Pronounced Seasonal Diet Diversity Expansion of Golden Eagles (Aquila chrysaetos) in Northern Greece during the Non-Breeding Season: The Role of Tortoises
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sampling Sites
2.2. Prey Item Sampling
2.3. Prey Item Identification and Counting
2.4. Spatial Structure and Environmental Parameters
2.5. Field Observations
2.6. Data Analysis
3. Results
3.1. Golden Eagle Diet
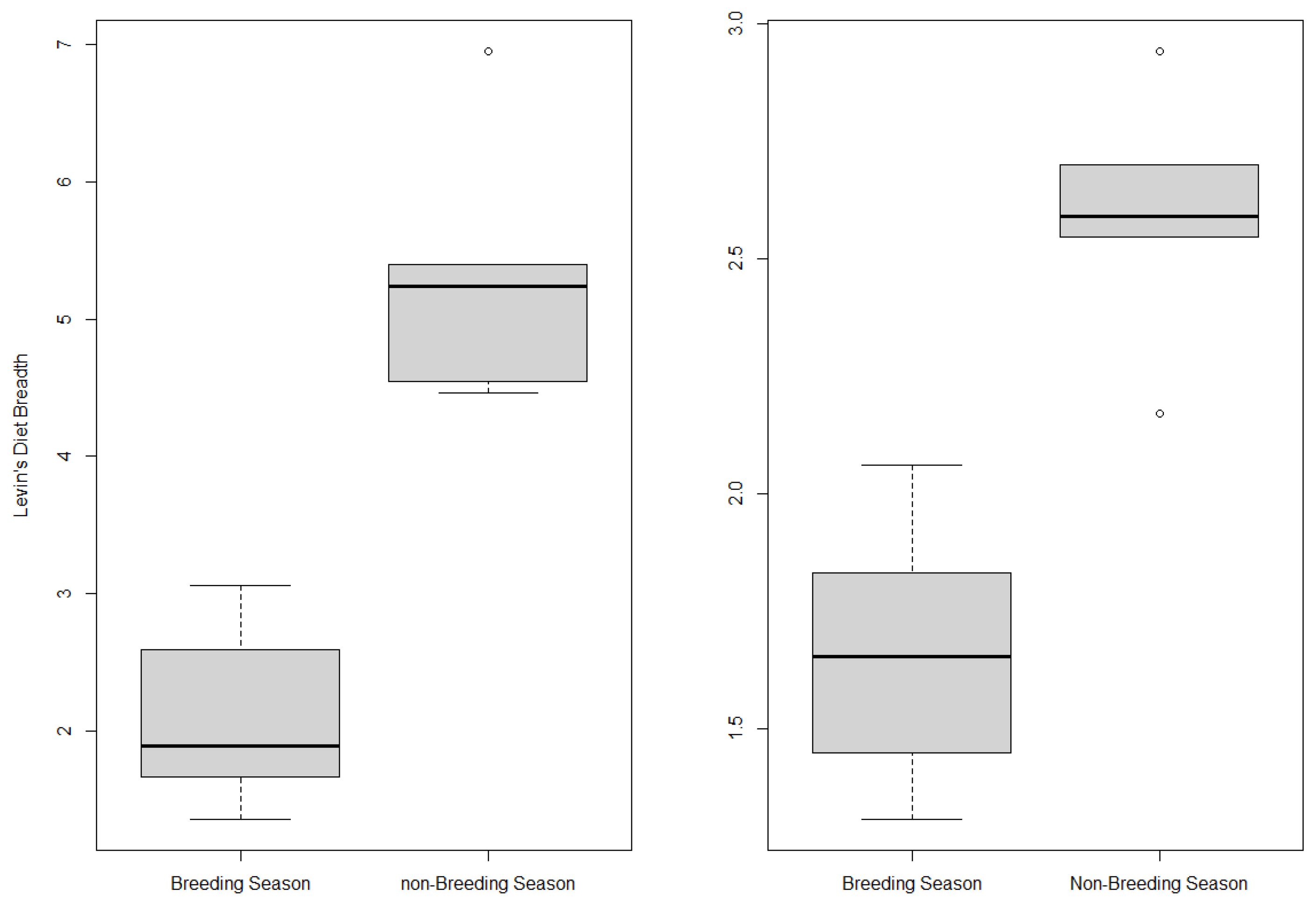
3.2. Seasonal Variation of Golden Eagle Diet
3.3. Relationships of Golden Eagle Diet to Spatial and Habitat Characteristics
3.4. Field Observations
4. Discussion
4.1. Golden Eagle Diet
4.2. Seasonal Variation of Eagle Diet
4.3. Habitat Variables and Diet
4.4. Tortoise Dependence
4.5. Methodological Insights
4.6. Conservation Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Class Category | Class | Order | Family | N | % | Lowest Taxonomic Level Identified |
|---|---|---|---|---|---|---|
| Mammals all other | Mammalia | - | - | 4 | 0.5 | |
| Mammals all other | Cheiroptera | Molossidae | 1 | 0.1 | Tandarida teniotis b (1) | |
| Hedgehogs | Eulipotyphla | Erinaceidae | 34 | 4.1 | Erinaceus concolor (35) | |
| Mammals all other | Eulipotyphla | Soricidae | 3 | 0.4 | - | |
| Hares | Lagomorpha | Leporidae | 15 | 1.8 | Lepus capensis (15) | |
| Mammals all other | Rodentia | Muridae | 15 | 1.8 | Mus musculus (1), Apodemus sp (3), Rattus sp (2), unidentified (9) | |
| Glirids and Sciurids | Rodentia | Gliridae | 13 | 1.6 | Glis glis | |
| Glirids and Sciurids | Rodentia | Sciuridae | 10 | 1.2 | Sciurus vulgaris | |
| Carnivores | Carnivora | Canidae | 16 | 1.9 | Canis sp nb (2), V vulpes (14) | |
| Carnivores | Carnivora | Mustelidae | 29 | 3.5 | M Meles b (2), Martes foina (19), Martes sp (5), Mustela nivalis b (2), M putorius b (1) | |
| Carnivores | Carnivora | Felidae | 2 | 0.2 | Felis catus nb | |
| Ungulates | Artiodactyla | Bovidae | 15 | 1.8 | Caprini (5), Capra hircus (7), Ovis ariesnb (2), Bos taurus b (1) | |
| Ungulates | Artiodactyla | Cervidae | 3 | 0.4 | Capreolus capreolus nb | |
| Ungulates | Artiodactyla | Suidae | 2 | 0.2 | Sus domestica b | |
| Ungulates | Perissodactyla | Equidae | 1 | 0.1 | Equus sp b | |
| Birds all other | Aves | - | - | 11 | 1.3 | |
| Warerbirds | Pelecaniformes | Phalacrocoracidae | 1 | 0.1 | Phalacrocorax carbo b | |
| Waterbirds | Anseriformes | Anatidae | 2 | 0.2 | Anas strepera b (1) | |
| Raptors and Owls | Accipitriformes | Accipitridae | 2 | 0.2 | Buteo buteo b (1) | |
| Raptors and Owls | Falconiformes | Falconidae | 1 | 0.1 | ||
| Birds all other | Galliformes | Phasianidae | 3 | 0.4 | Alectoris graeca b (2), C coturnix b (1) | |
| Waterbirds | Charadriiformes | Laridae | 16 | 1.9 | Larus michahellis (14), Larus sp (2) | |
| Thrushes and pigeons | Columbiformes | Columbidae | 12 | 1.5 | Columba palumbus (5), C livia domestica (5), Streptopelia turtur b (1), unidentified 1 | |
| Birds all other | Apodiformes | Apodidae | 1 | 0.1 | Apus melba b | |
| Birds all other | Caprimulgiformes | Caprimulgidae | 2 | 0.2 | Caprimulgus europaeus b | |
| Raptors and owls | Stringiformes | Stringidae | 1 | 0.1 | Athene noctua nb | |
| Birds all other | Passeriformes | - | 9 | 1.1 | ||
| Birds all other | Passeriformes | Alaudidae | 1 | 0.1 | Galerida cristata nb | |
| Thrushes and pigeons | Passeriformes | Turdidae | 22 | 2.7 | Turdus philomelos 8, T merula 5, Turdus spnb (5), T viscivorus 3, T pilaris 1 | |
| Birds all other | Passeriformes | Paridae | 1 | 0.1 | Cyanistes caeruleus nb | |
| Birds all other | Passeriformes | Sturnidae | 7 | 0.8 | Sturnus vulgaris | |
| Corvids | Passeriformes | Corvidae | 18 | 2.2 | Garrulus glandarius (15), Corvus cornix nb (2), Pica pica (1) | |
| Birds all other | Passeriformes | Fringillidae | 2 | 0.2 | ||
| Snakes and lizards | Reptilia | Squamata | Anguidae | 24 | 2.9 | Pseudopous apodus |
| Snakes and lizards | Squamata | Lacertidae | 6 | 0.7 | Lacerta spp. 6, Podarcis spp 1 | |
| Snakes and lizards | Serpentes | - | 3 | 0.4 | ||
| Snakes and lizards | Ophidia | Colubridae | 7 | 0.8 | Elaphe situla b (2), Dolichophis caspius (4), Platyceps najadum nb (1) | |
