Characteristics and Drivers of Soil Organic Carbon Saturation Deficit in Karst Forests of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Areas
2.2. Research Methodology
2.2.1. Sample Site Selection and Vegetation Survey
2.2.2. Soil Sample Collection and Processing
2.2.3. Litter Sample Collection and Processing
2.2.4. SOC Fraction
2.2.5. Methods for Determination of Soil Sample Indicators
2.3. Data Processing and Analysis
3. Results
3.1. Characteristics of Changes in SOC Content
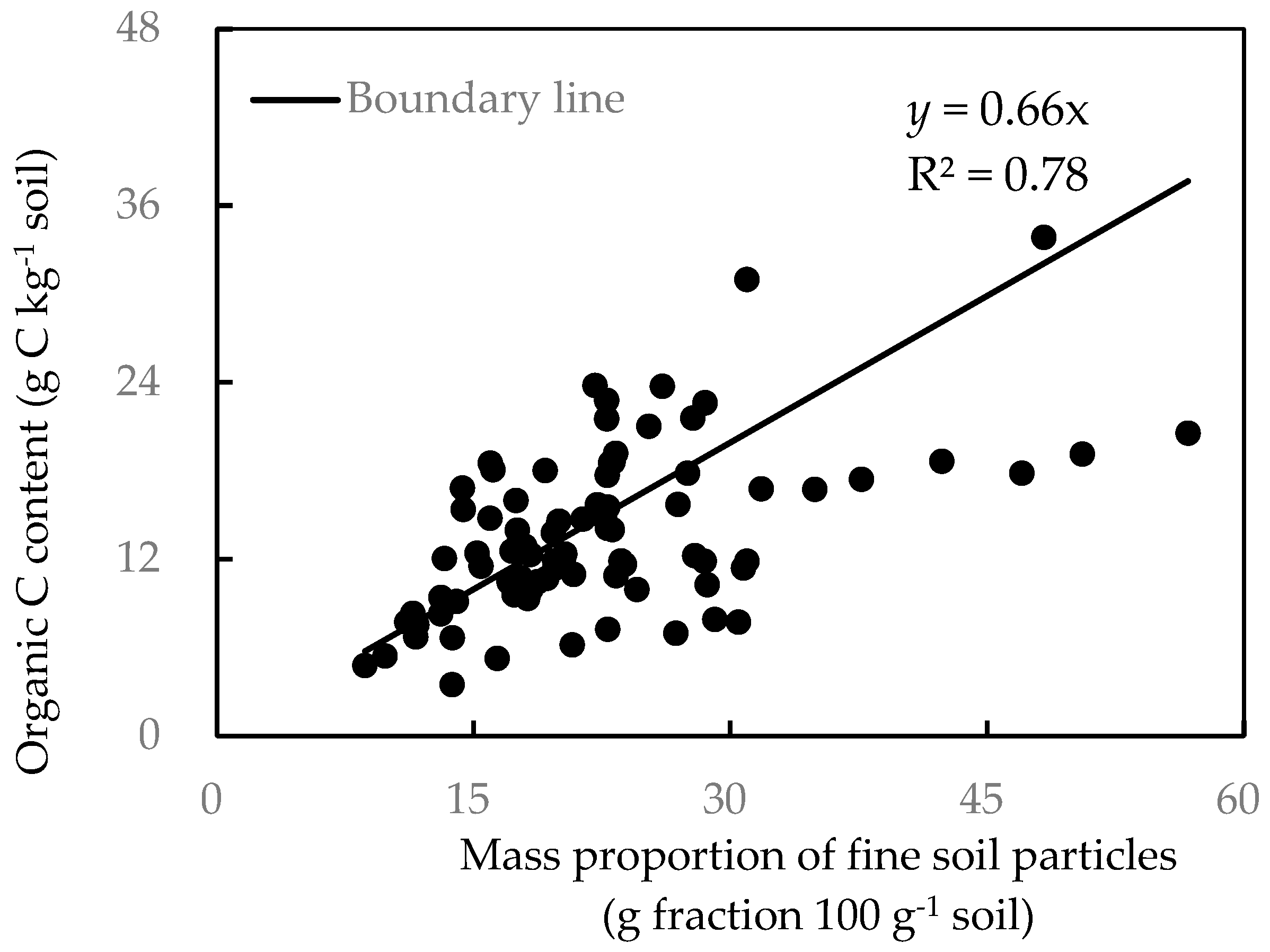
3.2. CSD Characteristics
3.3. Analysis of the Main Drivers of CSD
4. Discussion
4.1. Characteristics of the Changes in SOC Content
4.2. Characteristics of the Changes in CSD
4.3. Analysis of the Main Drivers of the CSD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Panja, P. Deforestation, Carbon dioxide increase in the atmosphere and global warming: A modelling study. Int. J. Model. Simul. 2019, 3, 209–219. [Google Scholar] [CrossRef]
- Samaneh, T.; Shamsollah, A.; Mojtaba, Z. Digital mapping of soil organic carbon using ensemble learning model in Mollisols of Hyrcanian forests, northern Iran. Geoderma Reg. 2020, 20, e00256. [Google Scholar] [CrossRef]
- Mojtaba, Z.; Garosi, Y.; Reza, O.H.; Ayoubi, S.; Taghizadeh, M.R.; Scholten, T.; Xu, M. Improving the spatial prediction of soil organic carbon using environmental covariates selection: A comparison of a group of environmental covariates. Catena 2021, 208, 105727. [Google Scholar] [CrossRef]
- Zhu, J.X.; Hu, H.F.; Tao, S.L.; Chi, X.L.; Jiang, L.; Ji, C.J.; Zhu, J.L.; Tang, Z.Y.; Pan, Y.D.; Richard, A.B. Carbon stocks and changes of dead organic matter in China’s forests. Nat. Commun. 2017, 1, 151. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.J.; Duan, M.S.; Deng, Z. Using an extended theory of planned behaviour to explain willingness towards voluntary carbon offsetting among Chinese consumers. Ecol. Econ. 2021, 2, 174–185. [Google Scholar] [CrossRef]
- Sayer, E.J.; Baxendale, C.; Birkett, A.J.; Bréchet, L.M.; Castro, B.; Deirdre, K.B.; Luis, L.S.; Chadtip, R. Altered litter inputs modify carbon and nitrogen storage in soil organic matter in a lowland tropical forest. Biogeochemistry 2020, 1, 23–34. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhu, J.; Shi, L.; Fu, Q.L.; Hu, H.Q.; Huang, Q.Y. Influence mechanisms of iron, aluminum and manganese oxides on the mineralization of organic matter in paddy soil. J. Environ. Manag. 2021, 301, 10–20. [Google Scholar] [CrossRef]
- Almeida, F.J.; Souza, I.F.; Hurtarte, C.C.; Teixeira, P.P.C.; Inagaki, T.M.; Silva, I.R.; Mueller, C.W. Forest litter constraints on the pathways controlling soil organic matter formation. Soil Biol. Biochem. 2021, 4, 142–153. [Google Scholar] [CrossRef]
- West, T.O.; Six, J. Considering the influence of sequestration duration and carbon saturation on estimates of soil carbon capacity. Clim. Chang. 2007, 80, 25–41. [Google Scholar] [CrossRef]
