Effect of Hermaphrodite–Gynomonoecious Sexual System and Pollination Mode on Fitness of Early Life History Stages of Offspring in a Cold Desert Perennial Ephemeral
Abstract
:1. Introduction
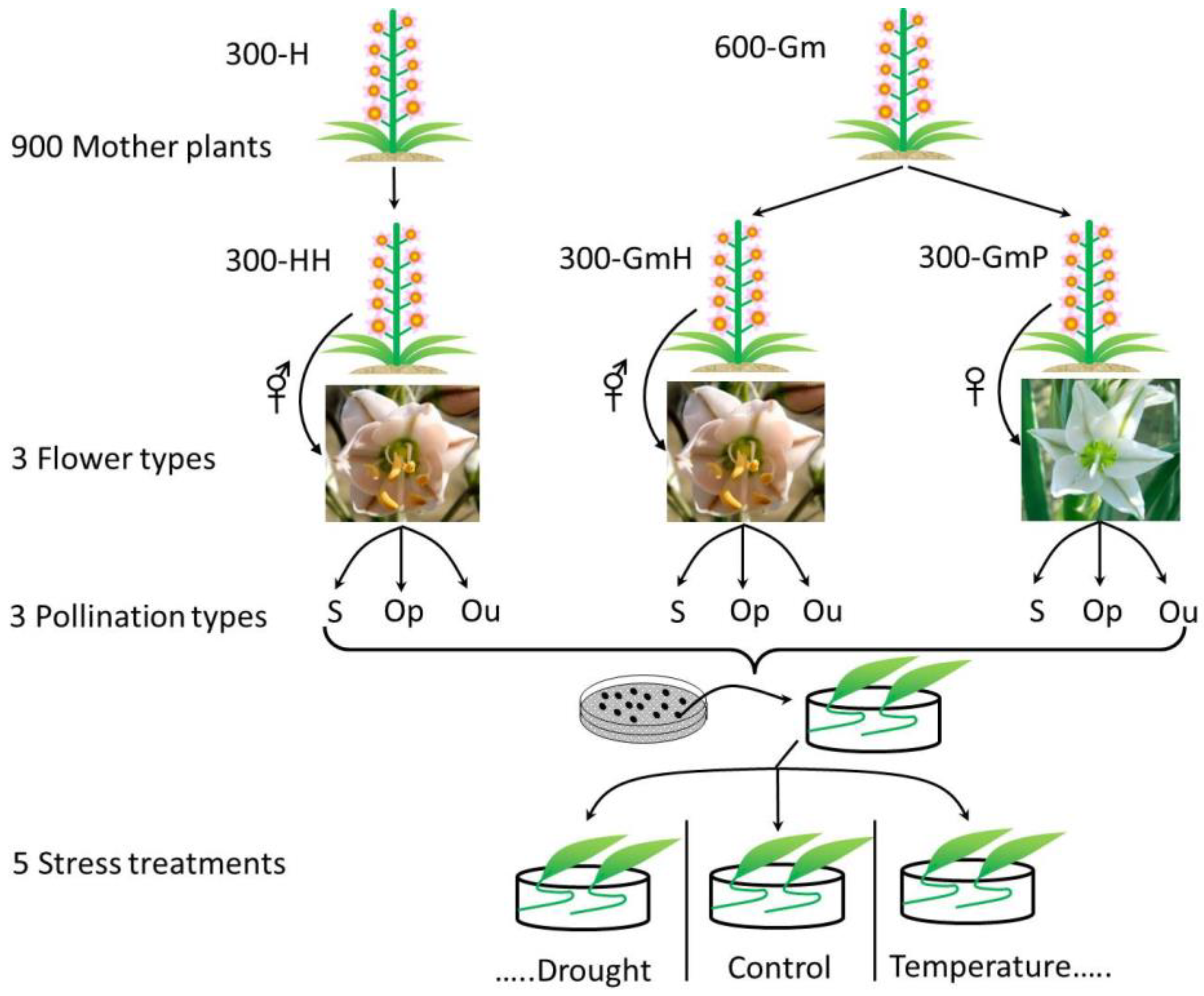
2. Materials and Methods
2.1. Study Species and Seed Collection Site
2.2. Pollination Type
2.3. Seed and Seedling Traits Measured
2.3.1. Seed Set
2.3.2. Seed Mass