| Snakes and lizards | Ophidia | Psammophilidae | 3 | 0.4 | Malpolon insignitus | |
| Tortoises | Chelonia | Testudinidae | 511 | 61.8 | Eurotestudo hermanni (133), Testudo graeca (56), Testudo spp. (322) | |
| 827 |
Appendix B
| Prey Category | N of Instances | N of Successes | Breeding Season | Non–Breeding Season |
|---|---|---|---|---|
| Corvids | 4 | 0 | 3 | 1 |
| Raptors and owls | 3 | 0 | 2 | 1 |
| Thrushes and pigeons | 1 | 1 | 0 | 1 |
| Waterbirds | 1 | 0 | 1 | 0 |
| Other birds | 4 | 2 | 4 | 0 |
| Carnivores | 7 | 7 | 2 | 5 |
| Glirids and Sciurids | 1 | 1 | 1 | 0 |
| Hedgehogs | 2 | 2 | 1 | 1 |
| Hares | 1 | 1 | 1 | 0 |
| Ungulates | 7 | 5 | 1 | 6 |
| Other mammals | 1 | 1 | 2 | 2 |
| Snakes and lizards | 12 | 12 | 9 | 3 |
| Tortoises | 57 | 57 | 53 | 4 |
| Ungulates (carrion and offal) left on designated sites | 96 | 21 | 75 | |
References
- Newton, I. Population Ecology of Raptors; Poyser: Berkhamstead, UK, 1979. [Google Scholar]
- Watson, J.; Rae, S.R.; Stillman, R. Nesting Density and Breeding Success of Golden Eagles in Relation to Food Supply in Scotland. J. Anim. Ecol. 1992, 61, 543. [Google Scholar] [CrossRef]
- Fernandez, C. Effect of the Viral Haemorrhagic Pneumonia of the Wild Rabbit on the Diet and Breeding Success of the Golden Eagle Aquila Chrysaëtos (L.). Revue d’Écol. 1993, 48, 323–329. [Google Scholar]
- Clouet, M.; Gerard, J.-F.; Goar, J.-L.; Goulard, M.; González, L.; Rebours, I.; Faure, C. Diet and Breeding Performance of the Golden Eagle Aquila chrysaetos at the Eastern and Western Extremities of the Pyrenees: An Example of Intra-Population Variability. Ardeola 2017, 64, 347–361. [Google Scholar] [CrossRef]
- Cadahía, L.; López-López, P.; Urios, V.; Negro, J.J. Satellite telemetry reveals individual variation in juvenile Bonelli’s eagle dispersal areas. Eur. J. Wildl. Res. 2010, 56, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Resano-Mayor, J.; Hernández-Matías, A.; Real, J.; Parés, F.; Moleón, M.; Mateo, R.; Ortiz-Santaliestra, M.E. The influence of diet on nestling body condition of an apex predator: A multi-biomarker approach. J. Comp. Physiol. B 2016, 186, 343–362. [Google Scholar] [CrossRef]
- Rutz, C.; Bijlsma, R.G. Food-limitation in a generalist predator. Proc. R. Soc. B Boil. Sci. 2006, 273, 2069–2076. [Google Scholar] [CrossRef] [Green Version]
- Reif, V.; Tornberg, R.; Jungell, S.; Korpimäki, E. Diet variation of common buzzards in Finland supports the alternative prey hypothesis. Ecography 2001, 24, 267–274. [Google Scholar] [CrossRef]
- Watson, J. The Golden Eagle, 2nd ed.; Poyser: London, UK, 2010. [Google Scholar]
- Bedrosian, G.; Watson, J.W.; Steenhof, K.; Kochert, M.N.; Preston, C.R.; Woodbridge, B.; Williams, G.E.; Keller, K.R.; Crandall, R.H. Spatial and Temporal Patterns in Golden Eagle Diets in the Western United States, with Implications for Conservation Planning. J. Raptor Res. 2017, 51, 347–367. [Google Scholar] [CrossRef] [Green Version]
- Seguin, J.F.; Thibault, J.C.; Torre, J.; Bayle, P.; Vigne, J.D. The Diet of Young Golden Eagles Aquila chrysaetos in Corsica: Foraging in a Man-Made Mammal Fauna. Ardea 2001, 89, 527–535. [Google Scholar]
- Whitfield, D.P.; Reid, R.; Haworth, P.F.; Madders, M.; Marquiss, M.; Tingay, R.; Fielding, A.H. Diet specificity is not associated with increased reproductive performance of Golden Eagles Aquila chrysaetos in Western Scotland. Ibis 2009, 151, 255–264. [Google Scholar] [CrossRef]
- Takeuchi, T.; Shiraki, S.; Nashimoto, M.; Matsuki, R.; Abe, S.; Yatake, H. Regional and temporal variations in prey selected by Golden Eagles Aquila chrysaetos during the nestling period in Japan. Ibis 2006, 148, 79–87. [Google Scholar] [CrossRef]
- Tjernberg, M. Diet of the golden eagle Aquila chrysaetos during the breeding season in Sweden. Ecography 1981, 4, 12–19. [Google Scholar] [CrossRef]
- Högström, S.; Wiss, L. Diet of the Golden Eagle Aquila chrysaetos (L.) in Gotland, Sweden during the Breeding Season. Ornis Fennica 1992, 69, 39–44. [Google Scholar]
- Brown, J.L.; Bedrosian, G.; Keller, K.R. Habitat Associations of Golden Eagle Prey Inferred from Prey Remains at Nesting Sites in Utah, USA. J. Raptor Res. 2021, 55, 1–16. [Google Scholar] [CrossRef]
