Abstract
In the Russian Arctic seas and adjacent areas of the Arctic basin, 120 sites of siboglinid records are currently known. Individuals belonging to 15 species have been collected. The largest number (49.2%) of records were made in the Barents Sea, followed by the Laptev Sea (37.5%) and the Arctic basin (10 records; 8.3%). No siboglinids have been reported from the Chukchi Sea. The largest number of species has been identified in both the Laptev Sea and Arctic basin (seven species each). Seventy-eight percent of the records were discovered at water depths down to 400 m. Many of the siboglinid records in the Arctic seas of Russia are associated with areas of high hydrocarbon concentrations. In the Barents Sea, Nereilinum murmanicum has been collected near the largest gas fields. The records of Oligobrachia haakonmosbiensis, N. murmanicum, Siboglinum ekmani, Siboglinum hyperboreum, Siboglinum norvegicum, as well as two undetermined species of siboglinids are associated with the marginal areas of bottom gas hydrates where methane emissions can occur. The Arctic seas of Russia feature vast areas of permafrost rocks containing gas hydrates flooded by the sea. Under the influence of river runoff, gas hydrates dissociate, and methane emissions occur. Crispabrachia yenisey and Galathealinum karaense were found in the Yenisei estuary, and O. haakonmosbiensis was found in the Lena estuary.
1. Introduction
The Siboglinidae is a family of sedentary annelid worms whose representatives all lack a digestive tract. The nourishment of siboglinids is provided by symbiotic bacteria. Within Siboglinidae, four groups of organisms are distinguished, differing in habitat and type of symbiotic bacteria [1]. Representatives of the genus Osedax Rouse, Goffredi & Vrijenhoek, 2004 contain heterotrophic symbionts that gain energy by splitting lipids contained in cetacean skeletons [2,3,4]. Vestimentiferans inhabit hydrothermal oases and areas of cold hydrocarbon seeps and have chemoautotrophic sulfide-oxidizing symbionts [1,5,6,7]. Metagenomic studies show that vestimentiferan symbionts are able to combine autotrophic and heterotrophic nutrition, i.e., to nourish themselves mixotrophically [8,9]. Small and thin Monilifera worms settle on sunken wood, ropes, and cardboard but can also occur in a silty substrate [10,11,12,13,14,15]. Data on the nature of moniliferan symbionts differ: some authors believe that they have methane-oxidizing symbionts [16,17], and others believe that they have sulfide-oxidizing symbionts [18,19].
Species of the Frenulata group (Pogonophora sensu stricto) inhabit soft sediments throughout the world’s oceans in a wide range of depths—from sublittoral to hadal. Some species of Frenulata have been shown to harbor methane-oxidizing symbionts [20], while others harbor sulfide-oxidizing symbionts [21,22].
In areas of hydrocarbon seeps in the sediment column, the anaerobic oxidation of methane occurs using sulfates with the participation of free-living microorganisms [17,19,23,24,25,26,27,28,29,30]. This yields high concentrations of sulfide, which serves as an energy source for the sulfide-oxidizing symbionts of siboglinids.
The seas of the Russian Arctic sector are a region with large hydrocarbon resources [31,32,33,34,35,36,37]. This suggests a rich siboglinid fauna. This study provides an overview on the distribution of all known siboglinid records in the Arctic seas of Russia in connection with areas of hydrocarbon manifestations of various genesis. We expect that these data will allow us to understand the extent of our knowledge of the siboglinid fauna in the Arctic seas of Russia, which will serve as a basis for further faunal and hydrobiological studies. The obtained data may be useful for understanding to what degree the spreading of siboglinids is determined by the presence of methane seeps and gas hydrates.
2. Data Sources
Information on the distribution of siboglinids in the Russian sector of the Arctic was obtained from the analysis of literature sources [13,38,39,40,41,42,43,44,45,46]. We have also included data on new records of siboglinids in the St. Anna Trench in the Kara Sea and several new records of Oligobrachia haakonmosbiensis Smirnov, 2000 in the Laptev Sea, which have not yet been published [47,48,49]. These data were obtained from the collections of the Zoological Institute of the Russian Academy of Sciences and the P.P.Shirshov Institute of Oceanology of the Russian Academy of Sciences. Unpublished data on new records were obtained from collections during the Arctic expeditions of the NIS “Academician Mstislav Keldysh” in 2017–2021. In all expeditions, the material was collected in the same way, namely, with a Van Veen dredge with a capture area of 0.1 m2. All selected material is fixed in 2.5% glutaraldehyde and 96% alcohol.
3. Overview of Siboglinid Records
- Oligobrachia haakonmosbiensis Smirnov, 2000
The type locality of O. haakonmosbiensis is the area of the Haakon Mosby mud volcano in the Norwegian Sea [13]. Within the region under consideration here, individuals of O. haakonmosbiensis are known in three seas: the Barents Sea, the Laptev Sea, and the East Siberian Sea, as well as in the Arctic Basin outside the official borders of the Barents Sea (Figure 1A,C,D). Within the described area, O. haakonmosbiensis occupies a very wide depth range (26–2166 m; Figure 2). Most of the records are concentrated in depths between 26 and 200 m (Figure 2).
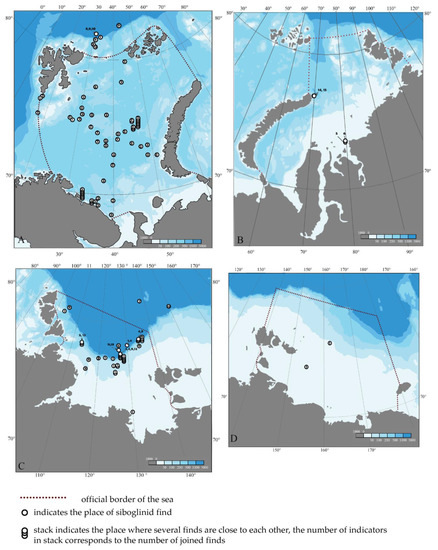
Figure 1.
Distribution maps of siboglinid records in the Arctic seas of Russia. (A)—the Barents Sea, (B)—the Kara Sea, (C)—the Laptev Sea, (D)—the East Siberian Sea. The numbers indicate the records of the following siboglinid species: 1—Oligobrachia haakonmosbiensis, 2—Nereilinum murmanicum, 3—Nereilinum squamosum, 4—Polarsternium rugellosum, 5—Crispabrachia yenisey, 6—Galathealinum karaense, 7—Polybrachia gorbunovi, 8—Siboglinum ekmani, 9—Siboglinum hyperboreum, 10—Siboglinum norvegicum, 11—Siboglinum sp., 12—Sclerolinum contortum, 13—Siboglinidae sp. 1, 14—Siboglinidae sp. 2, 15—Siboglinidae sp. 3.
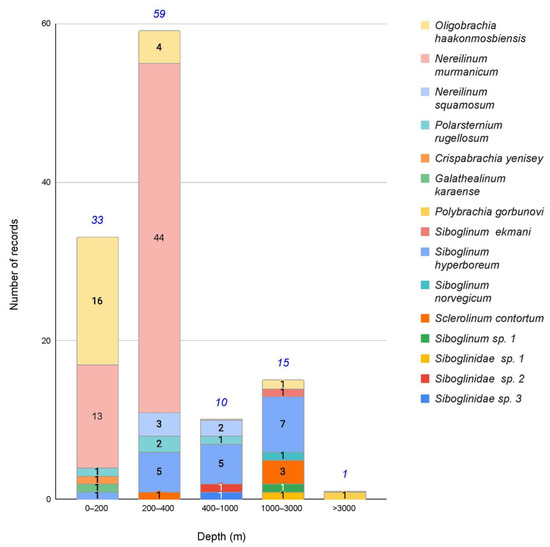
Figure 2.
Distribution of siboglinid records by depth. The numbers indicate the number of records at a given depth range. The number of records of the corresponding species are indicated in black; the total number of siboglinid finds of the current depth range is indicated in blue italics.
In the Barents Sea, O. haakonmosbiensis is known from two records: one located south of the Svalbard archipelago on the border with the Norwegian Sea, and the other in the western trough on the border with the Medvezhinsko-Nadezhdinskaya Bank at depths of 380 and 350 m, respectively. Outside the Barents Sea, north of its official border, to the northeast of the Svalbard archipelago in the Arctic Basin, the deepest record for the species stems from 2166 m (Figure 1).
In the Laptev Sea, O. haakonmosbiensis is widely distributed in the shelf part at depths of 50–300 m, mostly from 50–100 m (Figure 1C). O. haakonmosbiensis is the only representative of siboglinids in the East Siberian Sea (Figure 1D). There, it was found at two stations (26 and 48 m depth). These are the shallowest locations within its known range. The records of O. haakonmosbiensis in the East Siberian Sea are the easternmost records of siboglinids in the Russian part of the Arctic (none yet found in the Chukchi Sea).
- One location in the Laptev Sea coincides with the landfill where methane flares were registered [13].
