Chickadee Poecile atricapillus and Carolina Chickadee Poecile carolinensis (~11.5 km over a decade) may be associated with a change in mean minimum winter temperature, a limiting factor for Carolina Chickadee [52]. Future predictions based on a warmer climate suggest P. atricapillus could lose a significant fraction of its range [59], and Billerman et al. [60] made a similar prediction for the Red-naped Sapsucker Sphyrapicus nuchalis. More robust evidence of a direct link between climate change and hybrid zone movement, such as demonstrating phenotype-dependent changes in survival, fecundity, or dispersal probabilities associated with a change of temperature, would be helpful to gauge this type of prediction based on the association between species distribution and climate.
3.1.1. A Role for Interspecific Competition?
While patterns of species coexistence can thus be modified in response to niche tracking, the central tenet of Prediction 1 is that climate-driven range shifts may be impaired by interspecific interactions, potentially resulting in range loss and population decline that cannot be explained by climatic change alone. Examples in the literature tend to focus on the so-called ‘push’ scenario (e.g., [42]; but see also [23] for a possible case of apparent competition affecting Hippolais warblers). For mountaintop species, the decline of optimal habitat due to climate warming may be compounded by the arrival of warm-adapted competitors tracking their climatic niche [28]. When a highland species is subordinate to a dominant downslope competitor, the former may be ‘pushed’ upslope at a pace faster than predicted from abiotic changes alone. Conversely, dominant high-elevation species may act as ‘kings of the hill’ preventing subordinate downslope species from tracking their niche [61].
The hypothesis that interspecific competition plays a role in constraining species’ elevational distributions, a premise to the ‘push’ and ‘kings of the hill’ scenarios, is well supported ([62,63]; but see also, e.g., [64,65]). For instance, multiple studies have found that song playback triggers aggressive interspecific responses in one or both species replacing one another along an elevational gradient (e.g., [42,61,66]; but see also [8], and discussion below), and Freeman et al. [67] provided convincing evidence that direct territorial aggression plays an important role in setting elevational range limits among closely related species along the Manu Transect in southeastern Peru. In addition, ecological release manifested by an increase in elevational range in the absence of congeners is frequently reported on both islands and isolated mountaintops ([63] and references therein; [68,69]; but see also [70]).
Although data support the view that mountaintop species’ ranges are shrinking as they shift towards higher elevation [48], to the best of our knowledge, no study has provided irrefutable evidence for the ‘push’ hypothesis [9]. The difficulty of teasing apart the role of competition and other forces at play makes it a challenging proposition. A possible example is given by Freeman and Montgomery [42] who reported asymmetrical interspecific aggression between the apparently dominant Swainson’s Thrush Catharus ustulatus, living at lower elevations, and the declining, cold-adapted Bicknell’s Thrush C. bicknelli which is restricted to mountaintops in the northeastern United States and adjacent regions of Canada. Because an aggressive response to playback occurred only at high elevations, where both species were in contact, this behavior was likely a learned response to the presence of a competitor, a pattern also found in other similar studies (e.g., [61,66]). Competition with Swainson’s Thrush could thus contribute to recent population declines and local extirpations of Bicknell’s Thrush, a trend that could be accentuated in the future in response to climate warming [42], although the authors warn that the relative importance of competition in driving these dynamics is unknown.
Asymmetric interspecific aggression with apparent dominance of the low-elevation species was also reported in three species-pairs of passerines in New Guinea [66], two species-pairs of Catharus thrushes in Costa Rica [61], and one pair of bulbuls Pycnonotidae in Borneo [8]. However, the same studies also identified species-pair replacing each other exhibiting symmetric levels of interspecific aggression (a pair of Henicorhina Wood-wren in Costa Rica) or a complete absence of aggression (a pair of White-eye Zosteropidae species in Borneo and two pairs of passerines in New Guinea, although in the latter case, the pair were separated by a ‘no man’s land’ gap suggesting a lack of actual interactions). Lastly, a species-pair of Parus tit, whose patterns of aggression were consistent with the ‘king of the hills’ scenario, was identified by Barve and Dhondt [71].
3.1.2. Can We Make Predictions?
A meta-analysis conducted on four vertebrate classes (including birds) using levels of interspecific aggression as a measure of competitive dominance suggested that, contrary to the Darwin-MacArthur hypothesis, low-elevation species may not typically be better competitors than related high-elevation species [9]. Instead, patterns of interspecific aggression appear related to body size, and body size may be idiosyncratically related to elevational range [9]. Generalizations of future competitive outcomes in a changing climate are further complicated by the facts that: (i) the existence and degree of symmetric or asymmetric interspecific aggression, which are relatively easy to measure and frequently used as proxies for interspecific competition, provide at best an imperfect measure of the ability of two species to coexist in sympatry ([8,72]; and (ii) interspecific competition is only one of the many factors contributing to the elevational range. Some of these factors are species-specific, including habitat preference [73], thermoregulatory cost [74], body size, dispersal ability, and territoriality [75], while others, such as predation and parasitism, occur at the community level (e.g., [76]). Furthermore, all these effects show substantial variation across regions [75], and species may be differentially affected by other sources of disturbance, such as anthropogenic land-use change; (iii) increased sympatry driven by climate warming may not lead to population decline of one or both competitors if mechanisms of niche partitioning, such as differential foraging strategies in allopatry and sympatry, facilitate coexistence [77]; and (iv) more hypothetically, adaptive introgression may alter the long-established patterns of relative dominance on either side of the hybrid zone, leading to unexpected hybrid zone movement in the face of climate change (see [78] for a possible case in a Callipepla quail hybrid zone and [79] for a discussion on Ammodramus sparrows).
3.2. Prediction 2: Human Activities Predictably Alter the Competitive Dynamics between Dominant and Subordinate Species
3.2.1. Overall Support for Prediction 2
Many studies reveal that human-mediated expansion of dominant competitors negatively impacts subordinate ones. For instance, the increased abundance of the hyper-aggressive East Australian Noisy Miner in response to land-use change negatively impacts all small-bodied bird species ([24,80]; see also Discussion). On the Swedish island of Öland, the Collared Flycatcher Ficedula albicollis, which colonized ~ 60 years ago, is rapidly replacing the Pied Flycatcher F. hypoleuca via competition over nest sites, with F. albicollis being dominant, and via asymmetry in recruitment probability and hybridization costs, raising the possibility that F. hypoleuca will be driven to local extinction [81,82]. The Pied Flycatcher also appears to be limited by competition with more distantly related resident species such as the Great Tit and Blue Tit. In both cases, warmer temperatures accentuate the negative effects of competition for the Pied Flycatcher [38,81].
One of the best examples of species replacement mediated by interspecific competition in a context of human-made habitat transformation is the replacement of Mountain Bluebirds Sialis currucoides by Western Bluebirds S. mexicana [83]. In the late 1930s, Western Bluebirds were extirpated from the state of Montana due to changes in logging and agricultural practices that reduced the availability of mature trees with nest cavities [83]. While Mountain Bluebirds we also affected by these habitat changes, this species persisted in less affected higher elevation areas before recolonizing lower areas following the widespread implementation of nest box programs in the valleys [83]. More recently, the availability of nest boxes also allowed the more aggressive Western Bluebirds to recolonize their historical range where they are rapidly displacing Mountain Bluebirds from lower elevation areas. Complete species replacement typically occurs in 10 years or less [83].
Another well-documented case study concerns the competitive dynamics between the Barred Owl and the Spotted Owl. Spotted Owls have suffered a dramatic decline over the past century due to the extensive logging of mature and old-growth conifer forests, although other factors such as urbanization and, more recently, high-severity wildfires, have also played a role [84,85]. However, habitat loss alone cannot explain the magnitude of population declines observed in the Northern Spotted Owl, and the influence of competition with expanding Barred Owls has recently received a lot of attention [85]. The westward expansion of Barred Owls into regions previously occupied solely by Spotted Owls appears to have been facilitated by habitat modification by European settlers who suppressed fires historically set by Native Americans and planted trees in the Great Plains [86]. Substantial evidence suggests that Spotted Owls are now negatively affected by Barred Owls due to competition for habitat and prey and, to a lesser extent, hybridization as well as occasional predation events (e.g., [87,88]). For instance, by comparing the northern Sierra Nevada, where Barred Owl density was low, and the Pacific Northwest, where Barred Owl density was high, Wood et al. [89] determined that the overlap in foraging habitat selection and diet of these two species was high and independent of Barred Owl density, while interspecific hybridization was frequent only when Barred Owl was rare. A meta-analysis of 11 study areas suggested that the presence of Barred Owls negatively affected Northern Spotted Owl primarily by decreasing apparent survival and increasing local territory extinction rates [90]. In fact, the only Spotted Owl population that was either demographically stable or slightly increasing benefited from the lethal removal of Barred Owls as part of a removal study [90] and a larger-scale removal experiment revealed a strong positive effect on survival of sympatric Spotted Owls [91]. Since Barred Owls are larger and display higher levels of vocal and physical aggression during agonistic interspecific interactions, interference competition is a plausible contributing mechanism [92,93]. The fact that Barred Owls can occupy old-growth forests while also being more tolerant of forest fragmentation [92,94] suggests that Spotted Owls do not have any refuge to escape competition and incur the highest cost due to anthropogenic habitat disturbance. Altogether, these findings are predictive of the ongoing and accelerating competitive exclusion of Spotted Owls by expanding Barred Owls [89,90,92,95].
We also found support for the second component of Prediction 2 stating that relaxed competition following the decline of dominant species favors the expansion of subordinate competitors. In the Himalayas, the Cinereous Tit Parus cinereus, which is subordinate to the Green-backed Tit P. monticolis, appears to be able to expand its range upslope by occupying sub-optimal habitats for the dominant competitor, namely man-modified habitats where Green-backed Tits are present at reduced density [71]. Perhaps the most compelling example of competitive release and compensatory response is provided by studies of ‘professional’ antbirds in the Neotropics which rely on the army ant Eciton burchellii to flush their prey (e.g., [43]). In Panama’s guild, both the Ocellated Antbird Phaenostictus mcleannani (largest and socially dominant) and Bicolored Antbird Gymnopithys leucaspis (mid-sized and subordinate to Ocellated) are obligate ant-followers requiring a loose territorial system, while the Spotted Antbird Hylophylax naevioides (smallest and subordinate to the Ocellated and Bicolored Antbirds) is normally a facultative follower, foraging at ant swarms primarily when they pass through their territories [43]. However, on Barro Colorado Island, a land-bridge island formed after tropical forests were flooded to create Gatun Lake as part of the Panama Canal, the extinction of the Ocellated Antbird following a harsh dry season in 1969 was associated with an apparent relaxation of territorial defense in Spotted Antbirds (after a 20-year period). This behavioral flexibility, the authors argue, was the determinant for numerical and functional compensation manifested by a two-fold increase in density and a three-fold increase in metabolic biomass of Spotted Antbirds at ant swarms in Barro Colorado [43].
3.2.2. A More Complex Picture
Although the previous examples provide compelling support to Prediction 2, the capacity to use established competitive dominance levels to predict future outcomes is not absolute. For instance, an unexpected consequence of the loss of Ocellated Antbirds in Barro Colorado Island was that numerical and functional compensation benefitted a subordinate facultative follower, the Spotted Antbird, and not a larger and dominant obligate ant-follower, the Bicolored Antbird [43].