- Bakaloudis, D.E.; Vlachos, C.G. Feeding Habits and Provisioning Rate of Breeding Short-Toed Eagles Circaetus Gallicus in Northeastern Greece. J. Biol. Res. 2011, 16, 166–176. [Google Scholar]
- Vlachos, C.G.; Papageorgiou, N.K. Breeding Biology and Feeding of the Lesser Spotted Eagle Aquila Pomarina in Dadia Forest, North-Eastern Greece. In Eagle Studies; Meyburg, B.-U., Chancellor, R.D., Eds.; The World Working Group on Birds of Prey (WWGBP): Berlin, Germany, 1996; pp. 337–347. [Google Scholar]
- Dobrev, V.; Boev, Z.; Arkumarev, V.; Dobrev, D.; Kret, E.; Saravia, V.; Bounas, A.; Vavylis, D.; Nikolov, S.C.; Oppel, S. Diet is not related to productivity but to territory occupancy in a declining population of Egyptian Vultures Neophron percnopterus. Bird Conserv. Int. 2015, 26, 273–285. [Google Scholar] [CrossRef]
- Alivizatos, H.; Goutner, V. Feeding Habits of the Long-Legged Buzzard (Buteo Rufinus) during Breeding in Northeastern Greece. Isr. J. Zool. 1997, 43, 257–266. [Google Scholar] [CrossRef]
- Willemsen, R.E.; Hailey, A. Variation in adult survival rate of the tortoise Testudo hermanni in Greece: Implications for evolution of body size. J. Zool. 2001, 255, 43–53. [Google Scholar] [CrossRef]
- Handrinos, G.; Kastritis, A. Birds. In The Red Data Book of Threatened Animals in Greece; Legakis, A., Maragkou, P., Eds.; Hellenic Zoological Society: Athens, Greece, 2009; pp. 213–354, (In Greek with English Summaries). [Google Scholar]
- Ministry of Environment and Energy. Article 12 Reporting for the Implementation of the 2009/147/EC. 2019. Available online: https://cdr.eionet.europa.eu/Converters/run_conversion?file=gr/eu/art12/envxz8njg/GR_birds_reports_20191031-152940.xml&conv=612&source=remote#A091_B (accessed on 15 December 2021).
- Sidiropoulos, L. The Golden Eagle (Aquila chrysaetos, L.) in the Rhodope Mts: Modelling Densities, Distribution and Population Viability to Inform Conservation. Master’s Thesis, Division of Biology, Imperial College London, London, UK, 2012. [Google Scholar]
- Speybroeck, J.; Beukema, W.; Bok, B.; van der Voort, J.; Velikov, I. Field Guide to the Amphibians and Reptiles of Britain and Europe; Bloomsbury: London, UK, 2016. [Google Scholar]
- Sulkava, S.; Huhtala, K.; Rajala, P.; Tornberg, R. Changes in the Diet of the Golden Eagle Aquila chrysaetos and Small Game Populations in Finland in 1957–96. Ornis Fennica 1999, 76, 1–16. [Google Scholar]
- Featherbase. Featherbase: Online Avian Plumages Collection. Available online: https://www.featherbase.info/en/home (accessed on 6 November 2021).
- Brom, T.G. Microscopic Identification of Feathers and Feather Fragments of Palearctic Birds. Bijdragen Dierkunde 1986, 56, 181–204. [Google Scholar] [CrossRef] [Green Version]
- Teerink, B.J. Hair of West-European Mammals; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Papageorgiou, N.; Sfouggaris, A.; Vlachos, C.; Bakaloudis, D. Identification of Reptiles through Scale Morphology; Department of Forestry and the Natural Environment, Aristotle University of Thessaloniki (AUTh): Thessaloniki, Greece, 1993. [Google Scholar]
- Marti, C.D.; Bechard, M.; Jaksic, F.M. Food Habits. In Raptor Research and Management Techniques; Bildstein, K., Bird, D.M., Eds.; Hanckock House: Blaine, WA, USA, 2007; pp. 129–152. [Google Scholar]
- Katzner, T.E.; Bragin, E.A.; Knick, S.T.; Smith, A.T. Spatial structure in the diet of imperial eagles Aquila heliaca in Kazakhstan. J. Avian Biol. 2006, 37, 594–600. [Google Scholar] [CrossRef]
- Krebs, C. Ecological Methodology; Benjamin/Cummings: Menlo Park, CA, USA, 1999. [Google Scholar]
- European Environment Agency/European Union. Copernicus Land Monitoring Service. CORINE Land Cover Dataset 2018. Available online: https://www.eea.europa.eu/data-and-maps/data/copernicus-land-monitoring-service-corine (accessed on 10 December 2021).
- European Environment Agency/European Union. Copernicus Land Monitoring Service. High Resolution Forest Cover Dataset 2018. pp. 1–4. Available online: https://www.eea.europa.eu/data-and-maps/data/copernicus-land-monitoring-service- (accessed on 10 December 2021).
