1. Introduction
Birds sing to attract mates and defend territories, but their vocal signals are also important in maintaining group contacts, signaling food resources, making alarm calls in case of danger and begging for food sources [
1,
2,
3,
4]. It is therefore generally believed that birds have higher vocal activity earlier in the season when attracting mates and defending territories, and their acoustic activity decreases as the season progresses due to hormonal changes and parental care [
1,
5,
6]. In addition, vocalizations of birds are also affected by several intrinsic (e.g., eye size, body size and breeding status) [
5,
7,
8] and extrinsic factors (e.g., vegetation cover, moonlight, rainfall and temperature) [
6,
9,
10].
Some studies have demonstrated a trade-off between singing and foraging activities [
11,
12]. Consequently, because vocal activity require a lot of energy [
13,
14], most bird species prefer to vocalize at dawn and dusk, when light levels and temperatures are lower, resulting in fewer foraging opportunities and increased song transmission [
15,
16]. Similarly, some birds sing in the evening to ensure safe roosting sites [
17,
18]. Therefore, studies of diurnal and seasonal patterns of bird vocal activity may improve our understanding of the function of vocalizations, breeding activities, and the focal species’ ecology [
10,
19]. However, despite having extensive knowledge of the vocalization patterns of passerines, there is relatively little information available on the diurnal and seasonal patterns of brood parasitic species that depend on hosts to provide parental care to their chicks [
20,
21].
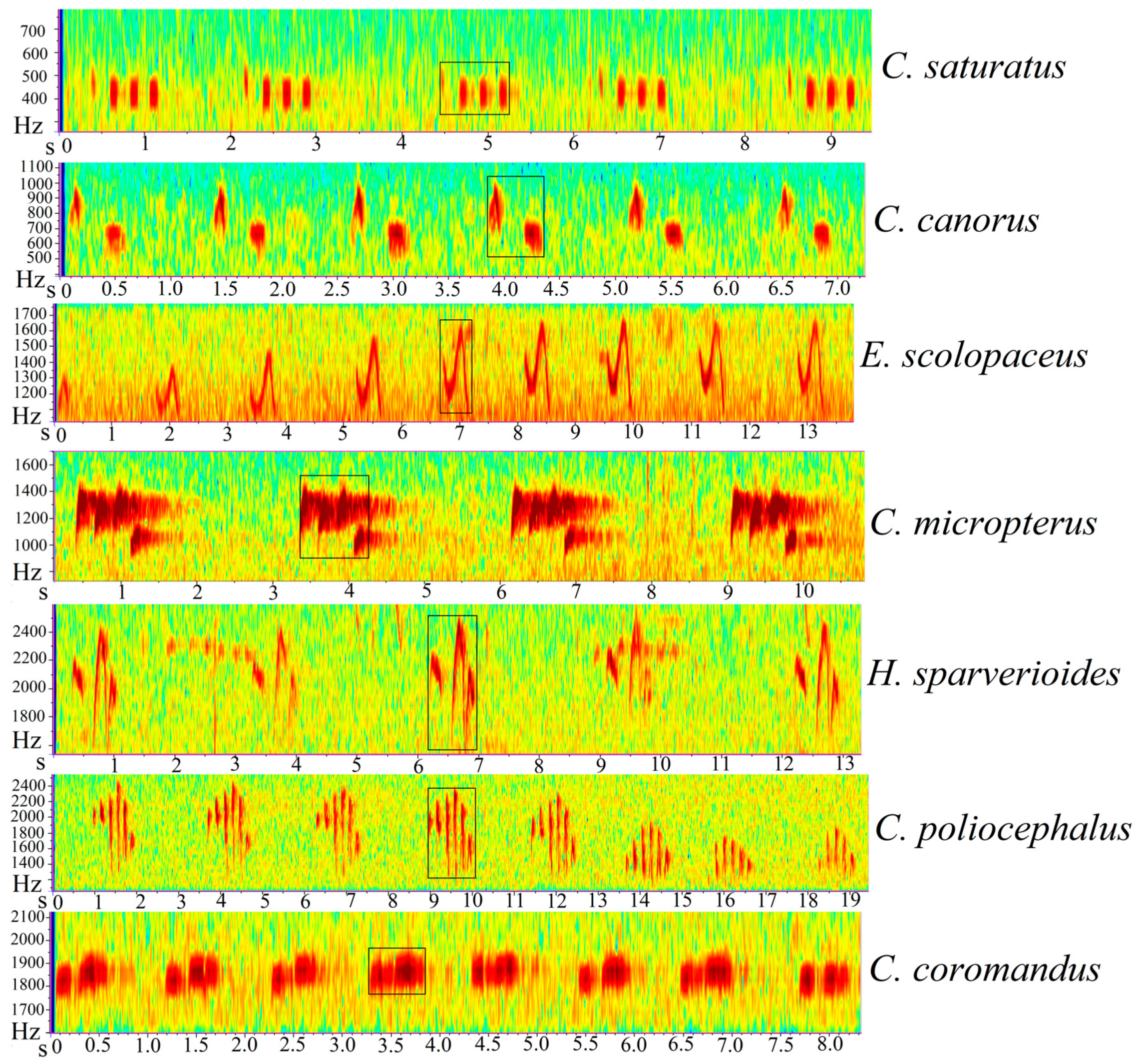
In the study, seven avian brood parasites were selected, they include Indian cuckoo (
Cuculus micropterus), Himalayan cuckoo (
Cuculus saturatus), Common cuckoo (
Cuculus canorus), Lesser cuckoo (
Cuculus poliocephalus), Asian koel (
Eudynamys scolopaceus), Large hawk-cuckoo (
Hierococcyx sparverioides), and Chestnut-winged cuckoo (
Clamator coromandus). Unlike passerines, they do not have well-developed syringeal muscles so their vocalizations are simple and monotonous [
22,
23,
24,
25,
26,
27,
28]. The spectrograms and frequency characteristics of the seven cuckoo species is shown in
Figure 1. These are avian brood parasites that lay their eggs in the nests of the host species and rely on them to raise their young [
22,
23,
24,
25,
26,
27,
28]. The females of several cuckoo species also call for conspecific and heterospecific communication and misdirecting host species, but their males produce a large number of calls over a complete breeding season. Thus, in our study we only focused on the male cuckoos’ calls. All these species are migratory and based on our acoustic data and some survey reports they arrive at our study sites during April and May [
29].
Acoustic communication is also essential in avian brood parasites [
2,
31], and previous research has shown that the vocal activity of Cuculidae serve similar functions of attracting mates and defending territories like passerines [
2,
3]. For example, during a playback experiment, Moskát and Hauber (2019) found that both male and female common cuckoos responded strongly to unknown bubbling calls and males approached the speaker more frequently than females [
2,
3]. Furthermore, female common cuckoo calls play an important role in intraspecific communication or competition and divert host species’ attention away from nest defense [
32,
33,
34,
35,
