Chara zhengzhouensis (Characeae, Charophyta), a New Freshwater Algal Species Described from North China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Morphological Observation
2.3. Herbarium
2.4. Sequence Amplification
2.5. Construction of Phylogenetic Tree
3. Results
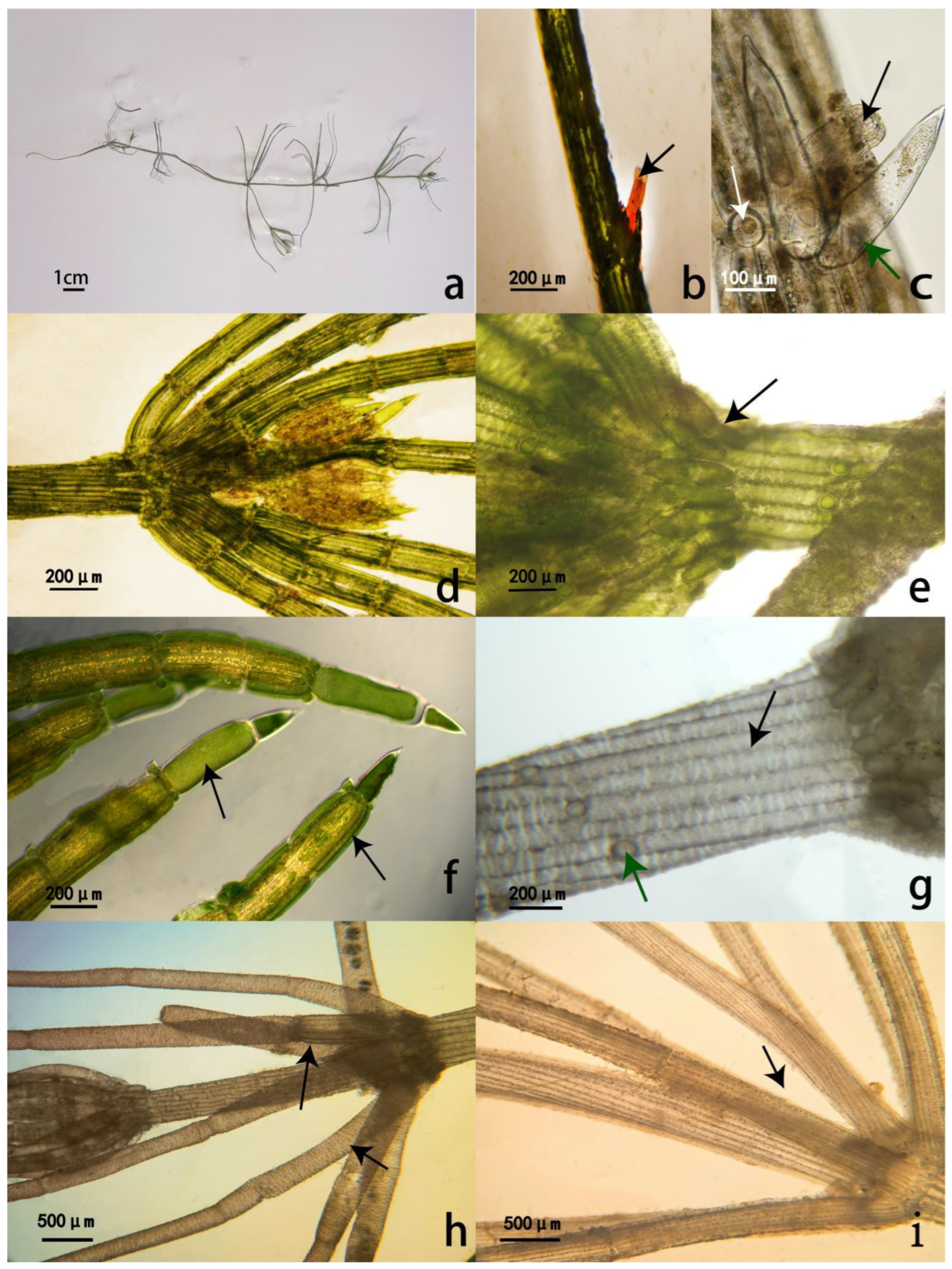
3.1. Morphological Description
3.2. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, B.P.; Kundu, B.C.; Sundaralingam, V.S.; Venkataraman, G.S. Charophyta; Indian Council of Agriculture Research: New Delhi, India, 1962. [Google Scholar]
- Blaženčić, J. Overview of the stoneworts (Charales) of Serbia with the estimation of the threat status. Bot. Serbica 2014, 38, 121–130. [Google Scholar]
- Qiu, Y.L. Phylogeny and evolution of charophytic algae and land plants. J. Syst. Evol. 2008, 46, 287–306. [Google Scholar]
- Lewis, L.A.; McCourt, R.M. Green algae and the origin of land plants. Am. J. Bot. 2004, 91, 1535–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, D.; Weber, K.; An, S.S.; Berning-Koch, W. Actin phylogeny identifies Mesostigma viride as a flagellate ancestor of the land plants. J. Mol. Evol. 1998, 47, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Hoham, R.W.; Bonome, T.A.; Martin, C.W.; Leebens-Mack, J.H. A cmibined 18S rDNA and rbcL phylogenetic analysis of Chlopomonas and Chlamydomonas (Chlorophyceae, Volvocales) emphasizing snow and other cold temperature habitats. J. Phycol. 2002, 38, 1051–1064. [Google Scholar] [CrossRef]
- Nishiyama, T.; Sakayama, H.; de Vries, J.; Buschmann, H.; Saint-Marcoux, D.; Ullrich, K.K.; Haas, F.B.; Vanderstraeten, L.; Becker, D.; Lang, D.; et al. The Chara genome: Secondary complexity and implications for plant terrestrialization. Cell 2018, 174, 448–464. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, I.; Pettolino, F.A.; Bacic, A.; Ralph, J.; Lu, F.; O’Neill, M.A.; Fei, Z.; Rose, J.K.C.; Domozych, D.S.; Willats, W.G.T. The charophycean green algae provide insights into the early origins of plant cell walls. Plant J. 2011, 68, 201–211. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Liu, Y.; Yang, L.; Shi, X.; Wang, H.; Diao, R.; Zhong, B. Origin and evolution of green plants in the light of key evolutionaryevents. J. Integr. Plant Biol. 2022, 64, 516–535. [Google Scholar]
- Proctor, V.W. Historical biogeography of Chara (Charophyta): An appraisal of the Braun-wood classification plus a falsifiable alternative for future consideration. J. Phycol. 1980, 16, 218–233. [Google Scholar] [CrossRef]