- McLeod, D.R.A.; Whitfield, D.P.; McGrady, M.J. Improving Prediction of Golden Eagle (Aquila chrysaetos) Ranging in Western Scotland Using GIS and Terrain Modeling. J. Raptor Res. 2002, 36, 70–77. [Google Scholar]
- Fielding, A.H.; Haworth, P.F.; Anderson, D.; Benn, S.; Dennis, R.; Weston, E.; Whitfield, D.P. A simple topographical model to predict Golden Eagle Aquila chrysaetos space use during dispersal. Ibis 2019, 162, 400–415. [Google Scholar] [CrossRef] [Green Version]
- NASA. EODIS ASTER Global Digital Elevation Model [Data Set]. NASA EOSDIS Land Processes DAAC. 2009. Available online: https://search.earthdata.nasa.gov/search/granules?p=C1711961296-LPCLOUD&pg[0][v]=f&pg[0][gsk]=-start_date&q=GDEM&tl=1644645645.19!3!! (accessed on 10 December 2021).
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Crawley, M.J. The R Book, 2nd ed.; Wiley: London, UK, 2013. [Google Scholar]
- Gastwirth, J.L.; Gel, Y.R.; Hui, W.; Lyubchich, V.; Miao, W.; Noguchi, K. Package ‘Lawstat’; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; Version 2.5-7; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. 2021. Available online: http://qgis.osgeo.org. (accessed on 10 December 2021).
- Grubac, B.R. The Golden Eagle (Aquila chrysaetos Chrysaetos) in South-Eastern Yugoslavia. Larus 1988, 38–39, 95–135. [Google Scholar]
- Kuzmanov, G.; Stoyanov, G.; Todorov, R. Sur la Biologie et la Protection de l’ Aigle Royal Aquila chrysaetos en Bulgarie. In Eagle Studies; Meyburg, B.-U., Chanchellor, R.D., Eds.; The World Working Group on Birds of Prey (WWGBP): Berlin, Germany, 1996; pp. 505–516. [Google Scholar]
- Georgiev, D.G. Diet of the Golden Eagle (Aquila chrysaetos) (Aves: Accipitridae) in Sarnena Sredna Gora Mountains (Bulgaria). Ecologia Balkanica 2009, 2, 95–98. [Google Scholar]
- Schweiger, A.; Fünfstück, H.-J.; Beierkuhnlein, C. Availability of optimal-sized prey affects global distribution patterns of the golden eagle Aquila chrysaetos. J. Avian Biol. 2014, 46, 81–88. [Google Scholar] [CrossRef]
- Handrinos, G.I. L’Aigle Royal en Grece. In Proceedings of the l’ Aigle Royal (Aquila chrysaetos) en Europe—Actes de 1er Colloque International sur l Aigle Royal en Europe; Maison de la Nature: Arvieux, France, 1986; pp. 18–22. [Google Scholar]
- Stara, K.; Sidiropoulos, L.; Tsiakiris, R. Bound Eagles, Evil Vultures and Cuckoo Horses. Preserving the Bio-Cultural Diversity of Carrion Eating Birds. Hum. Ecol. 2016, 44, 751–764. [Google Scholar] [CrossRef]
- Lyly, M.S.; Villers, A.; Koivisto, E.; Helle, P.; Ollila, T.; Korpimäki, E. Avian top predator and the landscape of fear: Responses of mammalian mesopredators to risk imposed by the golden eagle. Ecol. Evol. 2015, 5, 503–514. [Google Scholar] [CrossRef]
- Fielding, A.H.; Haworth, P.F.; Morgan, D.H.; Thompson, D.B.A.; Whitfield, D.P. The Impact of Golden Eagles (Aquila chrysaetos) on a Diverse Bird of Prey Assemblage. In Birds of Prey in a Changing Environment; The Stationary Office: Edinburgh, UK, 2003; pp. 221–244. [Google Scholar]
- Moleon, M.; Gil-Sanchez, J.M.; Real, J.; Sanchez-Zapata, J.A.; Bautista, J.; Sanchez-Clemot, J.F. Non-Breeding Feeding Ecology of Territorial Bonelli’s Eagles Hieraaetus fasciatus in the Iberian Peninsula. Ardeola 2007, 54, 135–143. [Google Scholar]
- Sánchez, R.; Margalida, A.; González, L.M.; Oria, J. Temporal and Spatial Differences in the Feeding Ecology of the Spanish Imperial Eagle Aquila adatberti during the Non-Breeding Season: Effects of the Rabbit Population Crash. Acta Ornithol. 2009, 44, 53–58. [Google Scholar] [CrossRef]
- Glasser, J.W. A Theory of Trophic Strategies: The Evolution of Facultative Specialists. Am. Nat. 1982, 119, 250–262. [Google Scholar] [CrossRef]
- Bakaloudis, D.E.; Vlachos, C.G.; Bontzorlos, V.A.; Papakosta, M.; Chatzinikos, E. European Hare (Lepus europaeus) Density Response in Mediterranean Ecosystems. In Proceedings of the 29th International Union of Game Biologists, Moscow, Russia, August 2009; Available online: https://www.researchgate.net/publication/260851278_EUROPEAN_HARE_LEPUS_EUROPAEUS_DENSITY_RESPONSE_IN_MEDITERRANEAN_ECOSYSTEMS (accessed on 10 December 2021).
- Sánchez-Zapata, J.A.; Calvo, J.F. Raptor distribution in relation to landscape composition in semi-arid Mediterranean habitats. J. Appl. Ecol. 1999, 36, 254–262. [Google Scholar] [CrossRef]
- Murgatroyd, M.; Avery, G.; Underhill, L.G.; Amar, A. Adaptability of a specialist predator: The effects of land use on diet diversification and breeding performance of Verreaux’s eagles. J. Avian Biol. 2016, 47, 834–845. [Google Scholar] [CrossRef]
- Malan, G.; Branch, W.R. Short Communications: Predation on Tent Tortoise and Leopard Tortoise Hatchlings by the Pale Chanting Goshawk in the Little Karoo. S. Afr. J. Zool. 1992, 27, 33–35. [Google Scholar] [CrossRef] [Green Version]
- Demerdzhiev, D.; Dobrev, D.; Isfendiyaroǧlu, S.; Boev, Z.; Stoychev, S.; Terziev, N.; Spasov, S. Distribution, Abundance, Breeding Parameters, Threats and Prey Preferences of the Eastern Imperial Eagle (Aquila heliaca) in European Turkey. Slovak Raptor J. 2014, 8, 17–25. [Google Scholar] [CrossRef]
- Shafaeipour, A. Nesting Season Diet of Golden Eagles (Aquila chrysaetos) in Western Iran. J. Raptor Res. 2015, 49, 303. [Google Scholar] [CrossRef]
- Varshavski, S.N. Feeding of Aquila chrysaetos in Southwestern Ust-Urt [in Russian]. Ornitologija 1968, 9, 146–149. [Google Scholar]
- Longshore, K.; Esque, T.; Nussear, L.; Johnson, D.R.; Simes, M.; Inman, R. An Assessment of Food Habits, Prey Availability, and Nesting Success of Golden Eagles within the Desert Renewable Energy Conservation Plan Area; California Energy Commission: Sacramento, CA, USA, 2017.
