Agricultural Land-Use Increases Floral Species Richness in Tropical Dry Forest and Savannah Ecosystems in West Africa
Abstract
:1. Introduction
2. Materials and Methods
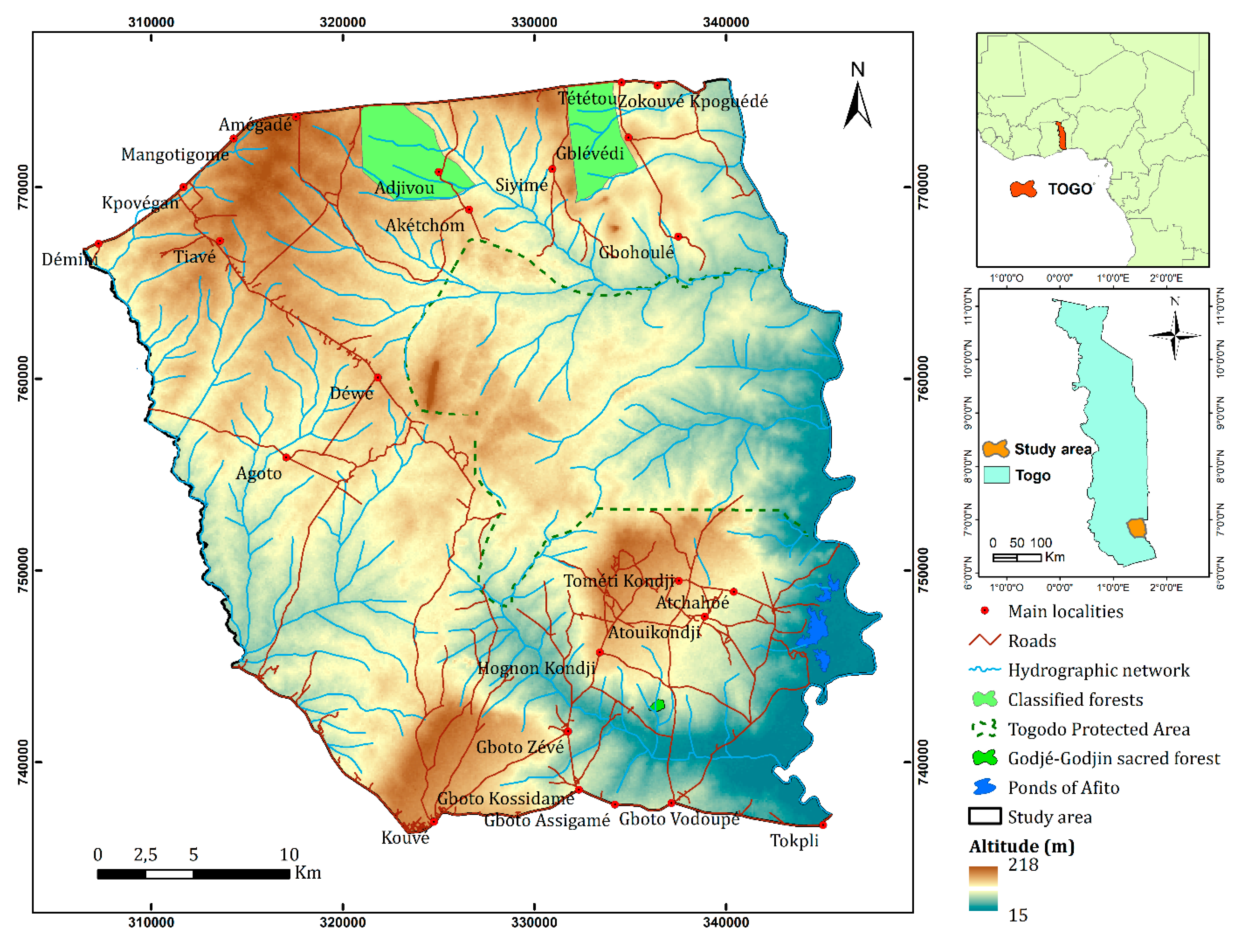
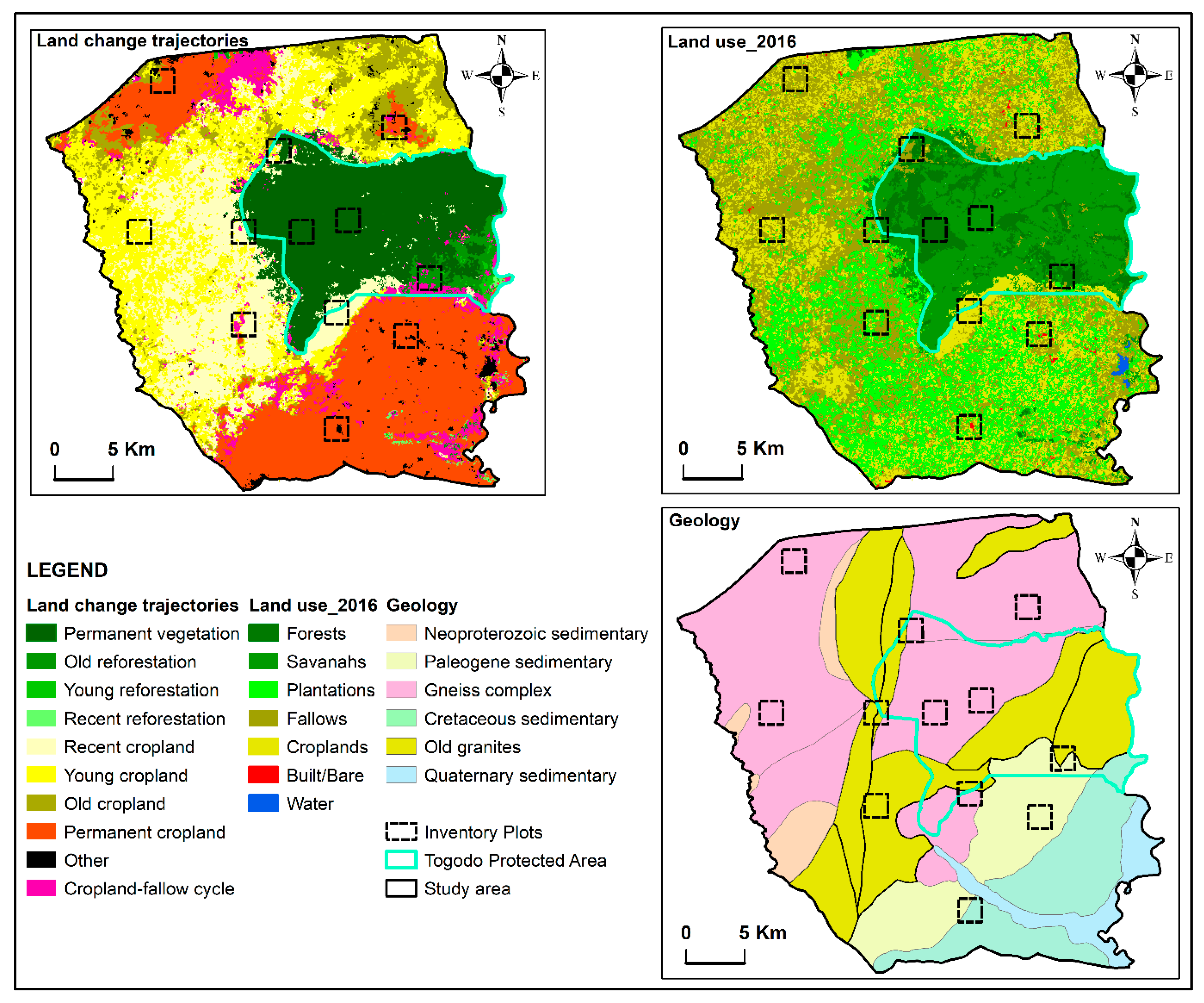
2.1. Materials: The Study Area and Sampling
2.1.1. Study Area
2.1.2. Stratified Sampling
2.2. Methods: Data Analysis
2.2.1. Total, Spatial, and Local Floral Diversities
2.2.2. Assessment of Plant Community Heterogeneity: The Species-Area Curve and the Concentration Curve or Pareto Curve
2.2.3. Assessment of the Vertical Structure of Plant Communities
2.2.4. Analysis of Native and Alien Species
3. Results
3.1. Total Diversity and Plant Communities’ Heterogeneity
3.2. Spatial Floral Diversity by Land-Use Type
3.3. Local Diversity by Land-Use Types
3.4. Native and Alien Species
4. Discussion
4.1. Total Diversity and Plant Communities’ Heterogeneity
4.2. Agricultural Land-Uses Influence on Spatial and Local Diversities
4.3. Effect of Agricultural Land-Use on the Vertical Structure of Plant Communities
4.4. Effect of Agricultural Land-Use on Native and Alien Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pairs | Pr Multcomp | Pr Wilco_bon | Pr Wilco_BH |
| FO-CH | 0.1656 | 0.024 * | 0.012 * |
| HA-CH | 0.9236 | 1 | 0.697 |
| JC-CH | 0.0797 | 0.696 | 0.099 |
| PA-CH | 0.2242 | 0.155 | 0.039 * |
| SA-CH | <0.001 *** | 5.00 × 10−7 *** | 5.00 × 10−7 *** |
| TK-CH | 0.0754 | 0.619 | 0.099 |
| HA-FO | 0.904 | 1 | 0.415 |
| JC-FO | 0.9725 | 1 | 0.515 |
| PA-FO | 0.9995 | 1 | 0.697 |
| SA-FO | 0.9399 | 1 | 0.415 |
| TK-FO | 1 | 1 | 0.95 |
| JC-HA | 0.9973 | 1 | 0.697 |
| PA-HA | 0.9803 | 1 | 0.51 |
| SA-HA | 0.1332 | 0.555 | 0.099 |
| TK-HA | 0.8596 | 1 | 0.515 |
| PA-JC | 0.9993 | 1 | 0.721 |
| SA-JC | 0.0478 * | 0.1 | 0.033 * |
| TK-JC | 0.9454 | 1 | 0.51 |
| SA-PA | 0.5625 | 1 | 0.214 |
| TK-PA | 0.9989 | 1 | 0.721 |
| TK-SA | 0.9189 | 1 | 0.51 |
References
