Phytoplankton Community Structure and Its Relationship with Environmental Factors in Nanhai Lake
Abstract
1. Introduction
2. Materials and Methods
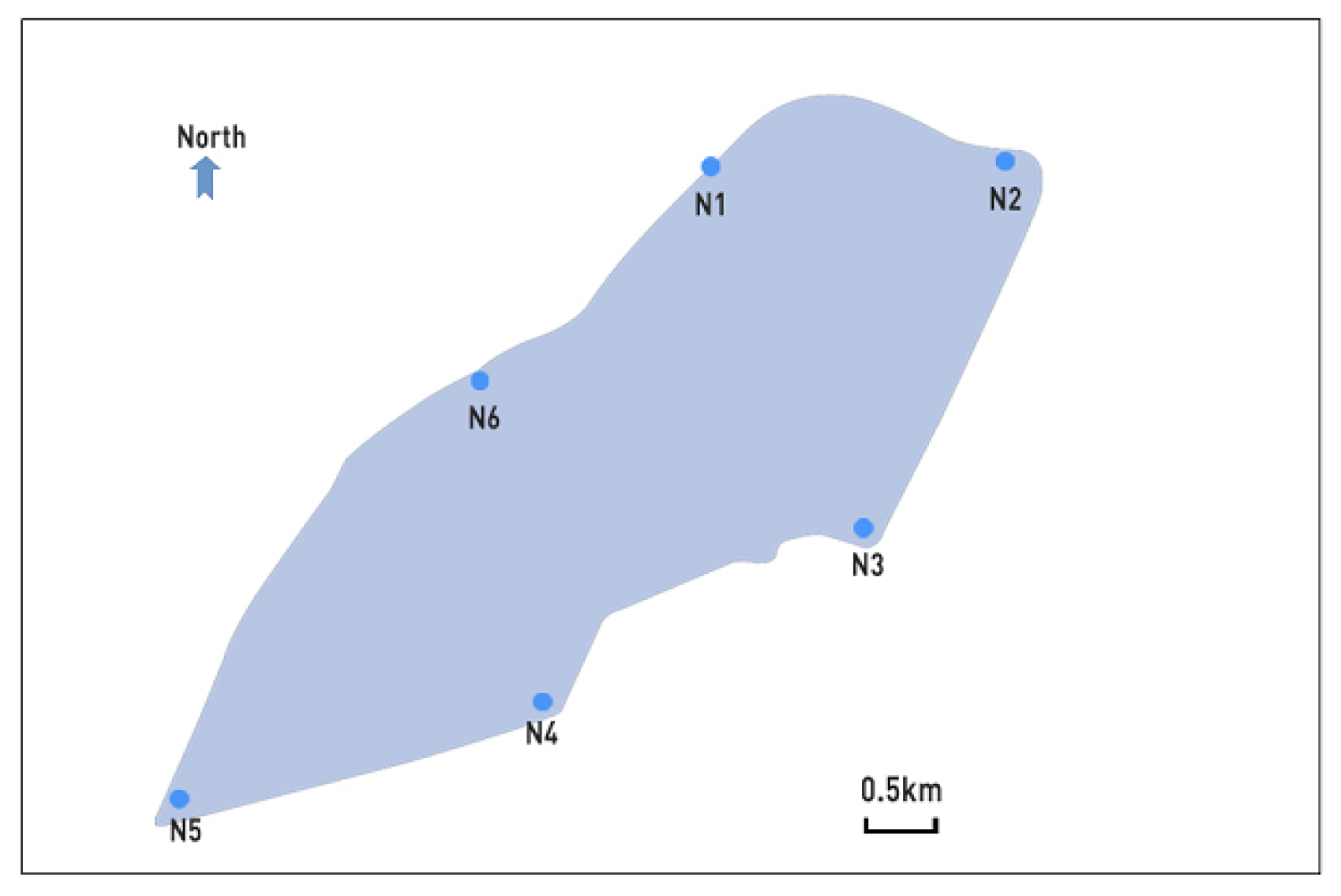
2.1. Sample Settings
2.2. Phytoplankton Collection Methods and Identification
2.3. Determination of Physical and Chemical Indicators
2.4. Data Analysis
2.4.1. Functional Group Classification and Biomass (B)
2.4.2. Bray–Curtis Heterogeneity Index (BC)
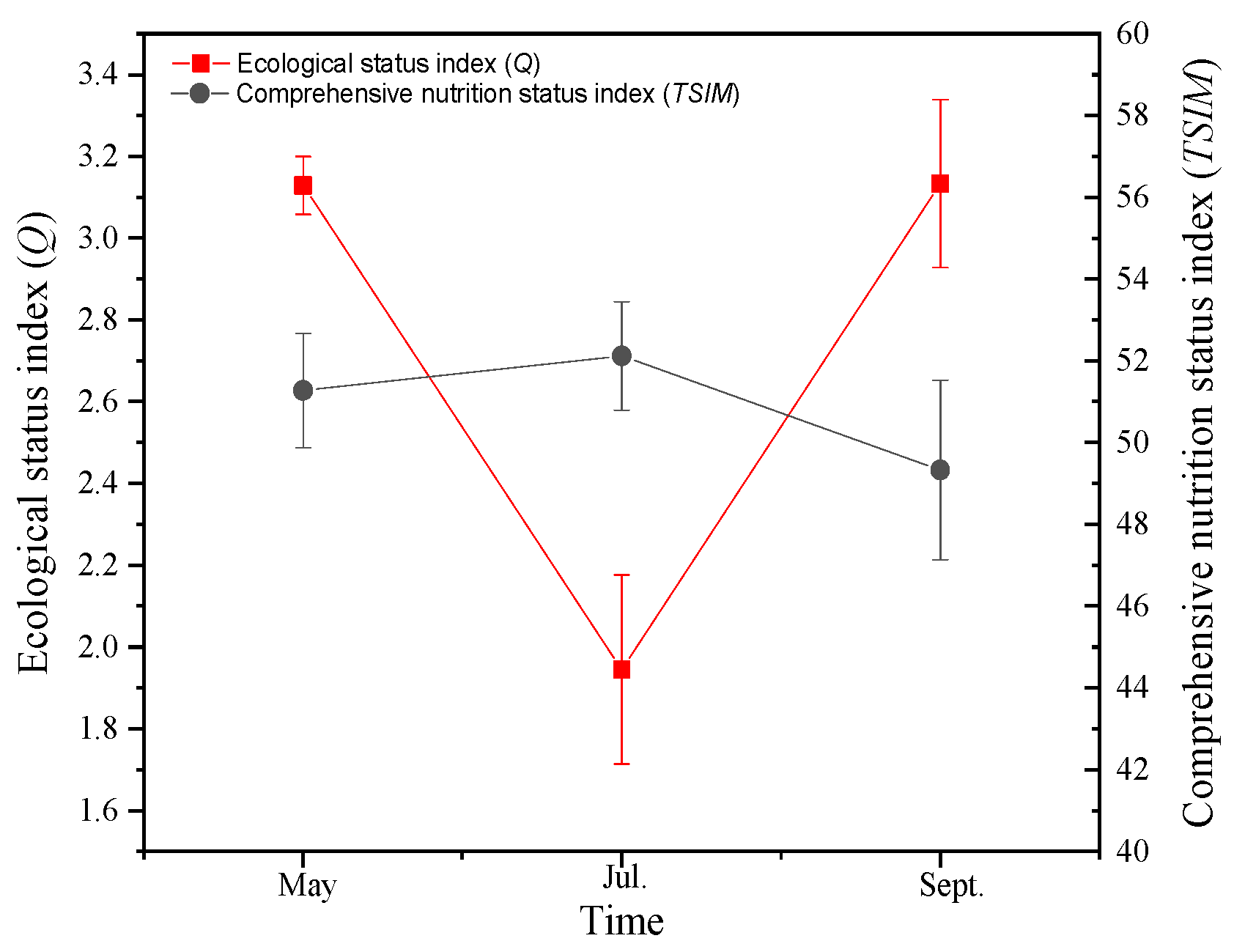
2.4.3. Comprehensive Nutrient Status Index (TSIM)
2.4.4. Ecological State Index (Q)
3. Results
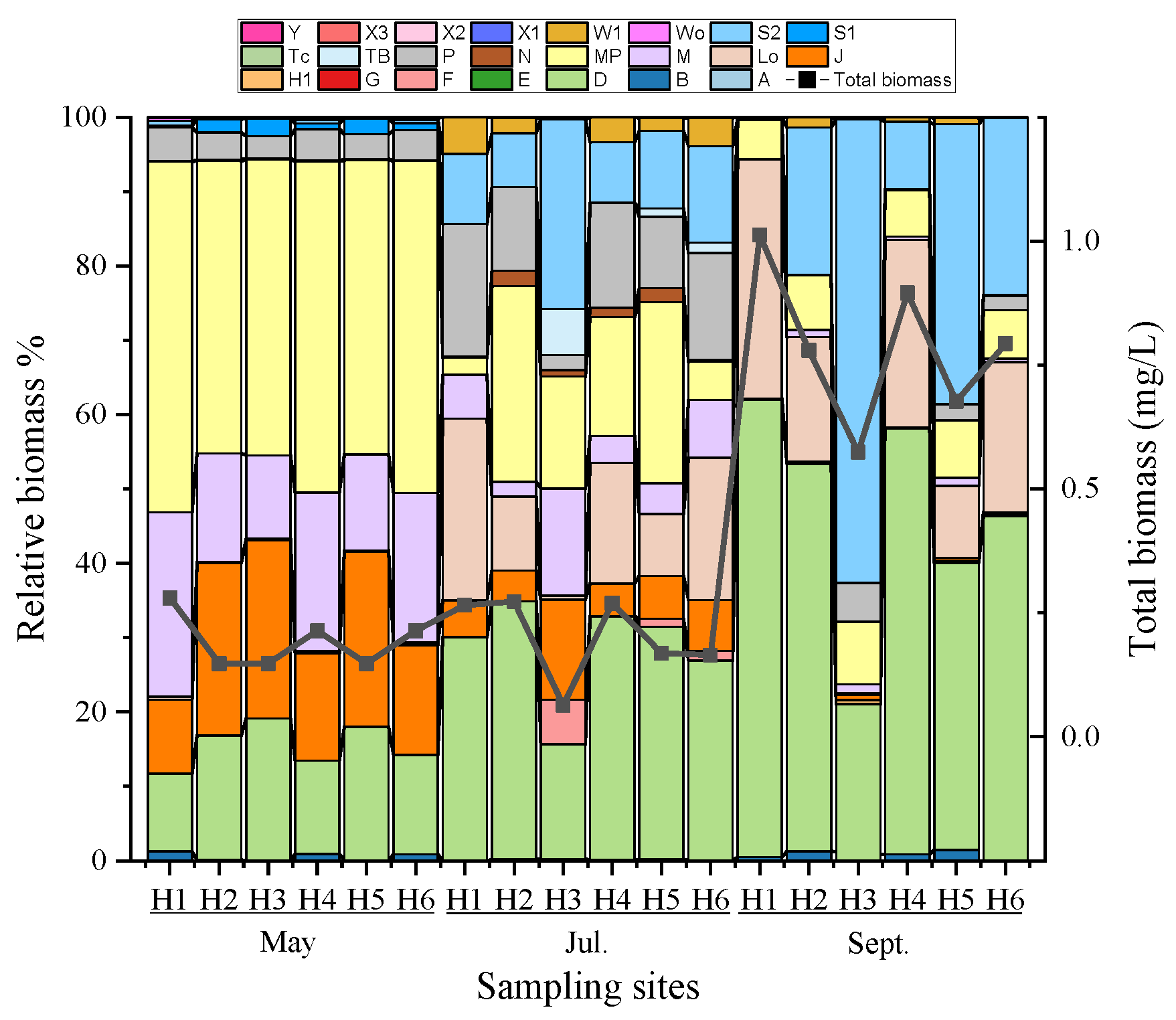
3.1. Division of Phytoplankton Species and Functional Groups
3.2. Water Quality Assessment Based on Phytoplankton Communities
3.2.1. Community Stability
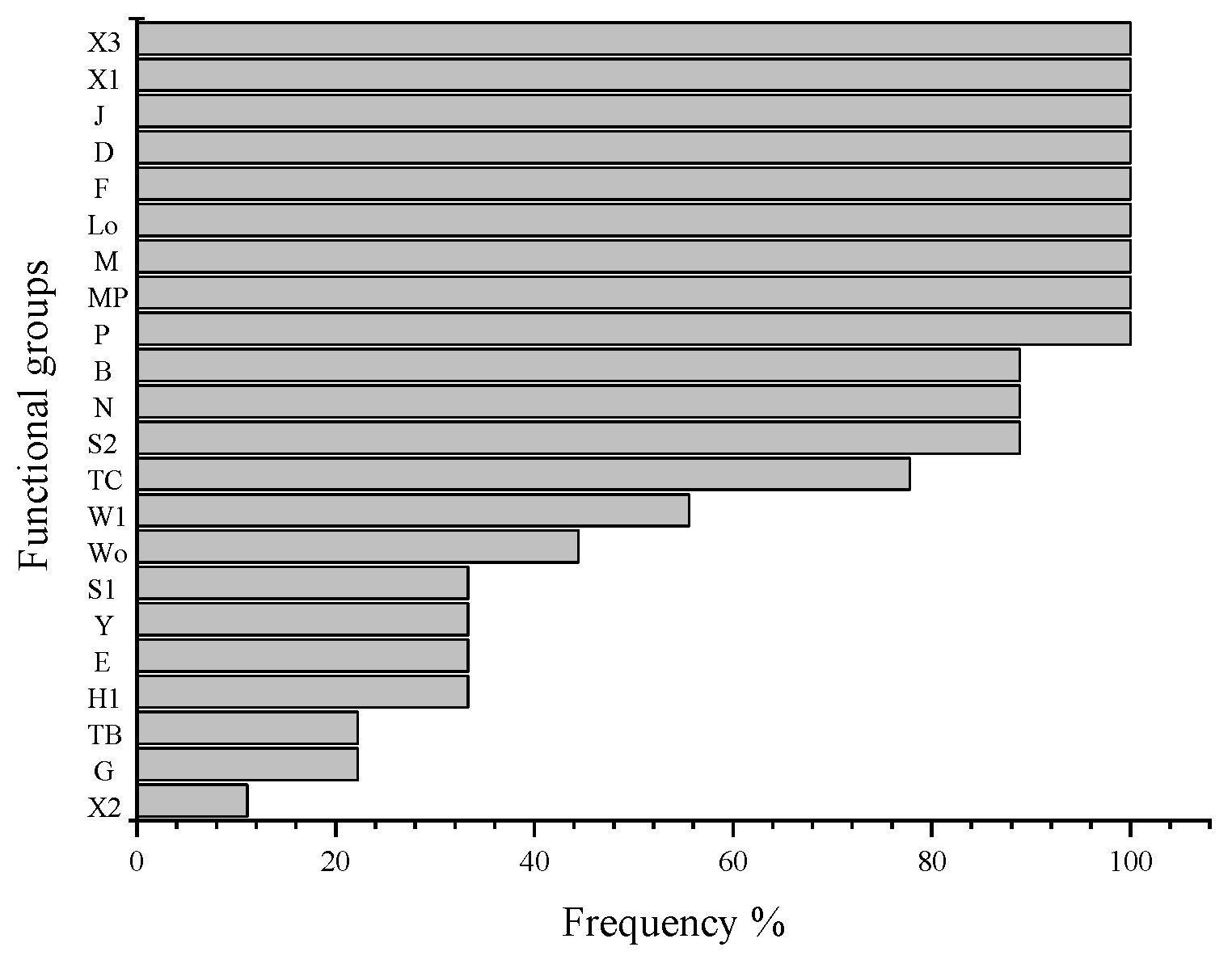
3.2.2. Ecological Status Index of Phytoplankton Functional Groups
3.2.3. Environmental Factors
3.3. Relationship between Phytoplankton and Environmental Factors
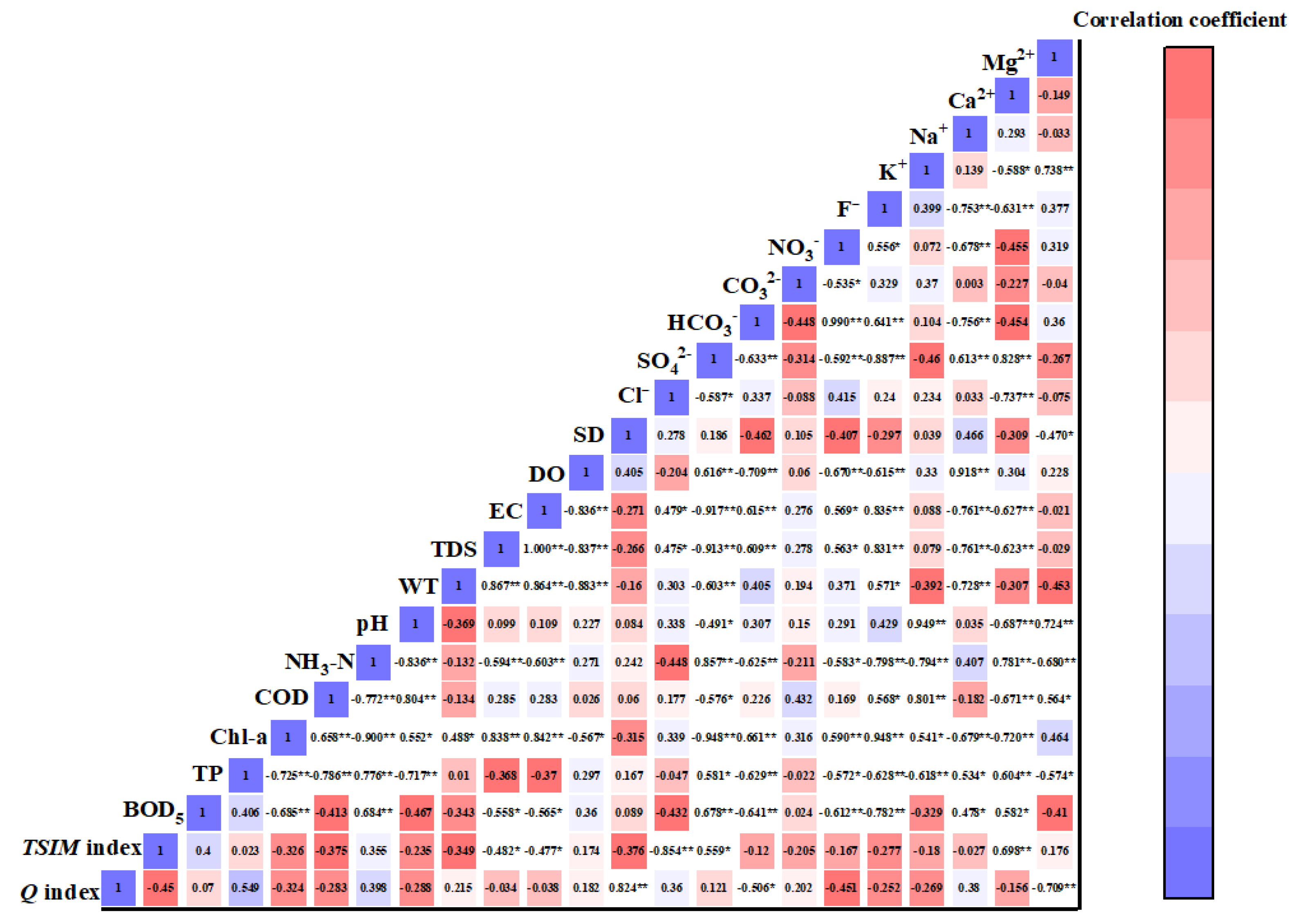
3.3.1. Relationship between Water Quality Assessment Indicators and Environmental Factors
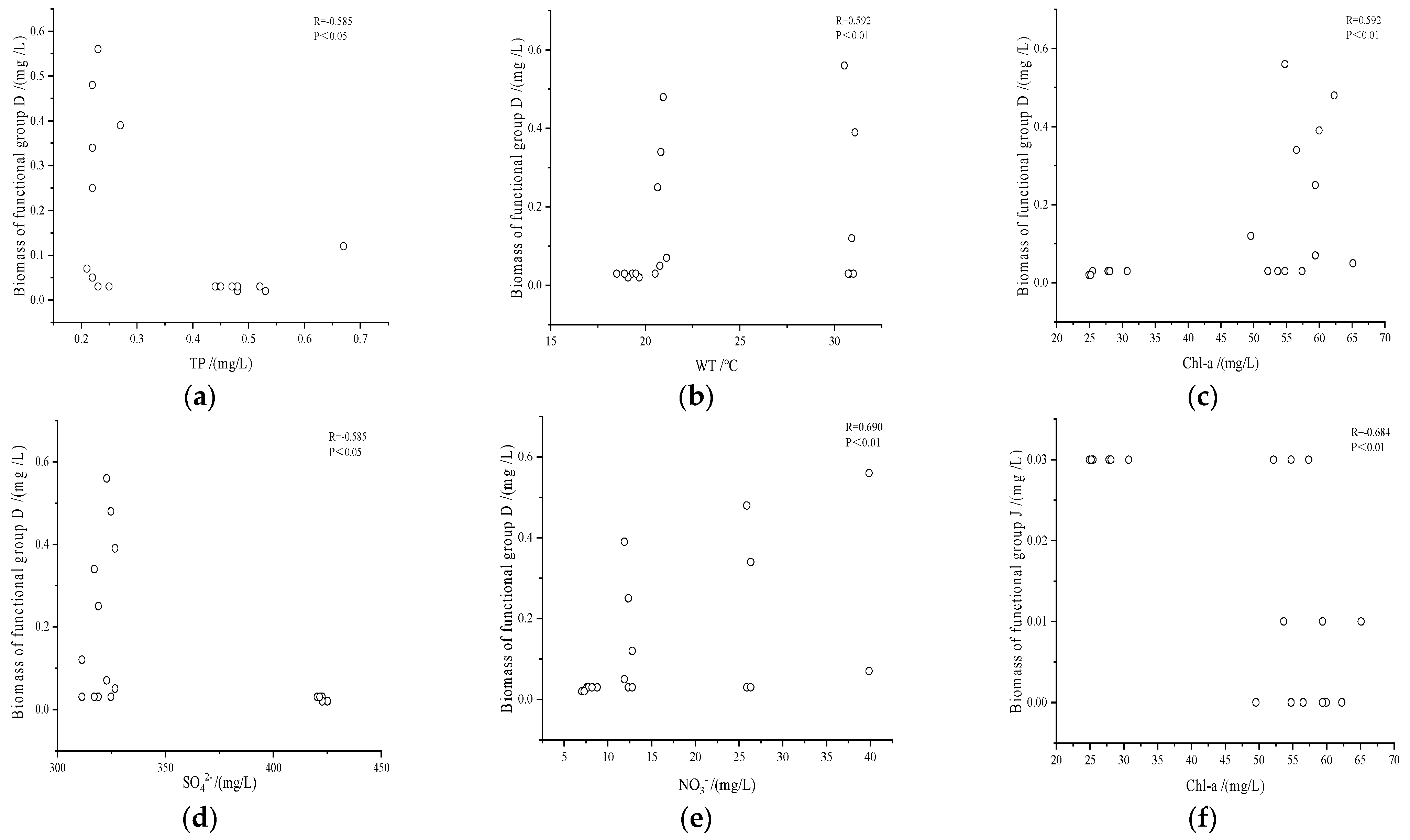
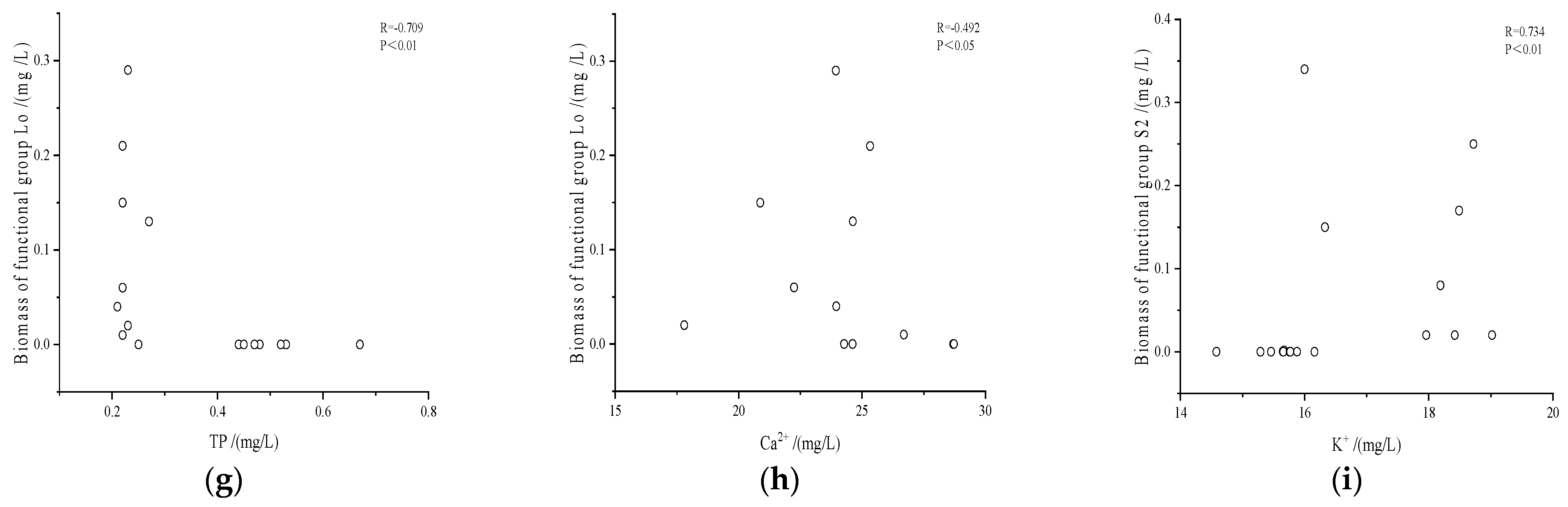
3.3.2. Spearman Correlation Analysis of Dominant Functional Groups and Environmental Factors
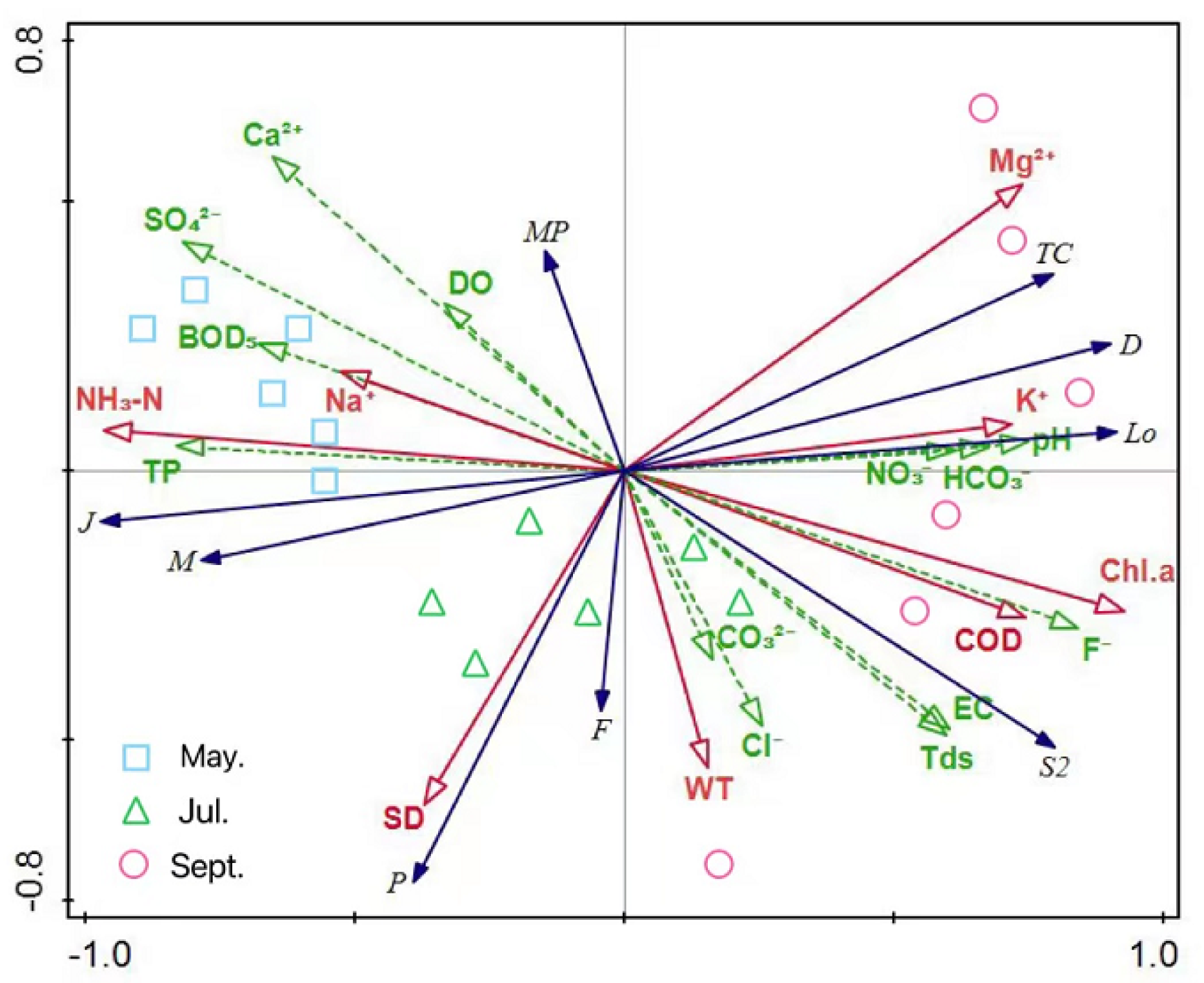
3.3.3. Redundancy Analysis of Functional Group Strength and Environmental Factors
4. Discussion
4.1. Characteristics of the Phytoplankton Community
4.2. The Relationship between Phytoplankton Functional Groups and Environmental Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eleanor, B.M.; Ian, D.J.; Emma, G. Biophysical interactions in phytoplankton. Encycl. Inland Waters 2022, 2, 154–162. [Google Scholar]
- Christophe, L.T.; Jonathan, D.; Emilie, P.; AnneLe, M.; Aurélien, J. Phytoplankton morpho−functional trait dataset from French water−bodies. Sci. Data 2021, 8, 40. [Google Scholar]
- Daniel, G.B.; Marlon, R.L.; Boris, W.; Sun, J.H.; Huang, Y. Global phytoplankton decline over the past century. Nature 2010, 466, 591–596. [Google Scholar]
- Laura, K.; Jana, K.G. Phytoplankton responses to marine climate change—An introduction. In Youmares 8 Oceans across Boundaries: Learning from Each Other; Springer: Cham, Switzerland, 2018; pp. 55–71. [Google Scholar]