2.3.3. Phenology of Embryo Growth
2.3.4. Seed Germination
2.3.5. Seedling Growth and Survival
Temperature
Water Stress
2.4. Effect of Inbreeding Depression on Seed Set, Mass, Germination and Seedling Growth and Survival
2.5. Multiplicative Fitness of Early Life History Traits
2.6. Data Analysis
3. Results
3.1. Seed Traits
3.2. Phenology of Embryo Growth
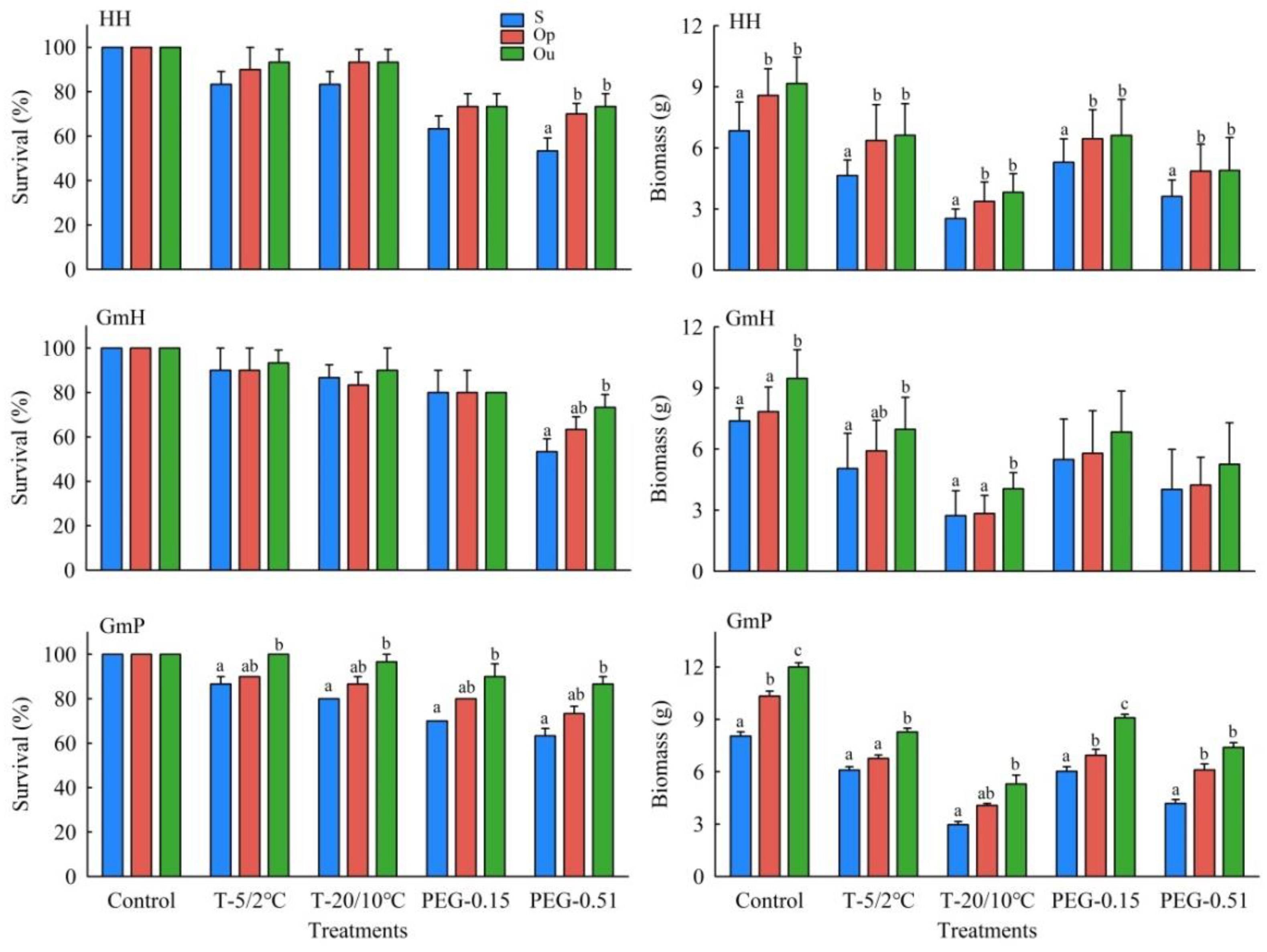
3.3. Seedling Traits