- Díaz, S.; Settele, J.; Brondízio, E.; Ngo, H.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.; Butchart, S.; et al. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES: Bonn, Germany, 2019; p. 39. [Google Scholar]
- Humphreys, A.M.; Govaerts, R.; Ficinski, S.Z.; Nic Lughadha, E.; Vorontsova, M.S. Global Dataset Shows Geography and Life Form Predict Modern Plant Extinction and Rediscovery. Nat. Ecol. Evol. 2019, 3, 1043–1047. [Google Scholar] [CrossRef]
- Knapp, W.M.; Frances, A.; Noss, R.; Naczi, R.F.C.; Weakley, A.; Gann, G.D.; Baldwin, B.G.; Miller, J.; McIntyre, P.; Mishler, B.D.; et al. Vascular Plant Extinction in the Continental United States and Canada. Conserv. Biol. 2021, 35, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E. Biodiversity: The Ravages of Guns, Nets and Bulldozers. Nature 2016, 536, 143. [Google Scholar] [CrossRef] [PubMed]
- Luyssaert, S.; Jammet, M.; Stoy, P.C.; Estel, S.; Pongratz, J.; Ceschia, E.; Churkina, G.; Don, A.; Erb, K.; Ferlicoq, M. Land Management and Land-Cover Change Have Impacts of Similar Magnitude on Surface Temperature. Nat. Clim. Chang. 2014, 4, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Fried, G. Apports Des Approches Fonctionnelles Pour l’évaluation Des Risques Associés Aux Changements de Végétation Induits Par Les Activités Humaines. HDR. Thesis, Université de Montpellier, Montpellier, France, 2019. [Google Scholar]
- Balima, L.H.; Nacoulma, B.M.I.; Bayen, P.; Kouamé, F.N.; Thiombiano, A. Agricultural Land Use Reduces Plant Biodiversity and Carbon Storage in Tropical West African Savanna Ecosystems: Implications for Sustainability. Glob. Ecol. Conserv. 2020, 21, e00875. [Google Scholar] [CrossRef]
- Tilman, D. Global Environmental Impacts of Agricultural Expansion: The Need for Sustainable and Efficient Practices. Proc. Natl Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Searchinger, T.D.; Estes, L.; Thornton, P.K.; Beringer, T.; Notenbaert, A.; Rubenstein, D.; Heimlich, R.; Licker, R.; Herrero, M. High Carbon and Biodiversity Costs from Converting Africa’s Wet Savannahs to Cropland. Nat. Clim. Chang. 