- Stewart, C.E.; Plante, A.F.; Paustian, K.P.; Conant, R.T.; Six, J. Soil carbon saturation: Linking concept and measurable carbon pools. Soil Sci. Soc. Am. J. 2008, 72, 379–392. [Google Scholar] [CrossRef]
- Feng, W.T.; Plante, A.F.; Six, J. Improving estimates of maximal organic carbon stabilization by fine soil particles. Biogeochemistry 2013, 112, 81–93. [Google Scholar] [CrossRef]
- Liu, G.H.; Fu, B.J.; Fang, J.Y. Carbon dynamics of Chinese forests and its contribution to global carbon balance. Acta Ecol. Sin. 2000, 5, 733–740. [Google Scholar]
- Sun, K.; Li, J.Q.; Yang, G.L.; Zhuo, M.; Hu, J. Effect of alterations in forest litter inputs on soil C and N storage distribution in pinus yunnanensis forest in central Yunnan Plateau. Acta Ecol. Sin. 2021, 41, 3100–3110. [Google Scholar] [CrossRef]
- Zhou, Y.R.; Yu, Z.L.; Zhao, S.D. Carbon storage and budget of major Chinese forest types. Chin. J. Plant Ecol. 2000, 24, 518–522. [Google Scholar]
- Huang, Z.S.; Yu, L.F.; Fu, Y.H.; Yang, R. Characteristics of carbon sequestration during natural restoration of Maolan karst forest ecosystems. Chin. J. Plant Ecol. 2015, 39, 554–564. [Google Scholar]
- Zhang, L.; Zhang, D.L.; Mao, Z.J. Characteristic mineralization of soil organic carbon in different successional series of broadleaved Korean pine forests in the temperate zone in China. Acta Ecol. Sin. 2017, 37, 6370–6378. [Google Scholar] [CrossRef]
- Lai, R.; Follett, R.F.; Stewart, B.A.; Kimble, J.M. Soil carbon sequestration to mitigate climate change and advance food security. Soil Sci. 2007, 172, 943–956. [Google Scholar] [CrossRef]
- Di, J.Y.; Xu, M.G.; Zhang, W.J.; Tong, X.G.; He, X.H.; Gao, H.J.; Liu, H.; Wang, B.R. Combinations of soil properties, carbon inputs and climate control the saturation deficit dynamics of stable soil carbon over 17-year fertilization. Sci. Rep. 2018, 8, 12653. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.F. Research on Ecological Process of Natural Restoration of Degraded Karst Forest. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 1998. [Google Scholar]
- Hamer, U.; Marschner, B. Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol. Biochem. 2005, 37, 445–454. [Google Scholar] [CrossRef]
- Rietz, D.N.; Haynes, R.J. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Hu, N.; Li, H.; Tang, Z.; Li, Z.F.; Li, G.C.; Jiang, Y.; Hu, X.M.; Lou, Y.L. Community size, activity and C:N stoichiometry of soil microorganisms following reforestation in a Karst region. Eur. J. Soil Biol. 2016, 73, 77–83. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhang, W.; Wang, K.L.; Pan, F.J.; Yang, S.; Shu, S.Y. Factors controlling accumulation of soil organic carbon along vegetation succession in a typical karst region in Southwest China. Sci. Total Environ. 2015, 521, 52–58. [Google Scholar] [CrossRef]
- Xiao, K.C.; He, T.G.; Chen, H.; Peng, W.X.; Song, T.Q.; Wang, K.L.; Li, D.J. Impacts of vegetation restoration strategies on soil organic carbon and nitrogen dynamics in a karst area, southwest China. Ecol. Eng. 2017, 101, 247–254. [Google Scholar] [CrossRef]
