Abstract
This is the first long-term study (monthly samples at two 4-year intervals: 1998 to 2001 and 2013 to 2016) on rotifers in a saline, deep lake. The pelagic rotifer assemblage of Lake Alchichica is simple and comprised by two species, both new and most likely endemic: Brachionus sp. Mexico (related to B. plicatilis) and Hexarthra sp. (related to H. jenkinae). Similar low species richness and composition are found in other saline lakes associated with salinity. Rotifers in Lake Alchichica were an irregular component of the zooplankton community. Rotifers’ overall abundance (471 ± 1211 ind m−2) and biomass (24 ± 63 mg DW m−2) were low; Brachionus sp. Mexico and Hexarthra sp. contributed similarly to the annual mean abundance (54% and 46%, respectively) and biomass (53% and 47%, respectively). Abundance and biomass were tightly coupled, but there was no regular pattern in their seasonal dynamics. When co-existing, Brachionus sp. Mexico showed a higher abundance than Hexarthra sp. The dominant (≈80%) phytoplankton biomass in Lake Alchichica, the large (35–63 µm) diatom Cyclotella alchichicana, is inedible for rotifers, thus rotifers most probably relied only on nanophytoplankton (≤20 µm). Seasonal and interannual differences in rotifers seem related to food availability (oligotrophy) and probably to biotic interactions (e.g., competition). Rotifer abundance and biomass values in 1998–2001 went down to 12.5% in 2013–2016. Climate change and stochastics events leading to pulses of the rotifers’ food, and biotic interactions seem to be the most plausible explanation.
1. Introduction
Lake Alchichica, on the easternmost region of the Mexican Altiplano, shows a remarkable degree of microendemisms for a small (≈2.4 km2) and deep (62 m) lake [1]. Ortega-Mayagoitia et al. [2] reported eighteen endemic species (from prokaryote to vertebrate) up to date. The planktonic community is not the exception, with the diatom Cyclotella alchichicana [3] and the calanoid copepod Leptodiaptomus garciai (Osorio-Tafall, 1942) [4] identified as microendemisms. Additionally, Lake Alchichica displays a remarkable low zooplankton species richness with just three species, one copepod—L. garciai—and two rotifers of two different families: Brachionidae and Hexarthridae. The Brachionus species inhabiting Lake Alchichica belongs to the B. plicatilis (Müller, 1786) complex, while the Hexarthra species of Lake Alchichica is related to H. jenkinae (De Beauchamp, 1932). The Brachionus species although previously identified as B. rotundiformis (Tschugunoff, 1921) [5], later, it was recognized as a different species not yet described but designated as Brachionus sp. ‘Mexico’ [6]. Both rotifer species, Brachionus sp. and Hexarthra sp., are most likely new species [7] and probably microendemic. Twenty years ago, the lack of genetic analyses hampered the exact species identification of Brachionus of Lake Alchichica, which may be a limitation of this study. However, recently it was confirmed that the Brachionus of Lake Alchichica is a single species by mean of COI sequences analysis, and applying three approaches (ABGD, PTP and GMYC) in DNA taxonomy on 30 sequences on adult Brachionus females [6]. Moreover, resting eggs obtained from the lake’s egg bank were analyzed using COI [8]. These results confirmed the information obtained from living organisms; there is only one Brachionus species in Lake Alchichica, a new and different species (Brachionus sp. México), included in the Brachionus plicatilis complex [6].
Alchichica is a warm-monomictic lake alternating a winter mixing period (late December-January to March-early April) with a long stratification season (late April-early December). Several papers show that thermal hydrodynamics are pretty regular and predictable (e.g., [9,10]). The primary production dynamics are tightly coupled with the lake’s hydrodynamics (e.g., [11,12]), with a biomass increase—winter diatom bloom—during the mixing season, a cyanobacterial (Nodularia aff. spumigena Mertens ex Bornet & Flahault, 1888) bloom at the onset of the stratification, and a deep chlorophyll maximum along the well stablished stratification. Besides, ref [13] found that large-size phytoplankton (>2 µm, e.g., the diatom Cyclotella alchichicana Oliva, Lugo, Alcocer & Cantoral-Uriza, 2006, 35–63 µm) dominate biomass throughout the year. Therefore, it seems that in addition to salinity, food availability could be limiting the zooplankton development in Lake Alchichica, particularly rotifers [14].
The seasonal (mixing and stratification), long-term hydrodynamical regularity of Lake Alchichica provides a unique opportunity to analyze if this consistency mirrors in the rotifer assemblage seasonal and interannual dynamics. Our primary goal was to identify the rotifer assemblage dynamic of the tropical, saline, deep Lake Alchichica and how the environmental characteristics control its temporal (seasonal and interannual) variation. For this purpose, we addressed the following research questions: (1) What are the annual (seasonal) dynamics of the rotifer assemblage composition and structure? (2) Does the annual dynamics of the rotifer assemblage composition and structure change interannually? (3) Do the environmental characteristics of the lake explain the annual and interannual dynamics of the rotifer assemblage composition and structure?
Our central hypothesis is that the regularity of the warm-monomixis thermal type largely controls the rotifer assemblage, resulting in regular annual and interannual dynamics. Specifically, we expected the regular hydrodynamical pattern to cause (1) larger abundance and biomass of rotifers associated with the winter mixing season and lower abundance and biomass along the stratification period, and (2) a long-term recurrent annual dynamic of the rotifer assemblage composition and structure. To answer the research questions and test the proposed hypotheses, our approach was to evaluate the annual and interannual changes in the water quality of the lake and rotifer composition, abundance and biomass using data derived from monthly monitoring of the water column within a range of 18 years in two 4-year intervals (1998–2001 and 2013–2016).
2. Materials and Methods
2.1. Study Area
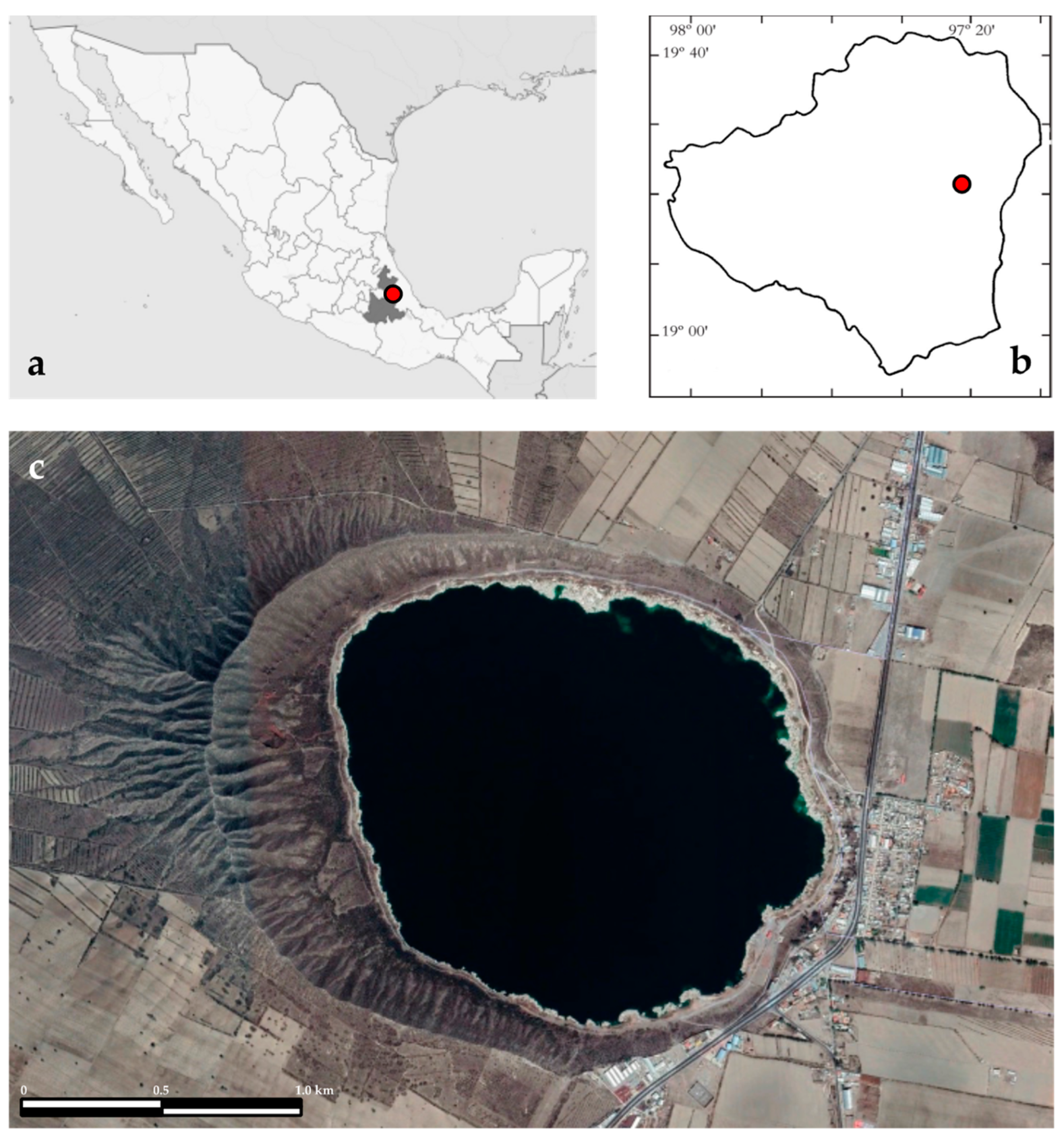
Lake Alchichica (19°24′ N, 97°24′ W, 2326 m a.s.l.) is in the Cuenca Oriental, an extensive endorheic basin of almost 5000 km2, on the border of the Puebla and Veracruz states (Figure 1). The lake has a surface area of 2.367 km2 (1.7 km of diameter), and a maximum depth of 62 m, and a mean of 48.4 m [15]. The lake holds ~115,000,000 m3 of saline (8.5–9 g L−1), NaCl type, alkaline (pH~9) waters [16]. This region has an arid climate with an annual evaporation of 1690 mm and a rainfall of less than 500 mm per year [13]. The air temperature fluctuates from −5.5 to 30 °C with an annual mean temperature of 14.4 °C [17].