- Vlachos, C.G.; Papageorgiou, N.; Bakaloudis, D.E. Effects of the Feeding Station Establishment on the Egyptian Vulture Neophron percnopterus in Dadia Forest, North Eastern Greece. In Holartctic Birds of Prey; Chancellor, R.D., Meyburg, B.-U., Ferrero, J.J., Eds.; ADENEX & WWGBP: Merida, Germany; Berlin, Germany, 1998; pp. 197–207. [Google Scholar]
- Grubac, B.R. Status & Biology of the Bearded Vulture Gypaetus barbatus Aureus in Macedonia. Birds Prey Bull. 1991, 4, 101–117. [Google Scholar]
- Margalida, A.; Bertran, J. Function and Temporal Variation in Use of Ossuaries by Bearded Vultures (Gypaetus barbatus) during the Nestling Period. Ornithology 2001, 118, 785–789. [Google Scholar] [CrossRef]
- Boire, D.; Nicolakakis, N.; Lefebvre, L. Tools and Brains in Birds. Behaviour 2002, 139, 939–973. [Google Scholar] [CrossRef] [Green Version]
- Ducatez, S.; Clavel, J.; Lefebvre, L. Ecological generalism and behavioural innovation in birds: Technical intelligence or the simple incorporation of new foods? J. Anim. Ecol. 2014, 84, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, L.; Nicolakakis, N. Forebrain Size and Innovation Rate in European Birds: Feeding, Nesting and Confounding Variables. Behaviour 2000, 137, 1415–1429. [Google Scholar] [CrossRef] [Green Version]
- Kitowski, I. Play behaviour and active training of Montagu’s harrier (Circus pygargus) offspring in the post-fledging period. J. Ethol. 2004, 23, 3–8. [Google Scholar] [CrossRef]
- Kitowski, I. Social learning of hunting skills in juvenile marsh harriers Circus aeruginosus. J. Ethol. 2008, 27, 327–332. [Google Scholar] [CrossRef]
- Capper, S. The Predation of Testudo Spp by Golden Eagles Aquila chrysaetos in Dadia Forest Reserve, NE Greece. Master’s Thesis, Department of Biology, University of Reading, Reading, UK, 1998. [Google Scholar]
- Hailey, A. The effects of fire and mechanical habitat destruction on survival of the tortoise Testudo hermanni in northern Greece. Biol. Conserv. 2000, 92, 321–333. [Google Scholar] [CrossRef]
- Kontsiotis, V.; Tsiompanoudis, A.C.; Bakaloudis, D.E. The influence of habitat structure on the European brown hare Lepus europaeus food habits in mountainous areas of northern Greece. Mammalia 2011, 75, 389–394. [Google Scholar] [CrossRef]
- Kienzle, E.; Kopsch, G.; Koelle, P.; Clauss, M. Chemical Composition of Turtles and Tortoises. J. Nutr. 2006, 136, 2053–2054. [Google Scholar] [CrossRef] [Green Version]
- Mertin, D.; Slamečka, J.; Ondruška, Ľ.; Zaujec, K.; Jurčík, R.; Gašparík, J. Comparison of Meat Quality between European Brown Hare and Domestic Rabbit. Slovak J. Anim. Sci. 2012, 45, 89–95. [Google Scholar]
- Dimaki, M.; Limperakis, P.; Maragkou, P. Reptiles. In The Red Data Book of Threatened Animals in Greece; Legakis, A., Maragkou, P., Eds.; Hellenic Zoological Society: Athens, Greece, 2009; pp. 179–510, (In Greek with English Summaries). [Google Scholar]
- Watson, J.; Leitch, A.F.; Rae, S.R. The diet of Golden Eagles Aquila chrysaetos in Scotland. Ibis 1993, 135, 387–393. [Google Scholar] [CrossRef]
- Haworth, P.F.; Mcgrady, M.J.; Whitfield, D.P.; Fielding, A.H.; Mcleod, D.R.A.; Haworth, P.F.; Mcgrady, M.J.; Whitfield, D.P.; Fielding, A.H. Ranging distance of resident Golden Eagles Aquila chrysaetos in western Scotland according to season and breeding status. Bird Study 2006, 53, 265–273. [Google Scholar] [CrossRef]
- Seguin, J.; Bayle, P.; Thibault, J.; Torre, J.; Vigne, J. A Comparison of Methods to Evaluate the Diet of Golden Eagle in Corsica. J. Raptor Res. 1998, 32, 314–318. [Google Scholar]
- Steenhof, K.; Kochert, M.N. Dietary Responses of Three Raptor Species to Changing Prey Densities in a Natural Environment. J. Anim. Ecol. 1988, 57, 37. [Google Scholar] [CrossRef]
- Preston, C.R.; Jones, R.E.; Horton, N.S.; Reston, C.H.R.P. Golden Eagle Diet Breadth and Reproduction in Relation to Fluctuations in Primary Prey Abundance in Wyoming’s Bighorn Basin. J. Raptor Res. 2017, 51, 334–346. [Google Scholar] [CrossRef]
- Ministry of Environment and Energy. Article 17 Reporting for the Implementation of the Habitats Directive. 2019. Available online: https://cdr.eionet.europa.eu/Converters/run_conversion?file=gr/eu/art12/envxz8njg/GR_birds_reports_20191031-152940.xml&conv=612&source=remote#A091_B (accessed on 15 December 2021).