- Huang, C.J. Main vegetation types and distribution characteristics in Yuntai Mountain, Shibing, Guizhou province of China. Guizhou For. Sci. Technol. 1995, 23, 26–30. [Google Scholar]
- Luo, X.J.; Li, Q.M.; Feng, Y.C.; Wang, L.H.; Jiang, Y.L. Biodiversity and floristic characteristics of orchids in Dashahe Nature Reserve, Daozhen, Guizhou province of China. Guizhou For. Sci. Technol. 2013, 41, 19–23. [Google Scholar]
- Dong, M. Plant clonal growth in heterogeneous habitats: Risk-spreading. Chin. J. Plant Ecol. 1996, 6, 543–548. [Google Scholar]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Nanjing, China, 2001; pp. 3–109. [Google Scholar]
- Guan, S.Y. Soil Enzymes and Research Methods; Agricultural Press: Beijing, China, 1986; pp. 294–297. [Google Scholar]
- Zhu, X.N. R Language; Renmin University of Chinese Press: Beijing, China, 2018; pp. 1–352. [Google Scholar]
- Wang, C.Q.; Xue, L.; Jiao, R.Z. Soil organic carbon fractions, C-cycling associated hydrolytic enzymes, and microbial carbon metabolism vary with stand age in Cunninghamia lanceolate (Lamb.) Hook plantations. For. Ecol. Manag. 2021, 48, 482–496. [Google Scholar] [CrossRef]
- Wu, Y.N.; Yu, L.F.; Zhang, L.M.; Liu, N.; Yan, L.B. Characteristics and influencing factors of soil carbon pool during vegetation restoration in Karst Plateau. Ecol. Environ. Sci. 2020, 29, 1935–1942. [Google Scholar] [CrossRef]
- Ge, T.D.; Yuan, H.Z.; Zhu, H.H.; Wu, X.H.; Nie, S.A.; Liu, C.; Tong, C.L.; Wu, J.S.; Brookes, P. Biological carbon assimilation and dynamics in a flooded rice-soil system. Soil Biol. Biochem. 2012, 48, 39–49. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 1992, 44B, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Elipe, B.; Carlos, G.; Noah, F.; David, J.E.; Matthew, A.B.; Sebastián, A.; Fernando, D.A.; Asmeret, A.B.; Nick, A.C.; Antonio, G.; et al. Global ecological predictors of the soil priming effect. Nat. Commun. 2019, 10, 3481. [Google Scholar]
- Zhang, L.M.; Xu, M.G.; Lou, Y.L.; Wang, X.L.; Qing, S.; Jiang, T.M.; Li, Z.F. Changes in Yellow paddy soil organic carbon fractions under long-term fertilization. Sci. Agric. Sin. 2014, 47, 3817–3825. [Google Scholar] [CrossRef]
- Chen, Z.M.; Wang, H.Y.; Liu, X.W.; Zhao, X.L.; Lu, D.J.; Zhou, J.M.; Li, C.Z. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice-wheat cropping system. Soil Tillage Res. 2017, 16, 121–127. [Google Scholar] [CrossRef]
- Lan, L.Y.; Yang, W.Q.; Wu, F.Z.; Liu, Y.W.; Guo, C.H.; Zhan, Y.; Tan, B. Effects of soil fauna on microbial community during litter decomposition of populus simonii and fargesia spathacea in the subalpine forest of western Sichuan, China. Chin. J. Appl. Ecol. 2019, 30, 2983–2991. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition. Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Elliott, E.T.; Paustian, K.; Frey, S.D. Modeling the Measurable or Measuring the Modelable: A Hierarchical Approach to Isolating Meaningful Soil Organic Matter Fractionations. In Evaluation of Soil Organic Matter Models; Nato Asiseries; Springer: Berlin/Heidelberg, Germany, 1996; Volume 2, pp. 161–179. [Google Scholar]