2015, 5, 481–486. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Kühn, I.; Wild, J.; Arianoutsou, M.; Bacher, S.; Chiron, F.; Didžiulis, V.; Essl, F. Disentangling the Role of Environmental and Human Pressures on Biological Invasions across Europe. Proc. Natl. Acad. Sci. USA 2010, 107, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Vilà, M.; Ibáñez, I. Plant Invasions in the Landscape. Landsc. Ecol. 2011, 26, 461–472. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien Species as a Driver of Recent Extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef] [PubMed]
- Raveloaritiana, E.; Wurz, A.; Grass, I.; Osen, K.; Soazafy, M.R.; Martin, D.A.; Faliniaina, L.; Rakotomalala, N.H.; Vorontsova, M.S.; Tscharntke, T.; et al. Land-Use Intensification Increases Richness of Native and Exotic Herbaceous Plants, but Not Endemics, in Malagasy Vanilla Landscapes. Divers. Distrib. 2021, 27, 784–798. [Google Scholar] [CrossRef]
- Burgess, N.; Hales, J.; Underwood, E.; Dinerstein, E.; Olson, D.; Itoua, I.; Schipper, J.; Ricketts, T.; Newman, K. Terrestrial Ecoregions of Africa and Madagascar: A Conservation Assessment; Island Press: Washington, DC, USA; Covelo, CA, USA; London, UK, 2004; ISBN 1-55963-364-6. [Google Scholar]
- CILSS. Landscapes of West Africa: A Window on a Changing World; CILSS: Ouagadougou, Burkina Faso, 2016. [Google Scholar]
- Adjonou, K.; Djiwa, O.; Kombate, Y.; Kokutse, A.D.; Kokou, K. Etude de La Dynamique Spatiale et Structure Des Forêts Denses Sèches Reliques Du Togo: Implications Pour Une Gestion Durable Des Aires Protégées. Int. J. Biol. Chem. Sci. 2010, 4, 168–183. [Google Scholar] [CrossRef]
- Triplet, P. Manuel de Gestion des Aires Protégées d’Afrique Francophone; Awely: Paris, France, 2009. [Google Scholar]
- Atsri, H.K.; Konko, Y.; Cuni-Sanchez, A.; Abotsi, K.E.; Kokou, K. Changes in the West African Forest-Savanna Mosaic, Insights from Central Togo. PLoS ONE 2018, 13, e0203999. [Google Scholar] [CrossRef] [Green Version]
