Alien Invasive Plant Effect on Soil Fauna Is Habitat Dependent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Variables
2.3. Vegetation Survey
2.4. Soil Fauna Survey
2.4.1. Soil Collembola
2.4.2. Soil Nematoda
2.5. Data Analyses
3. Results
3.1. Soil Variables
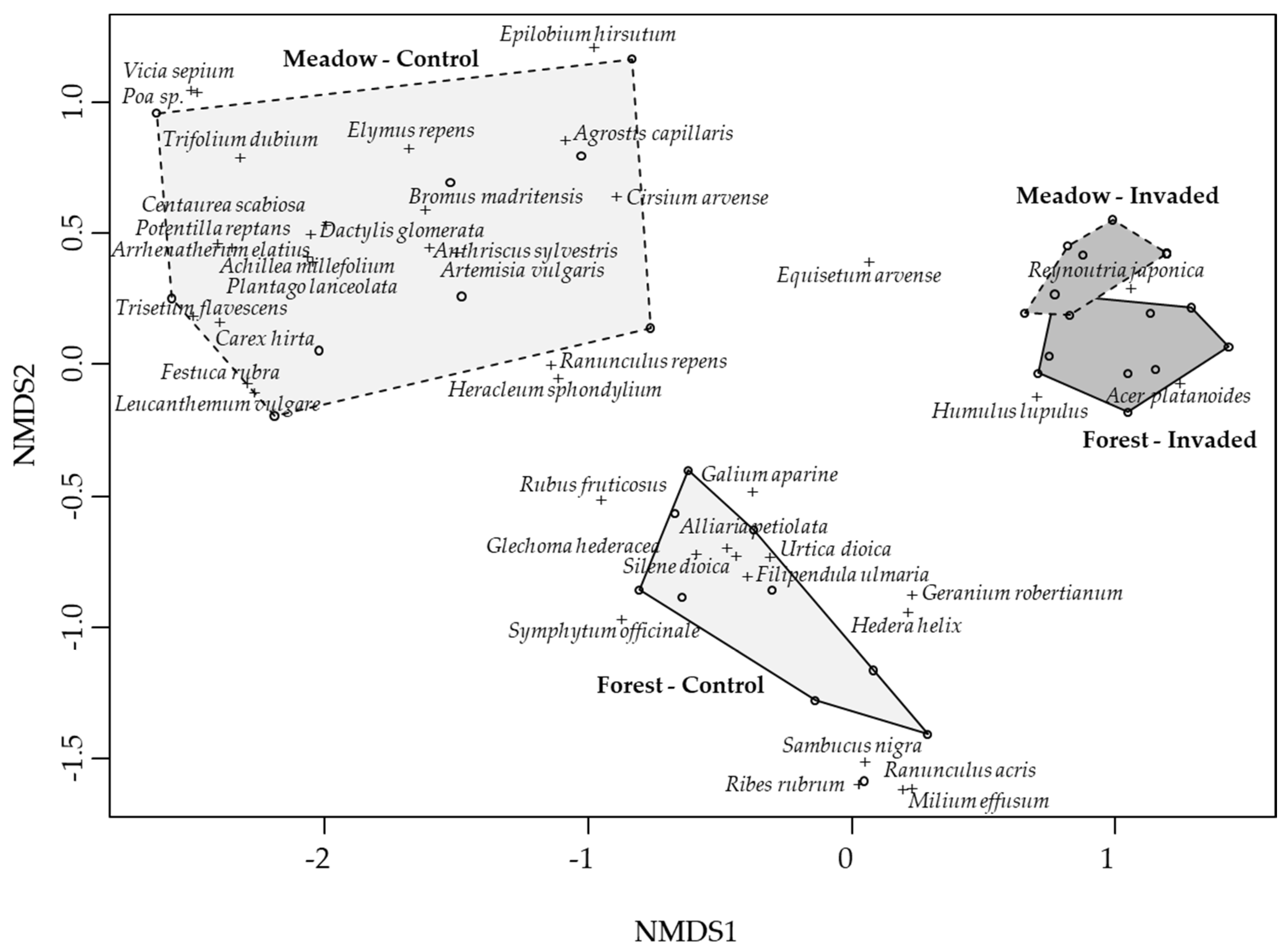
3.2. Plant Communities
3.3. Soil Fauna
3.4. Global Effect of Knotweed on Habitats
4. Discussion
4.1. Knotweed Effects on Native Plants and Soil Variables
4.2. Knotweed Effects on Collembola
4.3. Knotweed Effects on Nematoda
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Change Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Schirmel, J.; Bundschuh, M.; Entling, M.H.; Kowarik, I.; Buchholz, S. Impacts of invasive plants on resident animals across ecosystems, taxa, and feeding types: A global assessment. Glob. Change Biol. 2016, 22, 594–603. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Forey, E.; Lodhar, S.; Gopaul, S.; Boehmer, H.J.; Chauvat, M. A functional trait-based approach to assess the impact of an alien palm invasion on plant and soil communities on a South Pacific island. Austral Ecol. 2021, 46, 398–410. [Google Scholar] [CrossRef]
- McCary, M.A.; Mores, R.; Farfan, M.A.; Wise, D.H. Invasive plants have different effects on trophic structure of green and brown food webs in terrestrial ecosystems: A meta-analysis. Ecol. Lett. 2016, 19, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Abgrall, C.; Forey, E.; Chauvat, M. Soil fauna responses to invasive alien plants are determined by trophic groups and habitat structure: A global meta-analysis. Oikos 2019, 128, 1390–1401. [Google Scholar] [CrossRef]
- Wardle, D.A.; Peltzer, D.A. Impacts of invasive biota in forest ecosystems in an aboveground–belowground context. Biol. Invasions 2017, 19, 3301–3316. [Google Scholar] [CrossRef] [Green Version]
- Aldorfová, A.; Knobová, P.; Münzbergová, Z. Plant–soil feedback contributes to predicting plant invasiveness of 68 alien plant species differing in invasive status. Oikos 2020, 129, 1257–1270. [Google Scholar] [CrossRef]
- Martin, F.-M. The Study of the Spatial Dynamics of Asian Knotweeds (Reynoutria spp.) across Scales and its Contribution for Management Improvement. Ph.D. Thesis, Université Grenoble Alpes, Grenoble, France, 2019. [Google Scholar]
- Lavoie, C. The impact of invasive knotweed species (Reynoutria spp.) on the environment: Review and research perspectives. Biol. Invasions 2017, 19, 2319–2337. [Google Scholar] [CrossRef]