- Hailey, A.; Willemsen, R. Changes in the status of tortoise populations in Greece 1984–2001. Biodivers. Conserv. 2003, 12, 991–1011. [Google Scholar] [CrossRef]
- Tucker, G.M.; Evans, M.I. Habitats for Birds in Europe—A Conservation Strategy for the Wider Environment; BirdLife Conservation Series No. 6; BirdLife International: Cambridge, UK, 1997. [Google Scholar]
- Ruffault, J.; Curt, T.; Moron, V.; Trigo, R.M.; Mouillot, F.; Koutsias, N.; Pimont, F.; Martin-StPaul, N.; Barbero, R.; Dupuy, J.-L.; et al. Increased likelihood of heat-induced large wildfires in the Mediterranean Basin. Sci. Rep. 2020, 10, 13790. [Google Scholar] [CrossRef] [PubMed]
- Couturier, T.; Besnard, A.; Bertolero, A.; Bosc, V.; Astruc, G.; Cheylan, M. Factors determining the abundance and occurrence of Hermann’s tortoise Testudo hermanni in France and Spain: Fire regime and landscape changes as the main drivers. Biol. Conserv. 2014, 170, 177–187. [Google Scholar] [CrossRef]
- Türkozan, O.; Karaman, S.; Yılmaz, C.; Ülger, C. Daily movements and home range of Eastern Hermann’s Tortoise, Testudo hermanni boettgeri (Reptilia: Testudines). Zool. Middle East 2018, 65, 28–34. [Google Scholar] [CrossRef]
- Robinzon, B.; Nir, I.; Lapid, R. Growth and body composition in captive Testudo graeca terrestris fed with a high-energy diet. Appl. Herpetol. 2005, 2, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Hadjigeorgiou, I. Past, present and future of pastoralism in Greece. Pastor. Res. Policy Pract. 2011, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Triantakonstantis, D.P.; Kollias, V.J.; Kalivas, D.P. Forest Re-growth Since 1945 in the Dadia Forest Nature Reserve in Northern Greece. New For. 2006, 32, 51–69. [Google Scholar] [CrossRef]
- Poirazidis, K.; Bontzorlos, V.; Xofis, P.; Zakkak, S.; Xirouchakis, S.; Grigoriadou, E.; Kechagioglou, S.; Gasteratos, I.; Alivizatos, H.; Panagiotopoulou, M. Bioclimatic and environmental suitability models for capercaillie (Tetrao urogallus) conservation: Identification of optimal and marginal areas in Rodopi Mountain-Range National Park (Northern Greece). Glob. Ecol. Conserv. 2019, 17, e00526. [Google Scholar] [CrossRef]
- Moreira, F.; Russo, D. Modelling the impact of agricultural abandonment and wildfires on vertebrate diversity in Mediterranean Europe. Landsc. Ecol. 2007, 22, 1461–1476. [Google Scholar] [CrossRef]
- Sirami, C.; Brotons, L.; Burfield, I.; Fonderflick, J.; Martin, J.-L. Is land abandonment having an impact on biodiversity? A meta-analytical approach to bird distribution changes in the north-western Mediterranean. Biol. Conserv. 2008, 141, 450–459. [Google Scholar] [CrossRef]
- Pedrini, P.; Sergio, F. Golden Eagle Aquila chrysaetos density and productivity in relation to land abandonment and forest expansion in the Alps. Bird Study 2001, 48, 194–199. [Google Scholar] [CrossRef]
- Whitfield, D.P.; McLeod, D.R.A.; Fielding, A.H.; Broad, R.A.; Evans, R.J.; Haworth, P.F. The effects of forestry on golden eagles on the island of Mull, western Scotland. J. Appl. Ecol. 2001, 38, 1208–1220. [Google Scholar] [CrossRef]
- Ontiveros, D.; Pleguezuelos, J.M.; Caro, J. Prey density, prey detectability and food habits: The case of Bonelli’s eagle and the conservation measures. Biol. Conserv. 2005, 123, 19–25. [Google Scholar] [CrossRef]
- Nikolić, M.; Cvetković, J.; Stojadinović, D.; Crnobrnja-Isailović, J. Macro- and microhabitat preferences of eastern Hermann’s tortoise (Testudo hermanni boettgeri). Amphibia-Reptilia 2020, 41, 313–322. [Google Scholar] [CrossRef]
- Steenhof, K.; Kochert, M.N.; McDonald, T.L. Interactive Effects of Prey and Weather on Golden Eagle Reproduction. J. Anim. Ecol. 1997, 66, 350. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; Gregory, M.J.P.; Gordon, A.G.; McLeod, D.R.A.; Haworth, P.F. Complex effects of habitat loss on Golden Eagles Aquila chrysaetos. Ibis 2006, 149, 26–36. [Google Scholar] [CrossRef]
- Biswell, H. Prescribed Burning in California Wildlands Vegetation Management; University of California Press: Berkeley, CA, USA, 1989. [Google Scholar]
- Blondel, J.; Arronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Grove, A.T.; Rackham, O. The Nature of Mediterranean Europe—An Ecological History; Yale University Press: New Heaven, CT, USA, 2001. [Google Scholar]