- Valentin, J.L.; Leles, S.G.; Tenenbaum, D.R.; Figueiredoa, G.M. Frequent upwelling intrusions and rainfall events drive shifts in plankton community in a highly eutrophic estuary. Estuar. Coast. Shelf Sci. 2021, 257, 107387. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli−Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli−Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Xia, Y.F.; Hu, X.D.; Xu, J.X.; Li, Y.P.; Wu, S.S.; Wu, P.P. Seasonal succession of phytoplankton functional group and assessment of water quality in Lake Taihu. J. Lake Sci. 2019, 31, 134–146. [Google Scholar]
- Yang, W.H.; Shen, H.; Li, W.P.; Bu, N.L.; Zhang, M.Y. Phytoplankton community ecological characteristics and its relationship with environmental factors during non-freezing period in Nanhai Lake. Environ. Poll. Control 2020, 40, 395–400. [Google Scholar]
- Yu, L.H.; Wang, X.Y.; Li, W.P.; Gao, J.T.; Bao, J.Q.; Wang, J.N. Analysis and evaluation of water quality status in the Nanhai Wetland of Baotou. Environ. Chem. 2017, 36, 390–396. [Google Scholar]
- Jiang, Q.H.; Wang, J.N.; Li, W.P.; Li, X.; Jian, G.L.; Bu, N.L.; Miao, C.L. Temporal and spatial dynamics of phytoplankton in Nanhai Lake, Baotou, during the Ice−free Period. J. Hydrol. Ecol. 2020, 41, 30–36. [Google Scholar]
- Yang, W.H.; Shen, H.; Zhu, M.L.; Wang, Z.C.; Li, W.P.; Pan, T. Ecological characteristics of dominant phytoplankton community in Lake Nanhai (Baotou) during freezing−thawing period. J. Lake. Sci. 2020, 32, 450–461. [Google Scholar]
- Pan, T. Seasonal Succession Characteristics of Phytoplankton Functional Groups and Analysis of Its Influencing Factors of the Nanhai Lake; Inner Mongolia University Of Science & Technology: Baotou, China, 2019. [Google Scholar]
- Hu, H.J.; Wei, Y.X. The Freshwarter Algae of China: System, Ecology and Classification; Science Press: Beijing, China, 2006. [Google Scholar]
- Wehr, J.D.; Sheath, R.G. Freshwater Algae of North America: Ecology and Classification; Acacemic Press: New York, NY, USA, 2003. [Google Scholar]