- Flechtner, V.R.; Pietrasiak, N.; Lewis, L.A. Newly revealed diversity of green microalgae from wilderness areas of Joshua Tree National Park (JTNP). Monogr. West. N. Am. Nat. 2013, 6, 43–63. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.C.; Nowak, P.; Von Ammon, U.; Ballot, A. Species differentiation in the genus Chara (Charophyceae): Considerable phenotypic plasticity occurs within homogenous genetic groups. Eur. J. Phycol. 2016, 51, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, N.; Bhan, U. Middle Siwalik Charophyta from Mohand area, Dehradun Sub-Basin, NW Himalaya, India. J. Palaeontol. Soc. India 2021, 66, 1–11. [Google Scholar]
- Proschold, T.; Leliaert, F. Systematics of the green algae: Conflict of classic and modern approaches. Syst. Assoc. Spec. Vol. 2007, 75, 123. [Google Scholar]
- Schneider, S.C.; Rodrigues, A.; Moe, T.F.; Ballot, A. DNA barcoding the genus Chara: Molecular evidence recovers fewer taxa than the classical morphological approach. J. Phycol. 2015, 51, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Han, F.S.; Li, Y.Y. Flora Algarum Sinicarum Aquae Dulcis (Tomus 3): Charophyta; Science Press: Beijing, China, 1994. [Google Scholar]
- Qiu, L.C.; Ling, Y.J. Studies on distribution of Characeae in China. Acta Hydrobiol. Sin. 2007, 31, 755–759. [Google Scholar]
- Chen, K.X.; Lan, L.Q.; Liu, K.H.; Zhang, Z.X.; Cao, S.J.; Qing, R.W. A study of the characteristic charophyte and the classification status. J. Sichuan Univ. (Nat. Sci. Ed.) 2017, 54, 1107–1112. [Google Scholar]
- Ren, L.X.; Fang, Y.; Sun, S.; Bai, H.; Qing, R.W.; Lan, L.Q. Phylogenetic study of Charophyllaceae based on 18S rDNA, rbcL and atpB Sequences. J. Sichuan Univ. (Nat. Sci. Ed.) 2020, 57, 605–611. [Google Scholar]
- Drobnik, J. Zielnik i Zielnikoznastwo; PWN: Warszawa, Poland, 2007. [Google Scholar]
- Kalmbach, K. Herbarium Plant Collection Protocol. How to Prepare Herbarium Specimens? Denver Botanic Garden: Denver, CO, USA, 2011. [Google Scholar]
- Rybak, A.S. Revision of herbarium specimens of freshwater Enteromorpha-like Ulva (Ulvaceae, Chlorophyta) collected from Central Europe during the years 1849–1959. Phytotaxa 2015, 218, 1–29. [Google Scholar] [CrossRef]
- Chen, K.X.; Qing, R.W.; Fu, H.L.; Ren, L.X.; Liu, K.H.; Cao, S.J.; Lan, L.Q. Identification of a new characteristic species of Nitella (Charophyceae) from southwestern China based on morphology and molecular phylogeny. Plant Syst. Evol. 2018, 304, 123–132. [Google Scholar] [CrossRef]
- Li, Y.; Hou, K.L.; Zhang, J. The conparative analysis of atpB rbcL sequence in Alsophila obtained by direct and cloned sequencing. J. Anhui Agric. Sci. 2009, 37, 9405–9406. [Google Scholar]