- Diwediga, B.; Agodzo, S.; Wala, K.; Le, Q.B. Assessment of Multifunctional Landscapes Dynamics in the Mountainous Basin of the Mo River (Togo, West Africa). J. Geogr. Sci. 2017, 27, 579–605. [Google Scholar] [CrossRef]
- Badjana, H.M.; Helmschrot, J.; Selsam, P.; Wala, K.; Flügel, W.-A.; Afouda, A.; Akpagana, K. Land Cover Changes Assessment Using Object-based Image Analysis in the Binah River Watershed (Togo and Benin). Earth Space Sci. 2015, 2, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Akodéwou, A.; Oszwald, J.; Saïdi, S.; Gazull, L.; Akpavi, S.; Akpagana, K.; Gond, V. Land Use and Land Cover Dynamics Analysis of the Togodo Protected Area and Its Surroundings in Southeastern Togo, West Africa. Sustainability 2020, 12, 5439. [Google Scholar] [CrossRef]
- UICN/PACO. Evaluation de l’Efficacité de la Gestion des Aires Protégées: Aires Protégées du Togo; UICN-PACO: Ouagadougou, Burkina Faso, 2008; ISBN 978-2-8317-1130-0. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorologische Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated High-Resolution Grids of Monthly Climatic Observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Inderjit; Catford, J.A.; Kalisz, S.; Simberloff, D.; Wardle, D.A. A Framework for Understanding Human-driven Vegetation Change. Oikos 2017, 126, 1687–1698. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant Invasion across Space and Time: Factors Affecting Nonindigenous Species Success during Four Stages of Invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Gounot, M. Méthodes d’étude Quantitative de la Végétation; Masson: Paris, France, 1969. [Google Scholar]
- Alexandre, F.; Genin, A.; Godron, M.; Lecompte, M. Distribution des plantes et organisation de la végétation. Espace Géographique 1998, 27, 228–238. [Google Scholar] [CrossRef]
- Sylvain, J.P.; Aregba, A.; Assih-Edeou, P.; Castaing, C.; Chèvremont, P.; Collart, J.; Monciardini, C.; Marteau, P.; Ouassane, I.; Tchota, K. Notice Explicative de la Carte Géologique à 1: 200,000 Feuille Lomé 1986.