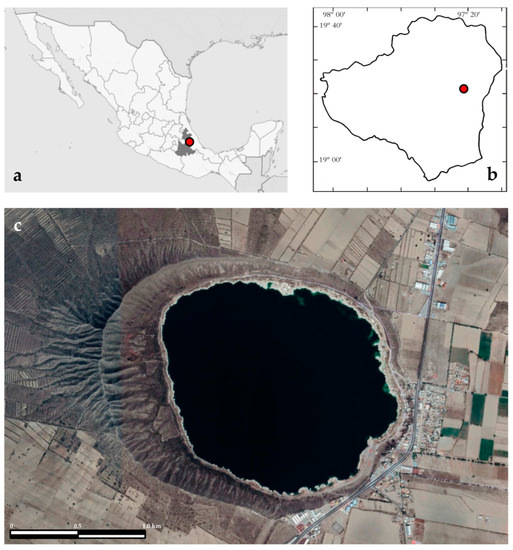
Figure 1.
Lake Alchichica in (a) Mexico and Puebla State and (b) Oriental basin. (c) Lake Alchichica (satellite photograph from Google Earth).
Lake Alchichica is warm-monomictic with a short mixing period (~3 months) and a long stratification period characterized by the rapid development of an anoxic hypolimnion [10]. Lake Alchichica is oligotrophic with concentrations of ≤5 μg chlorophyll-a L−1, 4.14 ± 0.69 μM total nitrogen, and 0.21 ± 0.04 μM total phosphorus in the mixed layer [18].
2.2. Field Sampling
Monthly campaigns were conducted in two 4-year intervals, from 1998 to 2001 and 2013 to 2016. Samplings were carried out in the central and deepest part of the lake. In situ vertical profiles (spatial resolution 1 m) of temperature and dissolved oxygen were measured with a Hydrolab (DS4 in 1998–2001, and DS5 in 2013–2016) multiparametric water quality probe. Additionally, photosynthetically active radiation (PAR) profiles were obtained with a Biospherical PNF-300 natural fluorescence profiler (discrete readings recorded every second as the sensor was lowered at a rate of about 1 m per 3–4 s, providing a vertical resolution of 25–30 cm). The extension of the euphotic zone (ZEU) was demarcated as the layer where PAR was≥ 0.1% of its surface value (SPAR). The mixed layer (ZMIX) was expressed as the upper layer mixed thoroughly by the wind to a uniform temperature. ZMIX is delimited by the top of the thermocline, which corresponds to the epilimnion while stratified, while during the mixing season, it corresponds to the entire water column.
A 6-L Niskin-type (1998 to 2001) and a 5-L Uwitec (2013 to 2016) water sampler bottles obtained duplicate water samples from ten water depths. At each sampling campaign, after recording the temperature, dissolved oxygen, and PAR profiles, the ten water depths were chosen to represent better the water column heterogeneity (e.g., thermo- and oxyclines).
The first set of ten samples was used for chlorophyll-a concentration (Chl-a) analysis: total (TChl-a) and size-fractionated Chl-a analysis. Samples for Chl-a were analyzed in a Turner Design 10-AU fluorometer following the EPA method 445.0 [19]. Chl-a were fractionated in two sizes: large (LChl-a ≥ 2 µm) and small (SChl-a < 2 µm, ≥0.7 µm) size fractions.
The second set of ten samples was used for the rotifer assemblage analysis. The ten water samples were filtered in situ (54 µm), and all organisms were concentrated in 50 mL vials and fixed with 4% formaldehyde for further analysis (identification, counting). The entire 50 mL concentrates were identified (i.e., Brachionus sp. Mexico or Hexarthra sp.) and counted (Sedgwick Rafter chamber under an optical microscope) in the laboratory and treated as a water-column integrated sample.
Rotifer biomass was evaluated as a biovolume calculated based on the geometric formulas proposed by [20]. Wet weight was estimated from the biovolume of each individual using a specific density of 1.0, and dry weight corresponds to 10% of wet weight [21]. Rotifer abundance and biomass values were water column integrated on aerial basis (i.e., m2), and expressed as individuals m−2 for abundance, and mg DW m−2 for biomass.
2.3. Statistical Analysis
The Spearman non-parametric correlation coefficient was used to correlate the monthly environmental variables (ZEU, ZMIX, temperature, and dissolved oxygen concentration) with the monthly water column integrated values of biological variables (TChl-a, LChl-a, and SChl-a and zooplankton (Brachionus sp. Mexico and Hexarthra sp. abundance, total rotifer abundance, Brachionus sp. Mexico and Hexarthra sp. biomass, and total rotifer biomass). All data were transformed to log10 (n + 1). p-values for the test were adjusted by the Bonferroni correction. The temporal dynamics of environmental and biological data were obtained using the monthly integrated data by year, by means of the Sigma Plot 12.0 software. A U-Mann-Whitney test was applied to compare environmental variables data in both periods using R 4.03 [22]. Trends in temporal dynamics were evaluated by the Mann-Kendall trend test [23]. For significance in annual differences, a Kruskal-Wallis test and a post-hoc Dunn test were applied [23].
3. Results
3.1. The Environment
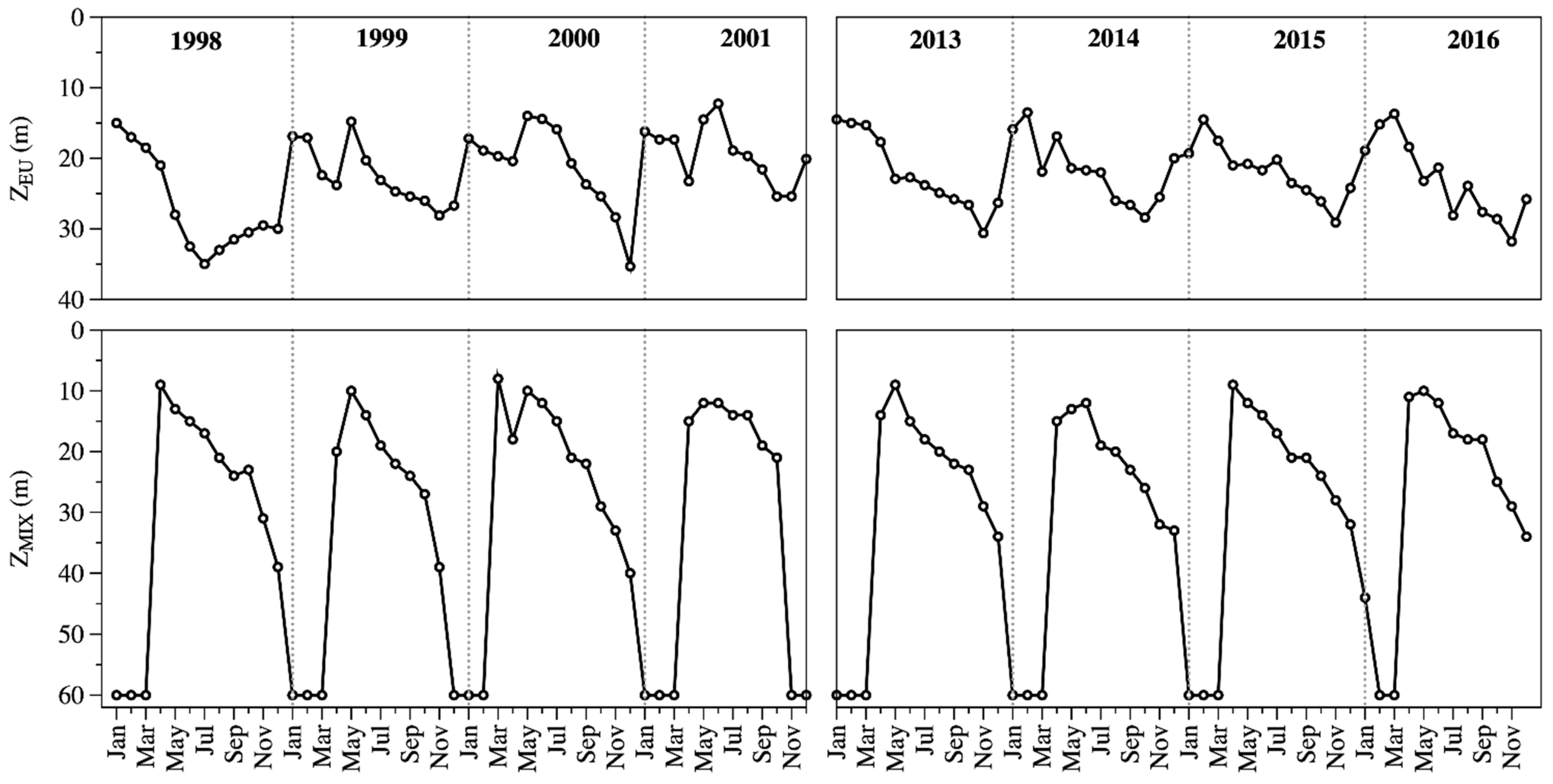
During the study periods, Lake Alchichica was a warm monomictic lake with a recurrent and predictable 3-months mixing season (January to March) and a large 9-months stratification season (April to December). The water column of Lake Alchichica was transparent (ZEU = 22.3 ± 5.4 m). There was a turbid-water phase with lower ZEU values during the mixing period (1998–2001: 19.0 ± 3.5 m, 2013–2016: 16.3 ± 2.6 m), and a clear-water phase with higher ZEU values during the stratification (1998–2001: 23.7 ± 6.3 m, 2013–2016: 24.2 ± 3.5 m) season (Figure 2). The average ZEU was 22.4 ± 6.0 m in 1998–2001 and 22.2 ± 4.8 m in 2013–2016. There was no statistical difference (p = 0.416) in the average values between both periods (Figure 2).