The complexity of mechanisms involved is illustrated by the dynamics of the hybrid zone between the Saltmarsh Sparrow Ammodramus caudacutus, a narrow niche specialist dependent on salt marshes, and the Nelson’s Sparrow A. nelsoni, which uses a broader range of habitats including grassland and brackish marshes [79]. Even though the Saltmarsh Sparrow may be better adapted to salt marshes and its larger size may give it a substantial competitive advantage over A. nelsoni for access to mates [96], recent temporal shifts in the Saltmarsh–Nelson’s Sparrow hybrid zone have occurred to the detriment of the former [97]. The authors speculated that extensive salt marsh loss due to sea level rise and other anthropogenic causes were responsible for the faster rate of decline of the Saltmarsh Sparrow which, in turn, explained the dynamics of the hybrid zone. If the authors’ interpretation is correct, it follows that global change may override long-established competitive dominance between these two species.
Finally, predictions need to rely not only on patterns of competitive dominance, but also on the dynamic of critical habitat components, which may be hard or impossible to do. For instance, Haynes et al. [98] reported that interspecific competition for nesting lakes between the Yellow-billed Loon Gavia adamsii and Pacific Loon G. pacifica on the Arctic Coastal Plain of Alaska led to a tenfold decrease of occupancy probability and a threefold decrease of colonization probability for the Pacific loon. However, current competitive outcomes may not be predictive of future outcomes in a warming climate, as they may depend on whether climate warming leads to a general expansion of lake surface area, which would favor Yellow-billed Loon, or an increase of lake drainage, which may favor Pacific loons [98].
3.3. Prediction 3: Introduced Exotic Bird Species May Supplant Native Species
3.3.1. The Importance of Interspecific Hybridization
The introduction of exotic bird species can lead to extinction of native species via interspecific competition and hybridization (extinction by introgression). For instance, introduced Mallards Anas platyrhynchos have had a particularly strong impact on native dabbling ducks across the world. In New Zealand, displacement by introduced Mallards of native Pacific Black Ducks A. superciliosa, locally known as Grey Ducks, may be the consequence of genetic introgression, resulting in the loss of pure Grey Ducks, direct interspecific competition, as well as competition between hybrids and natives [15]. Due to the formation of a hybrid swarm, the Pacific Black Duck is regarded as effectively extinct on the West Pacific Islands of Guam, Tinian, and Saipan [99], although Mallard colonization, in this case, might have been natural [100]. Additional examples include hybridization between feral domesticated Mallards and Hawaiian Ducks A. wyvilliana, constituting a major threat for the persistence of the Hawaiian endemic [101], and the fast-paced breakdown in American Black Duck genetic integrity following game-farm releases of Mallard in the continental US [102]. Negative consequences of interspecific hybridization and competition with exotics are not limited to Anas ducks, however; other examples include the declining White-headed Duck Oxyura leucocephala and Rock Partridge Alectoris graeca which are threatened by hybridization with the introduced Ruddy Duck Oxyura jamaicensis and Chukar A. chukar, respectively [44].
In the absence of introgressive hybridization, reduced fitness and population decline appear more likely than extinction, although local extinction events and displacement from certain habitat types are possible and may threaten the most vulnerable species (e.g., [103]). For instance, Shochat et al. [104] argue that in urban environments that are characterized by high productivity and predictability of resources, a few synanthropic species are favored to the detriment of native species that could otherwise adapt and thrive in such settings. Harm to native species can result not only from competition for nest sites and food and brood parasitism, but also possibly from acoustic competition as well (for a discussion of the latter, see [105]). For instance, in Israel introduced Common Myna Acridotheres tristis displace House Sparrows Passer domesticus from nest sites and prey on their chicks, reducing their breeding success and seemingly causing local population declines [106]. Competition for nests from feral Rock Pigeons Columba livia was the most frequent cause of breeding failure of a small seabird (Bulwer’s Petrel Bulweria bulwerii) on Tenerife, and in Belgium a negative association between numbers of the introduced Rose-ringed Parakeets Psittacula krameri and local Eurasian Nuthatches Sitta europaea was likely explained by competition over nesting cavities [107,108]. Competition for food can result in reduced foraging success, as in the case of the Seychelles Magpie Robin Copsychus sechellarum when foraging close to Domestic Hens and Common Myna, two species with a high percentage of dietary overlap with the Magpie Robin [109]. In England, the presence of the introduced Rose-ringed Parakeet at feeders significantly reduced feeding rates and increased vigilance among native garden birds such as Blue and Great Tits, a likely consequence of interference competition ([110]; note however that the authors also showed that some habituation may already have occurred in some native populations). Brood parasitism can also have a negative impact on local populations, as exemplified by the native Puerto Rican Vireo Vireo latimeri and the Shiny Cowbird Molothrus bonariensis which was introduced to Puerto Rico in 1955 [44]. Parasitism rates reached up to 83% in Guánica Forest, resulting in significantly lower hatching rate and nestling survival of vireos, and translating into sharp local population decline [111].
3.3.2. Introduced Birds: Not a Major Threat to Avian Diversity?
The introduction of exotic species does not always have observable negative consequences on local populations, however (e.g., [112,113,114]), and recent reviews suggest that introduced birds may not be a major threat to avian diversity globally. Baker et al. [44] reported that no population-level threat was identified in 72 out of 94 articles examining the impact of introduced bird species on native birds. Evans et al. [29], who assessed the impact of 58 alien bird species on 208 native bird species, found that that almost 70% of impacts sustained by native birds can be regarded as ‘weak’ (not causing population decline) and only 4% as ‘severe’ (causing native species extirpations), and that more impact resulted from predation (52% of all impacts) than from competition (33%). Cavity-nesting species such as woodpeckers were disproportionately affected by interspecific competition with alien birds and tended to suffer more often from population decline [29]. Using a different (but presumably overlapping) data set on the impact of 415 bird species with self-sustaining alien populations world-wide, Evans et al. [115] found that competition between alien birds and native species was the most frequently recorded impact mechanism with almost 45% of all recorded impacts, but that the competitive impacts of alien bird species tended to be low in comparison to the that of other mechanisms such as predation.
Overall, as pointed out by Bonter et al. [112] and Evans et al. [29], the negative effects resulting from competition with introduced species may be generally weaker than those resulting from the introduction of new predators or diseases, but they may also be more difficult to document. More empirical studies are thus needed to examine the impact of exotic species, including the magnitude of competition effects, while also accounting for multiple sources of uncertainty. For instance, a compelling example cited by two reviews [29,44], concerning the negative impact of Japanese White-eye Zosterops japonicus on native Hawaiian forest birds such as the endangered Hawaiʻi ʻakepa Loxops coccineus in Hakalau Forest NWR, has been contested. In the original articles [116,117], the authors suggested that a population of ʻakepa became non-viable in the early 2000s following an abrupt increase in White-eye. Contradicting that view however, long-term trends for native birds in the Hakalau Forest NWR suggest that ʻakepa numbers were either stable or increasing during that period, although the authors warn that the most recent data suggest emerging problems [118]. Similarly, extirpation of the native Cocos Buff-banded Rail Gallirallus philippensis andrewsi could have resulted from competition with the alien Green Junglefowl Gallus varius, as suggested by Evans et al. ([115]: used as the ‘Competition’ example in their Table 1), but also from several other factors such as habitat modification, predation by cats, rats, and humans, as well as competition with rats [115,119]. Hence, the actual contribution of competition with the junglefowl is unknown.
4. Discussion
4.1. Predictable ‘Winners’ and ‘Losers’
A better understanding of how biotic interactions mediate species responses to environmental disturbance is critical to understand and mitigate the negative consequences of global change to natural communities (e.g., [3]). In this review, we have provided numerous examples of population decline and displacement that appeared to be, at least in part, driven by interspecific competition, and were amplified by environmental changes associated with human activities (for counter examples, see, e.g., [44,112]). For instance, the negative impact of the Collared Flycatcher, Great Tit, and Blue Tit on the breeding density of the Pied flycatcher is accentuated by warmer temperatures [38,81], the displacement of subordinate competitors, such as Mountain Bluebird and Spotted Owl, may be preceded by habitat transformation favoring the expansion of dominant competitors such as Western Bluebird and Barred Owl (e.g., [83,85] and references therein), and introduced Mallards have led to the decline or extinction of native dabbling ducks across the world via interspecific competition and hybridization (e.g., [15]). Global change may thus alter the competitive dynamics among species belonging to various ecological groups in a non-random fashion, resulting in predictable ‘winners’ and ‘losers’ (such as generalists and specialists, respectively), and contributing to biotic homogenization [27,32].
4.2. Implications for Ecosystem Functioning
Biotic homogenization, including a massive reduction in species richness, is a major consequence of global change. Many species are declining while a much smaller number of expanding species which thrive in human-altered environments are replacing them [27]. Because of their contribution to critical ecosystem properties and services such as decomposition, pest control, pollination, and seed dispersal, birds are an important component of healthy ecosystems [120,121]. Thus, the loss of species and functional traits resulting from biotic homogenization is expected to negatively impact the ecosystem services rendered by birds as well as the ability of these ecosystems to be resilient in the face of future disturbances (e.g., [122,123,124,125,126]). In the following sections, we discuss findings supporting the view that interspecific competition may in some cases alter, or contribute to, the impact of global change forces on avian species richness and functional biodiversity. As the number of studies on this topic appears limited, we believe this is an area of promising research and discovery. A systematic review investigating the role of interspecific competition and other biotic interactions such as predation may be worthwhile.
Expanding (or invading) species may frequently have different habitat requirements than native species and thus benefit from anthropogenic land-use changes rather than outcompeting local native species in their own preferred habitats [127]. For example, native bird species in São Tomé were typically found in remote rainy forests at higher altitudes where they rely on carnivory and frugivory, whereas non-native species were typically associated with humanized lowland areas where they rely mostly on seeds which are more available there than in the best-preserved forests [128]. This does not mean, however, that interspecific competition cannot negatively influence species richness; for instance, Le Louarn et al. [129] showed that the presence of the invasive Rose-Ringed Parakeets at feeders was associated with a decrease in the number of species attending these feeders, a form of temporary exclusion due to interference from the parakeets.
In other cases, competitive exclusion can be permanent with the best example being provided by the East Australian Noisy Miner, an obligate colonial and co-operative breeder whose colonies can reach several hundred individuals [130]. The Noisy Miner has been referred to as a ‘reverse keystone’ species as it aggressively excludes almost all small-bodied bird species (<50 g) from its territories [24,80], one of which being the critically endangered Regent Honeyeater [131]. Potentially compounding this direct impact on small birds, the so-called ‘miner-tolerant’ species showing a positive association with Noisy Miners, including butcherbirds Cracticus spp., ravens Corvus spp., Laughing Kookaburras Dacelo novaeguineae and Black-backed Magpies Craticus tibicen, are typically large and aggressive species that prey on small birds and their young or eggs [132]. Competition from Noisy Miners and another aggressive congener, the Yellow-throated Miner Manorina flavigula, and not landscape structure, was found to be the main cause of taxonomic homogenization within a fragmented agricultural landscape [133], and Noisy Miner density was by far the strongest single predictor of small-bird richness and abundance in a study including 10 other environmental covariates [134]. Long-term data within a single forest showed that species richness of bird species smaller than Noisy Miners was tenfold greater, and their abundance at least twentyfold greater, outside miner colonies than within them [80]. A study conducted across a vast region from Victoria to Queensland found that Noisy Miners depressed occurrence of 57 of 71 small-bodied species [12]. A strong causal link between Noisy Miner colonies and depressed richness and abundance of smaller birds comes from both controlled and uncontrolled experiments showing a drastic increase in species richness and abundance after Noisy Miners were removed [131,135,136,137]. Multiple-season occupancy models showed that Noisy Miners increased extinction risk and decreased colonization probability of the species it excludes [138].
