Habitat Suitability of Eastern Sarus Crane (Antigone Antigone sharpii) in Ayeyarwady Delta, the Union of Myanmar
Abstract
1. Introduction
2. Materials and Methods
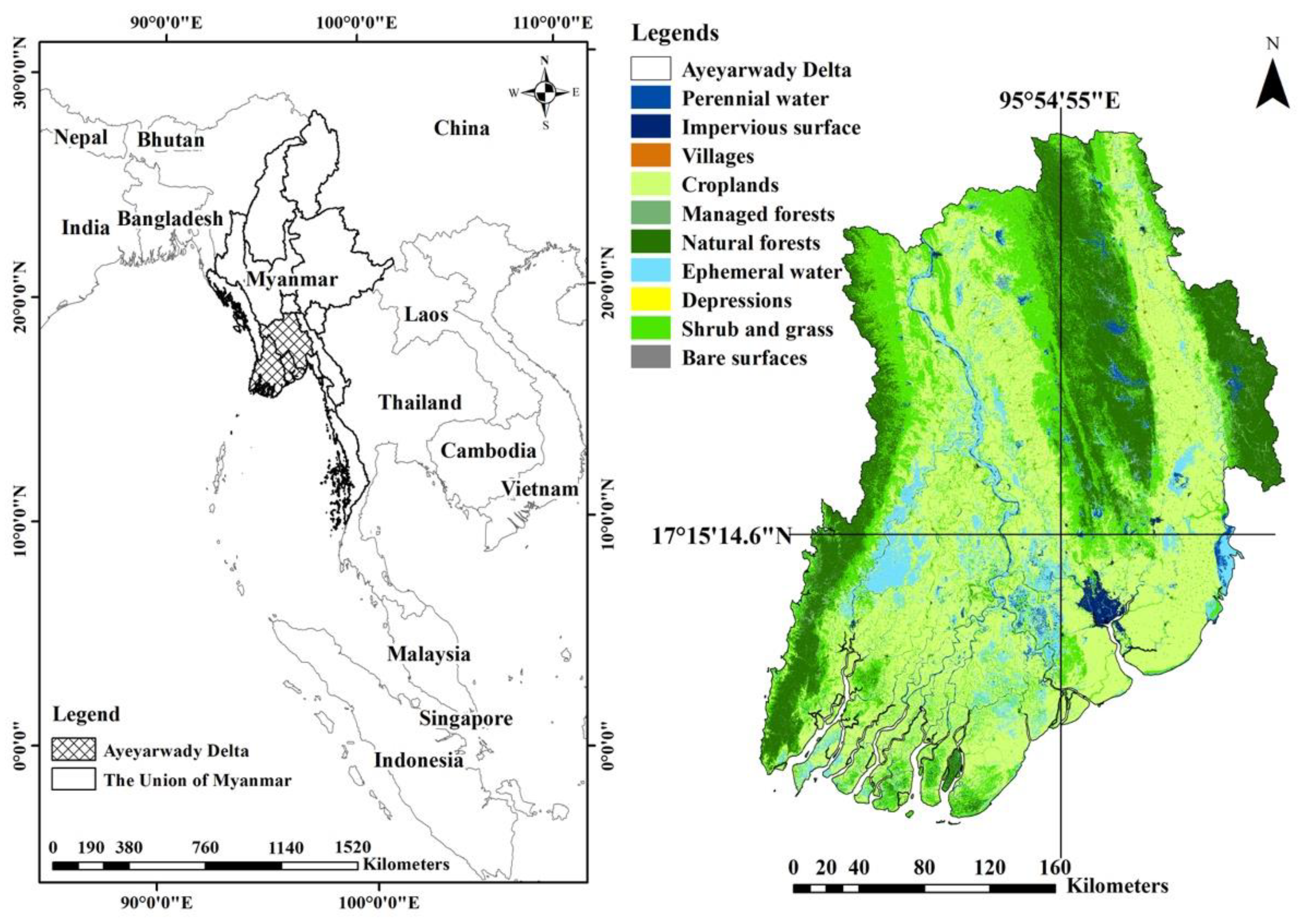
2.1. Study Area
2.2. Data Collection
2.3. Land Use and Land Cover Identification
2.4. Environmental Variables and Species Occurrence Data
2.5. Eastern Sarus Crane Suitable Habitat
2.6. Model Performance
2.7. Population Home Range
3. Results
3.1. Eastern Sarus Crane Habitat Suitability
3.2. Habitat Use Area
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Wang, X.; Li, J.; Kang, D. Integrating livestock grazing and integrating livestock grazing and Sympatric takin to evaluate the habitat suitability of Giant Panda in the Wanglang Nature Reserve. Animals 2021, 11, 2469. [Google Scholar] [CrossRef]
- Wei, F.; Nie, Y.; Miao, H.; Lu, H.; Hu, H. Advancements of the researches on biodiversity loss mechanisms. Chin. Sci. Bull. 2014, 59, 430–437. [Google Scholar] [CrossRef]
- Månsson, J.; Nilsson, L.; Hake, M. Territory size and habitat selection of breeding common cranes (Grus grus) in a boreal landscape. Ornis. Fennica. 2013, 90, 65–72. [Google Scholar]
- Pimm, S.L. Climate disruption and biodiversity. Curr. Biol. 2009, 19, R595–R601. [Google Scholar] [CrossRef]
- Ab Lah, N.Z.; Yusop, Z.; Hashim, M.; Mohd Salim, J.; Numata, S. Predicting the Habitat Suitability of Melaleuca cajuputi based on the MaxEnt Species Distribution Model. Forests 2021, 12, 1449. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.; Zhang, F.; Bempah, G.; Lu, C.; Lv, S.; Zhang, W.; Cui, P. Use of aquaculture ponds by globally endangered red crowned crane (Grus japonensis) during the wintering period in the Yancheng National Nature Reserve, a Ramsar wetland. Glob. Ecol. Conserv. 