- Adou Yao, C.Y.; N’Guessan, E.K. Diversité botanique dans le sud du parc national de Taï, Côte d’Ivoire. Afr. Sci. 2005, 1, 295–313. [Google Scholar]
- Cheek, M.; Cable, S. Plant Inventory for Conservation Management: The Kew-Earthwatch Programme in Western Cameroon, 1993–1996. In African rainforest and the conservation of biodiversity, Proceedings of the Limbe Conference, Limbe, Cameroun, 17–24 January 1997; Botanic Garden Meise: Cameroun, Africa, 1997; pp. 29–38. [Google Scholar]
- Hall, J.B.; Swaine, M.D. Distribution and Ecology of Vascular Plants in a Tropical Rain Forest. Forest Vegetation in Ghana. Springer: Dordrecht, The Netherlands, 1981. [Google Scholar]
- Hawthorne, W.D. Holes and the Sums of Parts in Ghanaian Forest: Regeneration, Scale and Sustainable Use. Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1996, 104, 75–176. [Google Scholar] [CrossRef]
- Amégnaglo, K.B.; Dourma, M.; Akpavi, S.; Akodewou, A.; Wala, K.; Diwediga, B.; Atakpama, W.; Agbodan, K.M.L.; Batawila, K.; Akpagana, K. Caractérisation des formations végétales pâturées de la zone guinéenne du Togo: Typologie, évaluation de la biomasse, diversité, valeur fourragère et régénération. Int. J. Biol. Chem. Sci. 2018, 12, 2065–2084. [Google Scholar] [CrossRef]
- Wala, K. La Végétation de La Chaîne de l’Atakora Au Bénin: Diversité Floristique, Phytosociologie et Impact Humain. Acta Botanica Gallica 2010, 157, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Braun-Blanquet, J.; Roussine, N.; Nègre, R. Les Groupements Végétaux de la France Méditerranéenne; Direction de la carte des groupements végétaux; CNRS: Montpellier, France, 1952. [Google Scholar]
- Brunel, J.-F.; Hiepko, P.; Scholz, H. Flore Analytique Du Togo: Phanérogames. Englera 1984, 4, 3–751. [Google Scholar] [CrossRef]
- Akoègninou, A.; Van der Burg, W.J.; Van der Maesen, L.J.G. Flore Analytique Du Bénin; Adjakidjè, V., Essou, J.P., Sinsin, B., Yédomonhan, H., Eds.; Backhuys Publishers: Cotonou, Benin; Wageningen, the Netherlands, 2006; ISBN 90-5782-181-8. [Google Scholar]
- Cayuela, L.; Stein, A.; Oksanen, J. Taxonstand: Taxonomic Standardization of Plant Species Names. R Package Version 2.1. R Foundation Stat. Comput. 2017, 2, 1–11. [Google Scholar]
- Réjou-Méchain, M.; Tanguy, A.; Piponiot, C.; Chave, J.; Hérault, B. Biomass: An R Package for Estimating Above-ground Biomass and Its Uncertainty in Tropical Forests. Methods Ecol. Evol. 2017, 8, 1163–1167. [Google Scholar] [CrossRef]
- Brillouin, L. Science and Information Theory, 2nd ed.; Academic Press: New York, NY, USA, 1962; ISBN 978-0-12-134950-9. [Google Scholar]
- Basharin, G.P. On a Statistical Estimate for the Entropy of a Sequence of Independent Random Variables. Theory Probab. Appl. 1959, 4, 333–336. [Google Scholar] [CrossRef]
- Strong, W.L. Biased Richness and Evenness Relationships within Shannon–Wiener Index Values. Ecol. Indic. 2016, 67, 703–713. [Google Scholar] [CrossRef]
- Barima, Y.S.S.; Assale, A.A.Y.; Andrieu, J. Caractérisation post conflits armés des perturbations dans la forêt classée du Haut-Sassandra en Côte d’Ivoire. Afr. Sci. 2016, 12, 66–82. [Google Scholar]