- Soh, S.; Makoto, S.; Antoine, D.M.Z.; Haruo, T.; Takashi, K.; Shinya, F. Forest understories controlled the soil organic carbon stock during the fallow period in African tropical forest: A 13C analysis. Sci. Rep. 2019, 9, 9835–9843. [Google Scholar] [CrossRef]
- Callesen, I.; Liski, J.; Raulund-Rasmussen, K.; Olsson, M.T.; Tau-Strand, L.V.; Westman, C.J. Soil carbon stores in Nordic well-drained forest soils—Relationships with climate and texture class. Glob. Chang. Biol. 2003, 9, 358–370. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Jörg, P.; Frauke, B.; Peter, S.; Uwe, G.; Edzard, H.; Arthur, R.; Bernd, S.; Margit, V.L.; Ingrid, K.K. Storage and drivers of organic carbon in forest soils of southeast Germany (Bavaria)-Implications for carbon sequestration. For. Ecol. Manag. 2013, 295, 162–172. [Google Scholar] [CrossRef]
- Guan, J.H.; Deng, L.; Zhang, J.G.; He, Q.Y.; Shi, W.Y.; Li, G.Q.; Du, S. Soil organic carbon density and its driving factors in forest ecosystems across a northwestern province in China. Geoderma 2019, 352, 1–12. [Google Scholar] [CrossRef]
- Schuman, G.E.; Janzen, H.H.; Herrick, J.E. Soil carbon dynamics and potential carbon sequestration by rangelands. Environ. Pollut. 2002, 116, 391–396. [Google Scholar] [CrossRef]
- Merabtene, M.D.; Faraoun, F.; Mlih, R.; Riad, D.; Ali, L.; Roland, B. Forest Soil Organic Carbon Stocks of Tessala Mount in North-West Algeria-Preliminary Estimates. Front. Environ. Sci. 2021, 120, 125–138. [Google Scholar] [CrossRef]
- Xu, J.H.; Gao, L.; Sun, Y.; Cui, X.Y. Distribution of mineral-bonded organic carbon and black carbon in forest soils of great Xing’an mountains, China and carbon sequestration potential of the soils. Acta Pedol. Sin. 2018, 55, 236–246. [Google Scholar] [CrossRef]
- Zhang, Q. Soil Carbon Distribution Characteristics and Carbon Sequestration Potential Estimation of Highway Shelterbelt in Taklimakan Desert; Northwest Agricultural & Forest University: Shanxi, China, 2019. [Google Scholar]
- Bertrand, I.; Delfosse, O.; Mary, B. Carbon and nitrogen mineralization in acidic, limed and calcareous agricultural soils: Apparent and actual effects. Soil Biol. Biochem. 2007, 39, 276–288. [Google Scholar] [CrossRef]
- Oren, A.; Steinberger, Y. Coping with artifacts induced by CaCO3-CO2-H2O equilibria in substrate utilization profiling of calcareous soils. Soil Biol. Biochem. 2008, 40, 2569–2577. [Google Scholar] [CrossRef]
- Di, J.Y. Characteristics and Driving Factors of Mineral Combined Organic Carbon Saturation Deficit in Typical Farmland Soils under Long-Term Fertilization; Chinese Academy of Agricultural Sciences: Beijing, China, 2017. [Google Scholar]