- Yang, L.; Liu, D.; Huang, Y.; Yang, Z.J.; Ji, D.B.; Song, L.X. Isotope analysis of the nutrient supply in Xiangxi Bay of the Three Gorges Reservoir. Ecol. Eng. 2015, 77, 65–73. [Google Scholar] [CrossRef]
- Peng, M.; Wu, J.W.; Li, L.X.; Tan, F.X.; He, Q.J.; Wu, Z.C.; Chai, Y. Characteristics of phytoplankton functional groups and their environmental drivers in Xiaowan Reservoir area of Lancang River during the summer of 2017–2019. Chin. J. Ecol. 2022, 41, 50–57. [Google Scholar]
- Zhao, W. Hydrobiology, 2nd ed.; China Agriculture Press: Beijing, China, 2016. [Google Scholar]
- Filstrup, C.T.; Hillebrand, H.; Heathcote, A.J.; Harpole, W.S.; Downing, J.A. Cyanobacteria dominance influences resource use efficiency and community turnover in phytoplankton and zooplankton communities. Ecol. Lett. 2014, 17, 464–474. [Google Scholar] [CrossRef]
- Wang, M.C.; Liu, X.Q.; Zhang, J.H. Evaluate method and classification standard on lake eutrophication. China Environ. Monit. 2002, 18, 47–49. [Google Scholar]
- Hu, R.; Lan, Y.Q.; Xiao, L.J.; Han, B.P. The concepts, classification and application of freshwater phytoplankton functional groups. J. Lake Sci. 2015, 27, 11–23. [Google Scholar]
- Becker, V.; Caputo, L.; Ordonez, J.; Marce, R.; Armengol, J.; Crossetti, L.O.; Huszar, L.M.V. Driving factors of the phytoplankton functional groups in a deep Mediterrane an reservoir. Water Res. 2010, 44, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.L. Characteristics of Phytoplankton Functional Groups in Dalihu Natural Reserve; Northeast Forestry University: Harbin, China, 2017. [Google Scholar]
- Jiang, Q.H.; Wang, J.N.; Li, W.P.; Li, X.; Bao, J.Q.; Wang, X.Y. Spring phytoplankton community structure in NanHai Wetland and its relationship with water quality. Res. Environ. Arid Land. 2018, 32, 166–171. [Google Scholar]
- Banu, K.; Rahmi, A.; Durali, D.; Osman, S. Temporal and seasonal variations in phytoplankton community structure in Uzuncayir Dam Lake (Tunceli, Turkey). Environ. Monit Assess 2020, 192, 105. [Google Scholar]
- Liu, P.; Wu, J.L. Source Analysis and comprehensive treatment of water pollution in Huaxi Watershed, Chongqing. Three Gorges Environ. Ecol. 2012, 34, 42–45. [Google Scholar]