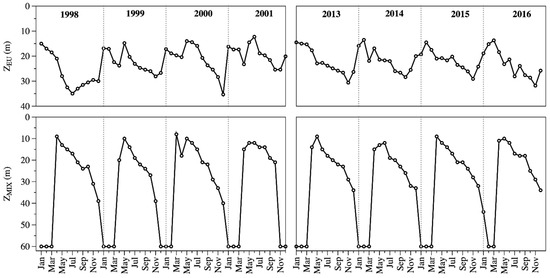
Figure 2.
Temporal dynamics of the euphotic zone (ZEU, up) and the mixing layer depth (ZMIX, down) in Lake Alchichica.
ZMIX ranged from being the whole water column (60 m) in the mixing period to eight meters (Figure 2). The ZMIX averaged 30 ± 19 m in 1998–2001 and 30 ± 18 m in 2013–2016. There was no statistical difference (p = 0.433) in the average values between both periods.
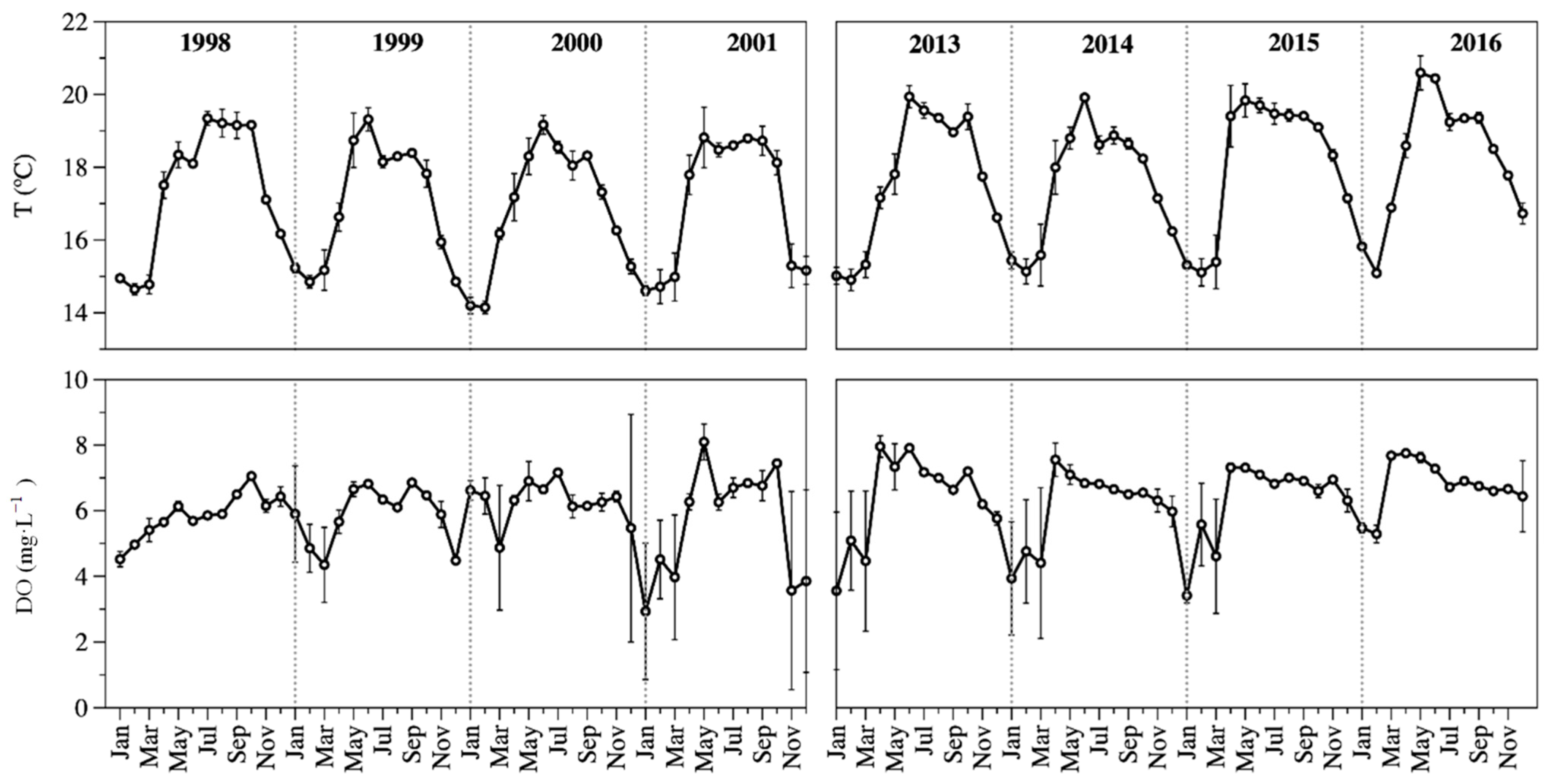
The ZMIX temperature (TMIX) was cold (14 to 21 °C), with the coldest values (15.4 ± 0.5 °C) associated with the winter mixing season, and the warmest (18.7 ± 1.1 °C) during stratification. Average TMIX was 17.1 ± 1.7 °C in 1998–2001 and 17.9 ± 1.7 °C in 2013–2016. There was a statistical difference (p = 0.003) in the average temperature between both periods (Figure 3).
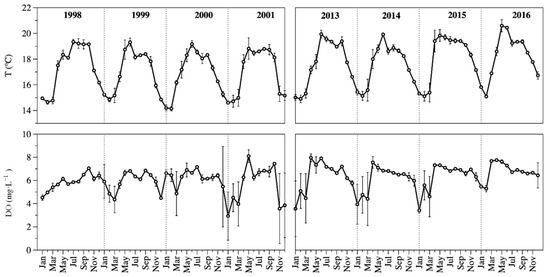
Figure 3.
Temporal dynamics of ZMIX temperature (up) and ZMIX DO concentration (down) in Lake Alchichica. (Average ± standard deviation).
The ZMIX was well oxygenated all year round (≈6 mg L−1). The DO concentration of the ZMIX (DOMIX) was high, close to saturation. The average DOMIX concentration was 5.9 ± 1.1 mg L−1 in 1998–2001 and 6.4 ± 1.1 mg L−1 in 2013–2016. There was a statistical difference (p = 0.002) in the average DO concentration between both periods (Figure 3).