- Upton, G.J.G. Fisher’s Exact Test. J. R. Stat. Soc. Ser. A 1992, 155, 395–402. [Google Scholar] [CrossRef]
- Amandier, L.; Wolff, A.; Godron, M.; Kadik, L. Calculs Comparés de Biodiversité Dans Quelques Forêts de Provence. Forêt Méditerranéenne 2012, 33, 151. [Google Scholar]
- Kadik, L.; Godron, M. Contribution à l’étude de La” Dégradation” de La Végétation Dans Les Pineraies de Pinus Halepensis Mill. D’Algérie et Dans Les Formations Dérivées». J. Bot. Soc. Bot. France 2004, 27, 9–19. [Google Scholar]
- Godron, M.; Kadik, L. La Mesure de La Biodiversité Spatiale. Symbioses 2003, 2003, 67–75. [Google Scholar]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.-Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and Estimators Linking Individual-Based and Sample-Based Rarefaction, Extrapolation and Comparison of Assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, Extrapolating, and Comparing Incidence-Based Species Accumulation Curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, T.C.; Ma, K.H.; Chao, A. INEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (Hill Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.M. Multcomp: Simultaneous Inference for General Linear Hypotheses. 2012. R package version. Available online: http://CRAN.R-project.org/package=multcomp (accessed on 13 December 2021).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package: Ordination Methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists. 2020. R Package Version. Available online: https://CRAN.R-project.org/package=vegan (accessed on 13 December 2021).
- Godron, M. Un Modèle Pour La Courbe Aire-Espèce. Le Naturaliste Canadien 1970, 97, 491–492. [Google Scholar]
- Godron, M. Écologie et Évolution Du Monde Vivant. L’échelle Crée Le Phénomène, Volume 2; L’Harmattan: Paris, France, 2012. [Google Scholar]
- Gosselin, M.; Laroussine, O. Biodiversité et Gestion Forestière: Connaître Pour Préserver: Synthèse Bibliographique; Cemagref; GIP Ecofor: Paris, France, 2004; ISBN 2-85362-620-2. [Google Scholar]
- Jacquard, P.; Daget, P.; Poissonnet, J.; Laroche, G. Expression de l’évolution du Potentiel de Production et la Composition Botanique d’une Formation Herbacée Dense; CNRS/CEPE: Montpellier, France, 1968. [Google Scholar]
- Daget, P.; Godron, M. Analyse Fréquentielle de l’écologie des Espèces Dans les Communautés, 1st ed.; Masson: Paris, France, 1982; ISBN 2-225-78090-0. [Google Scholar]
- Lafon, P. Sur la variabilité de la fréquence des formes dans un corpus. Mots 1980, 1, 127–165. [Google Scholar] [CrossRef] [Green Version]
- Connelly, L.M. Fisher’s Exact Test. MedSurg Nurs. 2016, 25, 58–60. [Google Scholar] [PubMed]
- Pyšek, P.; Pergl, J.; Essl, F.; Lenzner, B.; Dawson, W.; Kreft, H.; Weigelt, P.; Winter, M.; Kartesz, J.; Nishino, M.; et al. Naturalized Alien Flora of the World: Species Diversity, Taxonomic and Phylogenetic Patterns, Geographic Distribution and Global Hotspots of Plant Invasion. Preslia 2017, 89, 203–274. [Google Scholar] [CrossRef]
- R Development Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019.