- Ren, B.Q.; Xiang, X.G.; Chen, Z.D. Species identification of Alnus (Betulaceae) using nrDNA and cpDNA genetic markers. Mol. Ecol. Resour. 2010, 10, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Nan, F.R.; Lv, J.P.; Liu, Q.; Xie, S.L.; Feng, J. A new freshwater alga, Ulvella shanxiensis (Chlorophyta) described from China. Phytotaxa 2018, 340, 217–228. [Google Scholar] [CrossRef]
- Woubit, S.; Lorenzon, S.; Peyraud, A.; Manso-Silván, L.; Thiaucourt, F. A specific PCR for the identification of Mycoplasma capricolum subsp. capripneumoniae, the causative agent of contagious caprine pleuropneumonia (CCPP). Vet. Microbiol. 2004, 104, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Dempster, A.P.; Laird, N.M.; Rubin, D.B. Maximum likelihood from incomplete data via the EM algorithm. J. R. Stat. Soc.-Ser. B (Methodol.) 1977, 39, 1–22. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Posada, D. Using MODELTEST and PAUP* to select a model of nucleotide substitution. Curr. Protoc. Bioinform. 2003, 6, 1–14. [Google Scholar] [CrossRef]
- Brzeska, P.; Wozniczka, A.; Pelechaty, M.; Blindow, I. New records of Chara connivens P. Salzmann ex A. Braun 1835—An extremely rare and protected species in Polish brackish waters. Acta Soc. Bot. Pol. 2015, 84, 143–146. [Google Scholar] [CrossRef]
- Bryant, J.A.; Stewart, N. Phylum Chlorophyta. Order Charales. In The Freshwater Algal Flora of the British Isles. An Identification Guide to Freshwater and Terrestrial Algae, 2nd ed.; John, D.M., Whitton, B.A., Brook, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 742–765. [Google Scholar]
- Appelgren, K.; Snickars, M.; Mattila, J. Chara connivens Saltzm. ex A. Braun 1835 found in the Aland archipelago—A new species to Finland. Memo.-Soc. Pro Fauna Et Flora Fenn. 2004, 80, 11–13. [Google Scholar]
- Rybak, A.; Woyda-Ploszczyca, A. Ecology and distribution patterns of Chara connivens (Charophyta, Characeae) on the Canary Islands—The first record from Fuerteventura. Oceanol. Hydrobiol. Stud. 2019, 48, 368–380. [Google Scholar] [CrossRef]
- Bueno, N.C.; Prado, J.F.; Meurer, T.; Bicudo, C.E.D.M. New records of Chara (Chlorophyta, Characeae) for subtropical southern Brazil. Syst. Bot. 2011, 36, 523–541. [Google Scholar] [CrossRef]
- Pełechaty, M.; Pukacz, A.; Pełechata, A. Co-occurrence of two stoneworts of reverse ecological spectra in the same lake ecosystem. Habitat requirements of Chara delicatula Agardh and Chara globularis Thuillier in the context of bioindication. Pol. J. Environ. Stud. 2004, 13, 551–556. [Google Scholar]
- Cai, Z.; Yang, X.; Huang, T.; Zhu, W. A new similarity combining reconstruction coefficient with pairwise distance for agglomerative clustering. Inf. Sci. 2020, 508, 173–182. [Google Scholar] [CrossRef]