Relatively recently, Sen and co-authors [50,51] demonstrated that O. haakonmosbiensis represents a complex of cryptic species that are morphologically indistinguishable. Molecular phylogenetic analysis enables the distinguishing of three separate species. The type species O. haakonmosbiensis is distributed in the Norwegian Sea at depths 721–1303 m [13,50]. The second species of Oligobrachia CPL-clade has a predominantly Arctic distribution from the Svalbard archipelago on the border of the Norwegian and Barents Seas to the East Siberian Sea at depths of 26–380 m [50,52,53]. The third species is Oligobrachia sp. Vestnesa: it currently has a point range and is known only from two pockmarks in the region of the Vestnes ridge to the northwest of the Svalbard archipelago at a depth of 1200 m, where the type species O. haakonmosbiensis is also found [51]. All the records considered by us in this publication, from the Barents to the East Siberian Sea, are attributable to one of three cryptic species, namely, Oligobrachia CPL-clade [50,51,53]. The only record of the O. haakonmosbiensis in the Arctic basin northeast of Svalbard cannot yet be reliably attributed to one of the three cryptic species; it represents the deepest record at a depth of 2166 m ([40], collection of ZIN RAS).
- 2.
- Nereilinum murmanicum Ivanov, 1961
Among the regions considered by us, representatives of the species N. murmanicum are known only in the Barents Sea, where it is widely distributed within 69°5′–78°03′ N and 16°25′–51°01′ E (Figure 1A). The northern boundary of the N. murmanicum habitat is the southeastern part of the Perseus upland. To the west, the area is limited by the Medvezhinsko-Nadezhdinskaya upland on the border of the Barents and Norwegian Seas. The southernmost point falls on the southwestern part of the Murmansk upland and almost approaches the coast of the Kola Peninsula. In the east, the range is limited by the Central depression and the southeastern coast of the Southern Island of the Novaya Zemlya archipelago. The depth range at which N. murmanicum was found in the Barents Sea is 75.2–375.3 m; many of the records stem from 200 to 300 m (Figure 2) [40,42,54,55]. A total of 57 stations with records of this species are known within the Barents Sea (Figure 1A). All the records in the Barents Sea, except one, are confined to soft soils (silt, silty sand, clay), and the vast majority are on various silty soils, with a small number on clay soils. One record stems from a rocky ground [42,56]. In addition to the Barents Sea, this species was also reported in the Norwegian Sea, where it inhabits much greater depths of 990–1300 m [57].
- 3.
- Nereilinum squamosum Smirnov, 1999
The species is known from only a few records in two Arctic regions. One record in the Arctic basin is in the Barents Sea sector at a depth of 603 m (Figure 1A). Four stations are known from the Laptev Sea (Figure 1C), distributed along the shelf lower boundary and on the continental slope at depths of 240–560 m. These localities coincide with the habitats of several other siboglinid species (Polarsternium rugellosum Smirnov, 1999, Siboglinum hyperboreum Ivanov, 1960, Sclerolinum contortum Smirnov, 2000).
- 4.
- Polarsternium rugellosum Smirnov, 1999
This species is known only from the northeastern part of the Laptev Sea within 126°–130° E and 77°–78° N (Figure 1C) at a depth range of 100–556 m (Figure 2). The records coincide with the habitats of other siboglinid species: N. squamosum, S. hyperboreum, Sc. contortum.
- 5.
- Crispabrachia yenisey Karaseva, Rimskaya-Korsakova, Ekimova, Gantsevich, Kokarev, Kremnyov, Simakov, Udalov, Vedenin & Malakhov, 2021
The species is known from a single record in the Kara Sea in the estuary of the Yenisei River between Sibiryakov Island and the western shore of the Taimyr Peninsula at a depth of 28 m (Figure 1B).
- 6.
- Galathealinum karaense Smirnov, Zaitseva& Vedenin, 2020
This species, as in the case of the above-described C. yenisey, was discovered at the mouth of the Yenisei River at a depth of 25 m [43], whereby the records of both species were made relatively close to each other (Figure 1B).
- 7.
- Polybrachia gorbunovi (Ivanov, 1949)
Initially, this species was described by A.V. Ivanov [58] under the name Lamellisabella gorbunovi. Later, it was transferred to the genus Polybrachia [59]. It is known from a single record in the Arctic Basin in the Sadko Trench, opposite the Novosibirsk Islands (Figure 1C). The species was discovered during trawling on muddy soils at a depth of 3700–3800 m [38,60]. This record is the deepest of all Arctic siboglinids (Figure 2).
- 8.
- Siboglinum ekmani Jägersten, 1956
In the Arctic, this species is known only from one site in the Arctic Basin to the northeast of the Svalbard archipelago outside the Barents Sea at a depth of 2090–2166 m (Figure 1A). This record coincides with the discovery site of two more species of the genus: Siboglinum Caullery, 1914 (Siboglinum hyperboreum Ivanov, 1960 and Siboglinum norvegium Ivanov, 1960).
The Arctic record of this species is located in both the northernmost and easternmost parts of its ranges. The range mostly occupies the northern part of the Atlantic Ocean. The records of S. ekmani are distributed along the continental slope of North America down to the Caribbean Sea in the west and from the Norwegian Sea to the coast of Portugal in the east [12,61,62,63,64,65,66,67,68]. The species is one of the most eurybate among the frenulate pogonophorans and inhabits a depth range of 90–4485 m [12,62,64,66,67,68].
- 9.
- Siboglinum hyperboreum Ivanov, 1960
Within the Russian sector of the Arctic, representatives of this species were recorded in two areas in the Arctic Basin and the Laptev Sea. In the Arctic Basin, only two records are known from the northeast of Svalbard in the range of 30°–42° E to 80° N (Figure 1A). In the Laptev Sea, it is widely distributed from the west of the Severnaya Zemlya archipelago to the northeastern margin of the sea in the east (Figure 1C). In the Laptev Sea, the records stem from 55 to 2000 m. Most of the records in the Arctic occur at depths below 500 m (Figure 2). Some S. hyperboreum records in the Laptev Sea are located in the area with methane seeps [69].
Outside the Russian sector of the Arctic, the species has also been recorded in the Greenland Sea, with one record near the Greenland coast at 217 m and one in the Svalbard area at about 650 m [38,40,44,60,66]. Thus, S. hyperboreum has a purely Arctic range and eurybate distribution.
- 10.
- Siboglinum norvegicum Ivanov, 1960
This species is known from only a few records; one of them is in the Arctic Basin northeast of Svalbard in the Barents Sea at a depth of 2090–2166 m and coincides with the habitat of the above-mentioned S. ekmani and S. hyperboreum (Figure 1A). In addition to the Arctic Basin, the species is known from the Norwegian Sea, where it was recorded near the Shetland Islands at 120 m and off the coast of Norway at 165 m [38,60].
- 11.
- Siboglinum sp.
Representatives of Siboglinum sp. were recorded in the central part of the Laptev Sea at a depth of 1556–1560 m (Figure 1C). The species could not be determined due to the poor preservation of the material. At the same station, in addition to Siboglinum sp., a representative of Frenulata of the indeterminate genus Siboglinidae gen. sp. 1. was also found.
- 12.
- Sclerolinum contortum Smirnov, 2000
Sc. contortum within the Arctic region is known from several records in the Arctic Basin and the Laptev Sea. Two records are known in the Arctic Basin, the first being in the area adjacent to the Barents Sea to the northeast of the Svalbard archipelago (Figure 1A) and the second being in the East Siberian Sea area north of the Novosibirsk Islands (Figure 1C,D). The Arctic Basin records are confined to depths of 2000–2100 m (2). In the Laptev Sea, the species was found twice in its eastern and central parts at 310 and 1080 m. In three out of four cases, the presence of Sc. contortum coincides with the records of N. squamosum (Figure 1A,C).
Sc. contortum has a wide distribution outside the Arctic. It was found in the Norwegian Sea in the area of the Loki’s castle hydrothermal field within the mid-Arctic ridge at its maximum depths of 2340–2740 m [13,70,71]. It also occurs in the Gulf of Mexico [15,71]. The southernmost area of discovery is in the Atlantic sector of the Southern Ocean near the South Shetland and South Sandwich Islands.
- 13.
- Siboglinidae gen. sp. 1
These representatives of frenulate pogonophorans of uncertain taxonomic rank were found in the central part of the Laptev Sea at a depth of 1556–1560 m together with the undescribed species Siboglinum sp. mentioned above (Figure 1C). According to molecular phylogenetic analysis, Siboglinidae gen. sp. 1 is a sister genus to Crispabrachia [45].
- 14.
- Siboglinidae gen. sp. 2
A specimen with a single tentacle in a smooth tube was found in the Kara Sea near the northern tip of the Novaya Zemlya archipelago in the St. Anna Trench at a depth of 535.5 m (Figure 1B) [47]. The material is not described due to poor (incomplete) preservation.
- 15.
- Siboglinidae gen. sp. 3
A single specimen with two tentacles in a ringed tube was found in the Kara Sea in the St. Anna Trench together with Siboglinidae gen. sp. 2 indicated above (Figure 1B) [47]. The material is not described due to poor preservation.