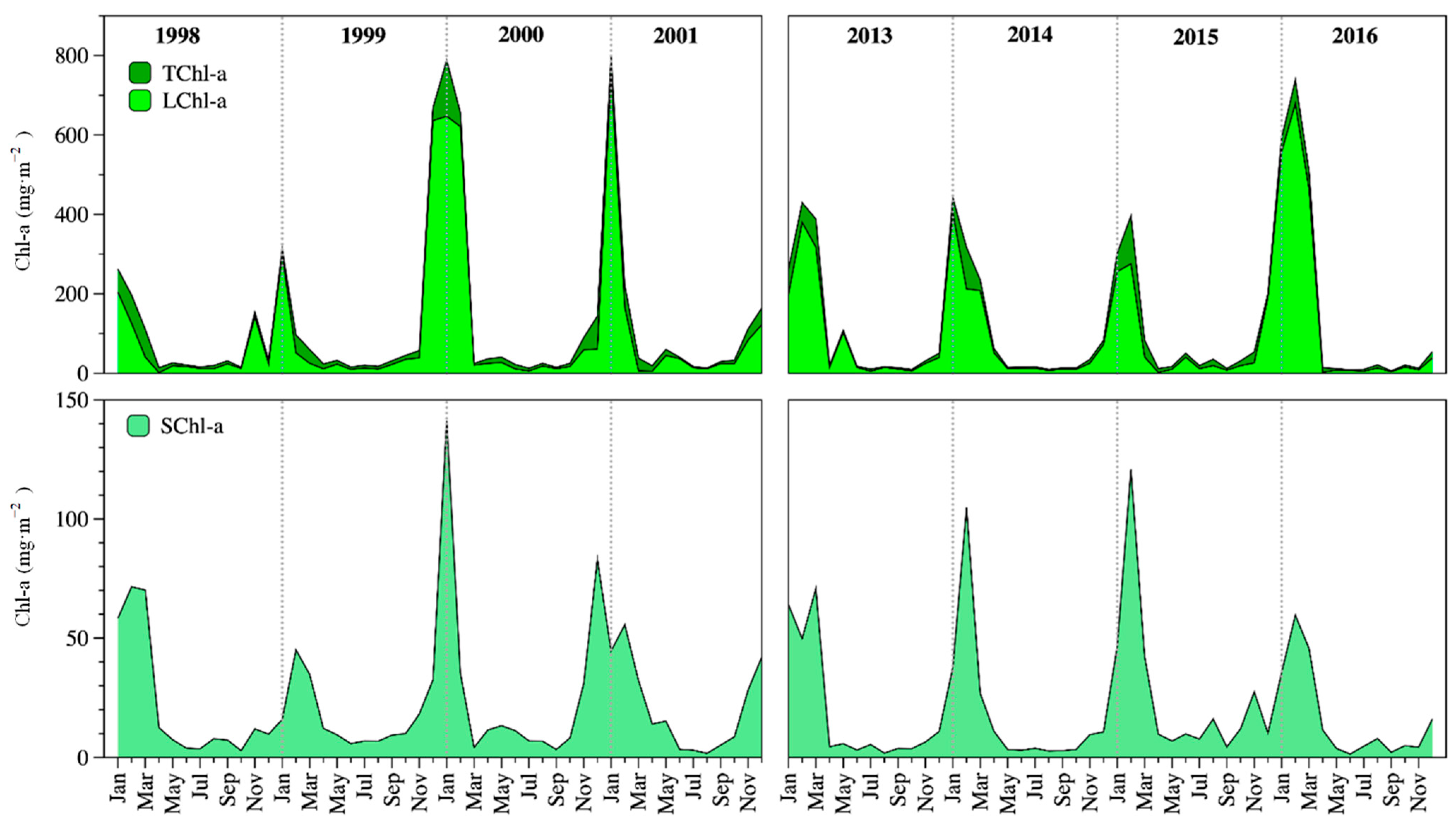
Lake Alchichica was oligotrophic. Total Chl-a concentration (TChl-a) in the ZMIX ranged from 8 to 857 mg m−2 (averaged 126 ± 194 mg m−2). Mixing was the most productive period with 361 ± 242 mg m−2. In contrast, the Chl-a concentration in the ZMIX averaged 48 ± 83 mg m−2 in the stratification season. Average TChl-a concentration was 122 ± 202 mg m−2 in 1998–2001 and 130 ± 189 mg m−2 in 2013–2016 (Figure 4). There was no statistical difference (p = 0.083) in the average TChl-a concentration between both periods.
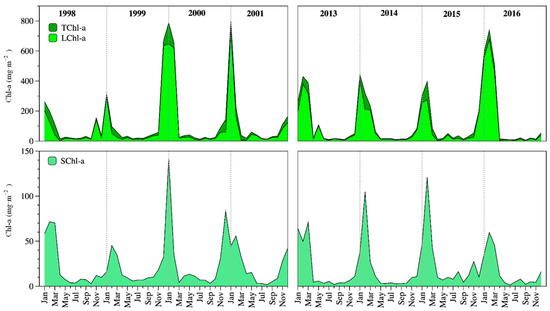
Figure 4.
Temporal dynamics of the ZMIX total (TChl-a, up), and fractionated Chl-a (LChl-a, up; SChl-a, down) in Lake Alchichica.
LChl-a contributed the highest percentage (≈80%) to the TChl-a concentration, while the SChl-a composed a minor percentage (≈20%). The temporal pattern of the TChl-a was similar to the large size phytoplankton fraction Chl-a (LChl-a) and the small size phytoplankton fraction (SChl-a). Average LChl-a was 96 ± 183 mg m−2 in 1998–2001 and 101 ± 162 mg m−2 in 2013–2016. Average SChl-a was 23 ± 27 mg m−2 in 1998–2001 and 20 ± 27 mg m−2 in 2013–2016 (Figure 4). There were no statistical differences in the average LChl-a (p = 0.232) or SChl-a (p = 0.081) concentrations between both periods.
3.2. The Rotifer Assemblage
The taxonomic list reported for Lake Alchichica along the 8-years (1998–2001 and 2013–2016 cycles) was limited to 2 species: Brachionus sp. Mexico and Hexarthra sp. Rotifers were absent in 21 out of 96 months, ≈22% of the eight years (1998–2001: ≈25%, 2013–2016: ≈19%). Brachionus sp. Mexico was undetected in 31.3% of the eight years (1998–2001: 29.2%, 2013–2016: 33.3%), while Hexarthra sp. in 45.8% of the eight years (1998–2001: 45.8%, 2013–2016: 45.8%).
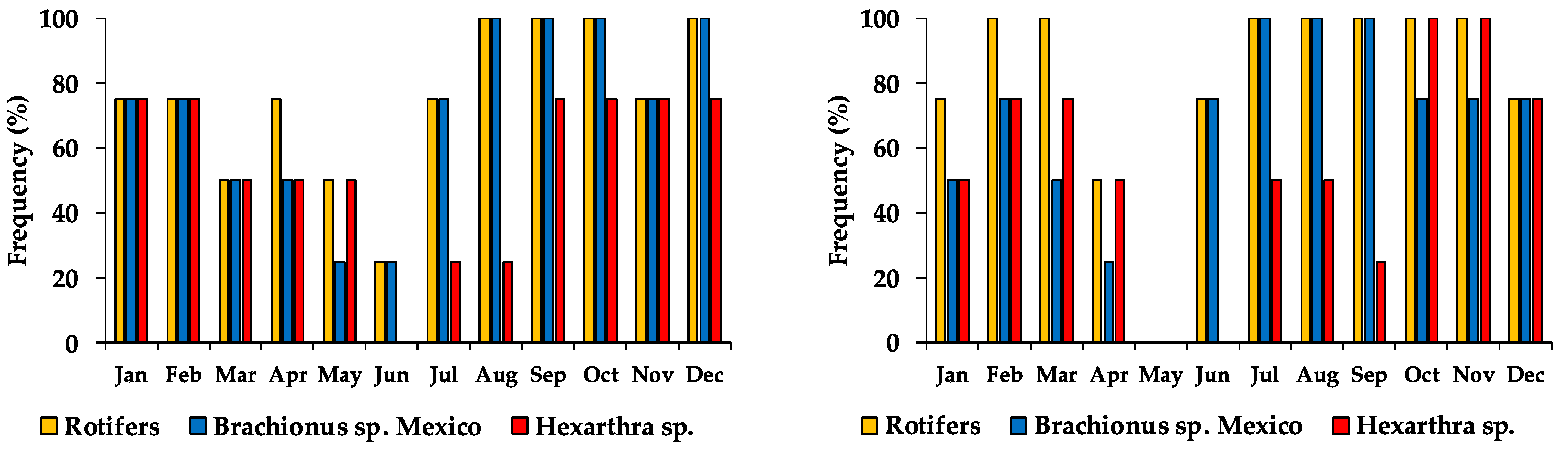
The rotifers’ appearance frequency in Lake Alchichica along the two four-years periods displayed a bimodal annual distribution curve with the lowest frequency of appearance in June (25%) and March and April (50%) in 1998–2001, and May (0%) and June (50%) in 2013–2015. Rotifer were mostly found during the mixing and early stratification period (January–April) and during the well-established and late stratification (August–December), while June (1998–2001) and May (2013–2016) showed the lowest rotifer occurrence (Figure 5). Brachionus sp. Mexico reached an average higher frequency of appearance with 70.8% (1998–2001) and 66.7% (2013–2016), while Hexarthra sp. only 54.2% in both periods.
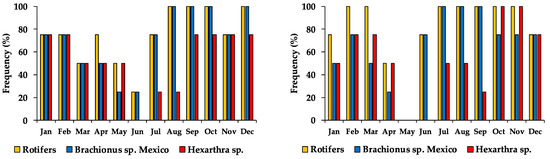
Figure 5.
Monthly frequency of appearance of rotifers in Lake Alchichica during 1998–2001 (left) and 2013–2016 (right). (100% of each bar = 4 appearances).
Rotifer abundance (Table 1) averaged 471,000 ± 1,211,000 ind m−2 with a range of 0 to 7,773,000 ind m−2. Brachionus sp. Mexico averaged 254,000 ± 776,000 ind m−2 (0–6,658,000 ind m−2) and Hexarthra sp. 217,000 ± 876,000 ind m−2 (0–7,700,000 ind m−2). When co-occurring, Brachionus sp. Mexico was more numerous (61%) than Hexarthra sp. (39%). Interestingly, while coinciding, the abundance rate was 6:1 either in favor of Brachionus sp. Mexico or Hexarthra sp.