- Abgrall, C.; Forey, E.; Mignot, L.; Chauvat, M. Invasion by Fallopia japonica alters soil food webs through secondary metabolites. Soil Biol. Biochem. 2018, 127, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Skubala, P.; Mierny, A. Invasive Reynoutria taxa as a contaminant of soil. Does it reduce abundance and diversity of microarthropods and damage soil habitat? Pestycydy 2009, 1–4, 57–62. [Google Scholar]
- Chauvat, M.; Perez, G.; Ponge, J.-F. Foraging patterns of soil springtails are impacted by food resources. Appl. Soil Ecol. 2014, 82, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Petersen, H. General aspects of collembolan ecology at the turn of the millennium: Proceedings of the Xth international colloquium on Apterygota, České Budějovice 2000: Apterygota at the beginning of the third millennium. Pedobiologia 2002, 46, 246–260. [Google Scholar] [CrossRef]
- Potapov, A.A.; Semenina, E.E.; Korotkevich, A.Y.; Kuznetsova, N.A.; Tiunov, A. V Connecting taxonomy and ecology: Trophic niches of collembolans as related to taxonomic identity and life forms. Soil Biol. Biochem. 2016, 101, 20–31. [Google Scholar] [CrossRef]
- Potapov, A.M.; Pollierer, M.M.; Salmon, S.; Šustr, V.; Chen, T. Multidimensional trophic niche revealed by complementary approaches: Gut content, digestive enzymes, fatty acids and stable isotopes in Collembola. J. Anim. Ecol. 2021, 92, 161–188. [Google Scholar] [CrossRef]
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Chauvat, M.; Wolters, V.; Dauber, J. Response of collembolan communities to land-use change and grassland succession. Ecography 2007, 30, 183–192. [Google Scholar] [CrossRef]
- da Silva, P.M.; Carvalho, F.; Dirilgen, T.; Stone, D.; Creamer, R.; Bolger, T.; Sousa, J.P. Traits of collembolan life-form indicate land use types and soil properties across an European transect. Appl. Soil Ecol. 2016, 97, 69–77. [Google Scholar] [CrossRef]
- Henneron, L.; Aubert, M.; Archaux, F.; Bureau, F.; Dumas, Y.; Ningre, F.; Richter, C.; Balandier, P.; Chauvat, M. Forest plant community as a driver of soil biodiversity: Experimental evidence from collembolan assemblages through large-scale and long-term removal of oak canopy trees Quercus petraea. Oikos 2017, 126, 420–434. [Google Scholar] [CrossRef]
- Yin, R.; Gruss, I.; Eisenhauer, N.; Kardol, P.; Thakur, M.P.; Schmidt, A.; Xu, Z.; Siebert, J.; Zhang, C.; Wu, G.-L. Land use modulates the effects of climate change on density but not community composition of Collembola. Soil Biol. Biochem. 2019, 138, 107598. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil--I. Fumigation with chloroform… V. A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 179. [Google Scholar] [CrossRef]