Importantly, the large-scale (>1 million km2) community shifts triggered by the despotic Miners may have far-reaching consequences beyond the observed depressed species richness in small birds due to the loss of foraging strategies and ecological functions provided by these smaller-bodied species, although more work is required to rigorously document such cascading effects [24]. For instance, exclusion of smaller insectivorous birds, which control herbivorous insect populations, can be linked to reduced tree condition and tree dieback in small forest remnants, while the alteration of pollinator assemblages resulting from the exclusion of small honeyeaters is likely to reduce the efficacy of pollination, gene flow and seed production, which could also negatively affect the long-term persistence of fragmented vegetation ([24,139,140], and references therein). Critically, the negative effect of interspecific competition is exacerbated by global change. First, Noisy Miners are most often found in disturbed habitats including lightly wooded agricultural land, the roadsides of cities and towns, edges of wooded habitats, or in open areas within or bordering forests and woodlands [130]. Hence, Noisy Miners are favored by the creation of edge habitats due to anthropogenic fragmentation of natural landscapes [24,80,141]. Second, structural simplification that results, e.g., from livestock and feral grazing [132,142], also facilitates detection and interception of potential competitors by miners [24,132]. Third, the warming of the climate also may advantage Noisy Miners as, unlike other smaller passerines, they appear immune to drought-induced degradation of the vegetation [143].
Such a well-studied and compelling example of the role played by aggressive interspecific competition is consistent with a negative effect of the introduced Red-vented Bulbul Pycnonotus cafer on the occurrence of nine native species of birds in New Caledonia [103] but contrasts with other studies suggesting a more subdued or elusive role of interspecific competition (e.g., [44] for a review). The effects of direct (e.g., land-use change) and indirect (e.g., modification of competitive dynamics among species) anthropogenic disturbances on species richness and functional diversity are often intricate and complex, they can be influenced by methodological choices, and may also vary across bird communities and ecosystems (see also Discussion below). For instance, habitat fragmentation in the Amazonian rainforest landscape led to reduced species richness and an overdispersion of functional traits in smaller fragments [144], which the authors interpreted as evidence for increased competition in smaller remnants driving an extinction bias toward more densely occupied regions of niche space. Conversely, Ulrich et al. [145] found little evidence that interspecific competition plays a major role in explaining the patterns of species abundance and functional diversity in small cloud forest fragments of Taita Hills (in southern Kenya).
More work is clearly needed to better grasp the relative influence of multiple factors, including interspecific competition, in driving the dynamics of disturbed communities and ecosystems. For instance, the loss of specialists affected by changing conditions such as landscape disturbance and fragmentation [146] may be compounded by increased competition with generalists [32], while the loss of specialists can also favor the expansion of generalists via relaxed competition with specialists [147]. Whatever the cause, the loss of specialists may lead to both taxonomic and functional homogenization [125,146], but the actual contribution of interspecific competition to the loss of specialists was not examined in these studies. Interestingly, Richmond et al. [148] used results from a simulation study to suggest that species-poor communities of generalists can, under certain circumstances, have equal or greater ecosystem function than species-rich communities of specialists. Experiments or field studies are needed to test this prediction.
4.3. Synthesis: Predictions Are Hampered by Multiple Sources of Uncertainty
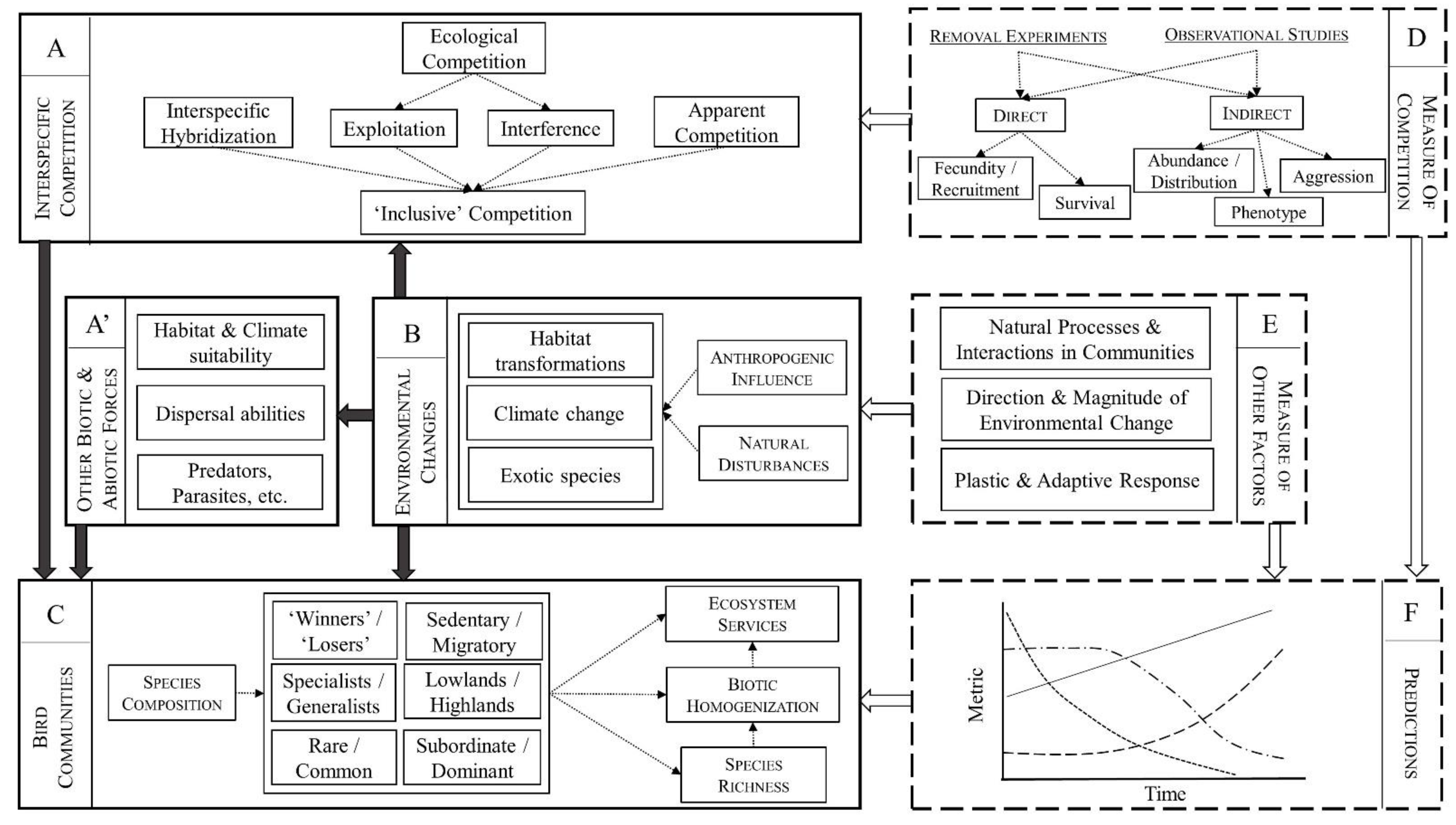
Interspecific competition (Figure 1—panel A) as well as other biotic interactions (Figure 1A’) may thus modulate species response to environmental changes (Figure 1B) and affect community-level responses including species composition, species richness, and ecosystem services (Figure 1C). However, predicting the consequences of environmental changes (Figure 1F) is hampered by multiple sources of uncertainty concerning the relative importance of interspecific competition and other forces, which result from: (i) vast disparity in definitions and methodologies across studies (Figure 1D); and (ii) uncertainty related to the intrinsic complexity of natural communities, the direction and magnitude of future changes, as well as the speed and efficacy of plastic and adaptive responses (Figure 1E). These elements are further discussed below.
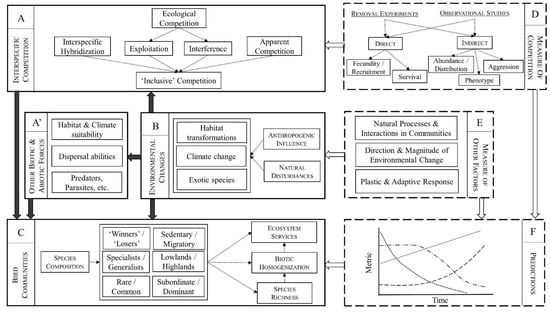
Figure 1.
Synthesis. Environmental changes (panel B) affect bird communities directly, through habitat transformation, climate change, and the introduction of exotic species, as well as indirectly, through a modification of dispersal abilities (A’) and biotic interactions such as competitive interactions (A+A’). Interspecific competition may thus modulate species response to environmental changes and affect community-level responses including species composition, species richness, and ecosystem services (C). However, predicting the consequences of environmental changes (F) is hampered by multiple sources of uncertainty (indicated by dashed boxes) concerning the relative importance of interspecific competition and other forces (D,E). Causal relationships are indicated by black arrows; measures and predictions are indicated by white arrows See text for details.
4.3.1. What Role for Interspecific Competition?
We discussed several well-studied examples where interspecific competition appeared to play a prominent role in the observed patterns of species replacement and biotic homogenization. For instance, the recent increase in density of the generalist Manorina honeyeaters following land-use change was responsible for the decline and loss of small and specialist species in Australia [133] and, as previously discussed, the expansion of the generalist Barred Owl into old-growth western forests occupied by the specialist Spotted Owl led to population declines and local extinctions of the latter. In both cases, differences in aggressiveness and competitive dominance coupled with a lack of refuge for habitat specialists might be responsible for the observed trends [93,133].
Despite these findings, we argue that, in general, the role played by interspecific competition remains poorly understood, for at least two main non-mutually exclusive reasons related to: (i) definition and methodology; and (ii) the complexity of natural communities (see Figure 1 for a schematic representation). A first issue is that the very definition, and thus measure, of interspecific competition varies between authors. Ecological competition itself may relate to competition for nest cavities, for food, and for territories, and all these components are rarely, if ever, studied simultaneously. More critically, interspecific hybridization and ecological competition are mostly regarded as independent forces in the literature (e.g., [82]), but the former can also be seen as a form of competition for shared resources such as mates and territories [149,150]. The consequences for species coexistence of hybridization and other interspecific reproduction interactions have recently gained a lot of attention [151,152,153,154]. Interspecific hybridization can result in reduced fitness caused by genetic swamping, where the rare form is replaced by hybrids, or demographic swamping, where the decline is due to the production of maladaptive hybrids [155]. Of course, interspecific competition over resources such as food and nest sites can occur at the same time as interspecific hybridization. The relative contribution of these two forces to population decline may vary along a continuum, from a predominance of ‘traditional’ ecological competition in Barred vs. Spotted Owls (e.g., [85]), a mixture of the two in Pied vs. Collared Flycatchers (e.g., [82]), and a major role to hybridization which seems to best explain the decline of Golden-winged Warbler Vermivora chrysoptera following the recent expansion of Blue-winged Warbler Vermivora cyanoptera (e.g., [156]).