- Kokou, K.; Atato, A.; Bellefontaine, R.; Kokutse, A.D.; Caballe, G. Diversité des forêts denses sèches du Togo (Afrique de l’Ouest). Rev. Écol. (Terre Vie) 2006, 61, 225–246. [Google Scholar]
- Scheel-Ybert, R. Stabilité de l’Écosystème sur le littoral sud-est du Brésil à l’holocène supérieur (5500–1400 ans BP). Les Pêcheurs-Cueilleurs-Chasseurs et le Milieu végétal: Apports de l’Anthracologie. Ph.D. Thesis, Univ. Montpellier II, Montpellier, France, 1998. [Google Scholar]
- Blanc, L.; Florès, O.; Molino, J.-F.; Gourlet-Fleury, S.; Sabatier, D. Diversité spécifique et regroupement d’espèces arborescentes en forêt guyanaise. Rev. For. Française 2003, LV, 131–146. [Google Scholar] [CrossRef] [Green Version]
- Estes, L.D.; Searchinger, T.; Spiegel, M.; Tian, D.; Sichinga, S.; Mwale, M.; Kehoe, L.; Kuemmerle, T.; Berven, A.; Chaney, N.; et al. Reconciling Agriculture, Carbon and Biodiversity in a Savannah Transformation Frontier. Phil. Trans. R. Soc. B 2016, 371, 20150316. [Google Scholar] [CrossRef] [Green Version]
- Oszwald, J.; Antoine, L.; De Sartre, X.A.; Marcello, T.; Gond, V. Analyse Des Directions de Changement Des États de Surface Végétaux Pour Renseigner La Dynamique Du Front Pionnier de Maçaranduba (Brésil) Entre 1997 et 2006. Télédétection 2010, 9, 97–111. [Google Scholar]
- Jara, T.; Hylander, K.; Nemomissa, S. Tree Diversity across Different Tropical Agricultural Land Use Types. Agric. Ecosyst. Environ. 2017, 240, 92–100. [Google Scholar] [CrossRef]
- Krishna, A.; Manjunath, G.O.; Rathod, R.; Kannur, S. Allelopathic Effect of Four Agroforestry Tree Species Leaf Leachates on Seed Germination of Certain Vegetable Crops. Karnataka J. Agric. Sci. 2010, 16, 430–433. [Google Scholar]
- Lacret, R.; Varela, R.M.; Molinillo, J.M.G.; Nogueiras, C.; Macías, F.A. Anthratectone and Naphthotectone, Two Quinones from Bioactive Extracts of Tectona Grandis. J. Chem. Ecol. 2011, 37, 1341–1348. [Google Scholar] [CrossRef]
- Hending, D.; Andrianiaina, A.; Maxfield, P.; Rakotomalala, Z.; Cotton, S. Floral Species Richness, Structural Diversity and Conservation Value of Vanilla Agroecosystems in Madagascar. Afr. J. Ecol. 2020, 58, 100–111. [Google Scholar] [CrossRef]
- Odum, E.P.; Odum, H.T. Fundamentals of Ecology, 2nd ed; Saunders Company: Philadelphia, PA, USA, 1959; ISBN B0018QZMS0. [Google Scholar]
- Grime, J.P. Competitive Exclusion in Herbaceous Vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Molino, J.-F.; Sabatier, D. Tree Diversity in Tropical Rain Forests: A Validation of the Intermediate Disturbance Hypothesis. Science 2001, 294, 1702–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackey, R.L.; Currie, D.J. A Re-Examination of the Expected Effects of Disturbance on Diversity. Oikos 2000, 88, 483–493. [Google Scholar] [CrossRef]
- Godron, M. L’étude du «grain» de la structure de la vegetation. Application à quelques exemples méditerranéens. Ecmed 1982, 8, 191–195. [Google Scholar] [CrossRef]
- Alexandre, F.; Génin, A. Les niveaux d’organisation du couvert végétal, question géographique et écologique. In Proceedings of the Les échelles pour les géographes et les autres, Avignon, France, 3–4 June 2010; pp. 59–65. [Google Scholar]