- Gong, P.; Guan, X.; Witter, E. A rapid method to extract ergosterol from soil by physical disruption. Appl. Soil Ecol. 2001, 17, 285–289. [Google Scholar] [CrossRef]
- Braun-Blanquet, J.; Roussine, N.; Nègre, R.; Emberger, L. Les Groupements Végétaux de la France Méditerranéenne; CNRS Edition: Paris, France, 1952. [Google Scholar]
- Macfadyen, A. Improved funnel-type extractors for soil arthropods. J. Anim. Ecol. 1961, 30, 171–184. [Google Scholar] [CrossRef]
- Gisin, H. Ökologie und Lebensgemeinschaften der Collembolen im Schweizerischen Exkursionsgebiet Basel, Inauguraldissertation; Universität Basel: Basel, Switzerland, 1943. [Google Scholar]
- Barker, K.R. Nematode Extraction and Bioassays; Barker, K., Barker, K.R., Carter, C.C., Sasser, J.N., Eds.; North Carolina State University Graphics: Raleigh, NC, USA, 1985. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Magnusson, A.; Skaug, H.; Nielsen, A.; Berg, C.; Kristensen, K.; Maechler, M.; van Bentham, K.; Bolker, B.; Brooks, M.; Brooks, M.M. Package ‘glmmTMB.’ R Packag. Version 0.2. 0. 2017. Available online: http://cran.uni-muenster.de/web/packages/glmmTMB/glmmTMB.pdf (accessed on 15 January 2021).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; O’Hara, R. Vegan: Community ecology package. R Packag. 2016, 2, 2–3. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Brousseau, P.-M.; Chauvat, M.; De Almeida, T.; Forey, E. Invasive knotweed modifies predator–prey interactions in the soil food web. Biol. Invasions 2021, 23, 1987–2002. [Google Scholar] [CrossRef]
- Mincheva, T.; Barni, E.; Varese, G.C.; Brusa, G.; Cerabolini, B.; Siniscalco, C. Litter quality, decomposition rates and saprotrophic mycoflora in Fallopia japonica (Houtt.) Ronse Decraene and in adjacent native grassland vegetation. Acta Oecol. 2014, 54, 29–35. [Google Scholar] [CrossRef]
- Urgenson, L.S.; Reichard, S.H.; Halpern, C.B. Community and ecosystem consequences of giant knotweed (Polygonum sachalinense) invasion into riparian forests of western Washington, USA. Biol. Conserv. 2009, 142, 1536–1541. [Google Scholar] [CrossRef]
- Mincheva, T.; Barni, E.; Siniscalco, C. From plant traits to invasion success: Impacts of the alien Fallopia japonica (Houtt.) Ronse Decraene on two native grassland species. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016, 150, 1348–1357. [Google Scholar] [CrossRef] [Green Version]