- He, S.H.; Ou, Y.T.; Zhao, L.; Ji, L.L.; Yang, S.Q.; Shi, J.Q.; Wu, Z.X. Analysis of phytoplankton community stability and influencing factors in a tributary of the Three Gorges Reservoir. Environ. Sci. 2021, 42, 3242–3252. [Google Scholar]
- Memet, V. Phytoplankton functional groups in a monomictic reservoir: Seasonal succession, ecological preferences, and relationships with environmental variables. Environ. Sci. Pollut. Res. Int. 2019, 26, 20439–20453. [Google Scholar]
- Liu, L.; Li, X.M.; Meng, Z.H.; Chen, K.; Hu, F.F.; Zhu, Y.J.; Yang, D.G. Succession characteristics of phytoplankton functional groups and water quality evaluation in Wuchang Lake. Chin. J. Ecol. 2022, 34, 202–214. [Google Scholar]
- Li, S.Q.; Li, W.; Guo, C.; Mai, Z.; Liao, C.S.; Liu, J.S.; Ge, X.C.; Yin, W.W.; Fan, Y.T. Phytoplankton community structure and its relationships with environmental factors in a new urban landscape lake. Biotic. Res. 2021, 43, 535–544. [Google Scholar]
- Zhao, L.L.; Zhu, G.W.; Chen, Y.F.; Li, W.; Zhu, M.Y.; Yao, X.; Cai, L.L. Thermal stratification and its influence factors in a large−sized and shallow Lake Taihu. Adv. Water Sci. 2011, 22, 844–850. [Google Scholar]
- Dong, C.Y.; Yu, Z.M.; Wu, Z.X.; Wu, C.J. Study on seasonal characteristics of thermal stratification in lacustrine zone of Lake Qiandao. Environ. Sci. 2013, 34, 2574–2581. [Google Scholar]
- Lin, J.; Su, Y.P.; Zhong, H.Z.; Chen, Y.Z.; Li, Y.F.; Lin, H. Vertical distribution of phytoplankton in a eutrophic reservoir, Shanzi Reservoir (Fujian) during summer stratification. J. Lake Sci. 2010, 22, 244–250. [Google Scholar]
- Fu, L.; Zhang, L.; Yu, J.J.; Zhou, C.; Haffner, D.G. Water stratification and its relevance to growth of algal community at backwater area in Three Gorges Reservoir. Chin. J. Environ. Eng. 2015, 9, 2265–2271. [Google Scholar]
- Li, Z.; Song, S.Q.; Li, C.W.; Yu, Z.M. Preliminary discussion on the phytoplankton assemblages and its response to the environmental changes in the Changjiang (Yangtze) River Estuary and its adjacent waters during the dry season and the wet season. Hai Yang Xue Bao 2017, 39, 124–144. [Google Scholar]