4. Discussion
The total number of siboglinid records in the Arctic seas of Russia and the adjacent sector of the central basin of the Arctic Ocean is 120. The largest number of records (59) falls on the Barents Sea (49.2%), followed by the Laptev Sea (45; 37.5%) (Figure 3). The Kara Sea accounts for 3.3% of the records, and the East Siberian Sea accounts for 1.7% (Figure 3).
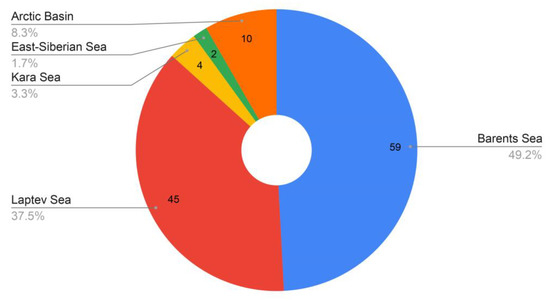
Figure 3.
Distribution of siboglinid records by seas.
No siboglinids have yet been reported from the Chukchi Sea. Ten records are known in the areas adjacent to the central Arctic Basin (8.3%). Based on current knowledge, the distribution of the number of records most likely reflects the study effort in the various Arctic parts. Nonetheless, one fact is clear. The largest number of siboglinid records has been recorded in the Barents Sea (probably reflecting the best knowledge of this region), while the largest number of species was found in the Laptev Sea and the Arctic Basin (Figure 4).
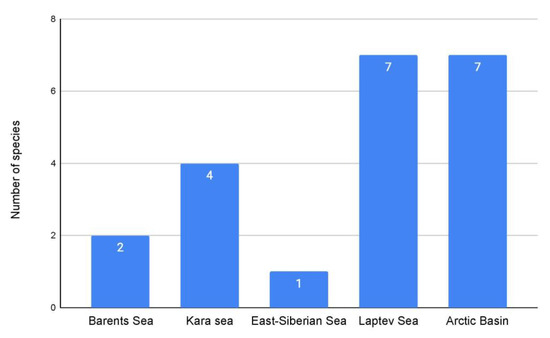
Figure 4.
Distribution of siboglinid species by seas.
Siboglinids are mainly deep-sea organisms whose representatives mostly inhabit bathyal depths [38,39,72]. In the Russian Arctic, most of the siboglinid records stem from the shelf (Figure 5), with 28% of all records at depths shallower than 200 m. The Barents Sea is characterized by a deep shelf, extending to depths of 300–400 m, reflecting a great glaciation setting in, followed by a sinking of the land in Quaternary time [73,74,75,76]. In this regard, most of the siboglinid records in the Barents Sea at depths down to 400 m are attributable to shelf habitats. In the Russian sector of the Arctic, 78% of the records are at depths down to 400 m (Figure 6). Taking into account our poor knowledge of the deep-water part of the Arctic Ocean, we can expect a higher number of siboglinid records at bathyal depths.
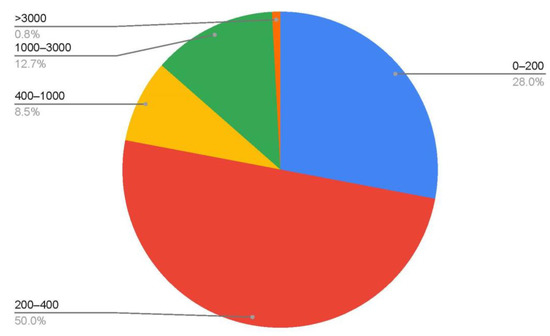
Figure 5.
Percentage distribution of siboglinid records by depth.
Many of the siboglinid records in the Russian Arctic sector are associated with areas featuring high hydrocarbon concentrations. An earlier analysis of the distribution of N. murmanicum showed that 82% of the total records of this species in the Barents Sea correspond to areas known as highly promising for oil and gas production [56,77]. N. murmanicum was found near the largest gas fields of the Barents Sea, namely, Shtokman, Ledovskoye, and Ledovoye [56].
The main hydrocarbon reserves in the world’s oceans are concentrated in the form of methane gas hydrates [35,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. Many siboglinid records in the Barents Sea and the adjoining Arctic Basin are confined to the areas of such gas hydrates (Figure 6A). The records of N. murmanicum are confined to gas hydrate deposits in the central part of the Barents Sea. The gas hydrate areas in the western part of the Barents Sea are associated with the records of both N. murmanicum and O. haakonmosbiensis (Figure 6A). The marginal gas hydrate deposit sites in the north Barents Sea and the adjoining Arctic basin, where methane emissions can occur, are associated with the records of O. haakonmosbiensis, N. murmanicum, S. ekmani, S. hyperboreum, and S. norvegicum (Figure 6A).
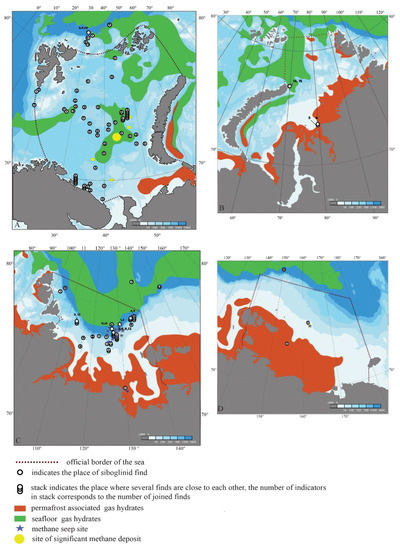
Figure 6.
Distribution map of siboglinid records in the Arctic seas of Russia with different methane occurrences (deposits, gas hydrates (by Solovyov et al., 1987 [98]), and methane seep sites (by Shakhova et al., 2015 [69])). (A)—the Barents Sea, (B)—the Kara Sea, (C)—the Laptev Sea, (D)—the East-Siberian Sea. The numbers indicate the records of the following siboglinid species: 1—Oligobrachia haakonmosbiensis, 2—Nereilinum murmanicum, 3—Nereilinum squamosum, 4—Polarsternium rugellosum, 5—Crispabrachia yenisey, 6—Galathealinum karaense, 7—Polybrachia gorbunovi, 8—Siboglinum ekmani, 9—Siboglinum hyperboreum, 10—Siboglinum norvegicum, 11—Siboglinum sp., 12—Sclerolinum contortum, 13—Siboglinidae sp. 1, 14—Siboglinidae sp. 2, 15—Siboglinidae sp. 3.
Gas hydrate deposits in the Russian Arctic are divided into bottom gas hydrates, which occupy deep-water areas of the Arctic Ocean, and gas hydrate deposits in the permafrost, which are located at shallow depths relatively close to the Russian Arctic coast [35,81,90,98,99,100,101,102,103,104]. Nonetheless, the bottom and permafrost gas hydrate areas in most of the Arctic seas of Russia are separated by extensive zones where gas hydrates are absent. This reflects either a lack of methane or a lack of thermobaric conditions necessary for the formation of clathrates.
The Kara Sea surpasses all other seas of the Russian Arctic in terms of hydrocarbon resources [31,105,106]. The Kara Sea siboglinid fauna remains very poorly studied. Frenulate pogonophorans have so far been found in only two areas here (Figure 6B). One of them is in the Yenisei Bay between Sibiryakov Island and the western shore of the Taimyr Peninsula [41,43,45]. In the former, both records stem from record-shallow depths for siboglinids. Crispabrachia yenisey was recorded at a depth of 28 m [41,45], while Galathealinum karaense was found at 25 m [43]. Siboglinids are stenohaline organisms and, as a rule, are not found in less salty waters [38]. The Yenisei Bay is characterized by strong a vertical stratification of salinity [107,108,109]. The surface average long-term value between Sibiryakov Island and the western coast of the Taimyr Peninsula around the records of C. yenisey and G. karaense is less than 5‰, whereas the bottom salinity here ranges from 30 to 32.5 ‰ [107,108,109]. The discovery site of C. yenisey and G. karaense is in an area where the methane concentration in the surface water layer reaches 130 nM, a peak value for the southern part of the Kara Sea [110]. The high concentrations of methane here reflect the degradation of permafrost gas hydrates under the influence of river runoff [100,110,111]. This process is intensive against the background of the Arctic general warming in the estuaries of Ob, Yenisei, Lena, and other large rivers of the Russian Arctic. The result is not only high concentrations of methane in the water but also the release of this greenhouse gas into the atmosphere [102,103,112,113,114,115,116,117,118].
A species closely related to G. karaense, namely, Galathealinum arcticum Southward, 1962, was found in the Canadian Arctic in the estuary area of the Mackenzie River at a depth of 36 m [119]. The estuarine areas of that river are also characterized by very strong salinity stratification: the values in the surface layer range from 1 to 10‰, but at 20 m, they exceed 31‰ in all seasons [120]. The Mackenzie River Delta and adjacent areas of the Beaufort Sea shelf are characterized by large gas hydrate deposits in the permafrost, which dissociate under the influence of river runoff under the conditions of Arctic warming and generate powerful methane flows [121,122,123,124,125,126,127,128,129,130].