- Bardon, C.; Poly, F.; el Zahar Haichar, F.; Le Roux, X.; Simon, L.; Meiffren, G.; Comte, G.; Rouifed, S.; Piola, F. Biological denitrification inhibition (BDI) with procyanidins induces modification of root traits, growth and N status in Fallopia x bohemica. Soil Biol. Biochem. 2017, 107, 41–49. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Stanek, M.; Frąc, M.; Oszust, K.; Woch, M.W.; Zubek, S. Invasive plant Reynoutria japonica produces large amounts of phenolic compounds and reduces the biomass but not activity of soil microbial communities. Sci. Total Environ. 2021, 767, 145439. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Kalisz, S.; Nuñez, M.A.; Wardle, D.A.; Wingfield, M.J. Biological invasions in forest ecosystems. Biol. Invasions 2017, 19, 3437–3458. [Google Scholar] [CrossRef]
- Perez, G.; Decaëns, T.; Dujardin, G.; Akpa-Vinceslas, M.; Langlois, E.; Chauvat, M. Response of collembolan assemblages to plant species successional gradient. Pedobiologia 2013, 56, 169–177. [Google Scholar] [CrossRef]
- Chauvat, M.; Zaitsev, A.S.; Wolters, V. Successional changes of Collembola and soil microbiota during forest rotation. Oecologia 2003, 137, 269–276. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant. Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Takeda, H. A 5 year study of pine needle litter decomposition in relation to mass loss and faunal abundances. Pedobiologia 1988, 32, 221–226. [Google Scholar]
- Faber, J.H.; Teuben, A.; Berg, M.P.; Doelman, P. Microbial biomass and activity in pine litter in the presence of Tomocerus minor (Insecta, Collembola). Biol. Fertil. Soils 1992, 12, 233–240. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takeda, H. Changes in feeding attributes of four collembolan populations during the decomposition process of pine needles. Pedobiologia 1995, 39, 155–169. [Google Scholar]
- Young-Mathews, A.; Culman, S.W.; Sánchez-Moreno, S.; O’Geen, A.T.; Ferris, H.; Hollander, A.D.; Jackson, L.E. Plant-soil biodiversity relationships and nutrient retention in agricultural riparian zones of the Sacramento Valley, California. Agrofor. Syst. 2010, 80, 41–60. [Google Scholar] [CrossRef] [Green Version]
- Briar, S.S.; Culman, S.W.; Young-Mathews, A.; Jackson, L.E.; Ferris, H. Nematode community responses to a moisture gradient and grazing along a restored riparian corridor. Eur. J. Soil Biol. 2012, 50, 32–38. [Google Scholar] [CrossRef]
- Čerevková, A.; Bobuľská, L.; Miklisová, D.; Renčo, M. A case study of soil food web components affected by Fallopia japonica (Polygonaceae) in three natural habitats in Central Europe. J. Nematol. 2019, 51, e2019-42. [Google Scholar] [CrossRef] [Green Version]