2020, 23, e01123. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, H.; Zhang, X.; Zhang, W.; Zhang, T.; Lu, C. Habitat changes in the most important stopover sites for the endangered red-crowned crane in China: A large-scale study. Environ. Sci. Pollut. Res. 2021, 28, 54719–54727. [Google Scholar] [CrossRef] [PubMed]
- BirdLife International. Species Factsheet: Antigone antigone. Available online: http://www.birdlife.org (accessed on 11 February 2022).
- Nevard, T.D.; Haase, M.; Archibald, G.; Leiper, I.; van Zalinge, R.N.; Purchkoon, N.; Siriaroonrat, B.; Latt, T.N.; Wink, M.; Garnett, S.T. Subspecies in the Sarus Crane Antigone antigone revisited; with particular reference to the Australian population. PLoS ONE 2020, 15, e0230150. [Google Scholar] [CrossRef]
- Tanee, T.; Chaveerach, A.; Anuniwat, A.; Tanomtong, A.; Pinthong, K.; Sudmoon, R.; Mokkamul, P. Molecular analysis for genetic diversity and distance of introduced Grus antigone sharpii L. to Thailand. Pakistan J. Biol. Sci. 2009, 12, 163–167. [Google Scholar] [CrossRef]
- Insee, J.; Kamolnorranath, S.; Baicharoen, S.; Chumpadang, S.; Sawasu, W.; Wajjwalku, W. PCR—Based method for sex identification of eastern sarus crane (Grus antigone sharpii): Implications for reintroduction programmes in Thailand. Zool. Sci. 2014, 31, 95–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- GBIF Secretariat. GBIF Backbone Taxonomy. Available online: https://www.gbif.org/species/5284517 (accessed on 11 February 2020).
