Molecular and Phytochemical Variability of Endemic Juniperus sabina var. balkanensis from Its Natural Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Essential Oil Isolation
2.3. GC-FID and GC/MS Analysis
2.4. DNA Extraction
2.5. Chloroplast Sequence Analysis
2.6. ISSR Analysis
2.7. Environmental Data
2.8. Statistical Analyses
3. Results
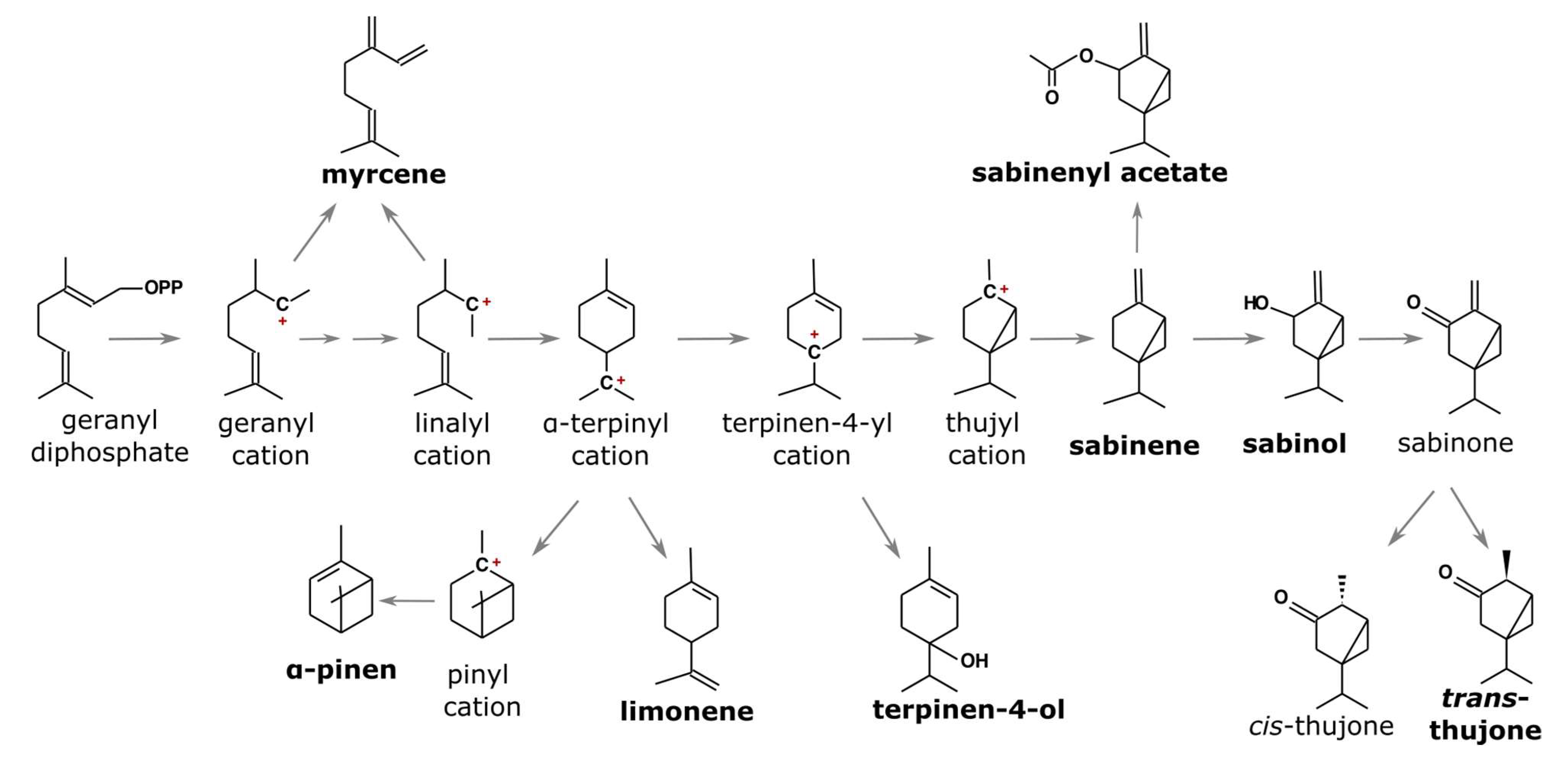
3.1. Essential Oil Composition
3.2. Essential Oil Variability
3.3. Genetic Variability
3.4. Environmental Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WFO. Juniperus sabina L. Published on the Internet. Available online: http://www.worldfloraonline.org/taxon/wfo-0000354994 (accessed on 21 November 2022).