- Godron, M.; Joly, H. Dictionnaire Du Paysage; CILF Les Dictio; Conseil International de la Langue Française: Paris, France, 2008. [Google Scholar]
- Shumi, G.; Schultner, J.; Dorresteijn, I.; Rodrigues, P.; Hanspach, J.; Hylander, K.; Senbeta, F.; Fischer, J. Land Use Legacy Effects on Woody Vegetation in Agricultural Landscapes of South-Western Ethiopia. Divers. Distrib. 2018, 24, 1136–1148. [Google Scholar] [CrossRef] [Green Version]
- Nacoulma, B.M.I.; Schumann, K.; Traoré, S.; Bernhardt-Römermann, M.; Hahn, K.; Wittig, R.; Thiombiano, A. Impacts of Land-Use on West African Savanna Vegetation: A Comparison between Protected and Communal Area in Burkina Faso. Biodivers Conserv. 2011, 20, 3341–3362. [Google Scholar] [CrossRef]
- Harvey, C.A.; Medina, A.; Sánchez, D.M.; Vílchez, S.; Hernández, B.; Saenz, J.C.; Maes, J.M.; Casanoves, F.; Sinclair, F.L. Patterns of Animal Diversity in Different Forms of Tree Cover in Agricultural Landscapes. Ecol. Appl. 2006, 16, 1986–1999. [Google Scholar] [CrossRef]
- Goudard, A. Fonctionnement Des Écosystèmes et Invasions Biologiques: Importance de La Biodiversité et Des Interactions Interspécifiques; Université Pierre et Marie Curie: Paris, France, 2007. [Google Scholar]
- Caillon, A.; Lavoué, M. Liste Hiérarchisée des Plantes Exotiques Envahissantes d’Aquitaine. Version 1.0; Conservatoire Botanique National Sud-Atlantique: Paris, France, 2016; p. 33. [Google Scholar]
- Grass, I.; Loos, J.; Baensch, S.; Batáry, P.; Librán-Embid, F.; Ficiciyan, A.; Klaus, F.; Riechers, M.; Rosa, J.; Tiede, J.; et al. Land-Sharing/-Sparing Connectivity Landscapes for Ecosystem Services and Biodiversity Conservation. People Nat. 2019, 1, 262–272. [Google Scholar] [CrossRef]
- Schlaepfer, M.A. Do Non-Native Species Contribute to Biodiversity? PLoS Biol. 2018, 16, e2005568. [Google Scholar] [CrossRef] [Green Version]







| CH | FO | HA | JC | PA | SA | TK | |
|---|---|---|---|---|---|---|---|
| ABO | −19 | 6 | 3 | 3 | 0 | 6 | 5 |
| ABU | 6 | 0 | 0 | 0 | 3 | 0 | 0 |
| HER | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| CH | FO | HA | JC | PA | SA | TK | |
|---|---|---|---|---|---|---|---|
| 1 | 14 | 0 | 23 | −16 | −4 | −12 | 0 |
| 2 | 5 | −12 | −26 | 4 | 0 | 11 | 6 |
| 3 | −28 | 22 | 5 | 5 | 9 | 0 | −8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akodéwou, A.; Godron, M. Agricultural Land-Use Increases Floral Species Richness in Tropical Dry Forest and Savannah Ecosystems in West Africa. Diversity 2022, 14, 106. https://doi.org/10.3390/d14020106
Akodéwou A, Godron M. Agricultural Land-Use Increases Floral Species Richness in Tropical Dry Forest and Savannah Ecosystems in West Africa. Diversity. 2022; 14(2):106. https://doi.org/10.3390/d14020106
Chicago/Turabian StyleAkodéwou, Amah, and Michel Godron. 2022. "Agricultural Land-Use Increases Floral Species Richness in Tropical Dry Forest and Savannah Ecosystems in West Africa" Diversity 14, no. 2: 106. https://doi.org/10.3390/d14020106