- Sun, Z.X.; Bai, H.Q.; Ye, H.C.; Zhuo, Z.Q.; Huang, W.J. Three-dimensional modelling of soil organic carbon density and carbon sequestration potential estimation in a dryland farming region of China. J. Geogr. Sci. 2021, 31, 1453–1468. [Google Scholar] [CrossRef]
- Dray, R.; Gorham, E. Litter production in forest of the world. Adv. Res. 1964, 2, 101–157. [Google Scholar]
- Wang, X.P.; Yang, X.; Yang, N.; Xin, X.J.; Qu, Y.B.; Zhao, L.X.; Gao, Y.B. Effects of litter diversity and composition on litter decomposition characteristics and soil microbial community. Acta Ecol. Sin. 2019, 39, 1–9. [Google Scholar] [CrossRef]
- Hartley, I.P.; Gatnett, M.H.; Hopkins, D.W.; Fletcher, B.J.; Sloan, V.L.; Phoenix, G.K.; Wookey, P.A. A potential loss of carbon associated with greater plant growth in the European Arctic. Nat. Clim. Chang. 2012, 12, 875–879. [Google Scholar] [CrossRef]
- Qin, Y.B.; Xin, Z.B.; Wang, D.M.; Xiao, Y.L. Soil organic carbon storage and its influencing factors in the riparian woodlands of a Chinese karst area. Catena 2017, 15, 21–29. [Google Scholar] [CrossRef]
- Chen, J.; Sinsabaugh, R.L. Linking microbial functional gene abundance and soil extracellular enzyme activity: Implications for soil carbon dynamics. Glob. Chang. Biol. 2021, 27, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.X.; He, H.B.; Cheng, W.X.; Zhen, B.; Wang, Y.; Zhang, X.D. Factors controlling soil organic carbon stability along a temperate forest altitudinal gradient. Sci. Rep. 2016, 6, 242–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.X.; Luan, Y.N.; Dai, W.; Wang, B.; Dai, A. Factors affecting soil organic carbon in a Phyllostachys edulis forest. J. For. Res. 2019, 30, 1487–1494. [Google Scholar] [CrossRef]


| Area | Succession Stage | Coordinates | Elevation (m) | Precipitation (mm) | Annual Mean Temperature (°C) | Aspect | Dominant Species | Soil Bedrock | Soil Type | Sample Size (m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Maolan | Herb stage | 108.03 25.26 | 840 | 1590.70 | 19.75 | NW | Pteridium revolutum, Imperata cylindrical var. major, Pogonatherum crinitum, Trisetum bifidum | Dolomite limestone | Clay, black limestone soil | 2 × 5 |
| Shrub stage | 107.94 25.30 | 820 | 1590.70 | 19.75 | SW | Pyracantha fortuneana, Nandina domestica, Lindera communis, Myrsine semiserrata, Clausena dunniana, Ulmus parvifolia | Dolomite limestone | Clay, black limestone soil | 4 × 10 | |
| Arbor stage | 107.95 25.29 | 840 | 1590.70 | 19.75 | SW | Swida wilsoniana, Machilus chienkweiensis, Lindera communis, Cladrastis platycarpa, Choerospondias axillaris | Dolomite limestone | Clay, black limestone soil | 20 × 20 | |
| Climax stage | 107.99 25.19 | 850 | 1590.70 | 19.75 | SW | Swida wilsoniana, Pittosporum brevicalyx, Cyclobalanopsis multiervis, Acerwangchii, Carpinus pubescens, Phoebe crassipedicella | Dolomite limestone | Clay, black limestone soil | 20 × 20 | |
| Yuntai Mountain | Herb stage | 108.12 27.18 | 873 | 1083.80 | 17.29 | SW | Awn, Ophiopogon japonicus, Ficus tikoua Bur., Athyrium dissitifolium | Carbonate rock | Clay, black limestone soil | 2 × 5 |