- Renčo, M.; Čerevková, A.; Homolová, Z. Nematode communities indicate the negative impact of Reynoutria japonica invasion on soil fauna in ruderal habitats of tatra national park in Slovakia. Glob. Ecol. Conserv. 2021, 26, e01470. [Google Scholar] [CrossRef]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 17, 293–302. [Google Scholar] [CrossRef]
- Vrchotová, N.; Šerá, B. Allelopathic properties of knotweed rhizome extracts. Plant Soil Environ. 2008, 54, 301–303. [Google Scholar] [CrossRef] [Green Version]
- Vrchotová, N.; Sera, B.; Triska, J. The stilbene and catechin content of the spring sprouts of Reynoutria species. Acta Chromatogr. 2007, 19, 21–28. [Google Scholar]
- Vastano, B.C.; Chen, Y.; Zhu, N.; Ho, C.-T.; Zhou, Z.; Rosen, R.T. Isolation and identification of stilbenes in two varieties of polygonum c uspidatum. J. Agric. Food Chem. 2000, 48, 253–256. [Google Scholar] [CrossRef]
- Zhang, P.; Li, B.; Wu, J.; Hu, S. Invasive plants differentially affect soil biota through litter and rhizosphere pathways: A meta-analysis. Ecol. Lett. 2019, 22, 200–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, J.P.; Bímová, K.; Mandák, B. Asexual spread versus sexual reproduction and evolution in Japanese Knotweed s.l. sets the stage for the “battle of the Clones”. Biol. Invasions 2009, 11, 1189–1203. [Google Scholar] [CrossRef]
- Morriën, E.; Duyts, H.; Van der Putten, W.H. Effects of native and exotic range-expanding plant species on taxonomic and functional composition of Nematodes in the soil food web. Oikos 2012, 121, 181–190. [Google Scholar] [CrossRef]
- Tanner, R.A.; Varia, S.; Eschen, R.; Wood, S.; Murphy, S.T.; Gange, A.C. Impacts of an invasive non-native annual weed, Impatiens glandulifera, on above-and below-ground invertebrate communities in the United Kingdom. PLoS ONE 2013, 8, e67271. [Google Scholar] [CrossRef] [Green Version]
- Maceda-Veiga, A.; Basas, H.; Lanzaco, G.; Sala, M.; De Sostoa, A.; Serra, A. Impacts of the invader giant reed (Arundo donax) on riparian habitats and ground arthropod communities. Biol. Invasions 2016, 18, 731–749. [Google Scholar] [CrossRef]
- Wardle, D.A. How plant communities influence decomposer communities. Biol. Divers. Funct. Soils 2005, 119–138. [Google Scholar] [CrossRef]
- Wardle, D.A. The influence of biotic interactions on soil biodiversity. Ecol. Lett. 2006, 9, 870–886. [Google Scholar] [CrossRef]

| Forest | Meadow | |||||
|---|---|---|---|---|---|---|
| z-Value | p-Value | Control | Invaded | Control | Invaded | |