In many cases, however, no attempt is made to tease apart different forms of competition (e.g., [20,38,50]). What is measured is thus a form of ‘inclusive competition’ (Figure 1A), where the negative consequence of one species on another is assessed independently of the mechanisms, which can include apparent competition, predation, cross-species transmission of parasites, and amensalism, in addition to ecological competition and hybridization (e.g., [157]; a distinction between coexistence and biotic interactions studies is discussed in [158]). When we factor in the possible interactions between these and other biotic forces, the larger web of interactions in the communities these species are part of, and the role of abiotic factors, it becomes apparent that evaluating the exact role of interspecific competition should be well beyond reach in most cases (see also Discussion below). In many studies, the role of interspecific competition is regarded as hypothetical, although authors often warn the impact could become stronger in the future (e.g., [159,160]). A good example is the homogenization of the scavenger guilds in North and South America following the range expansion of the Black Vulture Coragyps atratus which benefits from anthropogenic food sources such as rubbish dumps, cattle, and road kills [161]. In their study in South Argentina, the authors showed that carcass consumption is determined by species abundance rather than individual body size. Accordingly, the most abundant species tends to monopolize the resources, namely the Andean Condor Vultur gryphus in the mountains and the Black Vulture in human-modified plains [161]. Because females Andean Condor are often displaced from the mountain carcasses by the larger males, they may suffer disproportionately from competition with Black Vulture in the plains, which could negatively impact their feeding efficiency and lead to lower survival and a biased sex ratio [161].
With observational rather than experimental studies being the norm, another key issue is that alternative confounding factors cannot usually be excluded. Studies evaluating how the distribution, abundance, and/or phenotype of one species impacts another across space (e.g., [20]) and/or over time, including evolutionary time scale [162,163] typically fall in this category, although the latter may offer unique insights into the role of interspecific competition during lineage diversification [163]. Rare exceptions include removal experiments (see, e.g., [14,90,164,165]). Another caveat is that interspecific competition is rarely assessed and tested via its effects on fitness or demographic parameters such as survival, breeding success, immigration, or recruitment (but see [37,82,165]). Instead, indirect measures are often used, including shifts in distribution or habitat use in sympatry or syntopy and aggression for species pairs. Because habitat and range shifts in allopatry may reflect different environmental conditions rather than ecological release, accounting for habitat covariates is necessary (e.g., [166]). It remains, however, that key covariates may be elusive, potentially affecting the estimation of competition effects [20,98,166,167]. Since aggression cannot always be linked to a determined competitive outcome such as coexistence or exclusion [8,149], it may not come as a surprise that the seductive ‘push’ scenario of climate-driven and competition-accelerated extirpation of mountaintop species remains hypothetical to this day [9]. Comparative fitness measures in sympatry, ideally combined with removal experiments (e.g., [14]), may contribute meaningful data to answer this question [42]. Global citizen science databases such as eBIRD [168], which already accumulates two decades of distributional and abundance data, could provide further opportunities to test the central tenet of Prediction 1 that range shifts and population decline cannot be explained by climatic change alone and a significant contribution to the vital effort to connect niche tracking to community assembly theory [169]. By quantifying the ability of species to track their climatic niche over time, we may predict (but see also [169] for a discussion on the notion that the best trackers may be inferior resource competitors, at least in plants): (i) a greater disparity between competitors than between random pairs of species; and (ii) a positive correlation between the ability of species to track their climatic niche and their competitive ability. Note that a rigorous framework may be necessary to assess competitive dominance based on available data, including morphological and behavioral cues (e.g., [9,170]), or even abundance data (e.g., [145]), while also accounting for the fact that competitive hierarchies may vary in space or time (e.g., [171]).
4.3.2. Uncertainty Linked to Ecosystem Complexity and Future Changes
The intricate and complex nature of biological processes is another major impediment to a better understanding of the role of interspecific competition. Many abiotic and biotic factors, including interspecific competition with birds and other animals but also dispersal abilities, predators, and parasites to name a few, may act at different rates and over multiple spatial scales either independently, synergistically, or antagonistically, and contribute to population and community dynamics [26,41,172,173,174,175,176]. Isolating the influence of interspecific competition is thus extremely challenging, even without accounting for anthropogenic perturbations and global change (e.g., [8,166,177,178,179]). For instance, overdispersion of functional traits that can be used as a proxy for interspecific competition at the community level [144], may in some cases be driven by environmental filtering or dispersal limitations [145]. Another great example of such complexity is provided by Auer and Martin [180] who studied cascading effects of climate change on Elk Cervus canadensis, plants, and songbirds. These authors showed that changes in plant communities, driven by a climate-driven increase in herbivory, have affected the selection of nest sites by three songbird species. This resulted in a greater overlap of their nest sites which, in turn, was associated with an increase in nest predation rates, a case of apparent competition.
Faced with such complexity, many studies do not actually investigate the actual contribution of interspecific competition, but simply mention it as a possible cause of the observed trend or pattern (e.g., [146,147]). Those that do typically identify patterns consistent with interspecific competition without controlling for the many possible confounding factors (e.g., [181]) or explicitly contrast interspecific competition with a limited number of alternative factors such as landscape structure [133], habitat quality [85], or temperature seasonality (Janzen’s hypothesis: [69]). Using the relative explanatory power of these two predictors as a measure of their relative importance (e.g., [69]) may give a false sense of their actual importance if key covariates are left out. Using statistical designs allowing interspecific competition to be picked out from larger sets of potential confounding factors may be beneficial [17,64,169]. For instance, Guillaumet et al. [17] proposed a framework allowing confounding factors to be adjusted so as to maximize their influence on the variable of interest [17], potentially leading to conservative, rather than biased, estimates of the role of interspecific competition, while Ulrich et al. [145] describe an alternative approach where the maximum effect of interspecific competition on community assembly is estimated.
Interestingly, the extreme complexity of natural ecosystems can lead to unexpected and idiosyncratic responses [43,182]. For instance, contradicting the view that larger species should always suffer the heaviest consequences from habitat fragmentation, the large and despotic Noisy Miner is favored by habitat fragmentation and aggressively excludes small-bodied bird species from its territories [24], including the critically endangered Regent Honeyeater [131]. However, ecological release from abundant competitors severely affected by global change may sometimes benefit rarer species as measured by increases in occupancy, abundance, and biomass [121,183]. Another example concerns the replacement of specialists by generalists, which is not the only possible outcome of global change. For instance, the Collared Flycatcher is rapidly replacing the Pied Flycatcher in the Swedish island of Öland, even though the latter appears to have a wider niche breadth [82,184]. Competitive dominance, rather than the degree of specialization, may be a critical factor here, as the Collared Flycatcher is dominant in the competition over nest sites [81]. In Brazil’s southern grasslands, Staude et al. [185] found that the ratio of specialists to generalists in native grassland remnant patches increased with increasing agricultural cover in the landscape, suggesting that more intense competition in those remnants leads to the gradual exclusion of generalists. Whether populations of specialists can be sustained in the long term in these remnants remains to be seen. Finally, although larger species tend to be dominant competitors [9], exceptions seem to exist [160], particularly when abundance, rather than body size, determines the outcome of competition [161], and human-driven changes can favor the expansion or dominant species [83] or tilt the balance towards subordinate species [97]. Overall, predicting losers and winners is thus a difficult task, and today’s winner may be tomorrow’s loser in a fast-changing world [106].
In addition to the inherent complexity of natural ecological processes, uncertainty regarding future environmental conditions as well as the speed and efficacy of plastic and evolutionary responses to fast-changing environments and interspecific competition make predictions even more difficult (e.g., [98,169,186,187,188]). For instance, Bonnet-Lebrun et al. [189] suggest that the subordinate arctic-breeding Brünnich’s Guillemot Uria lomvia may in some cases escape competition from the dominant Common Guillemot U. aalge by foraging closer to sea ice in areas of sympatry, but such refugia may be compromised as sea ice continues to retreat in response to global warming. The competitive edge of resident species against migratory species (e.g., [35]) might be eroded if migrant species are able to alter their phenology, for instance by advancing pre-breeding migration dates [190] and/or evolving shorter migration routes, which may occur over only a few decades [191,192]. However, in an interesting twist to the story, the ability of migratory species to make these vital phenological adjustments may well be hampered by exploitation and interference competition with these resident species [36]. Alternatively, character displacement in ecological, phenological, and morphological traits can occur relatively fast and reduce the cost of interspecific competition and hybridization [11,184,193]. Simulation models [158] as well as accumulating empirical studies may offer insights into the relative frequency of these outcomes and the consequences for the conservation of avian species with contrasted life-history strategies.
Author Contributions
Both authors contributed equally to literature search. Alban Guillaumet wrote the first draft of the manuscript and both authors contributed to the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest and have no other ethic statement.