- Ren, H.; Tian, T.; Yang, Y.F.; Wang, Q. Spatial and temporal distribution of phytoplankton community and its relationship with environment factors in Nansha’s Rivers pearl river estuary. Acta Ecol. Sin. 2017, 37, 7729–7740. [Google Scholar]
- Xiao, L.J.; Wang, T.; Hu, R.; Han, B.P.; Wang, S.; Qian, X.; Padisák, J. Succession of phytoplankton functional groups regulated by monsoonal hydrology in a large Canyon−shaped Reservoir. Water Res. 2011, 45, 5009–5019. [Google Scholar] [CrossRef] [PubMed]
- Muhid, P.; Davis, T.W.; Bunn, S.E.; Burford, M.A. Effects of inorganic nutrients in recycled water on freshwater phytoplankton biomass and composition. Water Res. 2012, 47, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Lu, W.X.; Liang, Y.Y.; Li, J.; Fang, T.; Yang, K.; Gao, N. Community structure of phytoplankton and affecting factors in autumn in three Yangtze River−connected Lakes, Anhui China. Chin. J. Ecol. 2021, 40, 67–75. [Google Scholar]
- Blinn, D.W. Diatom community structure along physicochemical gradients in saline lakes. Ecology 1993, 74, 1246–1263. [Google Scholar] [CrossRef]
- Luo, M.N.; Sun, B.L.; Zhu, B.C.; Song, T.; Cai, K.; Lv, X.Y.; Zhang, Y.; Zhang, W.; Shi, J.Z.; Zhang, H.J.; et al. Distribution characteristics of phytoplankton functional groups and their relationship with environmental factors in Taihu Basin. J. Lake Sci. 2022, 34, 1471–1488. [Google Scholar]
- Wang, X.L.; Zhang, M.; Yin, J. Composition and influential factors of phytoplankton function groups in Lake Chaohu. J. Lake Sci. 2018, 30, 431–440. [Google Scholar]
- Paulino, J.G.N.; Esperanza, G.G.; José, R.A.F.; Cristina, D.M. A new predictive model for evaluating Chlorophyll-a concentration in Tanes Reservoir by using a Gaussian Process Regression. Water Resour. Manag. 2020, 34, 4921–4941. [Google Scholar]
- Li, X.C.; Yu, H.X.; Dou, H.S.; Pan, H.F.; Ma, C.X. Phytoplankton functional groups and related influencing factors in Hulun Lake and adjacent waters in spring. Chin. J. Fisher. 2020, 33, 31–41. [Google Scholar]
- Hui, H.K. Distribution characteristics of phytoplankton functional groups and its driving factors in Saline-alkali Lakes. Environ. Sci. Manag. 2019, 44, 126–131. [Google Scholar]