| Shrub stage | 108.16 27.13 | 865 | 1083.80 | 17.29 | NW | Bridelia tomentosa, Neillia sinensis Oliv., Viburnum dilatatum Thunb., Nothopanax davidii Franch.Harms | Carbonate rock | Loam, black limestone soil | 4 × 10 | |
| Arbor stage | 108.10 27.10 | 841 | 1083.80 | 17.29 | SW | Lindera communis Hemsl., Pistacia chinensis Bunge, Quercus acutissima Carr., Platycarya strobilacea | Carbonate rock | Loam, black limestone soil | 20 × 20 | |
| Climax stage | 108.11 27.12 | 875 | 1083.80 | 17.29 | NW | Cupressus funebris, Quercus dolicholepis, Platycarya strobilacea, Carpinus pubescens, Quercus phillyraeoides | Carbonate rock | Loam, black limestone soil | 20 × 20 | |
| Dashahe | Herb stage | 107.58 29.15 | 1371 | 1372.20 | 16.81 | NE | Imperata cylindrica, Carex capilliformis, R. setchuenensis | Carbonate rock | Clay, black limestone soil | 2 × 5 |
| Shrub stage | 107.57 29.10 | 1416 | 1372.20 | 16.81 | NE | Pyracantha fortuneana, Viburnum dilatatum Thunb., R. setchuenensis, Wild persimmon | Carbonate rock | Clay, black limestone soil | 4 × 10 | |
| Arbor stage | 108.01 29.12 | 1389 | 1372.20 | 16.81 | NE | Litsea elongata Benth., Machilus versicolora, Carpinus pubescens Burk., Fagus longipetiolata | Carbonate rock | Loam, black limestone soil | 20 × 20 | |
| Climax stage | 107.58 29.17 | 1304 | 1372.20 | 16.81 | N | Machilus pingii, Tetracentron sinense, Dipentodon sinicus, Davidia involucrata, Emmenopterys henryi | Carbonate rock | Loam, black limestone soil | 20 × 20 | |
| Nayong | Climax stage | 105.44 26.68 | 1861 | 1226.00 | 14.75 | NW | Davidia involucrata, Decaisnea insignis, Dipentodon sinicus, Cyclobalanopsis argyrotricha | Carbonate rock | Loam, black limestone soil | 20 × 20 |
| Pogang | Climax stage | 105.09 25.11 | 1280 | 1501.70 | 17.10 | N | Eucalyptus robusta, Platycarya strobilacea, Itoa orientalis Hemsl | Dolomite limestone | Loam, black limestone soil | 20 × 20 |
| Kuankuoshui | Climax stage | 107.06 28.18 | 1450 | 1029.40 | 15.91 | SW | Fagus longipetiolata, Emmenopterys henryi, Tulip poplar | Carbonate rock | Loam, black limestone soil | 20 × 20 |
| Huoyan mountain | Climax stage | 105.79 26.47 | 1680 | 1163.10 | 15.98 | W | Rhododendron stamineum, Birch, Oak | Carbonate rock | Loam, black limestone soil | 20 × 20 |
| Huanggu mountain | Climax stage | 108.78 27.54 | 1020 | 1542.00 | 17.56 | N | Fagus longipetiolata, Buxus sinica, Davidia involucrata, Hemlock | Carbonate rock | Loam, black limestone soil | 20 × 20 |
| Zijiang rift valley | Climax stage | 107.04 26.90 | 720 | 1169.00 | 14.13 | SW | Betula luminifera, Cinnamomum camphora, Pistacia chinensis, Liquidenbar formosana | Carbonate rock | Loam, black limestone soil | 20 × 20 |
| Bijia mountain | Climax stage | 106.14 25.12 | 1083 | 1062.70 | 20.53 | NW | Cyclobalanopsis oak, Carpinus pubescens, Celtis sinensis, Ormosia saxatilis | Carbonate rock | Loam, black limestone soil | 20 × 20 |