Table 1.
Rotifer abundance and biomass (average ± standard deviation) of Lake Alchichica, Puebla, measured in 1998–2001 and 2013–2016.
The eight years rotifer biomass (Table 1) averaged 24 ± 63 mg DW m−2 with a range of 0 to 4152 mg DW m−2. Brachionus sp. Mexico averaged 13 ± 38 mg DW m−2 (0–328 mg DW m−2) and Hexarthra sp. 11 ± 472 mg DW m−2 (0–698 mg DW m−2).
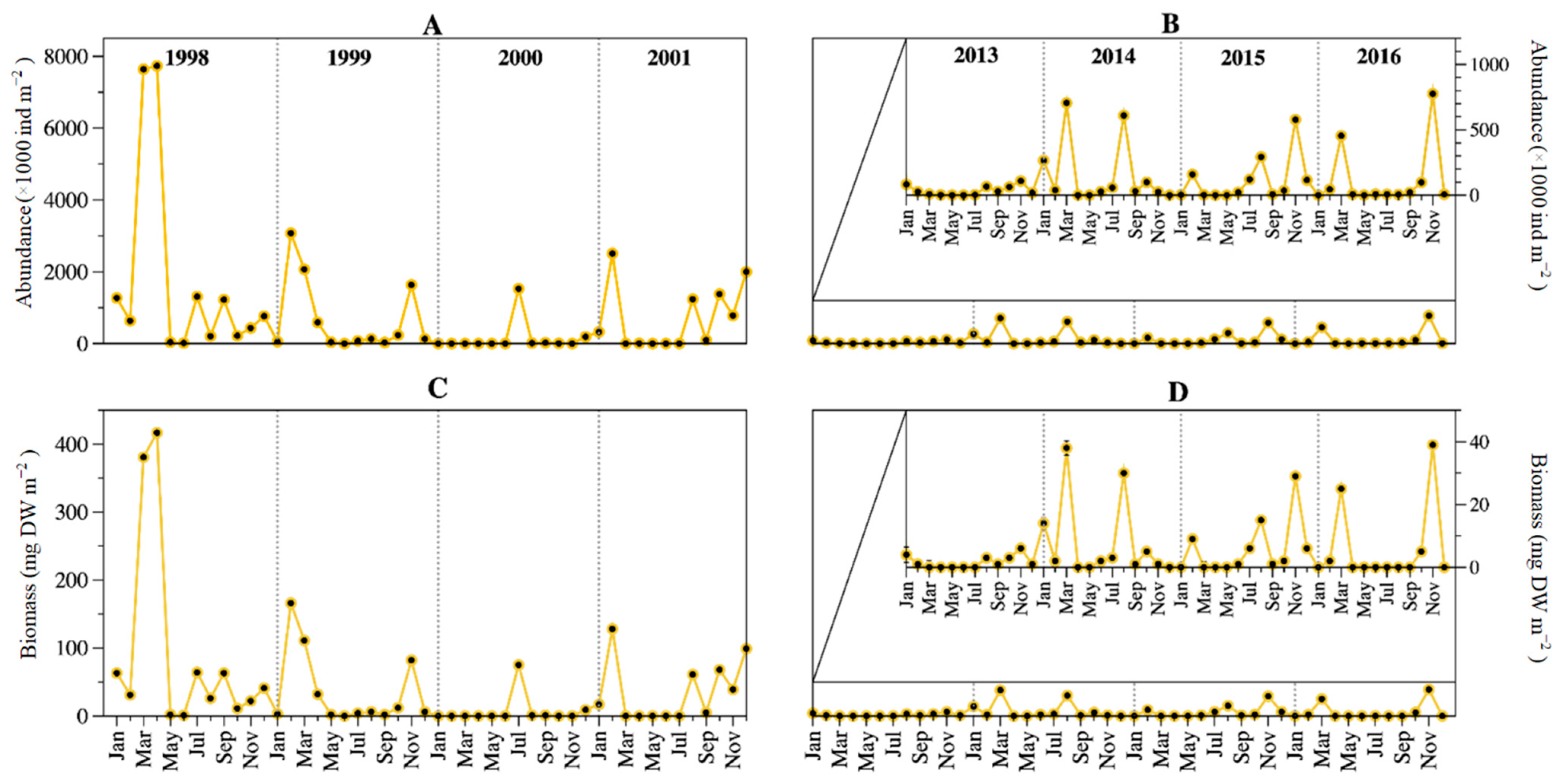
Besides the low abundance/biomass in May and June of both 4-years periods, there was not a regular pattern in the seasonal dynamics of Lake Alchichica rotifers when comparing both periods. Abundance and biomass dynamics were tightly coupled. Peaks at or above the 1998–2001 average values were found in February, March, July, and August, while at or above the 2013–2016 average values were found in January, March, August, and particularly November. Both 4-year periods coincided only in March and August (Figure 6).
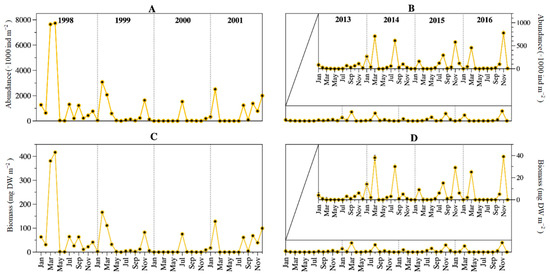
Figure 6.
Temporal dynamics of the rotifers’ abundance (A,B) and biomass (C,D) in Lake Alchichica during 1998–2001 and 2013–2016. (An amplification of the period is presented in (B,D) to better observe the temporal variation).
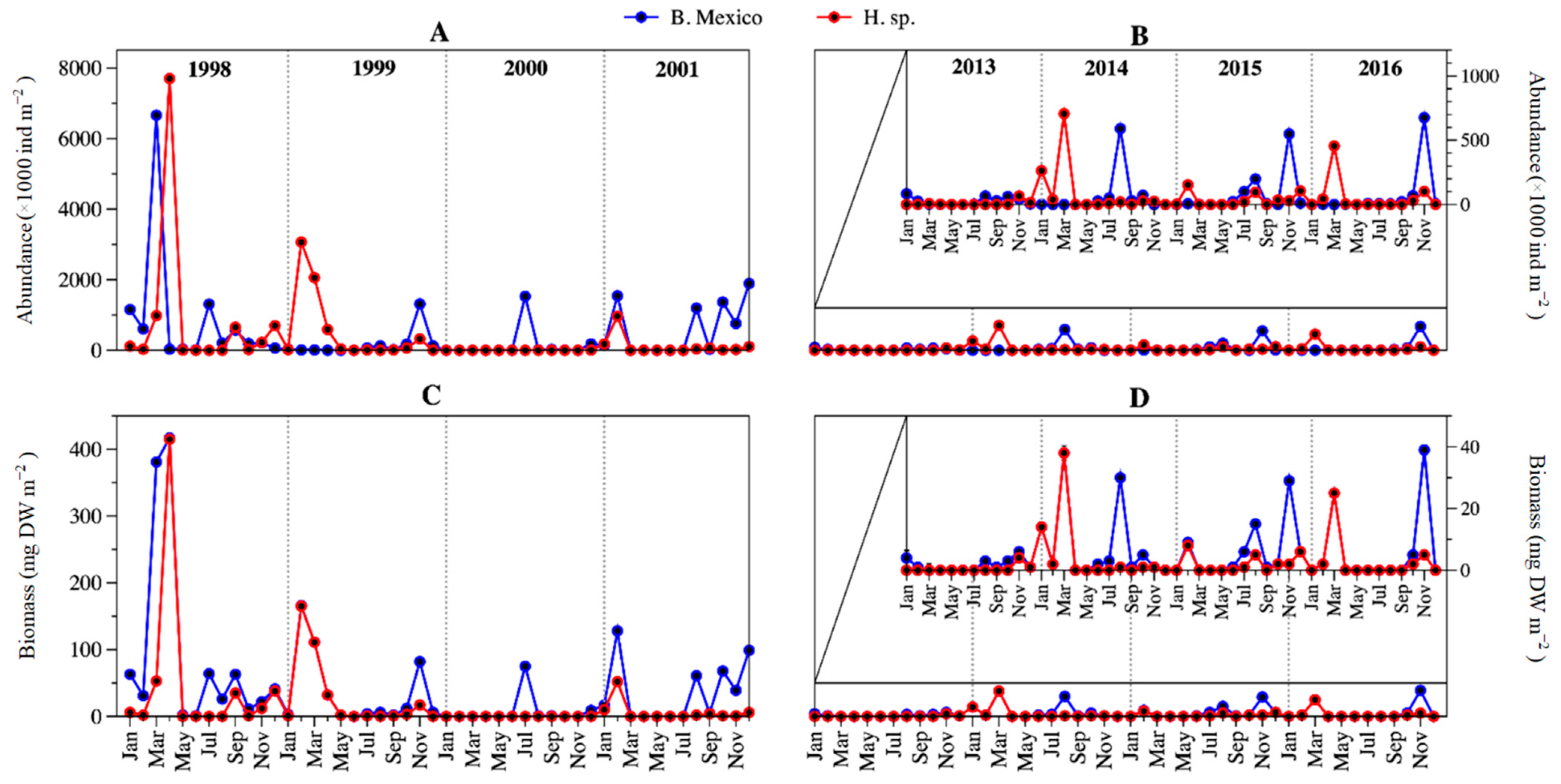
The abundance and biomass temporal dynamics of Brachionus sp. Mexico and Hexarthra sp. were different; abundance and biomass peaks of both species hardly coincided comparing both periods (Figure 7).