The area in which Siboglinidae gen. sp. 2 and Siboglinidae gen. sp. 3 were found (below 500 m depth, salinity exceeding 34‰) [47] corresponds to the southernmost section of the bottom gas hydrate distribution in the St. Anna Trench [98,99,104]. Warm and salty Atlantic water flows through the Fram Strait into the central depression of the Arctic Ocean and, further along the trench, enters the Kara Sea [46,131,132,133,134]. Existing models [35,135,136] demonstrate the potential dissociation of bottom gas hydrates in the St. Anna Trench, and the resulting methane stream serves as a source that ensures the survival of siboglinids.
The Laptev Sea also harbors some of the richest deposits of hydrocarbons [69,103,112,113,137,138,139]. Its southern part is characterized by gas hydrate deposits in the permafrost, whereas the deep-water northern part features extensive deposits of bottom gas hydrates [99]. Numerous methane seeps have been recorded on the outer part of the shelf in the northeast Laptev Sea [69,103,112,113]. To date, seven species of siboglinids have been reported in the Laptev Sea (including undetermined records), which is more than that in any other Russian Arctic sea (Figure 4). The distribution of siboglinids in the Laptev Sea is clearly correlated with areas of methane seeps of various geneses. The most widespread species in the Russian Arctic, O. haakonmosbiensis, was found in the Lena River estuary (Figure 6C), where the methane concentration of the water exceeds 1000 nm [117]. As in the case of the Yenisei and Mackenzie River mouths, high methane concentrations here reflect the degradation of permafrost under the influence of river runoff [69,103,110,117,138,140]. Siboglinid records in the northern part of the sea are associated with marginal areas of bottom gas hydrate deposits (Figure 6C). The discovery of P.gorbunovi in a section of the Arctic Basin adjacent to the Laptev Sea is associated with deep-sea gas hydrates (Figure 6C). The most numerous siboglinid records are confined to the field of methane seeps on the outer part of the Laptev Sea shelf (Figure 6C): all seven species recorded to date in the Laptev Sea were found in this area [139,141,142].
The East Siberian Sea is also among the most hydrocarbon-rich seas of the Russian Arctic. Methane seeps from underwater deposits cause high methane concentrations in the water column and strong emissions into the atmosphere [102,103,110,112,113,117,143,144]. Unfortunately, the siboglinid fauna of the East Siberian Sea remains poorly studied. Only two records of O. haakonmosbiensis are known here: on the shelf sections southeast and southwest of New Siberia Island (Figure 1D). One of those records was made at a depth of 26 m and is in the gas hydrate zone associated with coastal permafrost strata (Figure 6D). The methane emissions here are with the dissociation of gas hydrates of permafrost [69,102,103,110,115,117,137,138,145]. Another record at a depth of 48 m in the central part of the sea (Figure 6D) coincides with the landfill where methane flares have been registered [144]. In the deep-water part of the Arctic Basin adjacent to the East Siberian Sea, the presence of Sclerolinum contortum coincides with the marginal area of bottom gas hydrates.
The presented material shows a correlation of siboglinids records with various types of methane manifestations. As noticed above, in the Laptev Sea and in the Yenisei Bay, there are certain data on increased methane concentrations in places coinciding with the recordings of siboglinids [110,117]. In the East Siberian Sea and the Laptev Sea, some records coincide with methane flares [139,144]. For a general assessment of the possible effect of gas hydrates on the distribution of siboglinids across all seas of the Arctic seas of Russia, we present Solovyov’s data on the areas of gas hydrate content [96]. It should be noticed that methane can also come from many other sources—for example, from organic residues (such as whale falls and flooded wood). The presence of communities on submerged wood is very likely, at least in the area of P. gorbunovi records, since mollusks associated with submerged wood were collected at the same station [146,147].
5. Conclusions
Rich methane deposits have been identified in the Arctic seas of Russia and the adjacent part of the Arctic basin. This suggests the presence of a rich siboglinid fauna: gutless worms that live in symbiosis with chemoautotrophic bacteria that oxidize methane or hydrogen sulfide. Hydrogen sulfide is formed in areas of hydrocarbon seeps due to the anaerobic oxidation of methane in the sediment. Overall, the fauna of the Arctic seas and the Arctic Basin is not well studied. Nevertheless, siboglinids have been recorded in the basin and in all Arctic seas of Russia, except for the Chukchi Sea. The range of siboglinids is associated with hydrocarbon manifestations of various geneses. In the Barents Sea, most of the siboglinid records are associated with large gas and oil fields such as Shtokman, Ledovskoye, and Ledovoye. Elsewhere, they are associated with the marginal areas of bottom gas hydrates in the Arctic Basin, where hydrate dissociation generates methane flows. In the Laptev Sea and the East Siberian Sea, most of the siboglnid records are associated with areas of methane flares. The siboglinid records in the estuaries of large Siberian rivers, such as the Yenisei and Lena, are of particular interest. These records are associated with the emission of methane from permafrost rocks under the influence of river runoff.
Author Contributions
Conceptualization, N.P.K. and V.V.M.; Methodology, R.V.S. and A.A.U.; Validation, V.O.M. and M.M.G.; Formal analysis, N.P.K. and V.V.M.; Investigation, N.P.K., N.N.R.-K. and V.V.M.; Resources, R.V.S. and A.A.U.; Data curation, R.V.S. and A.A.U.; Writing—original draft preparation, N.P.K. and V.V.M.; Writing—review and editing, N.P.K., M.M.G., and V.V.M.; Visualization, N.P.K.; Supervision, V.V.M.; Project administration, V.V.M.; Funding acquisition, V.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 18-14-00141-P.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the colleagues from the P.P. Shirshov Institute of Oceanology Russian Academy of Sciences and the Zoological Institute, Russian Academy of Sciences for the availability of the collection data. We would like to thank Andrey Vedenin, Valentin Kokarev, and Miloslav Simakov for the work on the research cruises and the help in accessing the material. The authors thank the anonymous reviewers for their valuable comments and suggestions which allowed for the considerable improvement of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hilario, A.; Capa, M.; Dahlgren, T.G.; Halanych, K.M.; Little, C.T.; Thornhill, D.J.; Verna, C.; Glover, A.G. New perspectives on the ecology and evolution of siboglinid tubeworms. PLoS ONE 2011, 6, e16309. [Google Scholar] [CrossRef] [PubMed]
- Goffredi, S.K.; Orphan, V.J.; Rouse, G.W.; Jahnke, L.; Embaye, T.; Turk, K.; Lee, R.; Vrijenhoek, R.C. Evolutionary innovation: A bone-eating marine symbiosis. Environ. Microbiol. 2005, 7, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Rouse, G.W.; Goffredi, S.K.; Vrijenhoek, R. Osedax: Bone-eating marine worms with dwarf males. Science 2004, 305, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Goffredi, S.K.; Johnson, S.B.; Vrijenhoek, R.C. Genetic diversity and potential function of microbial symbionts associated with newly discovered species of Osedax polychaete worms. Appl. Environ. Microbiol. 2007, 73, 2314–2323. [Google Scholar] [CrossRef] [PubMed]