| Area | Succession Stage | pH | BD (g cm−3) | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | Lci (g C m−2) | Ca (g kg−1) | Ur (mg g−1 24 h−1) | Npa (mg g−1 24 h−1) | Sa (mg g−1 24 h−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maolan | Herb stage | 7.34 ± 0.08a | 1.31 ± 0.02a | 28.34 ± 2.80d | 1.57 ± 0.03d | 0.36 ± 0.01d | 12.27 ± 1.08d | 1.38 ± 0.05c | 0.09 ± 0.01c | 0.65 ± 0.08b | 7.08 ± 0.23b |
| Shrub stage | 7.63 ± 0.07a | 1.25 ± 0.01a | 65.30 ± 4.36c | 6.75 ± 0.43c | 1.01 ± 0.10b | 33.57 ± 2.44c | 3.19 ± 0.48b | 0.89 ± 0.48b | 2.62 ± 0.15a | 7.36 ± 0.14b | |
| Arbor stage | 7.23 ± 0.06a | 1.20 ± 0.01a | 85.22 ± 3.69b | 7.55 ± 0.11b | 0.77 ± 0.02c | 81.76 ± 2.23b | 4.83 ± 0.14a | 1.05 ± 0.11b | 2.16 ± 0.34a | 8.22 ± 0.22b | |
| Climax stage | 7.14 ± 0.27a | 1.02 ± 0.03b | 94.13 ± 3.51a | 8.42 ± 1.10aa | 1.21 ± 0.20a | 141.03 ± 2.53a | 4.83 ± 1.02a | 1.62 ± 0.45a | 2.79 ± 0.33a | 12.77 ± 0.73a | |
| Yuntai Mountain | Herb stage | 8.12 ± 0.05a | 1.43 ± 0.06a | 22.52 ± 1.23c | 1.88 ± 0.08d | 0.43 ± 0.01a | 23.33 ± 1.34c | 2.29 ± 0.09d | 0.35 ± 0.04c | 0.62 ± 0.04b | 0.75 ± 0.08c |
| Shrub stage | 7.94 ± 0.02a | 1.28 ± 0.03b | 41.46 ± 2.05b | 3.67 ± 0.22c | 0.64 ± 0.01a | 40.39 ± 2.19c | 4.49 ± 0.19c | 3.18 ± 0.09b | 2.20 ± 0.07a | 1.36 ± 0.12c | |
| Arbor stage | 7.97 ± 0.04a | 1.19 ± 0.04c | 58.84 ± 3.16b | 5.43 ± 0.25b | 0.57 ± 0.02a | 137.09 ± 6.90b | 6.63 ± 0.14a | 4.18 ± 0.30a | 2.26 ± 0.09a | 9.86 ± 1.99b | |
| Climax stage | 7.93 ± 0.06a | 1.17 ± 0.02c | 82.13 ± 2.48a | 6.75 ± 0.79a | 0.54 ± 0.03a | 181.11 ± 6.72a | 5.09 ± 0.74b | 4.25 ± 0.76a | 2.08 ± 0.24a | 12.83 ± 0.82a | |
| Dashahe | Herb stage | 7.49 ± 0.22a | 1.30 ± 0.02a | 18.35 ± 2.13d | 1.84 ± 0.03d | 0.51 ± 0.04a | 21.27 ± 8.71c | 2.26 ± 0.38b | 0.22 ± 0.04c | 0.55 ± 0.08c | 5.87 ± 2.12b |
| Shrub stage | 6.55 ± 0.04a | 1.18 ± 0.03b | 29.23 ± 3.05c | 2.33 ± 0.17c | 0.37 ± 0.01b | 29.25 ± 2.34c | 1.85 ± 0.13c | 0.35 ± 0.03c | 0.69 ± 0.03c | 4.67 ± 1.51b | |
| Arbor stage | 6.60 ± 0.10a | 1.22 ± 0.02b | 49.93 ± 3.52b | 3.77 ± 0.29b | 0.39 ± 0.01b | 98.86 ± 7.55b | 2.83 ± 0.25b | 0.74 ± 0.10b | 1.89 ± 0.14a | 10.60 ± 1.91a | |
| Climax stage | 7.74 ± 0.09a | 1.12 ± 0.01c | 78.75 ± 2.48a | 5.53 ± 0.67a | 0.69 ± 0.03a | 204.71 ± 12.24a | 3.20 ± 0.31a | 2.28 ± 0.75a | 1.12 ± 0.14b | 11.11 ± 1.74a |
| Area | Succession Stage | >250 μm Fraction C Content (g C kg−1 Soil) | 53–250 μm Fraction C Content (g C kg−1 Soil) | <53 μm Fraction C Content (g C kg−1 Soil) | Soil Organic C Content (g C kg−1 Soil) | Proportion of <53 μm C to Total Organic C (g Fraction 100g−1 Soil) |
|---|---|---|---|---|---|---|
| Maolan | Herb stage | 17.33 ± 0.62d | 7.50 ± 0.46d | 3.51 ± 0.38d | 28.34 ± 2.80d | 12.39 ± 0.55c |
| Shrub stage | 31.91 ± 1.50c | 22.77 ± 1.19c | 10.62 ± 0.68c | 65.30 ± 4.36c | 16.26 ± 0.43b | |
| Arbor stage | 39.49 ± 2.46b | 30.35 ± 2.55b | 15.38 ± 1.29b | 85.22 ± 3.69b | 18.05 ± 0.62a | |
| Climax stage | 43.77 ± 2.35aB | 33.19 ± 2.33aB | 17.17 ± 0.73aB | 94.13 ± 3.51aB | 18.24 ± 1.03aA | |