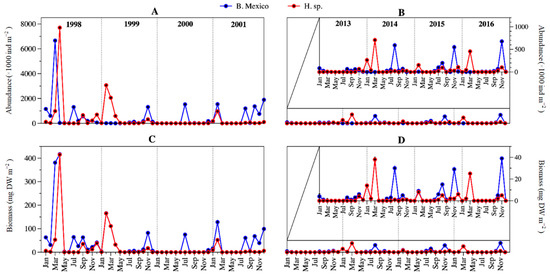
Figure 7.
Temporal dynamics of the Brachionus sp. Mexico and Hexarthra sp. abundance (A,B) and biomass (C,D) in Lake Alchichica during 1998–2001 and 2013–2016. (An amplification of the period is presented in (B,D) to better observe the temporal variation).
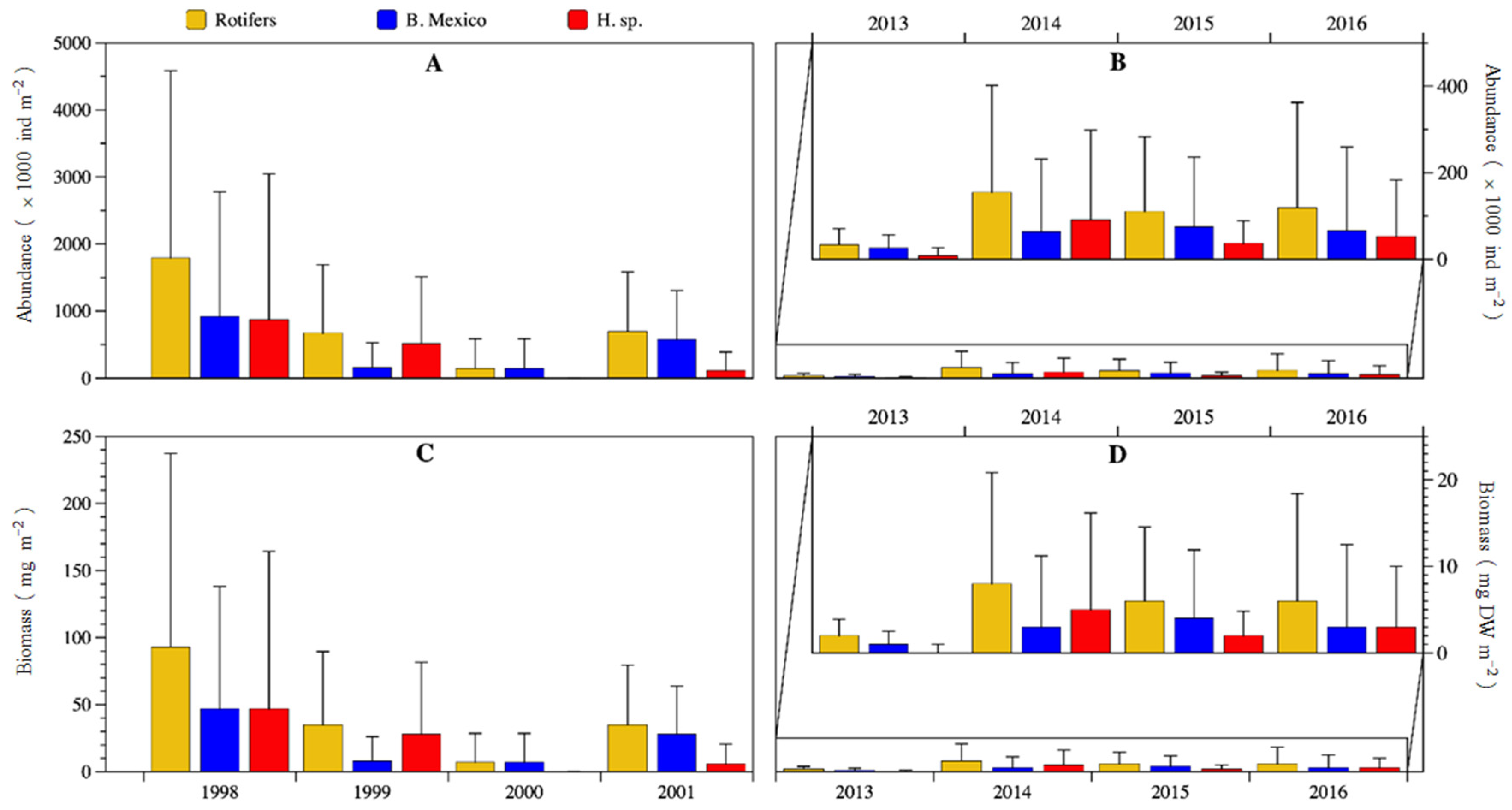
There was a statistically significant temporal decreasing trend from 1998 to 2016 (Man-Kendall test S = −18, p = 0.016) in the total rotifer biomass (Figure 8). Also, there was a large significative reduction in abundance and biomass from the 1998–2001 to the 2013–2016 period (U-Mann-Whitney test: abundance p = 0.02; biomass p = 0.017). Abundance decreased ≈87% from an average of 825,000 ± 1,632,000 ind m2 down to 105,000 ± 193,000 ind m−2; biomass decreased ≈87% from an average of 42.7 ± 84.6 mg DW m−2 down to 5.4 ± 9.9 mg DW m−2. Remarkably, 1998 was significantly different (much higher values, particularly in January, March, and April) as compared with 2000 (p = 0.00028), 2013 (p = 0.0077) and 2016 (p = 0.0079) (Dunn test post hoc probability values). When both 4-year periods were compared (U Mann-Whitney test), in the first period significant higher median values were found for Brachionus sp. Mexico abundance (p = 0.01) and biomass (p = 0.009), Hexarthra sp. biomass (p = 0.04) and total rotifers abundance (p = 0.02) and biomass (p = 0.002).
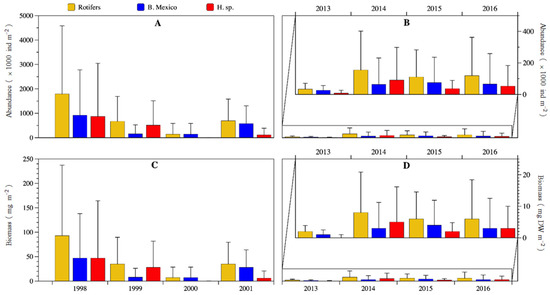
Figure 8.
Annual average abundance (A,B) and biomass (C,D) of rotifers of Lake Alchichica during 1998–2001 and 2013–2016. (An amplification of the period is presented in (B,D) to better observe the temporal variation).
Positive significant (p < 0.05) correlations were found for abundance and biomass of Hexarthra sp., and rotifer total abundance, and total biomass with ZMIX. Brachionus sp. Mexico abundance and biomass correlated positively with ZEU. Hexarthra sp. abundance and biomass were correlated positively with SChla. In contrast, Hexarthra sp. (abundance and biomass) had a significant negative correlation with TMIX (Table 2).

Table 2.
Non-parametric correlation coefficients (Spearman rho) between environmental and biological variables. Upper value: correlation coefficient; lower value: probability (Bonferroni correction); N = 96; only significative correlation (p < 0.05) are shown; negative correlations are in bold.
4. Discussion
The average TMIX augmented 0.8 °C over 18 years (Figure 3), this is ≈0.05 °C y−1, a rate which fits the [24] (i.e., 0.01 °C y−1) as indicative of climate change. Furthermore, the water temperature increase in Lake Alchichica fits the long-term (1996–2016) environmental temperature increasing trend reported by [25].
All described changes in environmental variables so far were slight; in such a way, it is hard to assign one of these variables to explain the significant change we found in the rotifer abundance and biomass between 1998–2001 and 2013–2016. Consequently, other factors must play a major role in regulating the seasonal and interannual rotifer variability in Lake Alchichica.
Salinity in saline lakes strongly assists in reducing rotifer species richness and favors species from the Brachionus plicatilis and Hexarthra jenkinae complexes. The low rotifer species richness (2 species) and composition (B. sp. Mexico and Hexarthra sp.) found in Lake Alchichica was similar to those reported for other saline lakes like [26,27] in Lakes La Alberca and Rincón de Parangueo, Guanajuato (B. polyodonta and H. inermis), by [28] in Lake Chitu, Ethiopia (B. plicatilis, and H. jenkinae), by [29] in Lake Pyramid, Nevada, USA (B. plicatilis, and H. jenkinae), and by [30] in Lake Werowrap, Australia (B. plicatilis, and H. jenkinae).