- Guo, L.L.; Zhu, W.; Li, M. Effect of major cations in water on the growth and polysaccharide contents of Microcystis aeruginosa. Ecol. Environ. Sci. 2013, 22, 1358–1364. [Google Scholar]
- Pan, C.M.; Liu, Y.; An, R.Z.; Ba, S. Phytoplankton in Mitika Wetland, Tibet, China: 2. Characteristics of functional groups and their relationship with environmental factors. J. Lake Sci. 2022, 34, 1115–1126. [Google Scholar]
- Chen, X.J.; Yang, J.; Du, G.S.; Liu, B. Relationships between environmental variables and seasonal succession in phytoplankton functional groups in the Guanting Reservoir. China Environ. Sci. 2016, 32, 74–81. [Google Scholar]
- Guo, X.Q.; Yang, Y.; Yu, Y.; Zhao, X.G.; Liu, X.B. Structural characteristics of and environmental impact on phytoplankton in Changli Gold Coast National Nature Reserve in summer 2019. J. Tianjin Univ. Sci. Technol. 2021, 36, 48–55. [Google Scholar]
- Goldman, J.C. Potential role of large oceanic diatoms in new primary production. Deep Sea Res. Part. I Oceanogr. Res. Papers 1993, 40, 159–168. [Google Scholar] [CrossRef]



| Serial Number | Index | Analytical Method |
|---|---|---|
| 1 | Ammonia Nitrogen (NH3−N) (mg/L) | Nessler’s Reagent Spectrophotometry |
| 2 | Total Phosphorus (TP) (mg/L) | Ammonium Molybdate Spectrophotometric Method |
| 3 | Chlorophyll a (Chl−a) (μg/L) | Acetone Extraction Method |
| 4 | Chemical Oxygen Demand (COD) (mg/L) | Rapid Digestion Method |
| 5 | Biochemical Oxygen Demand (BOD5) (mg/L) | Dilution and Seeding Method |
| 6 | Na+, Mg2+, K⁺, Ca2+ (mg/L) | ICP (Inductively Coupled Plasma Emission Spectrometer) (Agilent, 725−ES, USA) |
| 7 | Cl−, SO₄²−, CO₃²−, HCO₃− (mg/L) | Titrimetric Method |
| 8 | NO₃− (mg/L) | Spectrophotometric Method (Model−752) |
| 9 | Heavy metal ion (mg/L) | ICP (Inductively Coupled Plasma Emission Spectrometer) (Agilent, 725−ES, USA) |
| Functional Group | Typical Representatives | Habitat | Time | ||
|---|---|---|---|---|---|
| May | July | Sept. | |||
| A | Rhizosolenia sp. | Clear, often well−mixed, base−poor lakes | * | ||
| B | Cyclotella sp. | Vertically mixed, mesotrophic small–medium lakes | * | * | * |
| D | Synedra sp., Nitzschia sp. | Shallow, enriched turbid waters, including rivers | * | * | * |