| Total carbon (mg·g−1) | ||||||
| Knotweed | 1.14 | 0.25 | 5.84 ± 0.77 | 6.9 ± 1.47 | 4.77 ± 0.45 | 5.67 ± 0.38 |
| Habitat | −0.68 | 0.49 | ||||
| Knotweed × Habitat | −0.12 | 0.9 | ||||
| Total nitrogen (mg·g−1) | ||||||
| Knotweed | −1.48 | 0.14 | 0.39 ± 0.05 | 0.31 ± 0.05 | 0.28 ± 0.04 | 0.33 ± 0.03 |
| Habitat | −1.56 | 0.11 | ||||
| Knotweed × Habitat | 1.69 | 0.09 | ||||
| C:N ratio | ||||||
| Knotweed | 3.23 | 0.001 ** | 15.15 B ± 0.91 | 20.33 A ± 2.31 | 17.55 AB ± 1.27 | 17.73 AB ± 0.83 |
| Habitat | 0.97 | 0.33 | ||||
| Knotweed × Habitat | −2.2 | 0.03 * | ||||
| Nitrate (mg·g−1) | ||||||
| Knotweed | −0.56 | 0.58 | 1.61 AB ± 0.31 | 1.47 AB ± 0.23 | 0.67 B ± 0.2 | 1.56 A ± 0.12 |
| Habitat | −2.44 | 0.01 * | ||||
| Knotweed × Habitat | 2.86 | 0.004 ** | ||||
| Ammonium (mg·g−1) | ||||||
| Knotweed | −1.39 | 0.16 | 0.91 ± 0.23 | 0.65 ± 0.14 | 0.73 ± 0.08 | 0.59 ± 0.1 |
| Habitat | −0.12 | 0.91 | ||||
| Knotweed × Habitat | 0.15 | 0.88 | ||||
| pH | ||||||
| Knotweed | 0.55 | 0.59 | 6.97 ± 0.23 | 7.04 ± 0.26 | 7.56 ± 0.09 | 7.56 ± 0.04 |
| Habitat | 1.58 | 0.12 | ||||
| Knotweed × Habitat | −0.4 | 0.69 | ||||
| Microbial biomass (mgC·g−1) | ||||||
| Knotweed | −2.82 | 0.0049 ** | 0.23 A ± 0.04 | 0.14 B ± 0.03 | 0.2 AB ± 0.02 | 0.16 AB ± 0.02 |
| Habitat | −0.8 | 0.43 | ||||
| Knotweed × Habitat | 1.09 | 0.28 | ||||
| Ergosterol (μg·g−1) | ||||||
| Knotweed | −1.28 | 0.2 | 1.7 AB ± 0.5 | 1.1 B ± 0.3 | 3.1 A ± 0.4 | 2.0 AB ± 0.4 |
| Habitat | 1.98 | 0.048 * | ||||
| Knotweed × Habitat | −0.7 | 0.48 | ||||
| Humidity (%) | ||||||
| Knotweed | −1.1 | 0.27 | 33.4 A ± 1.6 | 31.5 A ± 1.4 | 24.5 B ± 1.8 | 29.0 AB ± 1.2 |
| Habitat | −3.71 | <0.001 *** | ||||
| Knotweed × Habitat | 2.54 | 0.011 * | ||||
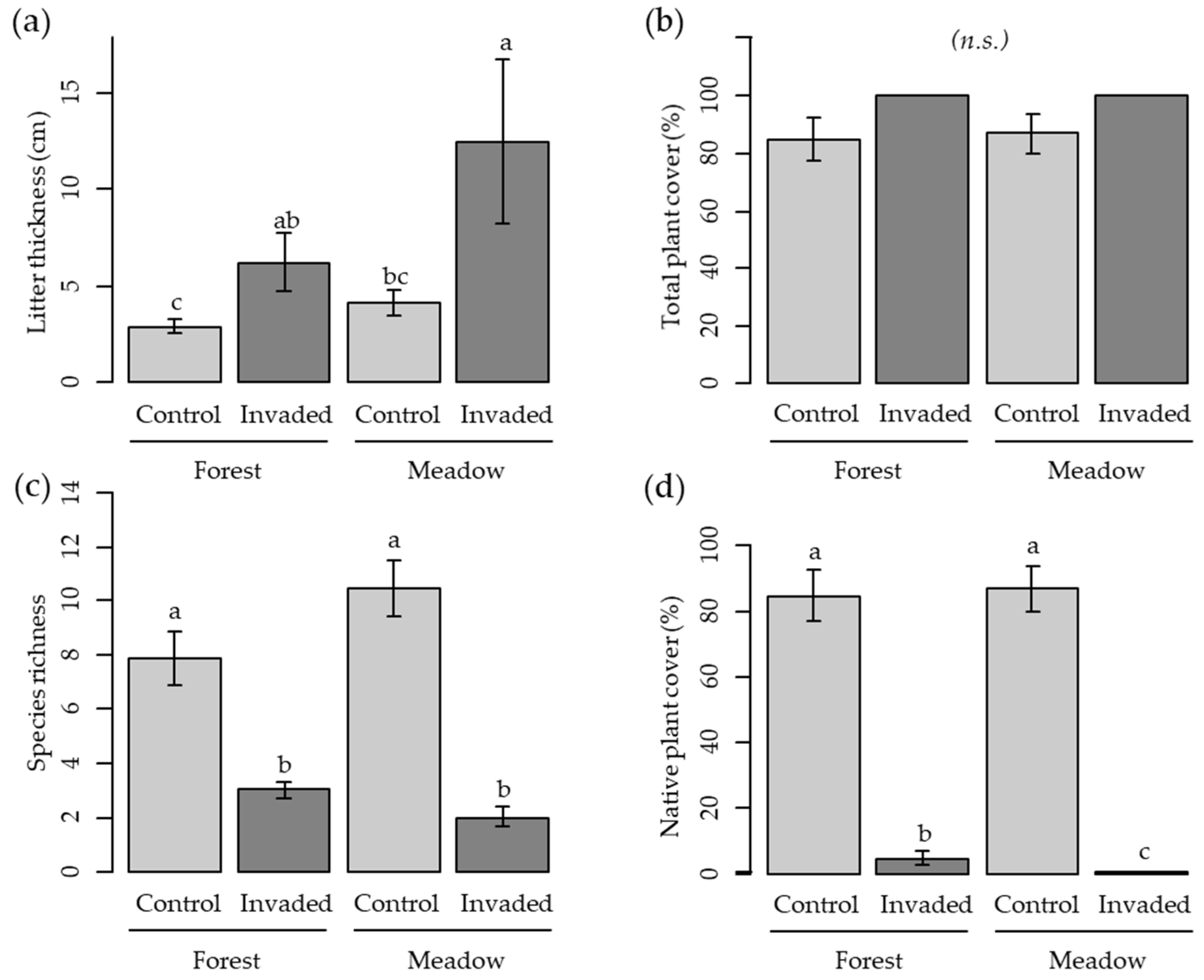
| Litter thickness (cm) | ||||||
| Knotweed | 2.71 | 0.0068 ** | 2.9 B ± 0.4 | 6.2 AB ± 1.5 | 4.1 B ± 0.7 | 12.4 A ± 4.3 |
| Habitat | 0.61 | 0.54 | ||||
| Knotweed × Habitat | 0.29 | 0.78 | ||||
| Forest | Meadow | |||||
|---|---|---|---|---|---|---|
| z-Value | p-Value | Control | Invaded | Control | Invaded | |
| Plant community variables | ||||||
| Species richness | ||||||
| Knotweed | −4.28 | <0.001 *** | 7.9 A ± 1 | 3.0 B ± 0.3 | 10.4 A ± 1.1 | 2.0 B ± 0.4 |
| Habitat | 1.49 | 0.14 | ||||
| Knotweed × Habitat | −2 | 0.045 * | ||||
| Total plant cover (%) | ||||||
| Knotweed | 2.26 | 0.024 * | 84.7 A ± 7.8 | 100 A ± 0 | 86.9 A ± 6.8 | 100 A ± 0 |
| Habitat | 0.25 | 0.8 | ||||
| Knotweed × Habitat | −0.20 | 0.84 | ||||
| Native plant cover (%) | ||||||