Fluctuations in rotifer abundance have been reported in other saline lakes and are associated with changes in salinity, primary production, or both [31,32,33,34]. However, Lake Alchichica’s salinity remained stable (8.5–9 g L−1) seasonally and long-term. Thus, salinity only affected the species composition of the zooplankton community of Lake Alchichica limiting it to the species that could live in it. Brachionus sp. Mexico and Hexarthra sp. are well-adapted to Lake Alchichica’s salinity since both cannot survive in freshwater [5,8].
Biological interactions could be considered by discarding the constant salinity as a factor affecting seasonal fluctuations in Lake Alchichica. Predation as an explanation of the rotifers’ irregularity is discarded. The only potential zooplankton predator is the silverside Poblana alchichica De Buen, 1945; however, this fish consumes mostly (70–80%) benthic prey and a low amount of zooplankton, particularly copepods but not rotifers [35].
Primary production was most likely the leading factor to explain the ample temporal fluctuations in rotifer abundance and biomass. It is known that resource limitation leads to suboptimal growth rates and affects competitive interactions [36]. By analyzing Chl-a concentration as a proxy of phytoplankton biomass, we found that the low values of Chl-a concentration did not change over 18 years (Figure 4). Nonetheless, the shortage of Chl-a (i.e., oligotrophic status), particularly in the SChl-a concentration (i.e., biomass dominated by large-sized phytoplankton) probably lead to strong food limitation on rotifers but does not explain the drastic reduction of the rotifer abundance and biomass from 1998–2001 to 2013–2016.
Phytoplankton composition and variation also enhances the low availability to rotifers at the onset of the stratification, May, and June displayed the lowest rotifer abundance. At the same time, Nodularia aff. spumigena blooms every year [37]. N. aff. spumigena is a large (20–2000 µm) filamentous cyanobacteria that, in addition, could produce cyanotoxins [38]. The unpalatability of these cyanobacteria (e.g., large size) and their toxicity most probably prevented their consumption by the Lake Alchichica rotifers. The large central diatom (35–63 µm) C. alchichicana dominates the phytoplankton biomass for the rest of the year and during the mixing period, between January and February, when blooms [39]. Although phytoplankton biomass increases, C. alchichicana is inedible for rotifers, as reported by [15]. Stochastic events in biotic and abiotic factors can momentarily benefit some species [40]. The latter could explain that rotifers’ availability of consumable resources occurs randomly.
In addition to resources, competition for food is a decisive factor that influences assemblage, abundance, and population dynamics [41,42]. Furthermore, there is a relationship between the dietary threshold and the body size in rotifers [43]. The food threshold concentration for zero population growth (C0) for Hexarthra sp. was twice as high as for Brachionus sp. Mexico [44]. Ortega-Mayagoitia et al. [14] found Brachionus sp. Mexico is a better competitor for food than Hexarthra sp., explaining that, in general, Brachionus sp. Mexico is more frequent (85%) and abundant than Hexarthra sp. (15%). Since both rotifer species rely on the same resource, it must be expected that competition for food [45] and differential resources consumption [46] lead to the displacement of one rotifer species, Hexarthra sp., most likely.
In addition to the competition between the two rotifers species, the continuous predominance of the calanoid copepod Leptodiaptomus garciai should also be considered. The throughout the year predominance of copepods in Lake Alchichica is due to the low food threshold compared to the two rotifers [44]. In addition, the copepods display higher internal energy reserves and lower loss rate of energy reserves when consumable resources are limited compared to the rotifers [15,47]. These ecophysiological characteristics give L. garciai a competitive advantage over B. sp. Mexico and Hexarthra sp. with which it coexists.
Finally, the correlation between the rotifers and the ZMIX most probably was related to nutrient release linked to a larger ZMIX. During the late stratification and the onset of the mixing period, the mixed layer expands. The nutrients trapped in the hypolimnion are released, favoring phytoplankton, and the subsequent zooplankton development followed with a bit of delay.
ZEU values were positively correlated with abundance and biomass of Brachionus sp. Mexico, but not Hexarthra sp. The highest ZEU values were found at the end of the well-established and the beginning of the late stratification. At the same time, ref [11] reported a peak of picoplankton developed in the upper 20 m, which might explain the rotifer abundance and biomass increase.
Hexarthra sp. abundance and biomass had a significant negative correlation (p < 0.05) with TMIX (Table 2). It means the lower abundance and biomass values of Hexarthra sp. were found during stratification with warmer and well-oxygenated ZMIX. During the circulation, the water column is colder and less oxygenated, particularly at the beginning of the circulation (i.e., resulting from the mixing between the anoxic hypolimnion and the oxygenated epilimnion). However, Chl-a concentration is higher, favoring higher abundances and biomasses of rotifers.
5. Conclusions
The rotifer species diversity of Lake Alchichica is restricted to two species, Brachionus sp. Mexico and Hexarthra sp.; both genera and the low observed species richness, have been previously reported from other saline lakes worldwide. Both rotifer species were scarce and intermittently present throughout the year. There was not a regular annual pattern. The highest frequencies of occurrence were in February, during the mixing period, and from August to October, at the end of the well-established and the beginning of the late stratification period.
The scarce food availability and the intense competition (among rotifers and with the calanoid copepod) for food seem to be responsible for the fluctuating and low presence and abundance of rotifers in Lake Alchichica on a seasonal basis and interannually.
A sharp decrease in the abundance and biomass of rotifers was found between 1998–2001 and 2013–2016. Climate change and stochastics events leading to pulses of the small-size phytoplankton, the rotifers’ food, and biotic interactions (competition among both species and with a better suited competitor, the calanoid copepod) seem to be the most plausible explanation.
Author Contributions
Conceptualization, R.F., J.A., A.L. and L.A.O.; Data curation, R.F., J.A. and S.G.-H.; Formal analysis, R.F., J.A., A.L. and L.A.O.; Funding acquisition, J.A.; Investigation, R.F., J.A., A.L., L.A.O. and S.G.-H.; Methodology, R.F., J.A., A.L., L.A.O. and S.G.-H.; Project administra-tion, L.A.O.; Resources, J.A.; Software, A.L. and L.A.O.; Supervision, R.F., J.A. and A.L.; Validation, R.F., J.A., A.L. and L.A.O.; Visualization, R.F., A.L. and L.A.O.; Writing—original draft, R.F., J.A., A.L. and L.A.O.; Writing—review & editing, R.F., J.A., A.L., L.A.O. and S.G.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Consejo Nacional de Ciencia y Tecnología (CONACYT) through the projects 25430-T, 34893-T and 103332, the Universidad Nacional Autónoma de México DGAPA/PAPIIT through the projects IN204597, IN215512, IN231820, and IN219220, and the Universidad Nacional Autónoma de México, FES Iztacala PAPCA 2013–2014, PAPCA 2014 and PAPCA 2000.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
We thank Laura Peralta for her field and laboratory support and Rolando Tirado Cruz for his laboratory support. We thank the anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions that significantly improved it.