References
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.R.; Collar, N.J. The future for Sundaic lowland forest birds: Long-term effects of commercial logging and fragmentation. Forktail 2002, 18, 127–146. [Google Scholar]
- Davis, A.J.; Jenkinson, L.S.; Lawton, J.H.; Shorrocks, B.; Wood, S. Making mistakes when predicting shifts in species range in response to global warming. Nature 1998, 391, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.E.; Urban, M.C.; Tewksbury, J.; Gilchrist, G.W.; Holt, R.D. A framework for community interactions under climate change. Trends Ecol. Evol. 2010, 25, 325–331. [Google Scholar] [CrossRef]
- Diamond, J.M. Distributional Ecology of New Guinea Birds: Recent ecological and biogeographical theories can be tested on the bird communities of New Guinea. Science 1973, 179, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.K.; Terborgh, J. Interspecific Aggression and Habitat Selection by Amazonian Birds. J. Anim. Ecol. 1995, 64, 1–11. [Google Scholar] [CrossRef]
- Jankowski, J.E.; Graham, C.H.; Parra, J.L.; Robinson, S.K.; Seddon, N.; Touchton, J.M.; Tobias, J.A. The role of competition in structuring tropical bird communities. Ornitol. Neotrop. 2012, 23, 97–106. [Google Scholar]
- Boyce, A.J.; E Martin, T.E. Interspecific aggression among parapatric and sympatric songbirds on a tropical elevational gradient. Behav. Ecol. 2019, 30, 541–547. [Google Scholar] [CrossRef]
- Freeman, B.G. Lower elevation animal species do not tend to be better competitors than their higher elevation relatives. Glob. Ecol. Biogeogr. 2019, 29, 171–181. [Google Scholar] [CrossRef]
- Price, T.D.; Kirkpatrick, M. Evolutionarily stable range limits set by interspecific competition. Proc. R. Soc. B: Boil. Sci. 2009, 276, 1429–1434. [Google Scholar] [CrossRef]
- Grant, P.R.; Grant, B.R. Evolution of Character Displacement in Darwin’s Finches. Science 2006, 313, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Mac Nally, R.; Bowen, M.; Howes, A.; McAlpine, C.; Maron, M. Despotic, high-impact species and the subcontinental scale control of avian assemblage structure. Ecology 2012, 93, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.E.; Cruz, A., Jr.; Grant, M.C.; Aid, C.S.; Strong, T.R. Field Experimental Evidence for Diffuse Competition Among Southwestern Riparian Birds. Am Nat. 1992, 140, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.R.; Martin, T.E. Ecological and fitness consequences of species coexistence: A removal experiment with wood warblers. Ecology 2001, 82, 189–206. [Google Scholar] [CrossRef]
- Quilodrán, C.; Austerlitz, F.; Currat, M.; Montoya-Burgos, J.I. Cryptic Biological Invasions: A General Model of Hybridization. Sci. Rep. 2018, 8, 2414. [Google Scholar] [CrossRef]
- Guillaumet, A.; Pons, J.M.; Godelle, B.; Crochet, P.-A. History of the Crested Lark in the Mediterranean region as revealed by mtDNA sequences and morphology. Mol. Phylogenet. Evol. 2006, 39, 645–656. [Google Scholar] [CrossRef]
- Guillaumet, A.; Ferdy, J.-B.; Desmarais, E.; Godelle, B.; Crochet, P.-A. Testing Bergmann’s rule in the presence of potentially confounding factors: A case study with three species of Galerida larks in Morocco. J. Biogeogr. 2007, 35, 579–591. [Google Scholar] [CrossRef]
- Guillaumet, A.; Gonin, J.; Prodon, R.; Crochet, P.-A. The geographic and seasonal dimensions of habitat use in Galerida larks: Implications for species coexistence and range limits. Ecography 2010, 33, 961–970. [Google Scholar] [CrossRef]
- Guillaumet, A.; Crochet, P.-A.; Pons, J.-M. Climate-driven diversification in two widespread Galerida larks. BMC Evol. Biol. 2008, 8, 32. [Google Scholar] [CrossRef]
- Guillaumet, A.; Leotard, G. Annoying neighbors: Multi-scale distribution determinants of two sympatric sibling species of birds. Curr. Zool. 2015, 61, 10–22. [Google Scholar] [CrossRef]
- Laiolo, P. From inter-specific behavioural interactions to species distribution patterns along gradients of habitat heterogeneity. Oecologia 2012, 171, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 2007, 16, 743–753. [Google Scholar] [CrossRef]
- Engler, J.O.; Rödder, D.; Elle, O.; Hochkirch, A.; Secondi, J. Data from: Species distribution models contribute to determine the effect of climate and interspecific interactions in moving hybrid zones. J. Evol. Biol. 2013, 26, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.; Grey, M.J.; Catterall, C.P.; Major, R.E.; Oliver, D.L.; Clarke, M.F.; Loyn, R.H.; Mac Nally, R.; Davidson, I.; Thomson, J.R. Avifaunal disarray due to a single despotic species. Divers. Distrib. 2013, 19, 1468–1479. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.; Gemmell, N.; A Rand, T.; Ewers, R.M. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef]
- Walther, G.-R. Community and ecosystem responses to recent climate change. Philos. Trans. R. Soc. B: Biol. Sci. 2010, 365, 2019–2024. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Urban, M.C.; Tewksbury, J.J.; Sheldon, K.S. On a collision course: Competition and dispersal differences create no-analogue communities and cause extinctions during climate change. Proc. R. Soc. Lond. 2012, 279, 2072–2080. [Google Scholar] [CrossRef]
- Evans, T.; Jeschke, J.M.; Liu, C.; Redding, D.W.; Şekercioğlu, Ç.; Blackburn, T.M. What factors increase the vulnerability of native birds to the impacts of alien birds? Ecography 2021, 44, 727–739. [Google Scholar] [CrossRef]
- Soares, F.C.; Leal, A.I.; Palmeirim, J.M.; de Lima, R.F. Niche differences may reduce susceptibility to competition between native and non-native birds in oceanic islands. Divers. Distrib. 2021, 27, 1507–1518. [Google Scholar] [CrossRef]
- Marvier, M.; Kareiva, P.; Neubert, M.G. Habitat destruction, fragmentation, and disturbance promote invasion by habitat generalists in a multispecies metapopulation. Risk Anal. 2004, 24, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2010, 9, 222–228. [Google Scholar] [CrossRef]
- Büchi, L.; Vuilleumier, S. Coexistence of Specialist and Generalist Species Is Shaped by Dispersal and Environmental Factors. Am. Nat. 2014, 183, 612–624. [Google Scholar] [CrossRef]
- Berthold, P.; Fiedler, W.; Schlenker, R.; Querner, U. 25-year study of the population development of Central European songbirds: A general decline, most evident in long-distance migrants. Naturwissenschaften 1998, 85, 350–353. [Google Scholar] [CrossRef]
- Ahola, M.P.; Laaksonen, T.; Eeva, T.; Lehikoinen, E. Climate change can alter competitive relationships between resident and migratory birds. J. Anim. Ecol. 2007, 76, 1045–1052. [Google Scholar] [CrossRef]
- Samplonius, J.M.; Bartošová, L.; Burgess, M.D.; Bushuev, A.V.; Eeva, T.; Ivankina, E.V.; Kerimov, A.B.; Krams, I.; Laaksonen, T.; Mägi, M.; et al. Phenological sensitivity to climate change is higher in resident than in migrant bird populations among European cavity breeders. Glob. Change Biol. 2018, 24, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Samplonius, J.M.; Both, C. Climate Change May Affect Fatal Competition between Two Bird Species. Curr. Biol. 2019, 29, 327–331. [Google Scholar] [CrossRef]
- Wittwer, T.; O’Hara, R.B.; Caplat, P.; Hickler, T.; Smith, H.G. Long-term population dynamics of a migrant bird suggests interaction of climate change and competition with resident species. Oikos 2015, 124, 1151–1159. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Freeman, B.G.; Class Freeman, A.M. Rapid upslope shifts in New Guinean birds illustrate strong distributional responses of tropical montane species to global warming. Proc. Natl. Acad. Sci. USA 2014, 111, 4490–4494. [Google Scholar] [CrossRef]
- Bocedi, G.; Atkins, K.E.; Liao, J.; Henry, R.C.; Travis, J.M.; Hellmann, J.J. Effects of local adaptation and interspecific competition on species’ responses to climate change. Ann. NY Acad. Sci. 2013, 1297, 83–97. [Google Scholar] [CrossRef]
- Freeman, B.G.; Montgomery, G. Interspecific aggression by the Swainson’s Thrush (Catharus ustulatus) may limit the distribution of the threatened Bicknell’s Thrush (Catharus bicknelli) in the Adirondack Mountains. Ornithol. Appl. 2016, 118, 169–178. [Google Scholar] [CrossRef]
- Touchton, J.M.; Smith, J.N.M. Species loss, delayed numerical responses, and functional compensation in an antbird guild. Ecology 2011, 92, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Harvey, K.J.; French, K. Threats from introduced birds to native birds. Emu-Austral Ornithol. 2014, 114, 1–12. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Thompson, F.R., III. Poleward shifts in winter ranges of North American birds. Ecology 2007, 88, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Tingley, M.W.; Monahan, W.B.; Beissinger, S.R.; Moritz, C. Birds track their Grinnellian niche through a century of climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 19637. [Google Scholar] [CrossRef]
- Freeman, B.G.; Lee-Yaw, J.A.; Sunday, J.M.; Hargreaves, A.L. Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Global. Ecol. Biogeogr. 2018, 27, 1268–1276. [Google Scholar] [CrossRef]
- Freeman, B.G.; Scholer, M.N.; Ruiz-Gutierrez, V.; Fitzpatrick, J.W. Climate change causes upslope shifts and mountaintop extirpations in a tropical bird community. Proc. Natl. Acad. Sci. USA 2018, 115, 11982–11987. [Google Scholar] [CrossRef]
- Tingley, M.W.; Koo, M.; Moritz, C.; Rush, A.C.; Beissinger, S.R. The push and pull of climate change causes heterogeneous shifts in avian elevational ranges. Glob. Change Biol. 2012, 18, 3279–3290. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Durant, J.M.; Fowler, M.S.; Matthysen, E.; Adriaensen, F.; Jonzén, N.; Chan, K.-S.; Liu, H.; De Laet, J.; Sheldon, B.C.; et al. Testing for effects of climate change on competitive relationships and coexistence between two bird species. Proc. R. Soc. B Boil. Sci. 2015, 282, 20141958. [Google Scholar] [CrossRef]
- Round, P.D.; Gale, G.A. Changes in the Status of Lophura Pheasants in Khao Yai National Park, Thailand: A Response to Warming Climate? Biotropica 2007, 40, 225–230. [Google Scholar] [CrossRef]
- Taylor, S.A.; White, T.A.; Hochachka, W.M.; Ferretti, V.; Curry, R.L.; Lovette, I. Climate-Mediated Movement of an Avian Hybrid Zone. Curr. Biol. 2014, 24, 671–676. [Google Scholar] [CrossRef]