- Felbeck, H. Chemoautotrophic potential of the hydrothermal vent tube worm, Riftia pachyptila Jones (Vestimentifera). Science 1981, 213, 336–338. [Google Scholar] [CrossRef]
- Cavanaugh, C.M.; Gardiner, S.L.; Jones, M.L.; Jannasch, H.W.; Waterbury, J.B. Prokaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones: Possible chemoautotrophic symbionts. Science 1981, 213, 340–342. [Google Scholar] [CrossRef]
- Distel, D.L.; Lane, D.J.; Olsen, G.J.; Giovannoni, S.J.; Pace, B.; Pace, N.R.; Stahl, D.A.; Felbeck, H. Sulfur-oxidizing bacterial endosymbionts: Analysis of phylogeny and specificity by 16S rRNA sequences. J. Bacteriol. 1988, 170, 2506–2510. [Google Scholar] [CrossRef]
- Robidart, J.C.; Bench, S.R.; Feldman, R.A.; Novoradovsky, A.; Podell, S.B.; Gaasterland, T.; Allen, E.E.; Felbeck, H. Metabolic versatility of the Riftia pachyptila endosymbiont revealed through metagenomics. Environ. Microbiol. 2008, 10, 727–737. [Google Scholar] [CrossRef]
- Reveillaud, J.; Anderson, R.; Reves-Sohn, S.; Cavanaugh, C.; Huber, J.A. Metagenomic investigation of vestimentiferan tubeworm endosymbionts from Mid-Cayman Rise reveals new insights into metabolism and diversity. Microbiome 2018, 6, 19. [Google Scholar] [CrossRef]
- Webb, M. A new bitentaculate pogonophoran from Hardanger fjorden, Norway. Sarsia 1964, 15, 49–56. [Google Scholar] [CrossRef]
- Webb, M. Additional notes on Sclerolinumbrattstromi (Pogonophora) and the establishment of a new family, Sclerolinidae. Sarsia 1964, 16, 47–58. [Google Scholar] [CrossRef]
- Southward, E.C. On some Pogonophora from the Caribbean and the Gulf of Mexico. Bull. Mar. Sci. 1972, 22, 739–776. [Google Scholar]
- Smirnov, R.V. Two new species of Pogonophora from the arctic mud volcano off northwestern Norway. Sarsia 2000, 85, 141–150. [Google Scholar] [CrossRef]
- Smirnov, R. Morphological characters and classification of the subclass Monilifera (Pogonophora) and the problem of evolution of the bridle in pogonophorans. Russ. J. Mar. Biol. 2008, 34, 359–368. [Google Scholar] [CrossRef]
- Eichinger, I.; Hourdez, S.; Bright, M. Morphology, microanatomy and sequence data of Sclerolinum contortum (Siboglindae, Annelida) of the Gulf of Mexico. Org. Divers. Evol. 2013, 13, 311–329. [Google Scholar] [CrossRef]
- Pimenov, N.; Savvichev, A.; Rusanov, I.; Lein, A.Y.; Ivanov, M. Microbiological processes of the carbon and sulfur cycles at cold methane seeps of the North Atlantic. Microbiology 2000, 69, 709–720. [Google Scholar] [CrossRef]
- Pimenov, N.; Savvichev, A.; Rusanov, I.; Lein, A.; Egorov, A.; Gebruk, A.; Moskalev, L.; Vogt, P. Microbial processes of carbon cycle as the base of food chain of Håkon Mosby Mud Volcano benthic community. Geo-Mar. Lett. 1999, 19, 89–96. [Google Scholar] [CrossRef]
- Xu, T.; Sun, Y.; Wang, Z.; Sen, A.; Qian, P.; Qiu, J. The morphology, mitogenome, phylogenetic position, and symbiotic bacteria of a new species of Sclerolinum (Annelida: Siboglinidae) in the South China Sea. Front. Mar. Sci. 2022, 8, 793645. [Google Scholar] [CrossRef]
- Lösekann, T.; Robador, A.; Niemann, H.; Knittel, K.; Boetius, A.; Dubilier, N. Endosymbioses between bacteria and deep-sea siboglinid tubeworms from an Arctic cold seep (Haakon Mosby Mud Volcano, Barents Sea). Environ. Microbiol. 2008, 10, 3237–3254. [Google Scholar] [CrossRef]
- Schmaljohann, R.; Flügel, H.J. Methane-oxidizing bacteria in Pogonophora. Sarsia 1987, 72, 91–98. [Google Scholar] [CrossRef]
- Southward, A.; Southward, E.C.; Dando, P.; Barrett, R.; Ling, R. Chemoautotrophic function of bacterial symbionts in small Pogonophora. J. Mar. Biol. Assoc. UK 1986, 66, 415–437. [Google Scholar] [CrossRef]
- Dando, P.; Southward, A.; Southward, E.; Barrett, R. Possible energy sources for chemoautotrophic prokaryotes symbiotic with invertebrates from a Norwegian fjord. Ophelia 1986, 26, 135–150. [Google Scholar] [CrossRef]
- Aharon, P.; Fu, B. Sulfur and oxygen isotopes of coeval sulfate–sulfide in pore fluids of cold seep sediments with sharp redox gradients. Chem. Geol. 2003, 195, 201–218. [Google Scholar] [CrossRef]
- Aharon, P.; Fu, B. Microbial sulfate reduction rates and sulfur and oxygen isotope fractionations at oil and gas seeps in deepwater Gulf of Mexico. Geochim. Cosmochim. Acta 2000, 64, 233–246. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, R.; Jørgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Joye, S.B.; Boetius, A.; Orcutt, B.N.; Montoya, J.P.; Schulz, H.N.; Erickson, M.J.; Lugo, S.K. The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chem. Geol. 2004, 205, 219–238. [Google Scholar] [CrossRef]
- Levin, L.A. Ecology of cold seep sediments: Interactions of fauna with flow, chemistry and microbes. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2005; pp. 11–56. [Google Scholar]
- Dattagupta, S.; Miles, L.L.; Barnabei, M.S.; Fisher, C.R. The hydrocarbon seep tubeworm Lamellibrachia luymesi primarily eliminates sulfate and hydrogen ions across its roots to conserve energy and ensure sulfide supply. J. Exp. Biol. 2006, 209, 3795–3805. [Google Scholar] [CrossRef]
- Regnier, P.; Dale, A.W.; Arndt, S.; LaRowe, D.E.; Mogollón, J.; Van Cappellen, P. Quantitative analysis of anaerobic oxidation of methane (AOM) in marine sediments: A modeling perspective. Earth-Sci. Rev. 2011, 106, 105–130. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, D.; Liang, Q.; Xia, Z.; Chen, L.; Chen, D. Impact of anaerobic oxidation of methane on the geochemical cycle of redox-sensitive elements at cold-seep sites of the northern South China Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 122, 84–94. [Google Scholar] [CrossRef]
- Bird, K.J.; Charpentier, R.R.; Gautier, D.L.; Houseknecht, D.W.; Klett, T.R.; Pitman, J.K.; Moore, T.E.; Schenk, C.J.; Tennyson, M.E.; Wandrey, C.R. Circum-Arctic resource appraisal: Estimates of undiscovered oil and gas north of the Arctic Circle. In Fact Sheet; Version 1.0 ed.; U. S. Geological Survey: Menlo Park, CA, USA, 2008. [Google Scholar] [CrossRef]
- Gautier, D.L.; Bird, K.J.; Charpentier, R.R.; Grantz, A.; Houseknecht, D.W.; Klett, T.R.; Moore, T.E.; Pitman, J.K.; Schenk, C.J.; Schuenemeyer, J.H. Oil and gas resource potential north of the Arctic Circle. Geol. Soc. Lond. Mem. 2011, 35, 151–161. [Google Scholar] [CrossRef]
- Spencer, A.M.; Embry, A.F.; Gautier, D.L.; Stoupakova, A.V.; Sørensen, K. An overview of the petroleum geology of the Arctic. Geol. Soc. Lond. Mem. 2011, 35, 1–15. [Google Scholar] [CrossRef]
- Gautier, D.L.; Bird, K.J.; Charpentier, R.R.; Grantz, A.; Houseknecht, D.W.; Klett, T.R.; Moore, T.E.; Pitman, J.K.; Schenk, C.J.; Schuenemeyer, J.H. Assessment of undiscovered oil and gas in the Arctic. Science 2009, 324, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Max, M.D.; Johnson, A.H.; Dillon, W.P. Natural Gas Hydrate-Arctic Ocean Deepwater Resource Potential; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Dmitrievsky, A.N.; Eremin, N.A.; Shabalin, N.A.; Kondratyuk, A.T.; Eremin, A.N. State and prospects for the development of hydrocarbon resources of the Arctic shelf Neft. Offshore 2017, 1, 32–42. (In Russian) [Google Scholar]
- Dmitrieva, D.; Romasheva, N. Sustainable Development of Oil and Gas Potential of the Arctic and Its Shelf Zone: The Role of Innovations. J. Mar. Sci. Eng. 2020, 8, 1003. [Google Scholar] [CrossRef]