- Zakkak, S.; Radovic, A.; Nikolov, S.C.; Shumka, S.; Kakalis, L.; Kati, V. Assessing the effect of agricultural land abandonment on bird communities in southern-eastern Europe. J. Environ. Manag. 2015, 164, 171–179. [Google Scholar] [CrossRef]
- Kati, V.; Foufopoulos, J.; Ioannidis, Y.; Papaioannou, H.; Poirazidis, K.; Lebrun, P. Diversity, ecological structure and conservation of herpetofauna in a Mediterranean area (Dadia National Park, Greece). Amphibia-Reptilia 2007, 28, 517–529. [Google Scholar] [CrossRef]
- Kati, V.; Poirazidis, K.; Dufrêne, M.; Halley, J.M.; Korakis, G.; Schindler, S.; Dimopoulos, P. Towards the use of ecological heterogeneity to design reserve networks: A case study from Dadia National Park, Greece. Biodivers. Conserv. 2010, 19, 1585–1597. [Google Scholar] [CrossRef]
- Herring, G.; Eagles-Smith, C.A.; Buck, J. Characterizing Golden Eagle Risk to Lead and Anticoagulant Rodenticide Exposure: A Review. J. Raptor Res. 2017, 51, 273–292. [Google Scholar] [CrossRef] [Green Version]
- Ganz, K.; Jenni, L.; Madry, M.M.; Kraemer, T.; Jenny, H.; Jenny, D. Acute and Chronic Lead Exposure in Four Avian Scavenger Species in Switzerland. Arch. Environ. Contam. Toxicol. 2018, 75, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, J.M.; Molleda, S.; Sanchez-Zapata, J.A.; Bautista, J.; Navas, I.; Godinho, R.; García-Fernández, A.J.; Moleón, M. From sport hunting to breeding success: Patterns of lead ammunition ingestion and its effects on an endangered raptor. Sci. Total Environ. 2017, 613–614, 483–491. [Google Scholar] [CrossRef]
- Nadjafzadeh, M.; Voigt, C.C.; Krone, O. Spatial, seasonal and individual variation in the diet of White-tailed Eagles Haliaeetus albicilla assessed using stable isotope ratios. Ibis 2015, 158, 1–15. [Google Scholar] [CrossRef]
- Bounas, A.; Ganoti, M.; Giannakaki, E.; Akrivos, A.; Vavylis, D.; Zorrilla, I.; Saravia, V. First confirmed case of lead poisoning in the endangered Egyptian Vulture (Neophron percnopterus) in the Balkans. Vulture News 2018, 70, 22. [Google Scholar] [CrossRef] [Green Version]
- Caballero, R.; Fernandez-Gonzalez, F.; Perez Badia, R.; Molle, G.; Roggero, P.P.; Bagella, S.; D’Ottavio, P.; Papanastasis, V.P.; Fotiadis, G.; Sidiropoulou, A.; et al. Grazing Systems and Biodiversity in Mediterranean Areas: Spain, Italy and Greece. Pastos 2009, 39, 9–152. [Google Scholar]
- Watson, J. The Golden Eagle and Pastoralism across Europe. In Birds and Pastoral Agriculture in Europe: Proceedings of the Second European Forum on Birds and Pastoralism, Port Erin, Isle of Man, UK, 26–30 October 1990; Curtis, D.J., Bignal, E.M., Curtis, M.A., Eds.; Joint Nature Conservation Committee: Inverness, UK; Scottish Chough Study Group: Bridgend, UK, 1991; pp. 56–57. [Google Scholar]
- Gjershaug, J.O.; Halley, D.; Stokke, B.G. Predefinitive Plumage in the Golden Eagle (Aquila chrysaetos): A Signal of Aggression or Submission? J. Raptor Res. 2019, 53, 431–435. [Google Scholar] [CrossRef]
- Sánchez-Zapata, J.A.; Eguía, S.; Blázquez, M.; Moleón, M.; Botella, F. Unexpected role of ungulate carcasses in the diet of Golden Eagles Aquila chrysaetos in Mediterranean mountains. Bird Study 2010, 57, 352–360. [Google Scholar] [CrossRef]
- Ntemiri, K.; Saravia, V.; Angelidis, C.; Baxevani, K.; Probonas, M.; Kret, E.; Mertzanis, Y.; Iliopoulos, Y.; Georgiadis, L.; Skartsi, D.; et al. Animal mortality and illegal poison bait use in Greece. Environ. Monit. Assess. 2018, 190, 488. [Google Scholar] [CrossRef] [PubMed]
- Velevski, M.; Nikolov, S.C.; Hallmann, B.; Dobrev, V.; Sidiropoulos, L.; Saravia, V.; Tsiakiris, R.; Arkumarev, V.; Galanaki, A.; Kominos, T.; et al. Population decline and range contraction of the Egyptian Vulture Neophron percnopterus in the Balkan Peninsula. Bird Conserv. Int. 2014, 25, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Sidiropoulos, L.; Tsiakiris, R.; Azmanis, P.; Galanaki, A.; Kastritis, A.; Stara, K.; Konstantinou, P.; Jerrentrup, H.; Xirouchakis, S.; Kominos, T. The Status of Vultures in Greece. In Vulture Conservation in the Balkan Peninsula and Adjacent Regions—10 Years of Research and Conservation; Andevski, J., Ed.; Vulture Conservation Foundation: Skopje, North Macedonia, 2013; pp. 20–23. [Google Scholar]
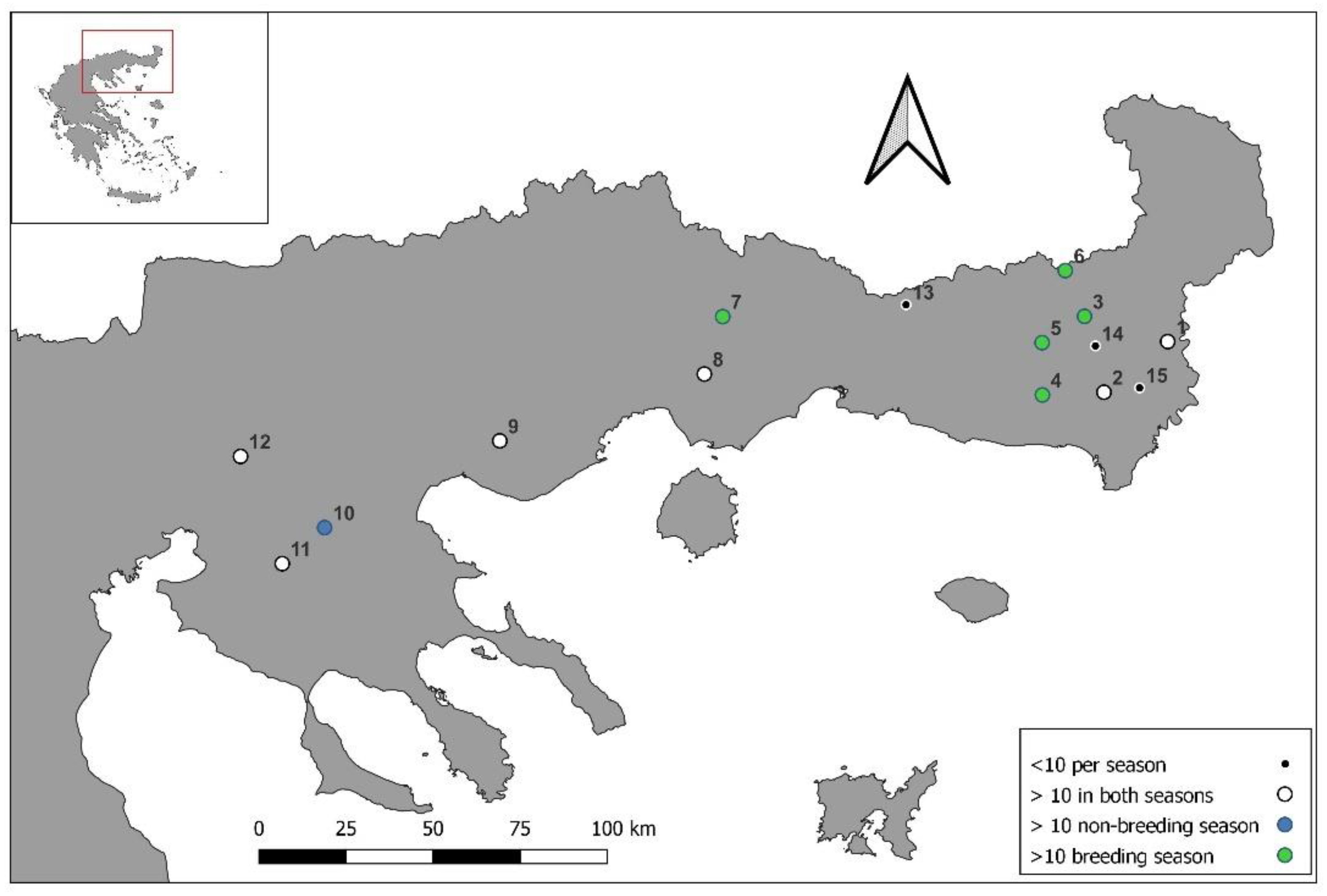

| Prey Categories | Code | Breeding (n = 11) | Non-Breeding (n = 7) | Annual | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | AFs | P | N | AFs | P | N | AF | P | ||