- Buchheim, M.A.; Müller, T.; Wolf, M. 18S rDNA sequence-structure phylogeny of the Chlorophyceae with special emphasis on the Sphaeropleales. Plant Gene 2017, 10, 45–50. [Google Scholar] [CrossRef]
- Zhang, S.T.; Bian, G.; Huang, H.M.; Chen, N.N.; Li, J.; Liu, H.; Zheng, W. Population studies of planktonic microalgae 18S rDNA in Fuzhou inland rivers. Chin. J. Forensic Med. 2012, 27, 197–200. [Google Scholar]
- Zhu, H.; Yi, X.; Zhu, S.; Wang, H.; Duan, Y.; Wang, X. Analysis on relationship and taxonomic status of some species in subg. Cerasus Koehne with chloroplast DNA atpB-rbcL fragment. Bull. Bot. Res. 2018, 38, 820–827. [Google Scholar]
- Moon, B.R.; Lee, O.M. Molecular phylogeny of the genera Staurastrum and Staurodesmus (Zygnematophyceae, Streptophyta) based on nuclear (18S rDNA) and chloroplast gene (atpB) sequences. Algae 2007, 22, 1–10. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, S.-Y.; Li, W.; Chen, J.-M.; Wang, Q.-F. Population genetic structure and phylogeographic patterns in the Chinese endemic species Sagittaria lichuanensis, inferred from cpDNA atpB–rbcL intergenic spacers. Botanique 2010, 88, 886–892. [Google Scholar] [CrossRef]
- Buchheim, M.A.; Sutherland, D.M.; Schleicher, T.; Förster, F.; Wolf, M. Phylogeny of Oedogoniales, Chaetophorales and Chaetopeltidales (Chlorophyceae): Inferences from sequence-structure analysis of ITS2. Ann. Bot. 2012, 109, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baytut, Ö.; Gürkanli, C.T.; Özkoç, İ.; Gönülol, A. Assessing 18S rDNA diversity of the chlorophytes among various freshwaters of the Central Black Sea Region. Turk. J. Fish. Aquat. Sci. 2013, 13, 811–818. [Google Scholar] [CrossRef]






| Parameters | Water Temperature | pH | Turbidity | Conductivity | Water Velocity |
|---|---|---|---|---|---|
| value | 16 °C | 7.5 | 1.9 ftu | 1042 μs.cm−1 | 0.15 m.s−1 |
| Parameters | Dissolved oxygen | Total nitrogen | Total phosphorus | Ammonia nitrogen | Water depth |
| value | 13.4 mg.L−1 | 234.1 mg.L−1 | 6.4 mg.L−1 | 32.6 mg.L−1 | 0.5 m |
| Primer | Sequence | Target | Direction |
|---|---|---|---|
| Chara-18s1 | 5′-CCGGAAAGCTGCAGGTCTAT-3′ | 18S rDNA | Forward |
| Chara-18s2 | 5′-CGGATAACTGGTGCGTCAAA-3′ | 18S rDNA | Reverse |
| Chara-r3 | 5′-CGGGCGAAATCAAAGGACAT-3′ | rbcL | Forward |
| Chara-r4 | 5′-GAAGAAGTAGGCCGTTGTCG -3′ | rbcL | Reverse |
| Chara-a5 | 5′-CTCGTGACTGGGATCAAGGT-3′ | atpB | Forward |
| Chara-a6 | 5′-GACTGATAACTCCGGAGCCA-3′ | atpB | Reverse |
| Gene | Model Selected | Base Frequencies | Rate Matrix |
|---|---|---|---|