- Lu, D.; Huang, H.; Wang, A.; Zhang, G. Genetic Evaluation of Juniperus sabina L. (Cupressaceae) in Arid and Semi-Arid Regions of China Based on SSR Markers. Forests 2022, 13, 231. [Google Scholar] [CrossRef]
- Adams, R.P.; Schwarzbach, A.E. Chloroplast capture in Juniperus sabina var. balkanensis RP Adams and AN Tashev, from the Balkan peninsula: A new variety with a history of hybridization with J. thurifera. Phytologia 2016, 98, 100–111. [Google Scholar]
- Farhat, P.; Siljak-Yakovlev, S.; Adams, R.P.; Bou Dagher Kharrat, M.; Robert, T. Genome size variation and polyploidy in the geographical range of Juniperus sabina L. (Cupressaceae). Bot. Lett. 2019, 166, 134–143. [Google Scholar] [CrossRef]
- Adams, R.P.; Farhat, P.; Shuka, L.; Siljak-Yakovlev, S. Discovery of Juniperus sabina var. balkanensis RP Adams and AN Tashev in Albania and relictual polymorphisms found in nrDNA. Phytologia 2018, 100, 187–194. [Google Scholar]
- Adams, R.P.; Boratynski, A.; Marcysiak, K.; Roma-Marzio, F.; Peruzzi, L.; Bartolucci, F.; Conti, F.; Mataraci, T.; Schwarzbach, A.E. Discovery of Juniperus sabina var. balkanensis RP Adams and AN Tashev in Macedonia, Bosnia-Herzegovina, Croatia and Central and Southern Italy and relictual polymorphisms found in nrDNA. Phytologia 2018, 100, 117–127. [Google Scholar]
- Adams, R.P.; Boratynski, A.; Mataraci, T.; Tashev, A.N.; Schwarzbach, A.E. Discovery of Juniperus sabina var. balkanensis R. P. Adams and A. N. Tashev in western Turkey (Anatolia). Phytologia 2017, 99, 22–31. [Google Scholar]
- Semerdjieva, I.B.; Shiwakoti, S.; Cantrell, C.L.; Zheljazkov, V.D.; Astatkie, T.; Schlegel, V.; Radoukova, T. Hydrodistillation Extraction Kinetics Regression Models for Essential Oil Yield and Composition in Juniperus virginiana, J. excelsa, and J. sabina. Molecules 2019, 24, 986. [Google Scholar] [CrossRef] [Green Version]
- Zheljazkov, V.D.; Cantrell, C.L.; Semerdjieva, I.; Radoukova, T.; Stoyanova, A.; Maneva, V.; Kačániová, M.; Astatkie, T.; Borisova, D.; Dincheva, I.; et al. Essential Oil Composition and Bioactivity of Two Juniper Species from Bulgaria and Slovakia. Molecules 2021, 26, 3659. [Google Scholar] [CrossRef]
- Farhat, P.; Siljak-Yakovlev, S.; Valentin, N.; Fabregat, C.; Lopez-Udias, S.; Salazar-Mendias, C.; Altarejos, J.; Adams, R.P. Gene flow between diploid and tetraploid junipers—Two contrasting evolutionary pathways in two Juniperus populations. BMC Evol. Biol. 2020, 20, 148. [Google Scholar] [CrossRef]
- Farhat, P.; Takvorian, N.; Avramidou, M.; Garraud, L.; Adams, R.P.; Siljak-Yakovlev, S.; Kharrat, M.B.D.; Robert, T. First evidence for allotriploid hybrids between Juniperus thurifera and J. sabina in a sympatric area in the French Alps. Ann. For. Sci. 2020, 77, 93. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Soliman, G.A.; Alqarni, M.H.; Hamad, A.M.; Foudah, A.I.; Alqasoumi, S.I. Chemical composition and protective effect of Juniperus sabina L. essential oil against CCl4 induced hepatotoxicity. Saudi Pharm. J. 2019, 27, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Khani, A.; Rashid, B.; Mirshekar, A. Chemical composition and insecticidal efficacy of Juniperus polycarpus and Juniperus sabina essential oils against Tribolium confusum (Coleoptera: Tenebrionidae). Int. J. Food Prop. 2017, 20, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Asgary, S.; Sahebkar, A.; Naderi, G.A.; Ardekani, M.R.S.; Kasher, T.; Aslani, S.; Airin, A.; Emami, S.A. Essential oils from the fruits and leaves of Juniperus sabina possess inhibitory activity against protein glycation and oxidative stress: An in vitro phytochemical investigation. J. Essent. Oil Res. 2013, 25, 70–77. [Google Scholar] [CrossRef]
- Doosti, F.; Bagherpasand, N.; Zolfagharian, F.; Sarabandi, S.; Emami, S.; Khayyat, H. Investigation of antioxidant activity of the essential oils of different parts of Juniperus sabina (Cupressaceae) by TBARS method in comparison with vitamin E. Res. Pharm. Sci. 2012, 7, 798. [Google Scholar]
- Asili, J.; Emami, S.A.; Rahimizadeh, M.; Fazly-Bazzaz, B.S.; Hassanzadeh, M.K. Chemical and Antimicrobial Studies of Juniperus sabina L. and Juniperus foetidissima Willd. Essential Oils. J. Essent. Oil Bear. Plants 2010, 13, 25–36. [Google Scholar] [CrossRef]
- Emami, S.A.; Shahidi, N.H.; Hassanzadeh-Khayyat, M. Antioxidant activity of the essential oils of different parts of Juniperus sabina L. and Juniperus foetidissima Willd (Cupressaceae). Int. J. Essent. Oil Ther. 2009, 3, 163–170. [Google Scholar]
- Rajčević, N.; Dodoš, T.; Novaković, J.; Kuzmanović, N.; Janaćković, P.; Marin, P. Are Environmental Factors Responsible for Essential Oil Chemotype Distribution of Balkan Juniperus communis var. saxatilis Populations? Plant Biosyst. 2022, 1–19. [Google Scholar] [CrossRef]
- Rajčević, N.; Dodoš, T.; Novaković, J.; Boršić, I.; Janaćković, P.; Marin, P.D. Differentiation of North-Western Balkan Juniperus communis L. (Cupressaceae) populations—Ecological and chemophenetic implications. J. Essent. Oil Res. 2020, 32, 562–570. [Google Scholar] [CrossRef]
- Rajčević, N.F.; Labus, M.G.; Dodoš, T.Z.; Novaković, J.J.; Marin, P.D. Juniperus phoenicea var. turbinata (Guss.) Parl. Leaf Essential Oil Variability in the Balkans. Chem. Biodivers. 2018, 15, e1800208. [Google Scholar]