| E | Dinobryon sp. | Usually small, oligotrophic, base−poor lakes or heterotrophic ponds | * | ||
| F | Kirchneriella sp., Oocystis sp., Selenastrum sp. | Clear epilimnia | * | * | * |
| G | Eudorina elegans sp. | Short, nutrient−rich water columns | * | ||
| H1 | Anabaena sp., Pseudanabaena sp., Aphanizomenon sp. | Dinitrogen−fixing Nostocaleans | * | ||
| J | Scenedesmus sp., Tetraedron sp., | Shallow, enriched lakes, ponds, and rivers | * | * | * |
| Coelastrum sp., Crucigenia sp., | |||||
| Pediastrum sp. | |||||
| Lo | Chroococcus sp., Merismopedia sp., | Summer epilimnia in mesotrophic lakes | * | * | * |
| Peridinium sp., Gymnodinium sp., | |||||
| Pinnularia sp. | |||||
| M | Microcystis sp. | Ideally mixed layers of small, eutrophic, low−latitude lakes | * | * | * |
| MP | Navicula sp., Cymbella sp., | Frequent agitation, turbidity, shallow water | * | * | * |
| Achnanthes sp. | |||||
| N | Cosmarium sp., Staurastrum sp. | Mesotrophic epilimnia | * | * | * |
| Tabellaria sp. | |||||
| P | Fragilaria sp., Melosira sp. | Eutrophic epilimnia | * | * | * |
| Closterium sp. | |||||
| TB | Gomphonema sp. | Rapid−flowing water | * | ||
| TC | Oscillatoria sp. | Nutrient−rich static water bodies, or slow−flowing rivers with algae outbreaks | * | * | * |
| S1 | Lyngbya sp. | Turbid mixed layers | * | * | |
| S2 | Spirulina sp., Synedra sp. | Shallow, turbid mixed layers | * | * | * |
| Wo | Chlamydomonas spp. | Rivers and ponds rich in organic matter or humus | * | * | |
| W1 | Euglena sp., Phacus sp. | Small organic ponds | * | ||
| X1 | Ankistrodesmus sp. | Shallow mixed layers in enriched conditions | * | * | * |
| X2 | Chroomonas sp. | Shallow, clear mixed layers in meso−eutrophic lakes | * | ||
| X3 | Chlorella sp., Schroederia sp., Ochromonas sp. | Shallow, clear, mixed layers | * | * | * |
| Y | Cryptomonas sp., Gymnodinium sp. | Usually, small, enriched lakes | * | * | |
| Community Turnover Value (BC) | D | MP | J | S2 | Lo | P | F | M | TB |
|---|---|---|---|---|---|---|---|---|---|
| May−July | 0.15 | 0.36 | 0.15 | 1.00 | 1.00 | 1.00 | 1.00 | 0.96 | 1.00 |
| July−Sept. | 0.22 | 0.43 | 1.00 | 0.54 | 0.39 | 1.00 | 1.00 | 1.00 | 1.00 |