| Birds | 53 | 8.9 | 0.91 | 54 | 31.2 | 1 | 107 | 13.4 | 0.92 | |
| Birds all other | B_o | 18 | 2.2 | 0.64 | 16 | 9.1 | 0.86 | 34 | 3.4 | 0.67 |
| Corvids | B_c | 11 | 3.1 | 0.73 | 7 | 3.7 | 0.71 | 18 | 3.7 | 0.75 |
| Raptors and Owls | B_ro | 2 | 0.1 | 0.18 | 2 | 0.8 | 0.29 | 4 | 0.3 | 0.25 |
| Thrushes and Pigeons | B_tp | 13 | 2.1 | 0.45 | 19 | 9.3 | 0.86 | 32 | 3.7 | 0.58 |
| Waterbirds | B_w | 9 | 1.3 | 0.36 | 10 | 8.2 | 0.86 | 19 | 2.4 | 0.5 |
| Mammals | 89 | 16.3 | 1 | 63 | 36.1 | 1 | 152 | 19.97 | 1 | |
| Carnivores | M_c | 25 | 4.8 | 0.73 | 20 | 11.1 | 0.86 | 45 | 6.1 | 0.83 |
| Glirids and Sciurids | M_gs | 14 | 2.4 | 0.36 | 6 | 2.4 | 0.43 | 20 | 2.7 | 0.5 |
| Hares | M_ha | 8 | 2.7 | 0.73 | 6 | 3.7 | 0.71 | 14 | 2.9 | 0.83 |
| Hedgehogs | M_he | 20 | 2.6 | 0.55 | 13 | 7.2 | 0.57 | 33 | 3.4 | 0.58 |
| Mammals all other | M_o | 11 | 2.6 | 0.64 | 9 | 6.4 | 0.86 | 20 | 3.4 | 0.75 |
| Ungulates | M_u | 11 | 1.2 | 0.36 | 9 | 5.3 | 0.57 | 20 | 1.5 | 0.42 |
| Reptiles | 479 | 7.8 | 1 | 59 | 32.8 | 1 | 538 | 65.6 | 1 | |
| Snakes and Lizards | R_sl | 30 | 4.6 | 0.73 | 12 | 8.5 | 0.57 | 42 | 6.0 | 0.75 |
| Tortoises | R_t | 449 | 70.3 | 1 | 47 | 24.2 | 1 | 496 | 60.7 | 1 |
| Total | 621 | 176 | 797 | 100 | ||||||
| Prey Category | Means ± SD (%) | Binomial Test of Proportions | Medians | Wilcoxon Matched Pairs Test | Simper Analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Breeding | Non-Breeding | x2 | p | Breeding/Non-Breeding | V | p | Contribution | p | |
| Birds all other | 2.2 ± 2.36 | 9.1 ± 6.16 | 11.4 | **** | 0.04/0.09 | 1 | * | 0.06 | * |
| Corvids | 3.1 ± 3.35 | 3.7 ± 3.24 | 2.11 | n/s | 0.01/0.03 | 0 | n/s | 0.02 | n/s |
| Raptors and Owls | 0.1 ± 0.35 | 0.8 ± 1.37 | 0.55 | n/s | 0/0 | 1 | n/s | 0.01 | n/s |
| Thrushes and Pigeons | 2.1 ± 3.65 | 9.3 ± 9.56 | 24.7 | **** | 0.027/0.07 | 3 | n/s | 0.07 | n/s |
| Waterbirds | 1.3 ± 2.77 | 8.2 ± 10.42 | 8.82 | *** | 0.01/0.03 | 0 | * | 0.07 | n/s |
| BIRDS | 8.9 ± 6.61 | 31.1 ± 13.27 | 55.98 | **** | 0.08/0.5 | 0 | ** | 0.31 | ** |
| Carnivores | 4.8 ± 5.60 | 11.1 ± 7.55 | 12.6 | **** | 0.03/0.11 | 1 | * | 0.08 | ** |
| Glirids and Sciurids | 2.4 ± 4.33 | 2.4 ± 3.29 | 0.35 | n/s | 0/0 | 1 | n/s | 0.02 | n/s |
| Hares | 2.7 ± 3.00 | 3.7 ± 3.22 | 2.45 | n/s | 0.01/0.04 | 2 | * | 0.03 | * |
| Hedgehogs | 2.6 ± 2.73 | 7.2 ± 9.78 | 4.99 | ** | 0.04/0.01 | 5 | n/s | 0.07 | n/s |
| Mammals all other | 2.6 ± 3.04 | 6.4 ± 5.57 | 4.97 | ** | 0.01/0.04 | 0 | * | 0.05 | n/s |
| Ungulates | 1.2 ± 1.85 | 5.28 ± 5.01 | 4.97 | ** | 0.01/0.08 | 0 | * | 0.05 | * |
| MAMMALS | 16.3 ± 10.16 | 36.0 ± 13.34 | 39.55 | **** | 0.14/0.52 | 0 | ** | 0.38 | *** |
| Snakes and Lizards | 4.6 ± 4.85 | 8.51 ± 13.21 | 0.72 | n/s | 0.04/0.01 | 14 | n/s | 0.07 | n/s |
| Tortoises | 70.2 ± 11.05 | 24.25 ± 11.18 | 119.4 | **** | 0.72/0.25 | 21 | ** | 0.4 | *** |
| REPTILES | 74.8 ± 11.57 | 32.9 ± 15.11 | 116.92 | **** | 0.91/0.49 | 21 | ** | 0.31 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidiropoulos, L.; Whitfield, D.P.; Astaras, C.; Vasilakis, D.; Alivizatos, H.; Kati, V. Pronounced Seasonal Diet Diversity Expansion of Golden Eagles (Aquila chrysaetos) in Northern Greece during the Non-Breeding Season: The Role of Tortoises. Diversity 2022, 14, 135. https://doi.org/10.3390/d14020135
Sidiropoulos L, Whitfield DP, Astaras C, Vasilakis D, Alivizatos H, Kati V. Pronounced Seasonal Diet Diversity Expansion of Golden Eagles (Aquila chrysaetos) in Northern Greece during the Non-Breeding Season: The Role of Tortoises. Diversity. 2022; 14(2):135. https://doi.org/10.3390/d14020135
Chicago/Turabian StyleSidiropoulos, Lavrentis, D. Philip Whitfield, Christos Astaras, Dimitris Vasilakis, Haralambos Alivizatos, and Vassiliki Kati. 2022. "Pronounced Seasonal Diet Diversity Expansion of Golden Eagles (Aquila chrysaetos) in Northern Greece during the Non-Breeding Season: The Role of Tortoises" Diversity 14, no. 2: 135. https://doi.org/10.3390/d14020135
APA StyleSidiropoulos, L., Whitfield, D. P., Astaras, C., Vasilakis, D., Alivizatos, H., & Kati, V. (2022). Pronounced Seasonal Diet Diversity Expansion of Golden Eagles (Aquila chrysaetos) in Northern Greece during the Non-Breeding Season: The Role of Tortoises. Diversity, 14(2), 135. https://doi.org/10.3390/d14020135