- Ivanov, A.V. Pogonophores; USSR Academy of Sciences Publishing House: Leningrad, Russia, 1960; Volume 75. (In Russian) [Google Scholar]
- Ivanov, A.V. Pogonophores and their geographical distribution. In Results of Science I Achievements of Oceanology; Zenkevich, E.A., Ed.; Academy of Sciences of the Soviet Union: Moscow, Russia, 1959; Volume 1, pp. 258–284. (In Russian) [Google Scholar]
- Smirnov, R.V. Systematics and Morphology of Pogonophores of the Arctic and Southern Oceans. Ph.D. Thesis, Zoological Institute of Russian, St. Petersburg, Russia, 2001. (In Russian). [Google Scholar]
- Rimskaya-Korsakova, N.N.; Karaseva, N.P.; Kokarev, V.N.; Simakov, M.I.; Gantsevich, M.M.; Malakhov, V.V. First Discovery of Pogonophora (Annelida, Siboglinidae) in the Kara Sea Coincide with the Area of High Methane Concentration. Dokl. Biol. Sci. 2020, 490, 25–27. [Google Scholar] [CrossRef]
- Kanafina, M.; Karaseva, N.; Zakharov, D.; Golikov, A.; Malakhov, V. New Data on Distribution of Nereilinum murmanicum (Annelida, Siboglinidae) in the Barents Sea. Dokl. Biol. Sci. 2022, 502, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, R.V.; Zaitseva, O.V.; Vedenin, A.A. A remarkable pogonophoran from a desalted shallow near the mouth of the Yenisey River in the Kara Sea, with the description of a new species of the genus Galathealinum (Annelida: Pogonophora: Frenulata). Zoosystematica Ross. 2020, 29, 138–154. [Google Scholar] [CrossRef]
- Smirnov, R.V. A new genus and two new species of pogonophora from the Arctic Ocean. Russ. J. Mar. Biol. 1999, 25, 312–319. [Google Scholar]
- Karaseva, N.; Rimskaya-Korsakova, N.; Ekimova, I.; Gantsevich, M.; Kokarev, V.; Kremnyov, S.; Simakov, M.; Udalov, A.; Vedenin, A.; Malakhov, V. A new genus of frenulates (Annelida: Siboglinidae) from shallow waters of the Yenisey River estuary, Kara Sea. Invertebr. Syst. 2021, 35, 857–875. [Google Scholar] [CrossRef]
- Kanafina, M.M.; Gabidullina, R.I.; Rimskaya-Korsakova, N.N.; Zakharov, D.V.; Sabirov, R.M.; Golikov, A.V. Functional morphology and ecology of the Arctic pogonophore Nereilinum murmanicum Ivanov, 1961 (Siboglinidae, Annelida). Proc. Kazan University. Nat. Sci./Uchenye Zap. Kazan. Universiteta. Seriya Estestv. Nauk. 2021, 163, 25–28. [Google Scholar] [CrossRef]
- Rimskaya-Korsakova, N.N.; Karaseva, N.P.; Osadchiev, A.A.; Semiletov, I.P.; Gantsevich, M.M.; Malakhov, V.V. Pogonophora (Annelida, Siboglinidae) record in the St. Anna Trough (Kara Sea) in the area of gas hydrate dissociation. Rep. Acad. Sci. 2022. in press. (In Russian) [Google Scholar]
- Karaseva, N.P.; Rimskaya-Korsakova, N.N.; Kokarev, V.N.; Simakov, M.I.; Smirnov, R.V.; Gantsevich, M.M.; Malakhov, V.V. Discovery of siboglinids (Annelida, Siboglinidae) in the Laptev Sea are confined to areas of methane seeps. Dokl. Biol. Sci. 2023. in press. (In Russian) [Google Scholar]
- Karaseva, N.P.; Rimskaya-Korsakova, N.N.; Kokarev, V.N.; Simakov, M.I.; Smirnov, R.V.; Gantsevich, M.M.; Malakhov, V.V. Discovery of siboglinids (Annelida, Siboglinidae) in the estuaries of the largest Arctic rivers are associated with permafrost gas hydrates. Dokl. Biol. Sci. 2023. in press. (In Russian) [Google Scholar]
- Sen, A.; Duperron, S.; Hourdez, S.; Piquet, B.; Léger, N.; Gebruk, A.; Le Port, A.-S.; Svenning, M.M.; Andersen, A.C. Cryptic frenulates are the dominant chemosymbiotrophic fauna at Arctic and high latitude Atlantic cold seeps. PLoS ONE 2018, 13, e0209273. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Didriksen, A.; Hourdez, S.; Svenning, M.M.; Rasmussen, T.L. Frenulate siboglinids at high Arctic methane seeps and insight into high latitude frenulate distribution. Ecol. Evol. 2020, 10, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, R.V. A revision of the Oligobrachiidae (Annelida: Pogonophora), with notes on the morphology and distribution of Oligobrachia haakonmosbiensis Smirnov. Mar. Biol. Res. 2014, 10, 972–982. [Google Scholar] [CrossRef]
- Karaseva, N.P.; Rimskaya-Korsakova, N.N.; Ekimova, I.A.; Kokarev, V.N.; Simakov, M.I.; Gantsevich, M.M.; Malakhov, V.V. The first discovery of pogonophores (Annelida, Siboglinidae) in the East Siberian Sea coincides with the areas of methane seeps. Dokl. Biol. Sci. 2021, 501, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V. Deux genres nouveaux de PogonophoresdiplobrachiauxNereilinum et Siboglinoides. Cah. Biol. Mar. 1961, 2, 381–397. [Google Scholar]
- Moskalev, L.I. Pogonophora in the Barents Sea. Doklady AN SSSR 1961, 137, 730–731. (In Russian) [Google Scholar]
- Karaseva, N.; Kanafina, M.; Gantsevich, M.; Rimskaya-Korsakova, N.; Zakharov, D.; Golikov, A.; Smirnov, R.; Malakhov, V. Distribution of Nereilinum murmanicum (Annelida, Siboglinidae) in the Barents Sea in the Context of Its Oil and Gas Potential. J. Mar. Sci. Eng. 2021, 9, 1339. [Google Scholar] [CrossRef]
- Flügel, H.J. A new species of Siboglinum (Pogonophora) from the North Atlantic and notes on Nereilinummurmanicum Ivanov. Sarsia 1990, 75, 233–241. [Google Scholar] [CrossRef]
- Ivanov, A.V. A new representative of the class Pogonophora. Zool. J. 1949, 28, 79–84. (In Russian) [Google Scholar]
- Ivanov, A.V. Neue Pogonophora aus dem nordwestlichen Teil des StillenOzeans. Zool. Jahrbücher Abt. Für Syst. Okol. Und Geogr. Tiere 1957, 85, 431–500. [Google Scholar]
- Ivanov, A.V. Pogonofory (Pogonophora); Fauna SSSR. 75; Pavovsky, E.N., Ed.; ANSSSR: Moscow, Russia; Leningrad, Russia, 1960. [Google Scholar]
- Little, C. A note on salinity tolerance in Siboglinumekmani (Pogonophora). Sarsia 1969, 38, 87–90. [Google Scholar] [CrossRef]
- Southward, E.C. Pogonophora of the northwest Atlantic: Nova Scotia to Florida. Smithson. Contrib. Zool. 1971, 88, 1–29. [Google Scholar] [CrossRef]
- Flugel, H.J.; Langhof, I. Pogonophora in the Skagerrak. Sarsia 1982, 67, 211–212. [Google Scholar] [CrossRef]
- Cunha, M.R.; Paterson, G.L.J.; Amaro, T.; Blackbird, S.; de Stigter, H.C.; Ferreira, C.; Glover, A.; Hilário, A.; Kiriakoulakis, K.; Neal, L.; et al. Biodiversity of macrofaunal assemblages from three Portuguese submarine canyons (NE Atlantic). Deep. Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 2433–2447. [Google Scholar] [CrossRef]
- Southward, E.C.; Southward, A.J. On some Pogonophora from the north-east Atlantic, including two new species. J. Mar. Biol. Assoc. United Kingd. 1958, 37, 627–632. [Google Scholar] [CrossRef]
- Dando, P.; Southward, A.; Southward, E.; Lamont, P.; Harvey, R. Interactions between sediment chemistry and frenulatepogonophores (Annelida) in the north-east Atlantic. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 966–996. [Google Scholar] [CrossRef]
- Jägersten, G. Investigatons on Siboglinumekmani, N. Sp., Encountered in Skagerak: With Some General Remarks on the Group Pogonophora. Zool. Bijdr. 1956, 31, 211–252. [Google Scholar]
- Webb, M. Notes on the distribution of Pogonophora in Norwegian fjords. Sarsia 1965, 18, 11–15. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Sergienko, V.; Lobkovsky, L.; Yusupov, V.; Salyuk, A.; Salomatin, A.; Chernykh, D.; Kosmach, D.; Panteleev, G. The East Siberian Arctic Shelf: Towards further assessment of permafrost-related methane fluxes and role of sea ice. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140451. [Google Scholar] [CrossRef]
- Lazar, C.S.; Dinasquet, J.; Pignet, P.; Prieur, D.; Toffin, L. Active Archaeal Communities at Cold Seep Sediments Populated by Siboglinidae Tubeworms from the Storegga Slide. Microb. Ecol. 2010, 60, 516–527. [Google Scholar] [CrossRef]