- Gee, J.M. Gene flow across a climatic barrier between hybridizing avian species, California and Gambel’s quail (Callipepla californica and C. gambelii). Evolution 2004, 58, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- DuBay, S.G.; Witt, C.C. Differential high-altitude adaptation and restricted gene flow across a mid-elevation hybrid zone in Andean tit-tyrant flycatchers. Mol. Ecol. 2014, 23, 3551–3565. [Google Scholar] [CrossRef]
- Vaughn, J.C.; Voelker, G.; Heatley, J.J. Glucose Concentrations in Closely Related Titmice (Baeolophus) Species Linked to Regional Habitat Differences Across an Avian Hybrid Zone. Open Ornithol. J. 2020, 13, 10–23. [Google Scholar] [CrossRef]
- Swenson, N.G. Gis-based niche models reveal unifying climatic mechanisms that maintain the location of avian hybrid zones in a North American suture zone. J. Evol. Biol. 2006, 19, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Carling, M.D.; Thomassen, H.A. The Role of Environmental Heterogeneity in Maintaining Reproductive Isolation between Hybridizing Passerina (Aves: Cardinalidae) Buntings. Int. J. Ecol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Buggs, R. Empirical study of hybrid zone movement. Heredity 2007, 99, 301–312. [Google Scholar] [CrossRef]
- McQuillan, M.A.; Rice, A.M. Differential effects of climate and species interactions on range limits at a hybrid zone: Potential direct and indirect impacts of climate change. Ecol. Evol. 2015, 5, 5120–5137. [Google Scholar] [CrossRef] [PubMed]
- Billerman, S.M.; Murphy, M.A.; Carling, M.D. Changing climate mediates sapsucker (Aves: Sphyrapicus) hybrid zone movement. Ecol. Evol. 2016, 6, 7976–7990. [Google Scholar] [CrossRef]
- Jankowski, J.E.; Robinson, S.K.; Levey, D.J. Squeezed at the top: Interspecific aggression may constrain elevational ranges in tropical birds. Ecology 2010, 91, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Surya, G.S.; Keitt, T.H. Altitudinal limits of Eastern Himalayan birds are created by competition past and present. PLoS ONE 2019, 14, e0217549. [Google Scholar] [CrossRef] [PubMed]
- Schumm, M.; White, A.E.; Supriya, K.; Price, T.D. Ecological Limits as the Driver of Bird Species Richness Patterns along the East Himalayan Elevational Gradient. Am. Nat. 2020, 195, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Elsen, P.R.; Tingley, M.W.; Kalyanaraman, R.; Ramesh, K.; Wilcove, D.S. The role of competition, ecotones, and temperature in the elevational distribution of Himalayan birds. Ecology 2017, 98, 337–348. [Google Scholar] [CrossRef]
- Boyce, A.J.; Shakya, S.; Sheldon, F.H.; Moyle, R.G.; Martin, T.E. Biotic interactions are the dominant drivers of phylogenetic and functional structure in bird communities along a tropical elevational gradient. Auk 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Freeman, B.G.; Class Freeman, A.M.; Hochachka, W.M. Asymmetric interspecific aggression in New Guinean songbirds that replace one another along an elevational gradient. Ibis 2016, 158, 726–737. [Google Scholar] [CrossRef]
- Freeman, B.G.; Tobias, J.A.; Schluter, D. Behavior influences range limits and patterns of coexistence across an elevational gradient in tropical birds. Ecography 2019, 42, 1832–1840. [Google Scholar] [CrossRef]
- Burner, R.C.; Boyce, A.J.; Bernasconi, D.; Styring, A.R.; Shakya, S.; Boer, C.; Rahman, M.A.; Martin, T.E.; Sheldon, F.H. Biotic interactions help explain variation in elevational range limits of birds among Bornean mountains. J. Biogeogr. 2020, 47, 760–771. [Google Scholar] [CrossRef]
- Freeman, B.G.; Strimas-Mackey, M.; Miller, E.T. Interspecific competition limits bird species’ ranges in tropical mountains. Science 2022, 377, 416–420. [Google Scholar] [CrossRef]
- Prodon, R.; Thibault, J.; Dejaifve, P. Expansion vs. compression of bird altitudinal ranges on a Mediterranean island. Ecology 2002, 83, 1294–1306. [Google Scholar] [CrossRef]
- Barve, S.; Dhondt, A.A. Elevational replacement of two Himalayan titmice: Interspecific competition or habitat preference? J. Avian Biol. 2017, 48, 1189–1194. [Google Scholar] [CrossRef]
- Freeman, B.G. Strong asymmetric interspecific aggression between two sympatric New Guinean robins. Ibis 2015, 158, 75–81. [Google Scholar] [CrossRef]
- Jones, S.E.I.; Tobias, J.A.; Freeman, R.; Portugal, S.J. Weak asymmetric interspecific aggression and divergent habitat preferences at an elevational contact zone between tropical songbirds. Ibis 2020, 162, 814–826. [Google Scholar] [CrossRef]
- Londoño, G.A.; Chappell, M.A.; Jankowski, J.E.; Robinson, S.K. Do thermoregulatory costs limit altitude distributions of Andean forest birds? Funct. Ecol. 2017, 31, 204–215. [Google Scholar] [CrossRef]
- Neate-Clegg, M.H.C.; Jones, S.E.I.; Tobias, J.A.; Newmark, W.D.; Şekercioǧlu, H. Ecological Correlates of Elevational Range Shifts in Tropical Birds. Front. Ecol. Evol. 2021, 9, 215. [Google Scholar] [CrossRef]
- Jankowski, J.E.; Londoño, G.A.; Robinson, S.K.; Chappell, M.A. Exploring the role of physiology and biotic interactions in determining elevational ranges of tropical animals. Ecography 2013, 36, 1–12. [Google Scholar] [CrossRef]
- Cimino, M.A.; Moline, M.A.; Fraser, W.R.; Patterson-Fraser, D.L.; Oliver, M.J. Climate-driven sympatry may not lead to foraging competition between congeneric top-predators. Sci. Rep. 2016, 6, srep18820. [Google Scholar] [CrossRef]
- Zonana, D.M. Mating On The Margins: The Impacts of Social Network Structure and Climate On Gene Flow In A Hybrid Zone Between California (Callipepla Californica) and Gambel’s Quail (Callipepla Gambelii). Ph.D. Thesis, University of Colorado, Boulder, CO, USA, 2019. [Google Scholar]
- Walsh, J.; Olsen, B.J.; Ruskin, K.J.; Shriver, W.G.; O’Brien, K.M.; Kovach, A.I. Extrinsic and intrinsic factors influence fitness in an avian hybrid zone. Biol. J. Linn. Soc. 2016, 119, 890–903. [Google Scholar] [CrossRef]
- Piper, S.D.; Catterall, C.P. A particular case and a general pattern: Hyperaggressive behaviour by one species may mediate avifaunal decreases in fragmented Australian forests. Oikos 2003, 101, 602–614. [Google Scholar] [CrossRef]
- Sætre, G.; Post, E.; Král, M. Can environmental fluctuation prevent competitive exclusion in sympatric flycatchers? Proc. R. Soc. Lond. 1999, 266, 1247–1251. [Google Scholar] [CrossRef]
- Vallin, N.; Rice, A.; Arntsen, H.; Kulma, K.; Qvarnstrom, A. Combined effects of interspecific competition and hybridization impede local coexistence of Ficedula flycatchers. Evol. Ecol. 2011, 26, 927–942. [Google Scholar] [CrossRef]
- Duckworth, R.A.; Badyaev, A.V. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl. Acad. Sci. USA 2007, 104, 15017–15022. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.J. Changes in the distribution and abundance of spotted owls during the past century. Stud. Avian Biol. 1994, 15, 293–300. [Google Scholar]
- Yackulic, C.B.; Bailey, L.L.; Dugger, K.M.; Davis, R.J.; Franklin, A.B.; Forsman, E.D.; Ackers, S.H.; Andrews, L.S.; Diller, L.V.; Gremel, S.A.; et al. The past and future roles of competition and habitat in the range-wide occupancy dynamics of Northern Spotted Owls. Ecol. Appl. 2019, 29, e01861. [Google Scholar] [CrossRef] [PubMed]
- Livezey, K.B. Range Expansion of Barred Owls, Part II: Facilitating Ecological Changes. Am. Midl. Nat. 2009, 161, 323–349. [Google Scholar] [CrossRef]
- Crozier, M.L.; Seamans, M.E.; Gutierrez, R.J.; Loschl, P.J.; Horn, R.B.; Sovern, S.G.; Forsman, E.D. Does the presence of barred owls suppress the calling behavior of spotted owls? Condor 2006, 108, 760–769. [Google Scholar] [CrossRef]
- Wiens, J.D.; Anthony, R.G.; Forsman, E.D. Competitive interactions and resource partitioning between northern spotted owls and barred owls in western Oregon. Wildl. Monogr. 2014, 185, 1–50. [Google Scholar] [CrossRef]
- Wood, C.M.; Kryshak, N.; Gustafson, M.; Hofstadter, D.F.; Hobart, B.K.; Whitmore, S.A.; Dotters, B.P.; Roberts, K.N.; Keane, J.J.; Sawyer, S.C.; et al. Density dependence influences competition and hybridization at an invasion front. Divers. Distrib. 2021, 27, 901–912. [Google Scholar] [CrossRef]
- Dugger, K.M.; Forsman, E.D.; Franklin, A.B.; Davis, R.J.; White, G.C.; Schwarz, C.J.; Burnham, K.P.; Nichols, J.D.; Hines, J.E.; Yackulic, C.B.; et al. The effects of habitat, climate, and Barred Owls on long-term demography of Northern Spotted Owls. Ornithol. Appl. 2015, 118, 57–116. [Google Scholar] [CrossRef]
- Wiens, J.D.; Dugger, K.M.; Higley, J.M.; Lesmeister, D.B.; Franklin, A.B.; Hamm, K.A.; White, G.C.; Dilione, K.E.; Simon, D.C.; Bown, R.R.; et al. Invader removal triggers competitive release in a threatened avian predator. Proc. Natl. Acad. Sci. USA 2021, 118, e2102859118. [Google Scholar] [CrossRef]
- Dugger, K.M.; Anthony, R.G.; Andrews, L.S. Transient dynamics of invasive competition: Barred owls, spotted owls, habitat, and the demons of competition present. Ecol. Appl. 2011, 21, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Van Lanen, N.J.; Franklin, A.B.; Huyvaert, K.P.; Reiser, R.F.; Carlson, P.C. Who hits and hoots at whom? Potential for interference competition between barred and northern spotted owls. Biol. Conserv. 2011, 144, 2194–2201. [Google Scholar] [CrossRef]
- Hamer, T.E.; Forsman, E.D.; Glenn, E.M. Home Range Attributes and Habitat Selection of Barred Owls and Spotted Owls in an Area of Sympatry. Condor 2007, 109, 750–768. [Google Scholar] [CrossRef]
- Long, L.L.; Wolfe, J.D. Review of the effects of barred owls on spotted owls. J. Wildl. Manag. 2019, 83, 1281–1296. [Google Scholar] [CrossRef]
- Walsh, J.; Shriver, W.G.; Olsen, B.J.; Kovach, A.I. Differential introgression and the maintenance of species boundaries in an advanced generation avian hybrid zone. BMC Evol. Biol. 2016, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Shriver, W.G.; Correll, M.D.; Olsen, B.J.; Elphick, C.S.; Hodgman, T.P.; Rowe, R.J.; O’Brien, K.M.; Kovach, A.I. Temporal shifts in the saltmarsh–Nelson’s sparrow hybrid zone revealed by replicated demographic and genetic surveys. Conserv. Genet. 2017, 18, 453–466. [Google Scholar] [CrossRef]
- Haynes, T.B.; Schmutz, J.A.; Lindberg, M.S.; Wright, K.G.; Uher-Koch, B.D.; Rosenberger, A.E. Occupancy of yellow-billed and pacific loons: Evidence for interspecific competition and Habitat mediated co-occurrence. J. Avian Biol. 2014, 45, 296–304. [Google Scholar] [CrossRef]
- Guay, P.-J.; Taysom, A.; Robinson, R.; Tracey, J.P. Hybridization between the Mallard and native dabbling ducks: Causes, consequences and management. Pac. Conserv. Biol. 2014, 20, 41–47. [Google Scholar] [CrossRef]