- Rajčević, N.; Janaćković, P.; Dodoš, T.; Tešević, V.; Marin, P.D. Essential-Oil Variability of Juniperus deltoides RP Adams along the East Adriatic Coast–How Many Chemotypes Are There? Chem. Biodivers. 2015, 12, 82–95. [Google Scholar] [CrossRef]
- Rajčević, N.; Janaćković, P.; Bojović, S.; Tešević, V.; Marin, P.D. Variability of the Needle Essential Oils of Juniperus deltoides RP Adams from Different Populations in Serbia and Croatia. Chem. Biodivers. 2013, 10, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.L.; Croteau, R. Monoterpene Biosynthesis. In Comprehensive Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 1999; pp. 97–153. ISBN 978-0-08-091283-7. [Google Scholar]
- Kshatriya, K. Thujone Biosynthesis in Western Redcedar (Thuja plicata). Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 2017. [Google Scholar]
- Wise, M.L.; Croteau, R. 2.05 Monoterpene Biosynthesis. In Comprehensive Natural Products Chemistry; Elsevier Science: Amsterdam, The Netherlands, 1999; pp. 57–93. ISBN 978-0-08-091283-7. [Google Scholar]
- Usman, L.A.; Oguntoye, O.S.; Ismaeel, R.O. Phytochemical Profile, Antioxidant and Antidiabetic Potential of Essential Oil From Fresh and Dried Leaves of Eucalyptus globulus. J. Chil. Chem. Soc. 2022, 67, 5453–5461. [Google Scholar] [CrossRef]
- Sabreena; Nazir, M.; Mahajan, R.; Hashim, M.J.; Iqbal, J.; Alyemeni, M.N.; Ganai, B.A.; Zargar, S.M. Deciphering allelic variability and population structure in buckwheat: An analogy between the efficiency of ISSR and SSR markers. Saudi J. Biol. Sci. 2021, 28, 6050–6056. [Google Scholar] [CrossRef] [PubMed]
- Boogar, A.R.; Salehi, H. ISSR-based genetic diversity assessment of five populations of Juniperus polycarpos K. Koch in southern habitats of Iran. Flower Ornam. Plants 2021, 5, 139–150. [Google Scholar]
- Hadian, J.; Raeisi, S.; Azizi, A.; Pezhmanmehr, M.; Sarkhosh, A. Genetic diversity of natural populations of medicinally valuable plant Satureja khuzistanica Jamzad based on ISSR markers. Braz. J. Bot. 2017, 40, 771–781. [Google Scholar] [CrossRef]
- Meloni, M.; Perini, D.; Filigheddu, R.; Binelli, G. Genetic variation in five Mediterranean populations of Juniperus phoenicea as revealed by inter-simple sequence repeat (ISSR) markers. Ann. Bot. 2006, 97, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.P. Junipers of the World: The Genus Juniperus; Trafford Publishing: Bloomington, IN, USA, 2011; ISBN 1-4269-5382-8. [Google Scholar]
- Barkworth, M.E.; Adams, R.P. Juniperus. In Flora of North America: Volume 2: Pteridophytes and Gymnosperms; Morin, N.R., Ed.; Oxford University Press: New York, NY, USA, 1993; Volume 2, pp. 412–420. ISBN 0-19-508242-7. [Google Scholar]
- Chaintreau, A. Simultaneous distillation–extraction: From birth to maturity—Review. Flavour Fragr. J. 2001, 16, 136–148. [Google Scholar] [CrossRef]
- Adams, R.P.; Schwarzbach, A.E. Taxonomy of Juniperus deppeana varieties and formas based on nrDNA (ITS), petN-psbM, trnS-trnG, trnD-trnT, trnL-trnF sequences. Phytologia 2013, 95, 161–166. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Asch, K. IGME 5000: 1: 5 Million International Geological Map of Europe and Adjacent Areas; BGR: Hannover, Germany, 2005. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001, 4, 9. [Google Scholar]
- Roldan-Ruiz, I.; Dendauw, J.; Van Bockstaele, E.; Depicker, A.; De Loose, M. AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol. Breed. 2000, 6, 125–134. [Google Scholar] [CrossRef]
- Meirmans, P.G. Genodive version 3.0: Easy-to-use software for the analysis of genetic data of diploids and polyploids. Mol. Ecol. Resour. 2020, 20, 1126–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronfort, J.; Jenczewski, E.; Bataillon, T.; Rousset, F. Analysis of population structure in autotetraploid species. Genetics 1998, 150, 921–930. [Google Scholar] [CrossRef]
- Meirmans, P.G.; Liu, S. Analysis of molecular variance (AMOVA) for autopolyploids. Front. Ecol. Evol. 2018, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Rudloff, E.V. Gas-liquid chromatography of terpenes: Part IX. The volatile oil of the leaves of Juniperus sabina L. Can. J. Chem. 1963, 41, 2876–2881. [Google Scholar] [CrossRef]
- Adams, R.P.; Nguyen, S.; Liu, J. Geographic Variation in the Leaf Essential Oils of Juniperus sabina L. and J. sabina var. arenaria (E.H. Wilson) Farjon. J. Essent. Oil Res. 2006, 18, 497–502. [Google Scholar] [CrossRef]
- Adams, R.P. Systematics of multi-seeded eastern hemisphere Juniperus based on leaf essential oils and RAPD DNA fingerprinting. Biochem. Syst. Ecol. 1999, 27, 709–725. [Google Scholar] [CrossRef]
- Adams, R.P.; Dembitsky, A.D.; Shatar, S. The Leaf Essential Oils and Taxonomy of Juniperus centrasiatica Kom., J. jarkendensis Kom., J. pseudosabina Fisch., Mey. & Ave-Lall., J. sabina L. and J. turkestanica Kom. from Central Asia. J. Essent. Oil Res. 1998, 10, 489–496. [Google Scholar]
- Adams, R.P.; Schwarzbach, A.E. The multi-seeded, entire leaf taxa of Juniperus, section Sabina: Inclusion of Juniperus microsperma. Phytologia 2013, 95, 118–121. [Google Scholar]
- Chatzopoulou, P.S.; Katsiotis, S.T. Chemical Investigation of Juniperus communis L. J. Essent. Oil Res. 1993, 5, 603–607. [Google Scholar] [CrossRef]
- Caramiello, R.; Bocco, A.; Buffa, G.; Maffei, M. Chemotaxonomy of Juniperus communis, J. sibirica and J. intermedia. J. Essent. Oil Res. 1995, 7, 133–145. [Google Scholar] [CrossRef]