- Pedersen, R.B.; Rapp, H.T.; Thorseth, I.H.; Lilley, M.D.; Barriga, F.J.; Baumberger, T.; Flesland, K.; Fonseca, R.; Früh-Green, G.L.; Jorgensen, S.L. Discovery of a black smoker vent field and vent fauna at the Arctic Mid-Ocean Ridge. Nat. Commun. 2010, 1, 126. [Google Scholar] [CrossRef] [PubMed]
- Southward, E.C. New Pogonophora from the northeast Pacific Ocean. Can. J. Zool. 1969, 47, 395–403. [Google Scholar] [CrossRef]
- Grønlie, O. Contributions to the Quaternary Geology of Novaya Zemlya. Report of the Scientific Results of the Norwegian Expedition to Novaya Zemlya 1921, Vol. 21; AW BroggersBoktrykkeri: Oslo, Norway, 1924. [Google Scholar]
- Panov, D. Geological structure of the Barents Sea in connection with the morphology of its coasts. Sci. Notes Mosc. State Univ. Ser Geogr. 1940, 48, 75–112. (In Russian) [Google Scholar]
- Matishov, G.G. Bottom geomorphology and the problem of Pleistocene glaciation of the Barents Sea shelf. Geomorphology 1977, 2, 91–98. (In Russian) [Google Scholar]
- Dobrovolsky, A.; Zalogin, B. Seas of the USSR; Publishing House of Moscow State University: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- Stoupakova, A.V. Structure and oil and gas content of the Barents-Kara shelf and adjacent territories. Geol. Oil Gas 2011, 6, 99–115. (In Russian) [Google Scholar]
- Trofimuk, A.; Makogon Yu Tolkachev, M. Gas hydrate deposits are a new reserve of energy resources. Geol. Oil Gas 1981, 10, 15–22. (In Russian) [Google Scholar]
- Trofimuk, A.; Chersky, N.; Tsarev, V. Gas hydrates are new sources of hydrocarbons. Priroda 1979, 1, 18–27. (In Russian) [Google Scholar]
- Trofimuk, A.; Chersky, N.; Tsarev, V. Peculiarities of natural gas accumulation in the hydrate formation zones of the World Ocean. Doklady AN SSSR 1973, 212, 931–934. (In Russian) [Google Scholar]
- Chersky, N.V.; Tsarev, V.P.; Nikitin, S.P. Research and Forecasting of Conditions for the Accumulation of Gas Resources in Gas Hydrate Deposits; Yakut Branch of the Siberian Branch of the USSR Academy of Sciences: Yakutsk, Russia, 1983. (In Russian) [Google Scholar]
- Kvenvolden, K.A. Potential effects of gas hydrate on human welfare. Proc. Natl. Acad. Sci. USA 1999, 96, 3420–3426. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Natural gas hydrate occurrence and issues. Ann. N. Y. Acad. Sci. 1994, 715, 232–246. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Ginsburg, G.; Soloviev, V. Worldwide distribution of subaquatic gas hydrates. Geo-Mar. Lett. 1993, 13, 32–40. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Methane hydrate—A major reservoir of carbon in the shallow geosphere? Chem. Geol. 1988, 71, 41–51. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Barnard, L.A. Hydrates of Natural Gas in Continental Margins: Environmental Processes: Model Investigations of Margin Environmental and Tectonic Processes. In Studies in Continental Margin Geology; AAPG: Tulsa, OK, USA, 1982. [Google Scholar]
- Ginsburg, G.; Solovyov, V. Submarine Gas Hydrates; VNII Okeanologia: Moscow, Russia, 1994. (In Russian) [Google Scholar]
- Ginsburg, G.; Gramberg, I.; Solovyov, V. Geology of Submarine Gas Hydrates. Sov. Geologiya. 1990, 11, 12–19. (In Russian) [Google Scholar]
- Ginsburg, G.; Ivanov, V.; Solovyov, V. Hydrates of Natural Gas in the Bowels of the World Ocean. Oil and Gas Content of the World Ocean; PGO “Sevmorgeologiya”: Leningrad, Russia, 1984; pp. 141–158. (In Russian) [Google Scholar]
- Cherskiy, N.; Tsarev, V.; Nikitin, S. Investigation and Prediction of Conditions of Accumulation of Gas Resources in Gas-Hydrate Pools (East Siberian Craton). Pet. Geol. Dig. Russ. Lit. Pet. Geol. 1984, 21, 71–76. [Google Scholar]
- Dillon, W.P. Gas hydrates in the ocean environment. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 473–486. [Google Scholar]
- Makagon, Y. Natural gas hydrates: Distribution, models, resources. Ross. Khimicheskij Zhurnal 2003, 47, 70–79. (In Russian) [Google Scholar]
- Klauda, J.B.; Sandler, S.I. Global distribution of methane hydrate in ocean sediment. Energy Fuels 2005, 19, 459–470. [Google Scholar] [CrossRef]
- Wallmann, K.; Pinero, E.; Burwicz, E.; Haeckel, M.; Hensen, C.; Dale, A.; Ruepke, L. The global inventory of methane hydrate in marine sediments: A theoretical approach. Energies 2012, 5, 2449–2498. [Google Scholar] [CrossRef]
- Pinero, E.; Marquardt, M.; Hensen, C.; Haeckel, M.; Wallmann, K. Estimation of the global inventory of methane hydrates in marine sediments using transfer functions. Biogeosciences 2013, 10, 959–975. [Google Scholar] [CrossRef]
- Liu, L.; Ryu, B.; Sun, Z.; Wu, N.; Cao, H.; Geng, W.; Zhang, X.; Jia, Y.; Xu, C.; Guo, L. Monitoring and research on environmental impacts related to marine natural gas hydrates: Review and future perspective. J. Nat. Gas Sci. Eng. 2019, 65, 82–107. [Google Scholar] [CrossRef]
- Gaidukova, O.; Misyura, S.; Strizhak, P. Key Areas of Gas Hydrates Study. Energies 2022, 15, 1799. [Google Scholar] [CrossRef]
- Solovyov, V.; Ginsburg, G.; Telepnev, E.; Mikhalyuk, Y.L. Cryogeothermy and Natural Gas Hydrates in the Depths of the Arctic Ocean; PGO “Sevmorgeologiya”: Leningrad, Russia, 1987. (In Russian) [Google Scholar]
- Solovyov, V.A.; Ginzburg, G.D. Arctic Seas of Russia. Conditions of gas-hydradic potential and potentially gas-hydrate-bearing water areas. In Geology and Mineral Resources of Russian Shelves Atlas; Nauchny Mir: Moscow, Russia, 2003; pp. 1–32. (In Russian) [Google Scholar]
- Collet, T.S.; Dallimore, S.R. Permafrost Associated Gas Hydrate. In Natural Gas Hydrate in Oceanic and Permafrost Environments; Max, M.D., Ed.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2003; pp. 43–58. [Google Scholar]
- Romanovskii, N.; Eliseeva, A.; Gavrilov, A.; Tipenko, G.; Hubberten, H.-W. The long-term dynamics of the permafrost and gas hydrate stability zone on rifts of the east Siberian arctic shelf (Report 1). Kriosf. Zemli 2005, 9, 42–53. (In Russian) [Google Scholar]
- Shakhova, N.; Semiletov, I.; Panteleev, G. The distribution of methane on the Siberian Arctic shelves: Implications for the marine methane cycle. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Shakhova, N.; Sergienko, V.; Semiletov, I. The contribution of the East Siberian shelf to the modern methane cycle. Bull. Russ. Acad. Sci. 2009, 79, 507–518. (In Russian) [Google Scholar] [CrossRef]
- Khimenkov, A.N.; Koshurnikov, A.V.; Stanilovskaya, Y.V. Geosystems of gas-saturated permafrost. Arct. Antarct. 2020, 2, 65–105. (In Russian) [Google Scholar] [CrossRef]
- Ananiev, V. Russia’s “hands do not reach” to the Arctic shelf. Oil Gas J. 2010, 5, 1–38. (In Russian) [Google Scholar]
- Kaminsky, V.D.; Chernykh, A.A.; Medvedeva TYuSuprunenko, O.I.; Suvorova, E.B. The Kara Sea is a promising testing ground for the study and development of hydrocarbon resources. Neft. Offshore 2020, 101, 82–89. (In Russian) [Google Scholar]
- Harms, I.; Hübner, U.; Backhaus, J.; Kulakov, M.; Stanovoy, V.; Stepanets, O.; Kodina, L.; Schlitzer, R. Salt intrusions in Siberian river estuaries: Observations and model experiments in Ob and Yenisei. In Siberian River Run-Off in the Kara Sea: Characterisation, Quantification, Variability, and Environmental Significance, Proceedings in Marine Science; 2003; pp. 27–45. Available online: https://core.ac.uk/reader/11750361 (accessed on 29 October 2022).