- Yamashina, Y. Notes on the Marianas mallard. Pac. Sci. 1948, 2, 121–124. [Google Scholar]
- Fowler, A.C.; Eadie, J.M.; Engilis, A., Jr. Identification of endangered Hawaiian ducks (Anas wyvilliana), introduced North American mallards (A. platyrhynchos) and their hybrids using multilocus genotypes. Conserv. Genet. 2008, 10, 1747–1758. [Google Scholar] [CrossRef]
- Mank, J.E.; Carlson, J.E.; Brittingham, M.C. A Century of Hybridization: Decreasing Genetic Distance Between American Black Ducks and Mallards. Conserv. Genet. 2004, 5, 395–403. [Google Scholar] [CrossRef]
- Thibault, M.; Vidal, E.; Potter, M.A.; Sanchez, T.; Brescia, F. The invasive Red-vented bulbul (Pycnonotus cafer) outcompetes native birds in a tropical biodiversity hotspot. PLoS ONE. 2018, 13, e0192249. [Google Scholar] [CrossRef] [PubMed]
- Shochat, E.; Lerman, S.B.; Anderies, J.M.; Warren, P.S.; Faeth, S.H.; Nilon, C.H. Invasion, Competition, and Biodiversity Loss in Urban Ecosystems. BioScience 2010, 60, 199–208. [Google Scholar] [CrossRef]
- Hopkins, J.M.; Edwards, W.; Laguna, J.M.; Schwarzkopf, L. An endangered bird calls less when invasive birds are calling. J. Avian Biol. 2020, 52, 1–9. [Google Scholar] [CrossRef]
- Colléony, A.; Shwartz, A. When the winners are the losers: Invasive alien bird species outcompete the native winners in the biotic homogenization process. Biol. Conserv. 2019, 241, 108314. [Google Scholar] [CrossRef]
- Strubbe, D.; Matthysen, E. Invasive ring-necked parakeets Psittacula krameri in Belgium: Habitat selection and impact on native birds. Ecography 2007, 30, 578–588. [Google Scholar] [CrossRef]
- Strubbe, D.; Matthysen, E. Experimental evidence for nest-site competition between invasive ring-necked parakeets (Psittacula krameri) and native nuthatches (Sitta europaea). Biol. Conserv. 2009, 142, 1588–1594. [Google Scholar] [CrossRef]
- Komdeur, J. Breeding of the Seychelles Magpie Robin Copsychus sechellarum and implications for its conservation. Ibis 1996, 138, 485–498. [Google Scholar] [CrossRef]
- Peck, H.L.; Pringle, H.E.; Marshall, H.H.; Owens, I.P.F.; Lord, A.M. Experimental evidence of impacts of an invasive parakeet on foraging behavior of native birds. Behav. Ecol. 2014, 25, 582–590. [Google Scholar] [CrossRef]
- Roach, M. Puerto Rican Vireo (Vireo latimeri), version 1.0. In Birds of the World; Schulenberg, T.S., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Bonter, D.N.; Zuckerberg, B.; Dickinson, J.L. Invasive birds in a novel landscape: Habitat associations and effects on established species. Ecography 2009, 33, 494–502. [Google Scholar] [CrossRef]
- Sol, D.; Bartomeus, I.; Griffin, A.S. The paradox of invasion in birds: Competitive superiority or ecological opportunism? Oecologia 2012, 169, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Vall-llosera, M.; Llimona, F.; de Cáceres, M.; Sales, S.; Sol, D. Competition, niche opportunities and the successful invasion of natural habitats. Biol. Invasions 2016, 18, 3535–3546. [Google Scholar] [CrossRef]
- Evans, T.; Kumschick, S.; Blackburn, T.M. Application of the Environmental Impact Classification for Alien Taxa (EICAT) to a global assessment of alien bird impacts. Divers. Distrib. 2016, 22, 919–931. [Google Scholar] [CrossRef]
- Freed, L.A.; Cann, R.L.; Bodner, G.R. Incipient extinction of a major population of the Hawaii akepa owing to introduced species. Evol. Ecol. Res. 2008, 10, 931–965. [Google Scholar]
- Freed, L.A.; Cann, R.L. Negative Effects of an Introduced Bird Species on Growth and Survival in a Native Bird Community. Curr. Biol. 2009, 19, 1736–1740. [Google Scholar] [CrossRef]
- Camp, R.J.; Pratt, T.K.; Gorresen, P.M.; Woodworth, B.L.; Jeffrey, J.J. Hawaiian forest bird trends: Using log-linear models to assess long-term trends is supported by model diagnostics and assumptions (reply to Freed and Cann 2013). Condor 2014, 116, 97–101. [Google Scholar] [CrossRef]
- Department of Climate Change, Energy, the Environment and Water of the Australian Government. Available online: https://www.dcceew.gov.au/environment/biodiversity/threatened/recovery-plans/buff-banded-rail-cocos-keeling-islands-gallirallus-philippensis-andrewsi-2006 (accessed on 7 October 2022).
- Whelan, C.J.; Şekercioğlu, H.; Wenny, D.G. Why birds matter: From economic ornithology to ecosystem services. J. Ornithol. 2015, 156, 227–238. [Google Scholar] [CrossRef]
- Inger, R.; Gregory, R.; Duffy, J.; Stott, I.; Voříšek, P.; Gaston, K.J. Common European birds are declining rapidly while less abundant species’ numbers are rising. Ecol. Lett. 2014, 18, 28–36. [Google Scholar] [CrossRef]
- García, D.; Martínez, D. Species richness matters for the quality of ecosystem services: A test using seed dispersal by frugivorous birds. Proc. R. Soc. B Boil. Sci. 2012, 279, 3106–3113. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jetz, W. The effect of range changes on the functional turnover, structure and diversity of bird assemblages under future climate scenarios. Glob. Change Biol. 2015, 21, 2917–2928. [Google Scholar] [CrossRef]
- Fricke, E.C.; Tewksbury, J.J.; Rogers, H.S. Defaunation leads to interaction deficits, not interaction compensation, in an island seed dispersal network. Glob. Change Biol. 2017, 24, e190–e200. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, G.; Wang, N.; Feng, G.; Yang, F.; Svenning, J.-C.; Yang, J. Taxonomic, phylogenetic and functional homogenization of bird communities due to land use change. Biol. Conserv. 2019, 236, 37–43. [Google Scholar] [CrossRef]
- Marcacci, G.; Westphal, C.; Wenzel, A.; Raj, V.; Nölke, N.; Tscharntke, T.; Grass, I. Taxonomic and functional homogenization of farmland birds along an urbanization gradient in a tropical megacity. Glob. Change Biol. 2021, 27, 4980–4994. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. Why do introduced species appear to devastate islands more than mainland areas? Pac. Sci. 1995, 49, 87–97. [Google Scholar]
- Soares, F.C.; Panisi, M.; Sampaio, H.; Soares, E.; Santana, A.; Buchanan, G.; Leal, A.I.; Palmeirim, J.M.; Lima, R. Land-use intensification promotes non-native species in a tropical island bird assemblage. Anim. Conserv. 2020, 23, 573–584. [Google Scholar] [CrossRef]
- Le Louarn, M.; Couillens, B.; Deschamps-Cottin, M.; Clergeau, P. Interference competition between an invasive parakeet and native bird species at feeding sites. J. Ethol. 2016, 34, 291–298. [Google Scholar] [CrossRef]
- Higgins, P.J.; Christidis, L.; Ford, H. Noisy Miner (Manorina melanocephala), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Crates, R.; Terauds, A.; Rayner, L.; Stojanovic, D.; Heinsohn, R.; Wilkie, C.; Webb, M. Spatially and temporally targeted suppression of despotic noisy miners has conservation benefits for highly mobile and threatened woodland birds. Biol. Conserv. 2018, 227, 343–351. [Google Scholar] [CrossRef]
- Val, J.; Eldridge, D.J.; Travers, S.K.; Oliver, I. Livestock grazing reinforces the competitive exclusion of small-bodied birds by large aggressive birds. J. Appl. Ecol. 2017, 55, 1919–1929. [Google Scholar] [CrossRef]
- Robertson, O.J.; McAlpine, C.; House, A.; Maron, M. Influence of Interspecific Competition and Landscape Structure on Spatial Homogenization of Avian Assemblages. PLoS ONE 2013, 8, e65299. [Google Scholar] [CrossRef]
- Thomson, J.R.; Maron, M.; Grey, M.J.; Catterall, C.P.; Major, R.E.; Oliver, D.L.; Clarke, M.F.; Loyn, R.H.; Davidson, I.; Ingwersen, D.; et al. Avifaunal disarray: Quantifying models of the occurrence and ecological effects of a despotic bird species. Divers. Distrib. 2015, 21, 451–464. [Google Scholar] [CrossRef]
- Grey, M.J.; Clarke, M.F.; Loyn, R.H. Initial changes in the avian communities of remnant eucalypt woodlands following a reduction in the abundance of noisy miners, Manorina melanocephala. Wildl. Res. 1997, 24, 631–648. [Google Scholar] [CrossRef]
- Grey, M.J.; Clarke, M.F.; Loyn, R.H. Influence of the Noisy Miner Manorina melanocephala on avian diversity and abundance in remnant Grey Box woodland. Pac. Conserv. Biol. 1998, 4, 55–69. [Google Scholar] [CrossRef]
- Debus, S.J.S. The effect of Noisy Miners on small bush birds: An unofficial cull and its outcome. Pac. Conserv. Biol. 2008, 14, 185–190. [Google Scholar] [CrossRef][Green Version]
- Mortelliti, A.; Ikin, K.; Tulloch, A.I.T.; Cunningham, R.; Stein, J.; Michael, D.; Lindenmayer, D.B. Surviving with a resident despot: Do revegetated patches act as refuges from the effects of the noisy miner (Manorina melanocephala) in a highly fragmented landscape? Divers. Distrib. 2016, 22, 770–782. [Google Scholar] [CrossRef]
- Bennett, J.M.; Clarke, R.H.; Thomson, J.R.; Mac Nally, R. Variation in abundance of nectarivorous birds: Does a competitive despot interfere with flower tracking? J. Anim. Ecol. 2014, 83, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Howes, A.; Mac Nally, R.; Loyn, R.; Kath, J.; Bowen, M.; McAlpine, C. Foraging guild perturbations and ecological homogenization driven by a despotic native bird species. Ibis 2014, 156, 341–354. [Google Scholar] [CrossRef]
- Bennett, J.M.; Clarke, R.H.; Horrocks, G.F.B.; Thomson, J.R.; Mac Nally, R. Climate drying amplifies the effects of land-use change and interspecific interactions on birds. Landsc. Ecol. 2015, 30, 2031–2043. [Google Scholar] [CrossRef]
- Howes, A.L.; Maron, M. Interspecific competition and conservation management of continuous subtropical woodlands. Wildl. Res. 2009, 36, 617–626. [Google Scholar] [CrossRef]
- Bennett, J.M.; Clarke, R.H.; Thomson, J.R.; Mac Nally, R. Fragmentation, vegetation change and irruptive competitors affect recruitment of woodland birds. Ecography 2015, 38, 163–171. [Google Scholar] [CrossRef]
- Bregman, T.P.; Lees, A.C.; Seddon, N.; Macgregor, H.E.A.; Darski, B.; Aleixo, A.; Bonsall, M.B.; Tobias, J.A. Species interactions regulate the collapse of biodiversity and ecosystem function in tropical forest fragments. Ecology 2015, 96, 2692–2704. [Google Scholar] [CrossRef]
- Ulrich, W.; Lens, L.; Tobias, J.; Habel, J.C. Contrasting Patterns of Species Richness and Functional Diversity in Bird Communities of East African Cloud Forest Fragments. PLoS ONE 2016, 11, e0163338. [Google Scholar] [CrossRef] [PubMed]