- Markó, G.; Novák, I.; Bernáth, J.; Altbäcker, V. Both Gas Chromatography and an Electronic Nose Reflect Chemical Polymorphism of Juniper Shrubs Browsed or Avoided by Sheep. J. Chem. Ecol. 2011, 37, 705–713. [Google Scholar] [CrossRef]
- Ottavioli, J.; Bighelli, A.; Casanova, J.; Tomi, F. Composition and Chemical Variability of Needle and Berry Oils from Corsican Juniperus communis var. communis. Nat. Prod. Commun. 2018, 13, 1043–1046. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.P. The serrate leaf margined Juniperus (Section Sabina) of the western hemisphere: Systematics and evolution based on leaf essential oils and Random Amplified Polymorphic DNAs (RAPDs). Biochem. Syst. Ecol. 2000, 28, 975–989. [Google Scholar] [CrossRef]
- Bettencourt, S.X.; Mendonça, D.; Lopes, M.S.; Rocha, S.; Monjardino, P.; Monteiro, L.; da Câmara Machado, A. Genetic diversity and population structure of the endemic Azorean juniper, Juniperus brevifolia (Seub.) Antoine, inferred from SSRs and ISSR markers. Biochem. Syst. Ecol. 2015, 59, 314–324. [Google Scholar] [CrossRef]
- Kim, E.-H.; Shin, J.-K.; Jeong, K.-S.; Lee, C.-S.; Chung, J.-M. Genetic variation and structure of Juniperus chinensis L. (Cupressaceae) in Korea. J. Ecol. Environ. 2018, 42, 14. [Google Scholar] [CrossRef] [Green Version]
- Saeed, S.; Barozai, M.Y.K.; Ahmed, A.; Tareen, R.B.; Ali, S.B.G.M. Impact of ecological diversity on genetic and phytochemical variation in Juniperus excelsa from high elevation zones of Quetta valley, Pakistan. Pak. J. Bot. 2017, 49, 201–206. [Google Scholar]
- Reim, S.; Lochschmidt, F.; Proft, A.; Tröber, U.; Wolf, H. Genetic structure and diversity in Juniperus communis populations in Saxony, Germany. Biodivers. Res. Conserv. 2016, 42, 9–18. [Google Scholar] [CrossRef] [Green Version]
- García, C.; Guichoux, E.; Hampe, A. A comparative analysis between SNPs and SSRs to investigate genetic variation in a juniper species (Juniperus phoenicea ssp. turbinata). Tree Genet. Genomes 2018, 14, 87. [Google Scholar] [CrossRef]
- Teixeira, H.; Rodríguez-Echeverría, S.; Nabais, C. Genetic Diversity and Differentiation of Juniperus thurifera in Spain and Morocco as Determined by SSR. PLoS ONE 2014, 9, e88996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobierajska, K.; Boratyńska, K.; Jasińska, A.; Dering, M.; Ok, T.; Douaihy, B.; Bou Dagher-Kharrat, M.; Romo, Á.; Boratyński, A. Effect of the Aegean Sea barrier between Europe and Asia on differentiation in Juniperus drupacea (Cupressaceae). Bot. J. Linn. Soc. 2016, 180, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Taib, A.; Morsli, A.; Chojnacka, A.; Walas, Ł.; Sękiewicz, K.; Boratyński, A.; Romo, À.; Dering, M. Patterns of genetic diversity in North Africa: Moroccan-Algerian genetic split in Juniperus thurifera subsp. africana. Sci. Rep. 2020, 10, 4810. [Google Scholar] [CrossRef] [Green Version]
- Rumeu, B.; Sosa, P.A.; Nogales, M.; González-Pérez, M.A. Development and characterization of 13 SSR markers for an endangered insular juniper (Juniperus cedrus Webb & Berth.). Conserv. Genet. Resour. 2013, 5, 457–459. [Google Scholar]
- Rumeu, B.; Vargas, P.; Jaén-Molina, R.; Nogales, M.; Caujapé-Castells, J. Phylogeography and genetic structure of the threatened Canarian Juniperus cedrus (Cupressaceae): Phylogeography of Macaronesian juniper. Bot. J. Linn. Soc. 2014, 175, 376–394. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, J.F.; Sánchez-Gómez, P.; Cánovas, J.L.; Hensen, I.; Aouissat, M. Influence of natural habitat fragmentation on the genetic structure of Canarian populations of Juniperus turbinata. Silva Fenn. 2017, 51, 1678. [Google Scholar] [CrossRef]
- Juan, A.; Fay, M.F.; Pastor, J.; Juan, R.; Fernández, I.; Crespo, M.B. Genetic structure and phylogeography in Juniperus oxycedrus subsp. macrocarpa around the Mediterranean and Atlantic coasts of the Iberian Peninsula, based on AFLP and plastid markers. Eur. J. For. Res. 2012, 131, 845–856. [Google Scholar] [CrossRef]
- Dzialuk, A.; Mazur, M.; Boratyńska, K.; Montserrat, J.M.; Romo, A.; Boratyński, A. Population genetic structure of Juniperus phoenicea (Cupressaceae) in the western Mediterranean Basin: Gradient of diversity on a broad geographical scale. Ann. For. Sci. 2011, 68, 1341–1350. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk, I.M.; Opgenoorth, L.; Luecke, Y.; Huck, S.; Ziegenhagen, B. Genetic support for perglacial survival of Juniperus communis L. in Central Europe. Holocene 2010, 20, 887–894. [Google Scholar] [CrossRef]
- Adams, R.P.; Schwarzbach, A.E.; Pandey, R.N. The concordance of terpenoid, ISSR and RAPD markers, and ITS sequence data sets among genotypes: An example from Juniperus. Biochem. Syst. Ecol. 2003, 31, 375–387. [Google Scholar] [CrossRef]
- Curto, M.; Nogueira, M.; Beja, P.; Amorim, F.; Schümann, M.; Meimberg, H. Influence of past agricultural fragmentation to the genetic structure of Juniperus oxycedrus in a Mediterranean landscape. Tree Genet. Genomes 2015, 11, 32. [Google Scholar] [CrossRef]

 —Central Balkan,
—Central Balkan,  —Resava gorge,
—Resava gorge,  —Mt. Bistra,
—Mt. Bistra,  —Mt. Korab,
—Mt. Korab,  —Mt. Prokletije,
—Mt. Prokletije,  —Mt. Biokovo,
—Mt. Biokovo,  —Mt. Velebit. Principle components given in the left-hand side corner: S—Sabinene, SA—trans-Sabinyl acetate, So—trans-Sabinol, T—terpinen-4-ol, A—Abieta-7,13-dien-3-one.