36]. In addition, although the calls of common cuckoo are simple and monotonous it is possible to identify individuals based on their call characteristics [
37], suggesting that different individuals may have specific functions of calls during the breeding season and individuals may also benefit from accessing the quality and behavioral conditions of other birds [
38]. Identification of individual calls is also helpful in accessing the life history and abundance of species in different areas [
39]. Until now, several studies have used different parameters of cuckoo species’ calls to classify species and individuals to identify the functions of calls [
2,
21,
37]. For example, Xia et al. (2019) identified significant differences in characteristics and functions of male common cuckoo’s two note and three note elements while Marton et al. (2021) demonstrated that bubbling calls of the female common cuckoo reduces host aggression to cuckoos at their nests [
35]. Nevertheless, only a few studies have focused on the temporal and seasonal patterns of calling activity of different cuckoo species over the entire breeding season [
20,
40,
41]. Unlike passerines, brood parasitic species do not have to decrease their vocal activity after pairing due to the lack of parental care. The temporal patterns of vocal activity may differ between passerines and non-passerines. Hence, more work is needed on non-passerine (brood parasitic species) in sub-tropics.
In addition, nocturnal calling behavior has also been observed in several brood parasitic species [
20,
40,
41], but this behavior varies among species, with some species calling only early in the breeding season, while others do so throughout the breeding season [
17,
20], possibly due to different functions of the night calls. Nonetheless, the occurrence and function of avian nocturnal vocalizations are poorly understood [
17]. Even though this type of research has now increased, with the use of acoustic recording technologies [
19,
42], we still need primary data from east Asia on the nocturnal calling of diurnal birds. Furthermore, these seven Cuculidae species overlap in geographical distribution and share similar food resources [
43], which may result in seasonal partitioning in call frequency.
In the study, we employed passive acoustic monitoring technology to record the vocal activity of Cuculidae species over an entire annual cycle. The main objectives of the study were (1) to investigate the diurnal and seasonal patterns of calling activity of these species to gain insights into the ecology of these species, to understand the functions of vocalizations and to identify periods of high calling activity; and (2) to examine whether there is an overlap in the temporal distribution of seven species’ seasonal calling activity. Most of the cuckoo species’ calls are thought to be used for attracting mates and for territorial competition [
2,
20,
44]. Based on this, we expected an increase in calling activity earlier in the season during mating, or two peaks, if the species breeds twice. Furthermore, vocal activity usually decreases after pairing in several bird species [
1], we also expected a gradual decrease in calling activity as the breeding season advanced. Earlier investigations on the daily patterns of several cuckoo species have shown peaks in calling activity at dawn and dusk [
20,
41], we also expected an increase in calling activity of most species at dawn and dusk if the purpose of calling is to attract mates and advertise territory.