- Ellis, D.H.; Gee, G.F.; Mirande, C.M. (Eds.) Cranes: Their Biology, Husbandry, and Conservation; Hancock House Pub Ltd.: Blaine, DC, USA, 1996. [Google Scholar]
- Meine, C. Summary Report on a Survey of Breeding Sarus Crane (Grus antigone) in Myanmar; The Myanmar Forestry Department and the Wildlife Conservation Society-Myanmar: Yangon, Myanmar, 1999. [Google Scholar]
- Archibald, G.; Sundar, K.; Barzen, J. A review of the three subspecies of sarus cranes Grus antigone. J. Ecol. Soc. 2003, 16, 5–15. [Google Scholar]
- Meine, C.; Archibald, G. The Cranes: Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland, 1996. [Google Scholar]
- Khosravi, R.; Hemami, M.R.; Malekian, M.; Flint, A.; Flint, L. Maxent modeling for predicting potential distribution of goitered gazelle in central Iran: The effect of extent and grain size on performance of the model. Turk. J. Zool. 2016, 40, 574–585. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Method Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Van Driel, W.F.; Alterra, T.N. Vulnerability and Resilience Assessment of the Ayeyarwady Delta in Myanmar: Full Assessment Phase. Delta Alliance Report no. 10, Bay of Bengal Large Marine Ecosystem (BOBLME) Project, Global Water Partnership (GWP) and Delta Alliance, Delft-Wageningen, USA, 2020. Available online: http://www.delta-alliance.org (accessed on 11 February 2020).
- Winn, M.S.; Triet, T.; Yi, A.M.; Naing, T.; Aung, A.; Myint, T.S.; Khine, K.; Cho, M.K. Population, breeding status, and habitats utilization of sarus crane Grus antigone in Ayeyarwady Region. J. Myanmar Acad. Art. Sci. 2019, 17, 257–266. [Google Scholar]
- Myanmar Information Management Unit. Ayeyarwady Region, Yangon Region & Bago Region Profiles 2019. Available online: http://themimu.info/states_regions (accessed on 11 February 2020).
- Davis, J.H.; Weadock, V. The Forests of Burma; Department of Botany, University of Florida: Gainesville, FL, USA, 1960. [Google Scholar]
- Aye, S.S. A Geographic Study of Primary Economic Activities in Kangyidaunt Township. Master’s Thesis, Pathein University, Pathein, Myanmar, 2016. [Google Scholar]
- Ministry of Environmental Conservation and Forestry. Budget Year 2017–2018; Moeyungyi Wetland Wildlife Sanctuary Activity Report; Ministry of Environmental Conservation and Forestry: Moeyungyi, Myanmar, 2018.
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climat. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Sundar, K.G. Are rice paddies suboptimal breeding habitat for sarus cranes in Uttar Pradesh, India? Condor 2009, 111, 611–623. [Google Scholar]
- Anderson, J.R. A Land Use and Land Cover Classification System for Use with Remote Sensor Data; US Government Printing Office: Washington, DC, USA, 1976. [Google Scholar]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. (Eds.) A Maximum Entropy Approach to Species Distribution Modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, New York, NY, USA, 4–8 July 2004; pp. 655–662. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecograph 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend, P.A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeograph. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Kumar, S.; Stohlgren, T.J. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ. 2009, 1, 94–98. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Myanmar Information Management Unit. Townships Information. 2020. Available online: http://themimu.info/mimu-township-profiles-dashboard (accessed on 11 February 2020).