| Knotweed | −4.32 | <0.001 *** | 84.7 A ± 7.8 | 4.9 B ± 1.9 | 86.9 A ± 6.8 | 0.4 C ± 0.3 |
| Habitat | −0.5 | 0.61 | ||||
| Knotweed × Habitat | −3.54 | <0.001 *** | ||||
| Collembola abundance | ||||||
| Knotweed | −2.5 | 0.013 * | 26,056 A ± 5594 | 13,333 A ± 3593 | 22,611 A ± 8924 | 32,055 A ± 9320 |
| Habitat | −0.74 | 0.46 | ||||
| Knotweed × Habitat | 3.21 | 0.0013 ** | ||||
| Epedaphic abundance | ||||||
| Knotweed | 1.48 | 0.14 | 556 B ± 155 | 556 AB ± 242 | 1722 A ± 657 | 556 B ± 194 |
| Habitat | 3.4 | <0.001 *** | ||||
| Knotweed x Habitat | −2.8 | 0.0052 ** | ||||
| Hemiedaphic abundance | ||||||
| Knotweed | −2.09 | 0.037 * | 15,278 A ± 3436 | 9611 A ± 2652 | 10,000 A ± 3123 | 19,333 A ± 5428 |
| Habitat | −0.49 | 0.62 | ||||
| Knotweed × Habitat | 2.59 | 0.0097 ** | ||||
| Euedaphic abundance | ||||||
| Knotweed | −1.52 | 0.13 | 10,167 ± 2981 | 4055 ± 823 | 10,889 ± 6628 | 12,167 ± 4349 |
| Habitat | −0.06 | 0.95 | ||||
| Knotweed × Habitat | 1.65 | 0.098 | ||||
| Forest | Meadow | |||||
|---|---|---|---|---|---|---|
| z-Value | p-Value | Control | Invaded | Control | Invaded | |
| Nematoda abundance | ||||||
| Knotweed | −1.43 | 0.15 | 351 ± 98 | 233 ± 69 | 941 ± 490 | 238 ± 58 |
| Habitat | 0.89 | 0.37 | ||||
| Knotweed × Habitat | −0.94 | 0.35 | ||||
| bacterial feeder | ||||||
| Knotweed | −1.4 | 0.16 | 224 ± 65 | 149 ± 52 | 655 ± 326 | 180 ± 55 |
| Habitat | 1.05 | 0.29 | ||||
| Knotweed × Habitat | −0.93 | 0.35 | ||||
| fungal feeder | ||||||
| Knotweed | −1.11 | 0.27 | 122 ± 35 | 81 ± 29 | 260 ± 155 | 52 ± 6 |
| Habitat | 0.44 | 0.66 | ||||
| Knotweed × Habitat | −0.38 | 0.71 | ||||
| plant feeder | ||||||
| Knotweed | −2.86 | 0.004 ** | 14 A ± 5 | 2 B± 0.8 | 24 A ± 13 | 4 AB ± 2 |
| Habitat | 0.28 | 0.78 | ||||
| Knotweed × Habitat | 1.04 | 0.3 | ||||
| omnivorous-predatory | ||||||
| Knotweed | −0.51 | 0.61 | 0.3 ± 0.3 | 0.2 ± 0.2 | 0.9 ± 0.7 | 0.3 ± 0.2 |
| Habitat | 0.45 | 0.66 | ||||
| Knotweed × Habitat | −0.62 | 0.54 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Almeida, T.; Forey, E.; Chauvat, M. Alien Invasive Plant Effect on Soil Fauna Is Habitat Dependent. Diversity 2022, 14, 61. https://doi.org/10.3390/d14020061
De Almeida T, Forey E, Chauvat M. Alien Invasive Plant Effect on Soil Fauna Is Habitat Dependent. Diversity. 2022; 14(2):61. https://doi.org/10.3390/d14020061
Chicago/Turabian StyleDe Almeida, Tania, Estelle Forey, and Matthieu Chauvat. 2022. "Alien Invasive Plant Effect on Soil Fauna Is Habitat Dependent" Diversity 14, no. 2: 61. https://doi.org/10.3390/d14020061