4. Discussion
This study indicated that all of the studied Cuculidae species exhibited diurnal and seasonal patterns of calling activity. Five species displayed a peak in vocal activity earlier in the season (late May or early June) and a gradual decline thereafter, except for
C. coromandus, which displayed two peaks in seasonal calling activity. This coincides with the findings of an earlier study in Korea, which found a decreasing trend in seasonal calling activity of cuckoo populations [
20] and suggested that the absence of parental care in cuckoo species may be a reason for such unimodal pattern [
33]. Furthermore, bird calls are not made randomly; instead, they are used for establishing territories and attracting mates [
2,
51]. Therefore, one function of calling earlier in the season might be to establish territories; for example, an earlier study documented that males of some cuckoo species reach the breeding grounds earlier than the females and defend their territories by calling higher [
20,
52]. However, in the Cuculidae species, the concept of exclusive territory is ambiguous, and some studies demonstrate the dominance hierarchy system [
53,
54,
55]. In any case, the availability of host nests at the appropriate stage is vital for avian brood parasitic species; hence, guarding to maintain exclusive access to host nests and reduce competition is highly important [
33,
56,
57].
In addition, brood-parasitic cuckoos are usually polygamous, and they make pairs only during copulation [
44,
58]. In our study, we found that
C. coromandus displayed two peaks in vocal activity in early June and early July, indicating that mate attraction is more important in this species, and they might call late in the season to attract additional females. In addition, this species produces two broods [
44], which means that peaks in vocal activity in the late season could indicate mating occurring later in the season since some females remain sexually available throughout the entire breeding season [
59].
Furthermore, at site-2, the number of calling activity of
E. scolopaceus and
H. sparverioides became higher earlier and decreased gradually later in the season compared to other sites. Differences in latitude may explain such variations in seasonal vocal patterns. For example, the vocal activity of these species were found to be lower in May at site-1 and site-3 compared to site-2, which is located at a lower elevation (
Tables S7 and S9). Considering the temperature difference of at least 4 degrees between lower and higher sites [
60], it is possible that more birds may arrive at site-1 and site-3 later in the season when the temperature becomes higher. In either case, as the number of sites is low and we have no data on bird density around recorders, it is difficult to conclude that low numbers of birds in late May caused decreased vocalizations.
Nonetheless, the vocal activity of
C. micropterus did not display any variation in the seasonal pattern. A most probable explanation for a constant seasonal singing pattern would be that, unlike other cuckoo species, this species’ calls were detected on low numbers (0.31%) in the recording spectrograms (
Table 1). Earlier studies have shown a positive relationship between vocal activity and population abundance of birds around recorders [
61,
62]. Unfortunately, we do not have actual data on bird abundance. Therefore, we have used data from our study area (
supplementary material: birdreport.csv) from 30 reports available on the bird report center of China (
http://www.birdreport.cn/home/search/page.html, accessed on 28 February 2022) between April and August and found that
C. canorus,
E. scolopaceus,
H. sparverioides and
C. coromandus appeared 10, 5, 4 and 1 time respectively, whereas the other three species appeared twice, which may indicate that the abundance of
C. micropterus is not as low as estimated from the call number of species. We propose that this might be due to the heterogeneous distribution and abundance of host species [
63,
64]. For example, the hosts of
C. micropterus include
Cyanopica cyanus,
Pycnonotus sinensis and
Turdus merula [
65,
66]. Based on our personal experience, we know that the aforementioned host species prefer to stay in human dominated areas and the sampling sites we selected in YNNR have very little human activities. Alternatively, the singing rate of several passerine species decreases when they feed their nestlings, illustrating the trade-off between energy distribution and singing performance [
67,
68]. However,
C. coromandus and
C. micropterus are brood parasites and depend on hosts to rear their offspring. This might be the reason why these species call later in the season.
Likewise, the vocal output of some brood-parasitic species is also triggered by the host species’ vocal output [
20] and they may time their activity to match the host species’ activity. To examine this, we checked the abundance of host species in different months in Li et al. (2017) [
46], and found that none of the host species of the
C. micropterus is dominant in our study area, and that the number of calls of these Cuckoo species is linked with host species’ abundance in the study area. For example,
Phylloscopus reguloides is the host of
C. saturates, and the seasonal distribution of the number of calls of
C. saturates is consistent with the actual abundance of
Phylloscopus reguloides, which is higher from April to June (see
Table 2 in Li et al. 2017 for a review [
46]). Similarly, peaks in calling activity of
C. poliocephalus and
C. canorus in earlier June correspond with the abundance of their host species
Urocissa erythrorhyncha and
Paradoxornis webbianus in the study area. Lastly,
Garrulax canorus and
Cettia fortipes are hosts of different cuckoo species and their actual abundance and number of calls lasts longer during the whole breeding season [
10,
46,
65,
66] (
Table S16).