—Mt. Velebit. Principle components given in the left-hand side corner: S—Sabinene, SA—trans-Sabinyl acetate, So—trans-Sabinol, T—terpinen-4-ol, A—Abieta-7,13-dien-3-one.
 —Central Balkan,
—Central Balkan,  —Resava gorge,
—Resava gorge,  —Mt. Bistra,
—Mt. Bistra,  —Mt. Korab,
—Mt. Korab,  —Mt. Prokletije,
—Mt. Prokletije,  —Mt. Biokovo,
—Mt. Biokovo,  —Mt. Velebit. Principle components given in the left-hand side corner: S—Sabinene, SA—trans-Sabinyl acetate, So—trans-Sabinol, T—terpinen-4-ol, A—Abieta-7,13-dien-3-one.
—Mt. Velebit. Principle components given in the left-hand side corner: S—Sabinene, SA—trans-Sabinyl acetate, So—trans-Sabinol, T—terpinen-4-ol, A—Abieta-7,13-dien-3-one.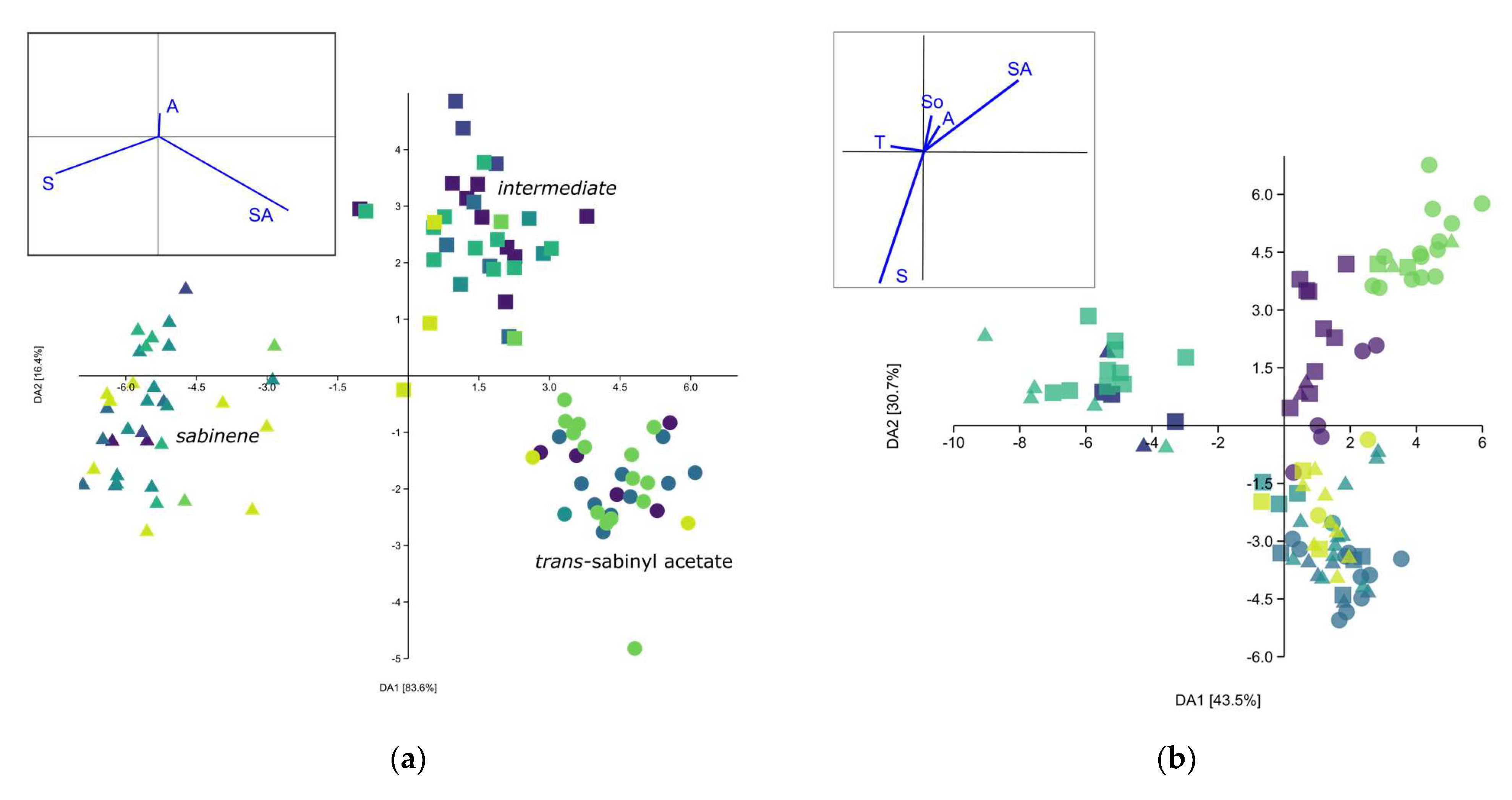
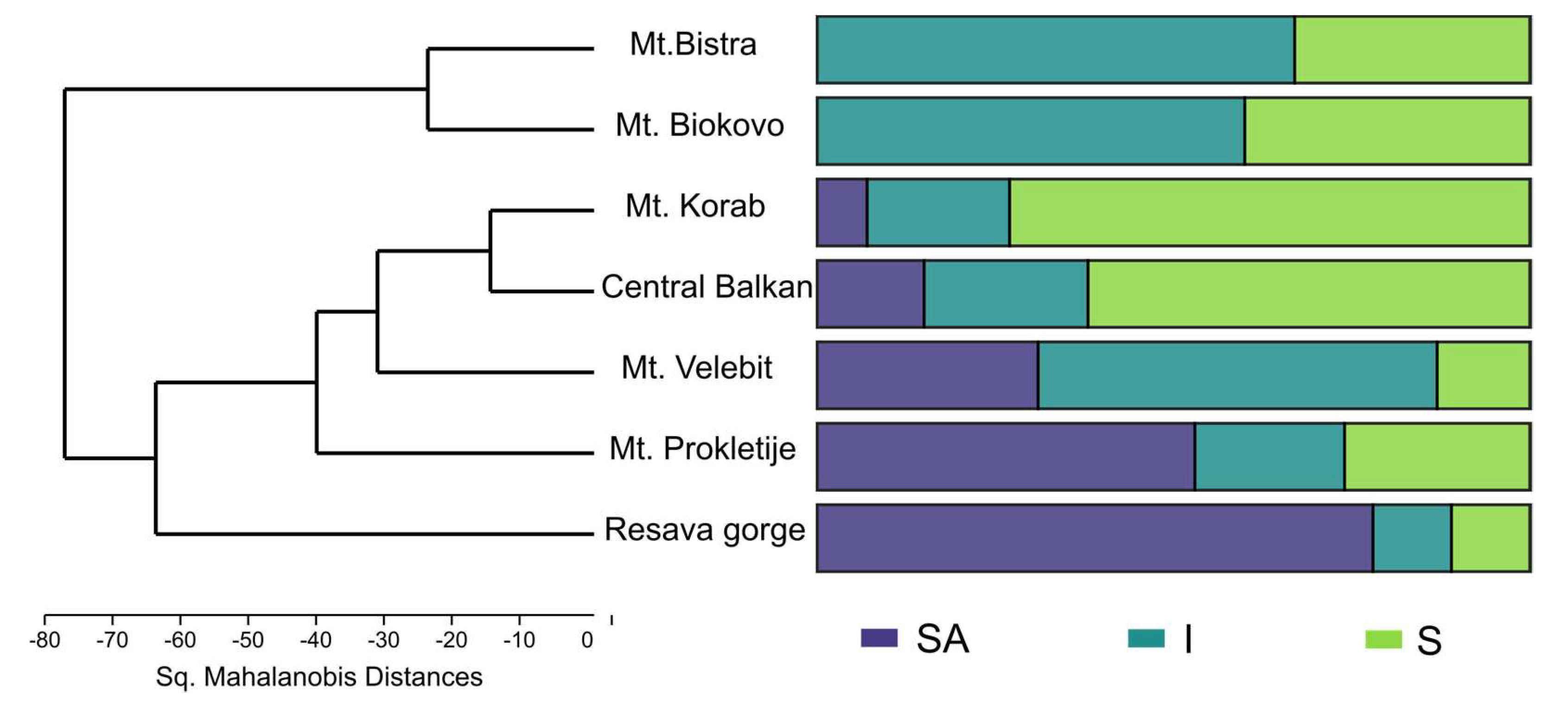
 —Central Balkan,
—Central Balkan,  —Resava gorge,
—Resava gorge,  —Mt. Bistra,
—Mt. Bistra,  —Mt. Korab,
—Mt. Korab,  —Mt. Prokletije,
—Mt. Prokletije,  —Mt. Biokovo,
—Mt. Biokovo,  —Mt. Velebit; triangles —sabinene chemotype, squares—intermediate chemotype, dots—trans-sabinyl acetate chemotype; (b) NJ tree, Nei’s genetic distances.
—Mt. Velebit; triangles —sabinene chemotype, squares—intermediate chemotype, dots—trans-sabinyl acetate chemotype; (b) NJ tree, Nei’s genetic distances.
 —Central Balkan,
—Central Balkan,  —Resava gorge,
—Resava gorge,  —Mt. Bistra,
—Mt. Bistra,  —Mt. Korab,
—Mt. Korab,  —Mt. Prokletije,
—Mt. Prokletije,  —Mt. Biokovo,
—Mt. Biokovo,  —Mt. Velebit; triangles —sabinene chemotype, squares—intermediate chemotype, dots—trans-sabinyl acetate chemotype; (b) NJ tree, Nei’s genetic distances.