| Heavy Metal Concentration | May | Jul. | Sept. |
|---|---|---|---|
| Al (mg/L) | 0.0050 ± 0.0035 | 0.0163 ± 0.0009 | 0.0225 ± 0.0053 |
| Ba (mg/L) | 0.0850 ± 0.0035 | 0.1010 ± 0.0014 | 0.0765 ± 0.0025 |
| Sr (mg/L) | 0.6050 ± 0.0177 | 0.3252 ± 0.0037 | 0.2180 ± 0.0035 |
| Ag (mg/L) | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| As (mg/L) | 0.0038 ± 0.0003 | 0.0115 ± 0.0004 | 0.0100 ± 0.0007 |
| Cd (mg/L) | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| Cr (mg/L) | 0.0001 ± 0.0000 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| Fe (mg/L) | 0.0023 ± 0.0003 | 0.0275 ± 0.0018 | 0.0050 ± 0.0014 |
| Mn (mg/L) | 0.0003 ± 0.0000 | 0.0105 ± 0.0011 | 0.0050 ± 0.0014 |
| Pb (mg/L) | 0.0001 ± 0.0000 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| Co (mg/L) | 0.0002 ± 0.0000 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| Cu (mg/L) | 0.0004 ± 0.0000 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| Li (mg/L) | 0.0519 ± 0.0009 | 0.0651 ± 0.0004 | 0.0485 ± 0.0011 |
| Ni (mg/L) | 0.0012 ± 0.0000 | 0.0010 ± 0.0000 | 0.0010 ± 0.0000 |
| Se (mg/L) | 0.0001 ± 0.0000 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| Zn (mg/L) | 0.0002 ± 0.0001 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, D.; Guo, Z.; Wei, W.; Bi, J.; Wang, Z.; Ji, X. Phytoplankton Community Structure and Its Relationship with Environmental Factors in Nanhai Lake. Diversity 2022, 14, 927. https://doi.org/10.3390/d14110927
Gong D, Guo Z, Wei W, Bi J, Wang Z, Ji X. Phytoplankton Community Structure and Its Relationship with Environmental Factors in Nanhai Lake. Diversity. 2022; 14(11):927. https://doi.org/10.3390/d14110927
Chicago/Turabian StyleGong, Donghui, Ziqing Guo, Wenxue Wei, Jie Bi, Zhizhong Wang, and Xiang Ji. 2022. "Phytoplankton Community Structure and Its Relationship with Environmental Factors in Nanhai Lake" Diversity 14, no. 11: 927. https://doi.org/10.3390/d14110927
APA StyleGong, D., Guo, Z., Wei, W., Bi, J., Wang, Z., & Ji, X. (2022). Phytoplankton Community Structure and Its Relationship with Environmental Factors in Nanhai Lake. Diversity, 14(11), 927. https://doi.org/10.3390/d14110927