- Dolgopolova, E.N. Regularities in the motion of water and sediments at the mouth of a river of estuarine-deltaic type: Case study of the Yenisei River. Water Resour. 2015, 42, 198–207. [Google Scholar] [CrossRef]
- Gebhardt, A.C.; Schoster, F.; Gaye-Haake, B.; Beeskow, B.; Rachold, V.; Unger, D.; Ittekkot, V. The turbidity maximum zone of the Yenisei River (Siberia) and its impact on organic and inorganic proxies. Estuar. Coast. Shelf Sci. 2005, 65, 61–73. [Google Scholar] [CrossRef]
- Shakhova, N.E.; Semiletov, I.P.; Belcheva, N.N. Great Siberian rivers as sources of methane on the Arctic shelf. Dokl. Earth Sci. 2007, 415, 734–736. [Google Scholar] [CrossRef]
- Guo, L.; Semiletov, I.; Gustafsson, Ö.; Ingri, J.; Andersson, P.; Dudarev, O.; White, D. Characterization of Siberian Arctic coastal sediments: Implications for terrestrial organic carbon export. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Leifer, I.; Salyuk, A.; Rekant, P.; Kosmach, D. Geochemical and geophysical evidence of methane release over the East Siberian Arctic Shelf. J. Geophys. Res. Ocean. 2010, 115. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Salyuk, A.; Yusupov, V.; Kosmach, D.; Gustafsson, Ö. Extensive methane venting to the atmosphere from sediments of the East Siberian Arctic Shelf. Science 2010, 327, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Shakhova, N.; Alekseev, V.; Semiletov, I. Forecast of methane emissions on the East Siberian shelf. Rep. Acad. Sci. 2010, 430, 533–536. (In Russian) [Google Scholar]
- Sergienko, V.; Lobkovskiy, L.; Semiletov, I.; Dudarev, O.; Dmitrevskiy, N.; Shakhova, N.; Romanovskiy, N.; Kosmach, D.; Nikolskiy, D.; Nikiforov, S. Underwater permafrost degradation and destruction of hydrates of the shelf of the Eastern Arctic seas as a possible cause of “methane catastrophe”: Some results comprehensive research in 2011. Dokl. Akad. Nauk. 2012, 445, 330–335. (In Russian) [Google Scholar]
- Bussmann, I. Distribution of methane in the Lena Delta and Buor Khaya Bay, Russia. Biogeosciences 2013, 10, 4641–4652. [Google Scholar] [CrossRef]
- Anisimov, O.A.; Zaboykina, Y.u.G.; Kokorev, V.A.; Yurganov, L.N. Possible causes of methane emission on the shelf of the Eastern Arctic seas. Ice Snow 2014, 54, 69–81. (In Russian) [Google Scholar]
- Ruppel, C.D.; Kessler, J.D. The interaction of climate change and methane hydrates. Rev. Geophys. 2017, 55, 126–168. [Google Scholar] [CrossRef]
- Southward, E.C. A new species of Galathealinum (Pogonophora) from the Canadian Arctic. Can. J. Zool. 1962, 40, 385–389. [Google Scholar] [CrossRef]
- Macdonald, R.W.; Yu, Y. The Mackenzie Estuary of the Arctic Ocean. In Estuaries; Wangersky, P.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 91–120. [Google Scholar]
- Majorowicz, J.; Osadetz, K.; Safanda, J. Gas Hydrate Formation and Dissipation Histories in the Northern Margin of Canada: Beaufort-Mackenzie and the Sverdrup Basins. J. Geophys. Res. 2012, 2012, 17. [Google Scholar] [CrossRef]
- Majorowicz, J.; Safanda, J.; Osadetz, K. Inferred gas hydrate and permafrost stability history models linked to climate change in the Beaufort-Mackenzie Basin, Arctic Canada. Clim. Past 2012, 8, 667–682. [Google Scholar] [CrossRef]
- Osadetz, K.G.; Morrell, G.R.; Dixon, J.; Dietrich, J.R.; Snowdon, L.R.; Dallimore, S.R.; Majorowicz, J.A. Beaufort Sea–Mackenzie Delta basin: A review of conventional and nonconventional (gas hydrate) petroleum reserves and undiscovered resources. Geol. Surv. Can. 2005, 585, 1–19. [Google Scholar]
- Majorowicz, J.A.; Hannigan, P.K. Natural Gas Hydrates in the Offshore Beaufort–Mackenzie Basin—Study of a Feasible Energy Source II. Nat. Resour. Res. 2000, 9, 201–214. [Google Scholar] [CrossRef]
- Bily, C.; Dick, J.W.L. Naturally Occurring Gas Hydrates in the Mackenzie Delta, N.W.T.1. Bull. Can. Pet. Geol. 1974, 22, 340–352. [Google Scholar] [CrossRef]
- Majorowicz, J.; Osadetz, K. Gas hydrate distribution and volume in Canada. AAPG Bull. 2001, 85, 1211–1230. [Google Scholar] [CrossRef]
- McLellan, P.; Gillen, K.; Podetz, C.; Dallimore, S.; Inoue, T.; Hancock, S. In situ stresses in the Mallik area. Bull. Geol. Surv. Can. 2005, 585, 133. [Google Scholar]
- Bellefleur, G.; Riedel, M.; Brent, T.; Wright, F.; Dallimore, S.R. Implication of seismic attenuation for gas hydrate resource characterization, Mallik, Mackenzie Delta, Canada. J. Geophys. Res. Solid Earth 2007, 112, B10311. [Google Scholar] [CrossRef]
- Majorowicz, J.; Osadetz, K.; Safanda, J. Onset and Stability of Gas Hydrates under Permafrost in an Environment of Surface Climatic Change in the Beaufort-Mackenzie basin-Past and Future. In AGU Fall Meeting Abstracts, U34A-02, Proceedings of the 6th International Conference on Gas Hydrates: Vancouver, BC, Canada, 6–10 July 2008; Available online: https://ui.adsabs.harvard.edu/abs/2008AGUFM.U34A..02M/abstract (accessed on 28 October 2022).
- Osadetz, K.G.; Chen, Z. A re-evaluation of Beaufort Sea-Mackenzie Delta basin gas hydrate resource potential: Petroleum system approaches to non-conventional gas resource appraisal and geologically-sourced methane flux. Bull. Can. Pet. Geol. 2010, 58, 56–71. [Google Scholar] [CrossRef]
- Schauer, U.; Loeng, H.; Rudels, B.; Ozhigin, V.K.; Dieck, W. Atlantic water flow through the Barents and Kara Seas. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 2281–2298. [Google Scholar] [CrossRef]
- Lien, V.S.; Trofimov, A.G. Formation of Barents Sea branch water in the north-eastern Barents Sea. Polar Res. 2013, 32, 18905. [Google Scholar] [CrossRef]
- Dmitrenko, I.A.; Rudels, B.; Kirillov, S.A.; Aksenov, Y.O.; Lien, V.S.; Ivanov, V.V.; Schauer, U.; Polyakov, I.V.; Coward, A.; Barber, D.G. Atlantic water flow into the Arctic Ocean through the St. Anna Trough in the northern Kara Sea. J. Geophys. Res. Ocean. 2015, 120, 5158–5178. [Google Scholar] [CrossRef]
- Osadchiev, A.; Viting, K.; Frey, D.; Demeshko, D.; Dzhamalova, A.; Nurlibaeva, A.; Gordey, A.; Krechik, V.; Spivak, E.; Semiletov, I. Structure and Circulation of Atlantic Water Masses in the St. Anna Trough in the Kara Sea. Front. Mar. Sci. 2022, 9, 915674. [Google Scholar] [CrossRef]
- Reagan, M.T.; Moridis, G.J.; Elliott, S.M.; Maltrud, M. Contribution of oceanic gas hydrate dissociation to the formation of Arctic Ocean methane plumes. J. Geophys. Res. Ocean. 2011, 116. [Google Scholar] [CrossRef]
- Giustiniani, M.; Tinivella, U.; Jakobsson, M.; Rebesco, M. Arctic ocean gas hydrate stability in a changing climate. J. Geol. Res. 2013, 2013, 783969. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Chuvilin, E. Understanding the Permafrost–Hydrate System and Associated Methane Releases in the East Siberian Arctic Shelf. Geosciences 2019, 9, 251. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Gustafsson, O.; Sergienko, V.; Lobkovsky, L.; Dudarev, O.; Tumskoy, V.; Grigoriev, M.; Mazurov, A.; Salyuk, A.; et al. Current rates and mechanisms of subsea permafrost degradation in the East Siberian Arctic Shelf. Nat. Commun. 2017, 8, 15872. [Google Scholar] [CrossRef]
- Baranov, B.; Galkin, S.; Vedenin, A.; Dozorova, K.; Gebruk, A.; Flint, M. Methane seeps on the outer shelf of the Laptev Sea: Characteristic features, structural control, and benthic fauna. Geo-Mar. Lett. 2020, 40, 541–557. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I. Characteristic features of carbon cycle in the shallow shelf of the eastern sector of Russian Arctic. Environ. Clim. Changes Catastr. 2008, 4, 167–181. [Google Scholar]
- Sirenko, B.; Denisenko, S.; Deubel, H.; Rachor, E. Deep water communities of the Laptev Sea and adjacent parts of the Arctic Ocean. In Fauna and Ecosystems of the Laptev Sea and Adjacent Deep Waters of the Arctic Part I St-Petersburg: ZIN RAS; 2004; Volume 54, pp. 28–73. Available online: https://www.researchgate.net/publication/303284538_Deep_Water_Communities_of_the_Laptev_Sea_and_Adjacent_Parts_of_the_Arctic_Ocean (accessed on 28 October 2022). (In Russian)
- Vedenin, A.; Galkin, S.; Mironov, A.N.; Gebruk, A. Vertical zonation of the Siberian Arctic benthos: Bathymetric boundaries from coastal shoals to deep-sea Central Arctic. PeerJ 2021, 9, e11640. [Google Scholar] [CrossRef]
- Anderson, B.; Hancock, S.; Wilson, S.; Enger, C.; Collett, T.; Boswell, R.; Hunter, R. Formation pressure testing at the Mount Elbert Gas Hydrate Stratigraphic Test Well, Alaska North Slope: Operational summary, history matching, and interpretations. Mar. Pet. Geol. 2011, 28, 478–492. [Google Scholar] [CrossRef]
- Ambrosimov, A.K. Methane seeps and hydrophysical anomalies of the East Siberian Sea as a response to climate change. Ecol. Syst. Devices 2020, 48–53. (In Russian) [Google Scholar] [CrossRef]
- Anisimov, O. Potential feedback of thawing permafrost to the global climate system through methane emission. Environ. Res. Lett. 2007, 2, 045016. [Google Scholar] [CrossRef]
- Nekhaev, I.O. Skenea profunda (Vetigastropoda: Skeneidae) in the central Arctic. Ruthenica 2022, 32, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Krol, E.N.; Nekhaev, I.O. Redescription of Leptogyra bujnitzkii (Gorbunov, 1946) comb. nov., the first representative of the gastropod subclass Neomphaliones from the high Arctic. Zootaxa 2020, 4759, 446–450. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).