—Mt. Velebit; triangles —sabinene chemotype, squares—intermediate chemotype, dots—trans-sabinyl acetate chemotype; (b) NJ tree, Nei’s genetic distances.
| Location | Country | Longitude | Latitude | Altitude | Incl. 1 | Exp. 2 | S 3 | BEOU |
|---|---|---|---|---|---|---|---|---|
| Mt. Velebit | Croatia | 44.245 | 15.810 | 1350 | 35 | SW | Sa | 17213 |
| Mt. Biokovo | Croatia | 43.299 | 17.077 | 700 | 30 | E | Li | 17094 |
| Mt. Prokletije | Montenegro | 42.510 | 19.834 | 1300 | 15 | S | Li | 17409 |
| Mt. Korab | Albania | 41.786 | 20.546 | 1250 | 45 | W | Li | 17837 |
| Mt. Bistra | North Macedonia | 41.594 | 20.665 | 1450 | 60 | S | Si | 17838 |
| Resava gorge | Serbia | 44.085 | 21.681 | 720 | 65 | SW | Li | 17209 |
| Central Balkan | Bulgaria | 42.688 | 25.123 | 1350 | 25 | SE | Sa | 17403 |
| SA | I | S | ||||
|---|---|---|---|---|---|---|
| Compound | F | p | n = 32 | n = 34 | n = 35 | |
| 1 | α-Pinene | 10.38 | *** | 1.2 ± 0.6 a | 1.7 ± 1.0 a | 2.1 ± 0.8 |
| 2 | Sabinene | 180.6 | *** | 5.6 ± 5.4 | 11.0 ± 7.0 | 37.4 ± 9.2 |
| 3 | Myrcene | 19.6 | *** | 1.2 ± 1.1 a | 1.7 ± 1.2 a | 2.8 ± 0.9 |
| 4 | Limonene | 8.8 | *** | 0.6 ± 0.5 | 1.4 ± 1.2 a | 1.3 ± 0.8 a |
| 5 | cis-Thujone | 18.6 | *** | 1.5 ± 1.6 | 0.7 ± 0.5 | 0.1 ± 0.1 |
| 6 | trans-Thujone | 26.81 | *** | 0.9 ± 0.5 a | 1.1 ± 0.9 a | 0.1 ± 0.2 |
| 7 | trans-Sabinol | 19.52 | *** | 4.0 ± 3.7 a | 3.1 ± 2.6 a | 0.3 ± 0.4 |
| 8 | Terpinen-4-ol | 11.6 | *** | 0.8 ± 0.3 | 2.6 ± 2.3 a | 3.6 ± 3.4 a |
| 9 | Citronellol | 6.5 | *** | 0.5 ± 0.7 a | 0.5 ± 0.6 a | 1.0 ± 0.8 |
| 10 | Methyl citronellate | 3.1 | *** | 1.5 ± 1.6 a | 2.0 ± 1.7 ab | 2.5 ± 1.4 b |
| 11 | Bornyl acetate | 6.9 | *** | 0.2 ± 0.9 a | 2.4 ± 3.9 | 0.5 ± 1.9 a |
| 12 | trans-Sabinyl acetate | 383.4 | *** | 45.6 ± 6.7 | 24.6 ± 8.1 | 1.8 ± 4.0 |
| 13 | Germacrene D | 4.4 | *** | 0.8 ± 0.6 ab | 0.6 ± 0.3 a | 1.1 ± 0.8 b |
| 14 | γ-Cadinene | 6.177 | *** | 0.4 ± 0.3 b | 1.2 ± 1.2 a | 0.8 ± 0.9 ab |
| 15 | Elemol | 6.5 | *** | 1.5 ± 1.6 | 0.5 ± 0.6 a | 0.7 ± 0.9 a |
| 16 | Germacrene B | 4.6 | *** | 0.4 ± 0.6 a | 0.6 ± 0.6 ab | 0.9 ± 0.9 b |
| 17 | Germacrene D-4-ol | 6.1 | *** | 4.1 ± 2.2 a | 3.9 ± 2.9 a | 5.9 ± 2.5 |
| 18 | β-Oplopenone | 2.051 | - | 0.9 ± 0.5 a | 0.7 ± 0.4 a | 0.9 ± 0.4 a |
| 19 | α-Cadinol | 11.4 | *** | 2.7 ± 1.0 a | 2.8 ± 1.2 a | 3.8 ± 0.8 |
| 20 | Abietadiene | 3.5 | *** | 1.0 ± 0.5 a | 1.3 ± 0.7 ab | 1.5 ± 1.2 b |
| 21 | Abieta-7,13-dien-3-one | 3.6 | *** | 4.4 ± 3.6 b | 7.0 ± 5.7 a | 4.7 ± 3.4 ab |
| LT 1 | Position in Alignment | GenBank Accession Numbers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ind. | Chemotype | 7 | 148 | 460 | 466..483 | 664 | 709 | 752 | 761 | ||
| V1 | SA | 795 | G | C | G | + 2 | G | - | G | A | OP297053 |
| B1 | S | 802 | G | C | G | + | G | - | G | A | OP297042 |
| B4 | I | 802 | G | C | G | + | G | - | G | A | OP297043 |
| P10 | SA | 798 | G | C | G | + | G | - | G | A | OP297049 |
| P4 | I | 800 | G | C | G | + | G | - | G | A | OP297050 |
| K3 | S | 802 | G | C | G | + | G | - | G | A | OP297046 |
| K8 | SA | 802 | G | C | G | + | G | - | G | A | OP297047 |
| M1 | - | 799 | G | C | G | + | G | - | G | A | OP297048 |
| R1 | S | 802 | G | C | G | + | G | - | G | A | OP297051 |
| R7 | SA | 798 | G | C | G | + | G | - | G | A | OP297052 |
| C1 | S | 797 | G | C | G | + | G | - | G | A | OP297044 |
| C8 | SA | 802 | G | C | G | + | G | - | G | A | OP297045 |
| var. balkanensis 3 | - | G | C | G | + | G | - | G | A | MT136665.1 | |
| var. sabina 3 | - | A | T | T | - | A | A | A | T | MT136634.1 | |
| Name | Primer Sequence | Tm [°C] | TB | PB | MB | %PB | PIC |
|---|---|---|---|---|---|---|---|