- Devictor, V.; Julliard, R.; Clavel, J.; Jiguet, F.; Lee, A.; Couvet, D. Functional biotic homogenization of bird communities in disturbed landscapes. Glob. Ecol. Biogeogr. 2007, 17, 252–261. [Google Scholar] [CrossRef]
- Julliard, R.; Clavel, J.; Devictor, V.; Jiguet, F.; Couvet, D. Spatial segregation of specialists and generalists in bird communities. Ecol. Lett. 2006, 9, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Richmond, C.E.; Breitburg, D.L.; Rose, K.A. The role of environmental generalist species in ecosystem function. Ecol. Model. 2005, 188, 279–295. [Google Scholar] [CrossRef]
- Drury, J.P.; Cowen, M.C.; Grether, G.F. Competition and hybridization drive interspecific territoriality in birds. Proc. Natl. Acad. Sci. USA 2020, 117, 12923–12930. [Google Scholar] [CrossRef]
- Lipshutz, S.E. Interspecific competition, hybridization, and reproductive isolation in secondary contact: Missing perspectives on males and females. Curr. Zool. 2017, 64, 75–88. [Google Scholar] [CrossRef]
- Harr, B.; Price, T. Climate change: A hybrid zone moves north. Curr. Biol. 2014, 24, R230–R232. [Google Scholar] [CrossRef]
- Grether, G.F.; Peiman, K.S.; Tobias, J.; Robinson, B. Causes and Consequences of Behavioral Interference between Species. Trends Ecol. Evol. 2017, 32, 760–772. [Google Scholar] [CrossRef]
- Gómez-Llano, M.; Germain, R.M.; Kyogoku, D.; McPeek, M.A.; Siepielski, A.M. When Ecology Fails: How Reproductive Interactions Promote Species Coexistence. Trends Ecol. Evol. 2021, 36, 610–622. [Google Scholar] [CrossRef]
- Irwin, D.; Schluter, D. Hybridization and the Coexistence of Species. Am. Nat. 2022, 200, E93–E109. [Google Scholar] [CrossRef]
- Todesco, M.; Pascual, M.A.; Owens, G.L.; Ostevik, K.L.; Moyers, B.T.; Hübner, S.; Heredia, S.M.; Hahn, M.A.; Caseys, C.; Bock, D.G.; et al. Hybridization and extinction. Evol. Appl. 2016, 9, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Confer, J.L.; Hartman, P.; Roth, A. Golden-winged Warbler (Vermivora chrysoptera), version 1.0. In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Godsoe, W.; Holland, N.J.; Cosner, C.; Kendall, B.E.; Brett, A.; Jankowski, J.; Holt, R.D. Interspecific interactions and range limits: Contrasts among interaction types. Theor. Ecol. 2016, 10, 167–179. [Google Scholar] [CrossRef]
- de la Torre Cerro, R.; Holloway, P. A review of the methods for studying biotic interactions in phenological analyses. Methods Ecol. Evol. 2021, 12, 227–244. [Google Scholar] [CrossRef]
- Flint, P.L.; Mallek, E.J.; King, R.J.; Schmutz, J.A.; Bollinger, K.S.; Derksen, D.V. Changes in abundance and spatial distribution of geese molting near Teshekpuk Lake, Alaska: Interspecific competition or ecological change? Polar Biol. 2007, 31, 549–556. [Google Scholar] [CrossRef]
- Burnham, K.K.; Burnham, J.L.; Konkel, B.W. Status of Peregrine falcon and Gyrfalcon populations in Northwest Greenland. In Proceedings of the North Water Polynya Conference, Copenhagen, Denmark, 22–24 November 2017; pp. 101–103. [Google Scholar]
- Carrete, M.; Lambertucci, S.A.; Speziale, K.; Ceballos, O.; Travaini, A.; Delibes, M.; Hiraldo, F.; Donázar, J.A. Winners and losers in Human-made habitats: Interspecific competition outcomes in two Neotropical vultures. Anim. Conserv. 2010, 13, 390–398. [Google Scholar] [CrossRef]
- Drury, J.; Clavel, J.; Manceau, M.; Morlon, H. Data from: Estimating the effect of competition on trait evolution using maximum likelihood inference. Syst. Biol. 2016, 65, 700–710. [Google Scholar] [CrossRef]
- Quintero, I.; Landis, M.J. Interdependent Phenotypic and Biogeographic Evolution Driven by Biotic Interactions. Syst. Biol. 2019, 69, 739–755. [Google Scholar] [CrossRef]
- Davitt, G.; Maute, K.; Major, R.E.; McDonald, P.G.; Maron, M. Short-term response of a declining woodland bird assemblage to the removal of a despotic competitor. Ecol. Evol. 2018, 8, 4771–4780. [Google Scholar] [CrossRef]
- Powell, L.L.; Ames, E.M.; Wright, J.R.; Matthiopoulos, J.; Marra, P.P. Interspecific competition between resident and wintering birds: Experimental evidence and consequences of coexistence. Ecology 2020, 102, e03208. [Google Scholar] [CrossRef]
- Cadena, D.C.; Loiselle, B.A. Limits to elevational distributions in two species of emberizine finches: Disentangling the role of interspecific competition, autoecology, and geographic variation in the environment. Ecography 2007, 30, 491–504. [Google Scholar] [CrossRef]
- Reif, J.; Reifová, R.; Skoracka, A.; Kuczyński, L. Competition-driven niche segregation on a landscape scale: Evidence for escaping from syntopy towards allotopy in two coexisting sibling passerine species. J. Anim. Ecol. 2018, 87, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D.; Kelling, S. eBird: A citizen-based bird observation network in the biological sciences. Biol. Conserv. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Donahue, M.J. How phenological tracking shapes species and communities in non-stationary environments. Biol. Rev. 2021, 96, 2810–2827. [Google Scholar] [CrossRef] [PubMed]
- Willis, E.O. The behavior of ocellated antbirds. Smithson. Contrib. Zool. 1973, 144, 1–57. [Google Scholar] [CrossRef]
- Altshuler, D.L. Flight performance and competitive displacement of hummingbirds across elevational gradients. Am. Nat. 2006, 167, 216–229. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Hawkins, S.J.; Southward, A.J.; Burrows, M.T. Modeling the Response of Populations of Competing Species to Climate Change. Ecology 2008, 89, 3138–3149. [Google Scholar] [CrossRef]
- Ainley, D.G.; Hyrenbach, K.D. Top-down and bottom-up factors affecting seabird population trends in the California current system (1985–2006). Prog. Oceanogr. 2010, 84, 242–254. [Google Scholar] [CrossRef]
- Montoya, J.M.; Raffaelli, D. Climate change, biotic interactions and ecosystem services. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2013–2018. [Google Scholar] [CrossRef]
- Crawford, R.J.M.; Makhado, A.B.; Waller, L.J.; Whittington, P.A. Winners and losers—responses to recent environmental change by South African seabirds that compete with purse-seine fisheries for food. Ostrich 2014, 85, 111–117. [Google Scholar] [CrossRef]
- Yackulic, C.B. Competitive exclusion over broad spatial extents is a slow process: Evidence and implications for species distribution modeling. Ecography 2017, 40, 305–313. [Google Scholar] [CrossRef]
- Rodenhouse, N.L.; Matthews, S.N.; McFarland, K.P.; Lambert, J.D.; Iverson, L.R.; Prasad, A.; Sillett, T.S.; Holmes, R.T. Potential effects of climate change on birds of the Northeast. Mitig. Adapt. Strateg. Glob. Change 2007, 13, 517–540. [Google Scholar] [CrossRef]
- Matthews, S.N.; Iverson, L.R.; Prasad, A.M.; Peters, M.P. Changes in potential habitat of 147 North American breeding bird species in response to redistribution of trees and climate following predicted climate change. Ecography 2011, 34, 933–945. [Google Scholar] [CrossRef]
- Cruz, J.; Windels, S.K.; Thogmartin, W.E.; Crimmins, S.M.; Grim, L.H.; Larson, J.H.; Zuckerberg, B. Top-down effects of repatriating bald eagles hinder jointly recovering competitors. J. Anim. Ecol. 2019, 88, 1054–1065. [Google Scholar] [CrossRef]
- Auer, S.K.; Martin, T.E. Climate change has indirect effects on resource use and overlap among coexisting bird species with negative consequences for their reproductive success. Glob. Change Biol. 2012, 19, 411–419. [Google Scholar] [CrossRef]
- Remsen, J.V., Jr.; Graves, W.S. Distribution patterns of Buarremon brush-finches (Emberizinae) and interspecific competition in Andean birds. Auk 1995, 112, 224–236. [Google Scholar] [CrossRef]
- Schaefer, T.; Ledebur, G.; Beier, J.; Leisler, B. Reproductive responses of two related coexisting songbird species to environmental changes: Global warming, competition, and population sizes. J. Ornithol. 2006, 147, 47–56. [Google Scholar] [CrossRef]
- Furnas, B.J. Rapid and varied responses of songbirds to climate change in California coniferous forests. Biol. Conserv. 2020, 241, 108347. [Google Scholar] [CrossRef]
- Vallin, N.; Rice, A.M.; Bailey, R.I.; Husby, A.; Qvarnström, A. Positive feedback between ecological and reproductive character displacement in a young avian hybrid zone. Evolution 2012, 66, 1167–1179. [Google Scholar] [CrossRef]
- Staude, I.R.; Overbeck, G.E.; Fontana, C.S.; Bencke, G.A.; da Silva, T.W.; Mimet, A.; Pereira, H.M. Specialist Birds Replace Generalists in Grassland Remnants as Land Use Change Intensifies. Front. Ecol. Evol. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Charmantier, A.; Gienapp, P. Climate change and timing of avian breeding and migration: Evolutionary versus plastic changes. Evol. Appl. 2013, 7, 15–28. [Google Scholar] [CrossRef]
- Kopp, M.; Matuszewski, S. Rapid evolution of quantitative traits: Theoretical perspectives. Evol. Appl. 2013, 7, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Catullo, R.A.; Llewelyn, J.; Phillips, B.L.; Moritz, C.C. The Potential for Rapid Evolution under Anthropogenic Climate Change. Curr. Biol. 2019, 29, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Bonnet-Lebrun, A.-S.; Larsen, T.; Frederiksen, M.; Fox, D.; le Bouard, F.; Boutet, A.; Þórarinsson, Þ.L.; Kolbeinsson, Y.; Deville, T.; Ratcliffe, N. Effects of competitive pressure and habitat heterogeneity on niche partitioning between Arctic and boreal congeners. Sci. Rep. 2021, 11, 22133. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.B.; Barlow, C.R.; Bensusan, K.; Perez, C.; Willis, S.G. Phenological trends in the pre- and post-breeding migration of long-distance migratory birds. Glob. Change Biol. 2022, 28, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Bearhop, S.; Fiedler, W.; Furness, R.W.; Votier, S.C.; Waldron, S.; Newton, J.; Bowen, G.J.; Berthold, P.; Farnsworth, K. Assortative mating as a mechanism for rapid evolution of a migratory divide. Science 2005, 310, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Fiedler, W.; Wikelski, M.; Flack, A. “Closer-to-home” strategy benefits juvenile survival in a long-distance migratory bird. Ecol. Evol. 2019, 9, 8945–8952. [Google Scholar] [CrossRef] [PubMed]
- Rybinski, J.; Sirkiä, P.M.; McFarlane, S.E.; Vallin, N.; Wheatcroft, D.; Ålund, M.; Qvarnström, A. Competition-driven build-up of habitat isolation and selection favoring modified dispersal patterns in a young avian hybrid zone. Evolution 2016, 70, 2226–2238. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).