- USGS. DEM Data 2019. 2019. Available online: http://earthexplorer.usgs.gov (accessed on 11 February 2020).
- Hijmans, R.J. Cross-validation of species distribution models: Removing spatial sorting bias and calibration with a null model. Ecol. 2012, 93, 679–688. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeograph. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Young, N.; Carter, L.; Evangelista, P. A MaxEnt Model v 3. 3.3 e Tutorial (ArcGIS v10); Colorado State University: Fort Collins, CO, USA, 2011. [Google Scholar]
- Seaman, D.E.; Millspaugh, J.J.; Kernohan, B.J.; Brundige, G.C.; Raedeke, K.J.; Gitzen, R.A. Effects of sample size on kernel home range estimates. J. Wildl. Manage. 1999, 63, 739–747. [Google Scholar] [CrossRef]
- Van DerWal, J.; Shoo, L.P.; Graham, C.; Williams, S.E. Selecting pseudo-absence data for presence-only distribution modeling: How far should you stray from what you know? Ecol. Model. 2009, 220, 589–594. [Google Scholar] [CrossRef]
- Katuwal, H.B. Sarus crane in lowlands of Nepal: Is it declining really? J. Asia-Pac. Biodivers. 2016, 9, 259–262. [Google Scholar] [CrossRef]
- Shrestha, D. Distribution and habitat utilization of sarus crane (Grus antigone antigone Linnaeus, 1758) during dry season in Rupandehi district, Nepal. Master’s Thesis, Khwopa College, Bhaktapur, Nepal, 2015. [Google Scholar]
- Tomar, V.S.; Rout, S.; Choukesy, S. Current status of habitat and food resources use by Sarus crane (Grus antigone) in Faridpur tehsil under Bareilly District of Uttar Pradesh. J. Entomol. Zool. Stud. 2018, 5, 2018–2023. [Google Scholar]
- Wen, L.; Saintilan, N.; Yang, X.; Hunter, S.; Mawer, D. MODIS NDVI based metrics improve habitat suitability modelling in fragmented patchy floodplains. Remote Sens. Appl. Soc. Environ. 2015, 31, 85–97. [Google Scholar] [CrossRef]
- Torbick, N.; Chowdhury, D.; Salas, W.; Qi, J. Monitoring rice agriculture across Myanmar using time series Sentinel-1 assisted by Landsat-8 and PALSAR-2. Remote Sens. 2017, 9, 119. [Google Scholar] [CrossRef]
- Lwin, K.Z. Biostratigraphic Correlation of Apyauk Well and Nyaungdon Well, Myanmar. Master’s Thesis, East Yangon University, Yangon, Myanmar, 2017. [Google Scholar]
- Gaung, J.S. In Twante, Fish Farmers Yearn for Cleaner Water Supplies. Available online: https://www.mmtimes.com/business/5384-in-twante-fish-farmers-yearn-for-cleaner-water-supplies.html (accessed on 11 February 2020).
- Maneas, G.; Bousbouras, D.; Norrby, V.; Berg, H. Status and distribution of waterbirds in a natura 2000 area: The case of Gialova Lagoon, Messinia, Greece. Front. Ecol. Evol. 2020, 8, e501548. [Google Scholar] [CrossRef]
- Chaiyarat, R.; Youngpoy, N.; Kongsurakan, P.; Nakbun, S. Habitat preferences of reintroduced banteng (Bos javanicus) into the Salakphra Wildlife Sanctuary, Thailand. Wildl. Res. 2019, 46, 573–586. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Guo, Z.; Dai, L.; Wu, Y.; Liu, H.; Li, Y.; Chen, H.; Zhang, Y.; Zhao, Y.; et al. Integrating Maxent model and landscape ecology theory for studying spatiotemporal dynamics of habitat: Suggestions for conservation of endangered Red-crowned crane. Ecol. Indic. 2020, 116, e106472. [Google Scholar] [CrossRef]











| Habitat Suitability | Summer Season | Occurrence | Rainy Season | Occurrence | Winter Season | Occurrence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| km2 | % | Point | % | km2 | % | Point | % | km2 | % | Point | % | |
| MaxEnt | ||||||||||||