| Yuntai Mountain | Herb stage | 17.32 ± 1.12c | 3.31 ± 0.29d | 1.89 ± 0.19c | 22.52 ± 0.84d | 8.39 ± 0.21b |
| Shrub stage | 20.70 ± 1.23c | 16.70 ± 1.61c | 4.06 ± 0.21c | 41.46 ± 1.75c | 9.79 ± 0.32b | |
| Arbor stage | 27.58 ± 2.67b | 20.58 ± 3.31b | 10.68 ± 0.98b | 58.84 ± 4.61b | 18.15 ± 0.65a | |
| Climax stage | 38.06 ± 1.48aB | 27.97 ± 1.70aBC | 16.10 ± 2.48aB | 82.13 ± 2.08aC | 19.60 ± 0.24aA | |
| Dashahe | Herb stage | 14.69 ± 1.10c | 2.20 ± 0.21d | 1.46 ± 0.19c | 18.35 ± 0.97d | 7.96 ± 0.13c |
| Shrub stage | 17.46 ± 0.82c | 9.58 ± 0.96c | 2.19 ± 0.24c | 29.23 ± 0.84c | 7.49 ± 0.28c | |
| Arbor stage | 28.93 ± 2.63b | 15.25 ± 1.74b | 5.75 ± 0.52b | 49.93 ± 3.21b | 11.52 ± 0.44b | |
| Climax stage | 39.01 ± 2.01aB | 24.22 ± 2.13aC | 15.52 ± 0.81aB | 78.75 ± 2.45aCD | 19.71 ± 0.36aA | |
| Nayong | Climax stage | 69.21 ± 3.42A | 54.34 ± 4.51A | 23.56 ± 3.87A | 147.11 ± 6.55A | 18.42 ± 0.52A |
| Pogang | Climax stage | 41.69 ± 4.74B | 24.65 ± 2.58C | 15.44 ± 1.43B | 81.78 ± 8.89C | 18.88 ± 0.34A |
| Kuankuoshui | Climax stage | 29.06 ± 1.99C | 18.99 ± 1.23D | 11.45 ± 0.28C | 59.50 ± 4.79DE | 19.24 ± 1.17A |
| Huoyan mountain | Climax stage | 28.22 ± 1.53C | 12.18 ± 0.93E | 9.03 ± 1.73D | 49.43 ± 0.63E | 21.09 ± 0.70A |
| Huanggu mountain | Climax stage | 39.65 ± 1.56B | 20.52 ± 0.76D | 15.62 ± 1.77B | 75.79 ± 1.39CD | 20.61 ± 2.26A |
| Zijiang rift valley | Climax stage | 17.95 ± 0.69D | 15.80 ± 0.99DE | 8.30 ± 0.78D | 42.05 ± 1.85F | 19.74 ± 1.76A |
| Bijia mountain | Climax stage | 32.20 ± 2.15C | 20.95 ± 1.64D | 12.26 ± 1.49C | 65.41 ± 6.03D | 18.82 ± 0.25A |
| Different Regions | Regression Equation | Indicative Factor | p Value | Correction R2 |
|---|---|---|---|---|
| Maolan | y = −0.45x1 − 6.22x2 + 43.85x3 − 0.42x4 + 94.38 | y: CSD | 0.001 | 0.96 |
| x1: Lci | ||||
| x2: TN | ||||
| x3: TP | ||||
| x4: SOC | ||||
| Yuntai Mountain | y = −0.40x1 + 97.15 | y: CSD | 0.000 | 0.90 |
| x1: Lci | ||||
| Dashahe | y = −0.16x1 − 11.49x2 + 98.59 | y: CSD | 0.001 | 0.91 |
| x1: Lci | ||||
| x2: Npa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, Y.; Chen, J.; Feng, L.; Li, F.; Yu, L. Characteristics and Drivers of Soil Organic Carbon Saturation Deficit in Karst Forests of China. Diversity 2022, 14, 62. https://doi.org/10.3390/d14020062
Zhang L, Wang Y, Chen J, Feng L, Li F, Yu L. Characteristics and Drivers of Soil Organic Carbon Saturation Deficit in Karst Forests of China. Diversity. 2022; 14(2):62. https://doi.org/10.3390/d14020062
Chicago/Turabian StyleZhang, Limin, Yang Wang, Jin Chen, Ling Feng, Fangbing Li, and Lifei Yu. 2022. "Characteristics and Drivers of Soil Organic Carbon Saturation Deficit in Karst Forests of China" Diversity 14, no. 2: 62. https://doi.org/10.3390/d14020062
APA StyleZhang, L., Wang, Y., Chen, J., Feng, L., Li, F., & Yu, L. (2022). Characteristics and Drivers of Soil Organic Carbon Saturation Deficit in Karst Forests of China. Diversity, 14(2), 62. https://doi.org/10.3390/d14020062