Conflicts of Interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- Alcocer, J. Lago Alchichica: Una Joya de Biodiversidad; Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2019; ISBN 978-607-30-2278-1. [Google Scholar]
- Ortega-Mayagoitia, E.; Vilaclara, G.; Alcántara-Hernández, R.J.; Macek, M. Diversity and endemisms. In Lake Alchichica Limnology: The Uniqueness of a Tropical Maar Lake; Alcocer, J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Oliva, M.G.; Lugo, A.; Alcocer, J.; Cantoral-Uriza, E.A. Cyclotella alchichicana sp. nov. from a saline mexican lake. Diatom Res. 2006, 21, 81–89. [Google Scholar] [CrossRef]
- Montiel-Martínez, A.; Ciros-Pérez, J.; Ortega-Mayagoitia, E.; Elías-Gutiérrez, M. Morphological, ecological, reproductive and molecular evidence for Leptodiaptomus garciai (Osorio-Tafall 1942) as a valid endemic species. J. Plankton Res. 2008, 30, 1079–1093. [Google Scholar] [CrossRef]
- Sarma, S.S.S.; Elguea-Sánchez, B.; Nandini, S. Effect of Salinity on Competition between the Rotifers Brachionus rotundiformis Tschugunoff and Hexarthra jenkinae (De Beauchamp) (Rotifera). Hydrobiologia 2002, 474, 183–188. [Google Scholar] [CrossRef]
- Mills, S.; Alcántara-Rodríguez, J.A.; Ciros-Pérez, J.; Gómez, A.; Hagiwara, A.; Galindo, K.H.; Jersabek, C.D.; Malekzadeh-Viayeh, R.; Leasi, F.; Lee, J.S.; et al. Fifteen species in one: Deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 2017, 796, 39–58. [Google Scholar] [CrossRef]
- Ortega-Mayagoitia, E.; Alcántara-Rodríguez, R.J.; Lugo-Vázquez, A.; Montiel-Martínez, A.; Ciros-Pérez, J. The joys and challenges of living in a saline, oligotrophic, warm monomictic lake. In Lake Alchichica Limnology: The Uniqueness of a Tropical Maar Lake; Alcocer, J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Alcántara-Rodríguez, J.A.; Ciros-Pérez, J.; Ortega-Mayagoitia, E.; Serrania-Soto, C.R.; Piedra-Ibarra, E. Local adaptation in populations of a Brachionus group plicatilis cryptic species inhabiting three deep crater lakes in Central Mexico. Freshw. Biol. 2012, 57, 728–740. [Google Scholar] [CrossRef]
- Alcocer, J.; Lugo, A.; Escobar, E.; Sànchez, M.R.; Vilaclara, G. Water column stratification and its implications in the tropical warm monomictic Lake Alchichica, Puebla, Mexico. Verh. Int. Ver. Theor. Angew. Limnol. 2000, 27, 3166–3169. [Google Scholar] [CrossRef]
- Macek, M.; Alcocer, J.; Lugo Vázquez, A.; Martínez-Pérez, M.E.; Peralta Soriano, L.; Vilaclara Fatjó, G. Long term picoplankton dynamics in a warm-monomictic, tropical high altitude lake. J. Limnol. 2009, 68, 183–192. [Google Scholar] [CrossRef]
- Cuevas-Lara, J.D.; Alcocer, J.; Oseguera, L.A.; Quiroz-Martínez, B. Dinámica a largo plazo (1999–2014) de la productividad primaria fitoplanctónica en el Lago Alchichica. In Estado Actual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis a 2016; Paz, F., Torres, R., Eds.; Programa Mexicano del Carbono en Colaboración con la Universidad Autónoma del Estado de Hidalgo: Texcoco, Mexico, 2016; pp. 280–286. ISBN 978-607-96490-4-3. [Google Scholar]
- González Contreras, C.G.; Alcocer, J.; Oseguera, L.A. Phytoplankton chlorophyll a in the tropical deep Alchichica: A long-term record (1999–2010). Hidrobiológica 2015, 25, 347–356. [Google Scholar]
- Adame, M.F.; Alcocer, J.; Escobar, E. Size-fractionated phytoplankton biomass and its implications for the dynamics of an oligotrophic tropical lake. Freshw. Biol. 2008, 53, 22–31. [Google Scholar] [CrossRef]
- Ortega-Mayagoitia, E.; Ciros-Pérez, J.; Sánchez-Martínez, M. A story of famine in the pelagic realm: Temporal and spatial patterns of food limitation in rotifers from an oligotrophic tropical lake. J. Plankton Res. 2011, 33, 1574–1585. [Google Scholar] [CrossRef]
- Filonov, A.; Tereshchenko, I.; Barba-López, M.R.; Alcocer, J.; Ladah, L. Meteorological regime, local climate, and hydrodynamics of Lake Alchichica. In Lake Alchichica Limnology: The Uniqueness of a Tropical Maar Lake; Alcocer, J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Vilaclara, G.; Chávez, M.; Lugo, A.; González, H.; Gaytán, M. Comparative description of crater-lakes basic chemistry in Puebla State, Mexico. Verh. Int. Ver. Theor. Angew. Limnol. 1993, 25, 435–440. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Quinta; Instituto de Geografía, UNAM: Ciudad de México, Mexico, 2004; ISBN 9703210104. [Google Scholar]
- Ramírez-Olvera, M.A.; Alcocer, J.; Merino-Ibarra, M.; Lugo, A. Nutrient limitation in a tropical saline lake: A microcosm experiment. Hydrobiologia 2009, 626, 5–13. [Google Scholar] [CrossRef]
- Arar, E.J.; Collins, G.B. Method 445.0 In Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1997.
- Ruttner-Kolisko, A. Suggestions for biomass calculation of plankton rotifers. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1977, 8, 71–76. [Google Scholar]
- McCauley, E. The estimation of the abundance and biomass of zooplankton in samples. In A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters; Downing, J., Rigler, F.H., Eds.; Blackwell Scientific Pub: Oxford, UK, 1984; pp. 228–265. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, D. PAST: Paleontological Statistics Software Package for Education and Data Analysis; Paleontological Society: McLean, VA, USA, 2001. [Google Scholar]
- Intergovernmental Panel on Climate Change. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Silva-Aguilera, R.; Escolero, O.; Alcocer, J. Recent Climate of Serdán-Oriental Basin. In Lake Alchichica Limnology. The Uniqueness of a Tropical Maar Lake; Javier, A., Ed.; Springer: Cham, Switzerland, 2022; pp. 51–91. [Google Scholar]
- Green, J. Associations of zooplankton in six crater lakes in Arizona, Mexico and New Mexico. J. Zool. 1986, 208, 135–159. [Google Scholar] [CrossRef]
- Alcocer, J.; Hammer, U.T. Saline lake ecosystems of Mexico. Aquat. Ecosyst. Health Manag. 1998, 1, 291–315. [Google Scholar] [CrossRef]
- Green, J. Zooplankton associations in some Ethiopian crater lakes. Freshw. Biol. 1986, 16, 495–499. [Google Scholar] [CrossRef]
- Galat, D.L.; Lider, E.L.; Vigg, S.; Robertson, R.L. Limnology of a large, deep, North American terminal lake, Pyramyd Lake, Nevada, U.S.A. In Salt Lakes. Developments in Hydrobiology; Williams, W.D., Ed.; Springer: Dordrecht, The Netherlands, 1981; Volume 5, pp. 281–317. [Google Scholar]
- Walker, K.F. Studies on a saline lake ecosystem. Aust. J. Mar. Freshw. Res. 1983, 24, 21–72. [Google Scholar] [CrossRef]
- Hammer, U.T. A comparative study of primary production and related factors in four saline lakes in Victoria, Australia. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1981, 66, 701–743. [Google Scholar] [CrossRef]
- Khan, T.A. Limnology of four saline lakes in western Victoria, Australia II. Biological parameters. Limnologica 2003, 33, 327–339. [Google Scholar] [CrossRef]
- Toruan, R.L. Zooplankton community emerging from fresh and saline wetlands. Ecohydrol. Hydrobiol. 2012, 12, 53–63. [Google Scholar] [CrossRef]
- Afonina, E.Y.; Tashlykova, N.A. Plankton of saline lakes in southeastern Transbaikalia: Transformation and environmental factors. Contemp. Probl. Ecol. 2019, 12, 155–170. [Google Scholar] [CrossRef]
- Alcocer, J.; Arce, E.; Zambrano, L.; Chiappa-Carrara, X. Poblana alchichica: A threatened silverside species? Verh. Int. Ver. Theor. Angew. Limnol. 2010, 30, 1429–1432. [Google Scholar] [CrossRef]
- Tilma, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Oliva, M.G.; Lugo, A.; Alcocer, J.; Peralata, L.; Oseguera, L.A. Planktonic bloom-forming Nodularia in the saline Lake Alchichica, Mexico. Nat. Resour. Environ. Issues 2009, 15, 121–126. [Google Scholar]
- Engström-Öst, J.; Koski, M.; Schmidt, K.; Viitasalo, M.; Jónasdóttir, S.H.; Kokkonen, M.; Repka, S.; Sivonen, K. Effects of toxic cyanobacteria on a plankton assemblage: Community development during decay of Nodularia spumigena. Mar. Ecol. Prog. Ser. 2002, 232, 1–14. [Google Scholar] [CrossRef][Green Version]
- Ardiles, V.; Alcocer, J.; Vilaclara, G.; Oseguera, L.A.; Velasco, L. Diatom fluxes in a tropical, oligotrophic lake dominated by large-sized phytoplankton. Hydrobiologia 2012, 679, 77–90. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Padisák, J.; Sommer, U. Intermediate disturbance in the ecology of phytoplankton and the maintenance of species diversity: A synthesis. Hydrobiologia 1993, 249, 183–188. [Google Scholar] [CrossRef]
- Walz, N. Rotifer populations in plankton communities: Energetics and life history strategies. Experientia 1995, 51, 437–453. [Google Scholar] [CrossRef]
- Obertegger, U.; Flaim, G. Taxonomic and functional diversity of rotifers, what do they tell us about community assembly? Hydrobiologia 2018, 823, 79–91. [Google Scholar] [CrossRef]
- Stemberger, R.S.; Gilbert, J.J. Rotifer threshold food concentrations and the size-efficiency hypothesis. Ecology 1987, 68, 181–187. [Google Scholar] [CrossRef]
- Ciros-Pérez, J.; Ortega-Mayagoitia, E.; Alcocer, J. The role of ecophysiological and behavioral traits in structuring the zooplankton assemblage in a deep, oligotrophic, tropical lake. Limnol. Oceanogr. 2015, 60, 2158–2172. [Google Scholar] [CrossRef]
- Quintana, X.D.; Arim, M.; Badosa, A.; Blanco, J.M.; Boix, D.; Brucet, S.; Compte, J.; Egozcue, J.J.; de Eyto, E.; Gaedke, U.; et al. Predation and competition effects on the size diversity of aquatic communities. Aquat. Sci. 2015, 77, 45–57. [Google Scholar] [CrossRef]
- Ciros-Pérez, J.; Carmona, M.J.; Serra, M. Resource competition between sympatric sibling rotifer species. Limnol. Oceanogr. 2001, 46, 1511–1523. [Google Scholar] [CrossRef]
- Ortega-Mayagoitia, E.; Hernández-Martínez, O.; Ciros-Pérez, J. Phenotypic plasticity of life-history traits of a calanoid copepod in a tropical lake: Is the magnitude of thermal plasticity related to thermal variability? PLoS ONE 2018, 13, e0196496. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).