18S rDNA | TrN+I+G −lnL = 4623.7183 K = 7 AIC = 9261.4365 (I) = 0.5183 (G) = 0.7373 | freqA = 0.2429 freqC = 0.2113 freqG = 0.2840 freqT = 0.2617 | R(a) [A-C] = 1.0000 R(b) [A-G] = 2.3888 R(c) [A-T] = 1.0000 R(d) [C-G] = 1.0000 R(e) [C-T] = 10.4735 R(f) [G-T] = 1.0000 |
rbcL | GTR+I+G −lnL = 4215.2666 K = 10 AIC = 8450.5332 (I) = 0.4206 (G) = 0.8503 | freqA = 0.2972 freqC = 0.1468 freqG = 0.2121 freqT = 0.3439 | R(a) [A-C] = 2.6430 R(b) [A-G] = 5.7966 R(c) [A-T] = 2.4732 R(d) [C-G] = 1.9510 R(e) [C-T] =15.1225 R(f) [G-T] = 1.0000 |
atpB | GTR+G −lnL = 3441.4927 K = 9 AIC = 6900.9854 (I) = 0 (G) = 0.1286 | freqA = 0.3583 freqC = 0.1199 freqG = 0.1549 freqT = 0.3669 | R(a) [A-C] = 3.7343 R(b) [A-G] = 8.3657 R(c) [A-T] = 0.7536 R(d) [C-G] = 5.5236 R(e) [C-T] = 14.4017 R(f) [G-T] = 1.0000 |
| Character | C. zhengzhouensis | C. connivens | C. virgata |
|---|---|---|---|
| stipulodes | 2 rows, well developed, 190–240 μm long, sometimes degenerate | 2 rows, papillous | 2 rows, upper row well developed and acute, lower row short to papillous |
| cortex | dichotomous | triplostichous | triplostichous |
| spine cells | solitary, papillate | solitary, papillate | solitary, papillate |
| cortex of based segments of branchlets | corticate or ecorticate | corticate | corticate |
| bract cells | usually 5, papillate | 5–7, papillate | 5–6, inside well developed, outside papillate |
| ⚥ or ♂/♀ | dioecious | dioecious | monoecious |
| habitat | freshwater; ditch; oligotrophic water | freshwater or brackish water; ponds, ditches, and rice fields; mesotrophic to eutrophic water | freshwater or or occasionally brackish water; lakes to ditches and streams; oligotrophic water |
| distribution | Asia (China) | Asia, Africa, and Europe | Asia, Europe, North America, South America, Australia and New Zealand |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Q.; Liu, X.; Nan, F.; Liu, Q.; Lv, J.; Feng, J.; Li, R.; Xie, S. Chara zhengzhouensis (Characeae, Charophyta), a New Freshwater Algal Species Described from North China. Diversity 2022, 14, 864. https://doi.org/10.3390/d14100864
Song Q, Liu X, Nan F, Liu Q, Lv J, Feng J, Li R, Xie S. Chara zhengzhouensis (Characeae, Charophyta), a New Freshwater Algal Species Described from North China. Diversity. 2022; 14(10):864. https://doi.org/10.3390/d14100864
Chicago/Turabian StyleSong, Qingyuan, Xudong Liu, Fangru Nan, Qi Liu, Junping Lv, Jia Feng, Ruiyuan Li, and Shulian Xie. 2022. "Chara zhengzhouensis (Characeae, Charophyta), a New Freshwater Algal Species Described from North China" Diversity 14, no. 10: 864. https://doi.org/10.3390/d14100864