| JS 17 | 5′-TCC TCC TCC TCC TCC TCC-3′ | 61.3 | 6 | 6 | 0 | 100 | 0.334 |
| JS 01 | 5′-GAG AGA GAG AGA GAG AC-3′ | 49 | 4 | 3 | 1 | 75 | 0.195 |
| JS 16 | 5′-AGA GAG AGA GAG AGA GCC-3′ | 53.8 | 6 | 6 | 0 | 100 | 0.227 |
| JS 03 | 5′-CAC ACA CAC ACA CAC AG-3′ | 53 | 3 | 2 | 1 | 66.7 | 0.311 |
| JS 04 | 5′-AGC AGC AGC AGC GT-3′ | 54.9 | 5 | 5 | 0 | 100 | 0.298 |
| Total | 24 | 22 | 2 | 88.3 | 0.273 |
| Source | d.f. * | S.s. | VC | TV (%) | p |
|---|---|---|---|---|---|
| Within population | 14 | 36.67 | 2.62 | 72.8 | |
| Among populations | 4 | 19.28 | 0.73 | 20.4 | 0.001 |
| Among regions | 2 | 13.01 | 0.25 | 6.8 | 0.004 |
| Taxon 1 | Origin | Sex | Dominant Compounds (%) | Ref. |
|---|---|---|---|---|
| J. sabina | Canada | - | trans-Sabinyl acetate (38.0), sabinene (30.5), myrcene/cadinene (4.5) | [46] |
| Spain | - | Sabinene (38.1), trans-sabynil acetate (16.2), terpinen-4-ol (6.9) | [47] | |
| - | Sabinene (54.9), limonene (2.4), terpinen-4-ol (7.2) | [47] | ||
| Switzerland | - | trans-Sabinyl acetate (35.0), sabinene (34.8), limonene (3.0) | [47] | |
| Slovakia 2 | m | Sabinene (24.5), myrtenyl acetate (20.8), elemol (12.2) | [9] | |
| Bulgaria 2 | m | Sabinene (28.2), methyl eugenol (13.5), terpinen-4-ol (12.4) | [9] | |
| f | Myrtenyl acetate (23.0), sabinene (16.7), elemol (13.2) | [9] | ||
| m | Sabinene (31.0), elemol (13.7), terpinen-4-ol (13.6) | [9] | ||
| Iran | m | trans-Sabinyl acetate (46.2), sabinene (24.3), δ-cadinene (5.5) | [14] | |
| m | trans-Sabinyl acetate (30.3), sabinene (26.7), δ-cadinene (6.9) | [14] | ||
| m | Sabinene (21.5), α-pinene (14.7), γ-terpinene (6.8) | [16] | ||
| f | Sabinene (24.3), 4-terpineol (8.1), myrcene (5.4) | [16] | ||
| Kazakhstan | - | Sabinene (42.6), trans-sabynil acetate (15.9), α-pinene (15.8) | [47] | |
| - | Sabinene (36.8), α-pinene (17.2), cedrol (15.2) | [48] | ||
| Mongolia | - | Sabinene (46.5), trans-sabynil acetate (15.9), cedrol (13.2) | [47] | |
| - | Sabinene (50.0), trans-sabinyl acetate (18.3), terpinen-4-ol (3.1) | [47] | ||
| - | Sabinene (56.7), terpinen-4-ol (4.7), methyl citronellate (3.1) | [47] | ||
| China | - | Sabinene (39.0), trans-sabynil acetate (17.5), cedrol (15.8) | [49] | |
| - | Sabinene (46.5), trans-sabinyl acetate (15.9), cedrol (13.2) | [50] | ||
| var. arenaria | China | - | sabinene (57.1), α-pinene (3.8), germacrene D-4-ol (3.5) | [47] |
| var. erectopatens | China | - | Sabinene (33.3), unknown (13.2), elemol (3.7) | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajčević, N.; Dodoš, T.; Janković, S.; Janaćković, P.; Zheljazkov, V.D.; Marin, P.D. Molecular and Phytochemical Variability of Endemic Juniperus sabina var. balkanensis from Its Natural Range. Diversity 2022, 14, 1062. https://doi.org/10.3390/d14121062
Rajčević N, Dodoš T, Janković S, Janaćković P, Zheljazkov VD, Marin PD. Molecular and Phytochemical Variability of Endemic Juniperus sabina var. balkanensis from Its Natural Range. Diversity. 2022; 14(12):1062. https://doi.org/10.3390/d14121062
Chicago/Turabian StyleRajčević, Nemanja, Tanja Dodoš, Smiljana Janković, Pedja Janaćković, Valtcho D. Zheljazkov, and Petar D. Marin. 2022. "Molecular and Phytochemical Variability of Endemic Juniperus sabina var. balkanensis from Its Natural Range" Diversity 14, no. 12: 1062. https://doi.org/10.3390/d14121062
APA StyleRajčević, N., Dodoš, T., Janković, S., Janaćković, P., Zheljazkov, V. D., & Marin, P. D. (2022). Molecular and Phytochemical Variability of Endemic Juniperus sabina var. balkanensis from Its Natural Range. Diversity, 14(12), 1062. https://doi.org/10.3390/d14121062