| High | 2155.8 | 2.62 | 28 | 85 | 1028.7 | 1.2 | 26 | 76 | 2450 | 3 | 24 | 80 |
| Moderate | 2275.4 | 2.8 | 5 | 15 | 2011.3 | 2.5 | 6 | 18 | 2513.5 | 3.1 | 5 | 17 |
| Low | 3627.1 | 4.4 | 0 | 0 | 3734.3 | 4.5 | 2 | 6 | 4788.4 | 5.8 | 1 | 3 |
| Unsuitable | 74,673.5 | 90.8 | 0 | 0 | 75,482.4 | 91.8 | 0 | 0 | 72,504.6 | 88.1 | 0 | 0 |
| 95% MCP | 7014.6 | N/A | N/A | N/A | 7892.9 | N/A | N/A | N/A | 7783.9 | N/A | N/A | N/A |
| KDE | ||||||||||||
| 100 | 28,867 | N/A | N/A | N/A | 3601.3 | N/A | N/A | N/A | 43,780.1 | N/A | N/A | N/A |
| 95 | 9761.9 | N/A | N/A | N/A | 1238.1 | N/A | N/A | N/A | 13,839.5 | N/A | N/A | N/A |
| 90 | 7710.9 | N/A | N/A | N/A | 953.4 | N/A | N/A | N/A | 10,774.1 | N/A | N/A | N/A |
| 50 | 2485.2 | N/A | N/A | N/A | 223.2 | N/A | N/A | N/A | 3274.8 | N/A | N/A | N/A |
| Model | Summer (Training Sample = 24, Test Sample 7, Background Point = 10,024) | Rainy (Training Sample = 26, Test Sample 8, Background Point = 10,026) | Winter (Training Sample = 21, Test Sample 7, Background Point = 10,021) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Training AUC | Test Gain | Test AUC | Training AUC | Test Gain | Test AUC | Training AUC | Test Gain | Test AUC | |
| 0 | 0.987 | 3.034 | 0.987 | 0.988 | 3.96 | 0.994 | 0.989 | 2.08 | 0.959 |
| 1 | 0.986 | 2.295 | 0.968 | 0.988 | 2.814 | 0.977 | 0.986 | 2.293 | 0.967 |
| 2 | 0.988 | 1.749 | 0.976 | 0.991 | 2.73 | 0.975 | 0.987 | 2.241 | 0.964 |
| 3 | 0.984 | 2.269 | 0.977 | 0.988 | 2.911 | 0.981 | 0.986 | 2.351 | 0.968 |
| 4 | 0.986 | 2.829 | 0.982 | 0.99 | 2.352 | 0.973 | 0.986 | 2.39 | 0.97 |
| 5 | 0.987 | 2.265 | 0.985 | 0.989 | 3.603 | 0.989 | 0.984 | 2.467 | 0.971 |
| 6 | 0.989 | 2.327 | 0.955 | 0.989 | 2.81 | 0.981 | 0.983 | 2.358 | 0.97 |
| 7 | 0.988 | 2.34 | 0.992 | 0.988 | 3.63 | 0.993 | 0.99 | 1.297 | 0.942 |
| 8 | 0.989 | 2.086 | 0.983 | 0.99 | 2.697 | 0.978 | 0.985 | 2.438 | 0.97 |
| 9 | 0.988 | 1.551 | 0.948 | 0.988 | 3.243 | 0.989 | 0.982 | 2.888 | 0.985 |
| 10 | 0.988 | 2.59 | 0.977 | 0.988 | 2.999 | 0.986 | 0.986 | 2.358 | 0.967 |
| 11 | 0.985 | 2.74 | 0.968 | 0.991 | 2.487 | 0.974 | 0.985 | 2.134 | 0.962 |
| 12 | 0.988 | 2.083 | 0.973 | 0.993 | 2.283 | 0.97 | 0.98 | 2.794 | 0.983 |
| 13 | 0.988 | 2.8 | 0.967 | 0.992 | 2.712 | 0.97 | 0.984 | 2.492 | 0.974 |
| 14 | 0.989 | 2.253 | 0.989 | 0.994 | 1.94 | 0.96 | 0.984 | 2.483 | 0.971 |
| Average | 0.987 | 2.348 | 0.975 | 0.99 | 2.878 | 0.979 | 0.985 | 2.337 | 0.968 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latt, T.N.; Chaiyarat, R.; Choowaew, S.; Thongtip, N.; Stewart, T.N. Habitat Suitability of Eastern Sarus Crane (Antigone Antigone sharpii) in Ayeyarwady Delta, the Union of Myanmar. Diversity 2022, 14, 1076. https://doi.org/10.3390/d14121076
Latt TN, Chaiyarat R, Choowaew S, Thongtip N, Stewart TN. Habitat Suitability of Eastern Sarus Crane (Antigone Antigone sharpii) in Ayeyarwady Delta, the Union of Myanmar. Diversity. 2022; 14(12):1076. https://doi.org/10.3390/d14121076
Chicago/Turabian StyleLatt, Tin Nwe, Rattanawat Chaiyarat, Sansanee Choowaew, Nikorn Thongtip, and Thomas Neal Stewart. 2022. "Habitat Suitability of Eastern Sarus Crane (Antigone Antigone sharpii) in Ayeyarwady Delta, the Union of Myanmar" Diversity 14, no. 12: 1076. https://doi.org/10.3390/d14121076
APA StyleLatt, T. N., Chaiyarat, R., Choowaew, S., Thongtip, N., & Stewart, T. N. (2022). Habitat Suitability of Eastern Sarus Crane (Antigone Antigone sharpii) in Ayeyarwady Delta, the Union of Myanmar. Diversity, 14(12), 1076. https://doi.org/10.3390/d14121076