In this study, for
C. canorus and
C. poliocephalus, we only calculated the number of male calls. From Yoo et al. (2020) [
20] we found that the diurnal pattern was similar in both species and both sexes. The peak seasonal vocal activity of females lagged behind that of males by approximately 2 weeks for
C. canorus, and 1 week for
C. poliocephalus. Therefore, there may be little bias of seasonal distribution in our results, but this bias would be small because males typically produce calls much more frequently than females [
20].
Birds sing at dawn and dusk to attract mates or defend territories [
10,
40]. Our results indicated that all species exhibited significant variation during different hours except for
C. micropterus, which did not exhibit any variation. The increased vocal activity of
E. scolopaceus and
C. saturatus was observed at dawn and dusk. In contrast, the other four species displayed a high peak in calling activity in the morning, consistent with the findings of earlier studies [
10,
20,
40]. Furthermore, at these times, light levels are usually low for foraging, and low temperatures are also conducive to the transmission of songs, so these birds may take advantage of this time by singing as they have nothing to do [
1,
69]. In addition, several studies have shown that peak timings of activity act as a communication network [
42,
70], and birds can gather information about the quality, social rank, and age of other birds by eavesdropping on their vocal interactions. Because, at dawn and dusk, birds sing under standardized conditions involving temporary singing interactions among different territory owners these times may be more beneficial to birds in making subsequent decisions [
71,
72,
73,
74].
Bird species display varying nocturnal calling behaviors; some frequently call at night while others only sing infrequently at night [
17,
75]. In our study, we discovered that although most of the species were calling more frequently during the day from 4:00 to 20:00, they did not restrict their activity to this period; instead, some species (e.g.,
C. coromandus and
C. poliocephalus Figures S3 and S7) called throughout the night, and some (e.g.,
C. canorus;
Figure S6) sang during the night only earlier in the season. These variations in singing are often attributed to different functions of songs. For example, in Common Nightingales (
Luscinia megarhynchos), unmated males sing nocturnal songs throughout the breeding season to attract females, whereas mating males stop night singing after pairing later in the season [
5]. In addition to attracting migrating females, several other functions of the nocturnal song have also been hypothesized, including reduced acoustic competition, the favorable transmission of songs, increased illumination of moonlight and reduced predator exposure [
76,
77,
78,
79]. Further research using detailed field data to unravel the function of Cuculidae calls responsible for variation in calling patterns among species would be highly beneficial to understand how calls work.
The seven Cuculidae species occupy similar ecological niches and have similar food resources [
43] so they may partition acoustic space. We counted the number of calls of each species in each site; the results indicated that the composition of species is different in different sites, and
C. micropterus is the least dominant species followed by
C. coromandus. The seasonal distributions of vocal activity of two pairwise groups (
E. scolopaceus and
C. micropterus,
C. poliocephalus and
C. coromandus) overlap, and they also display a similar frequency range (
Figure S9). However, they do not overlap in temporal patterns of seasonal vocal activity, possibly due to
C. micropterus’s and
C. coromandus’s low activity. The other ten groups (
Table 9) overlapped in the seasonal distribution of vocal activity but varied in frequency (
Figure S9). Therefore, we can conclude that the vocal activity of species in a pairwise group would not overlap simultaneously in space, time, and frequency. These findings are consistent with previous research, which found that birds singing at the same frequency as insects alter their song start time to avoid acoustic masking [
80]. For example, a study in Peruvian bird assemblage indicated the significant negative correlation between spectral and temporal overlap [
81]. Another study demonstrated how four sympatric wren-warblers partitioned their acoustic signal space and song perch height, demonstrating that acoustic signal partitioning can occur along multiple axes [
82]. Our results also confirm that Cuculidae species have a flexible mechanism to avoid acoustic overlap.
In conclusion, we show that automatic recording is a valuable technique for studying the diurnal and seasonal patterns of vocal activity of Cuculidae species. Our results provided important insights into the diurnal and seasonal patterns of calling activity. However, further research with different population data is needed to determine which of the seasonal and diurnal song hypotheses best describes calling activity in Cuculidae species. Our study also showed that these parasitic species exhibit temporal partitioning in the seasonal distribution of vocalizations.