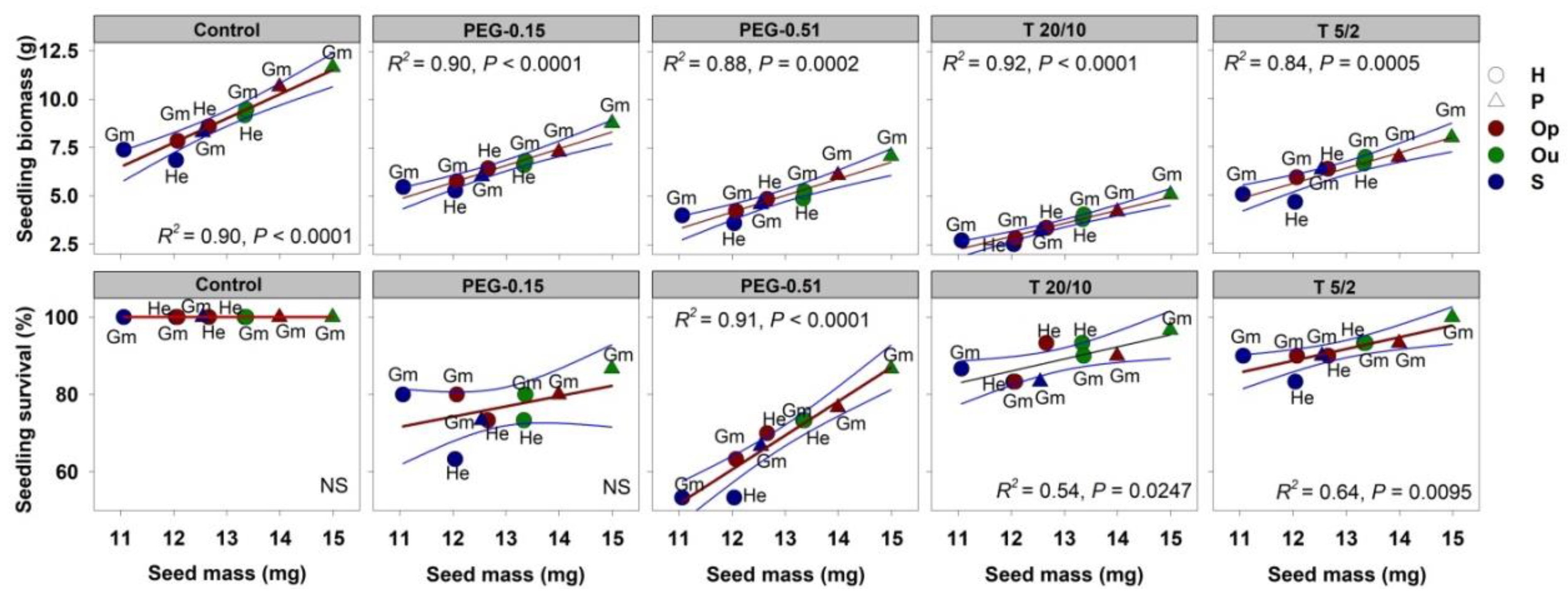
3.4. Effect of Seed Mass on Germination and Seedling Traits
3.5. Effect of Inbreeding Depression on Seed and Seedling Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwin, C. The Different Forms of Flowers on Plants of the Same Species; John Murray: London, UK, 1877. [Google Scholar]
- Yampolsky, C.; Yampolsky, H. Distribution of sex forms in the phanerogamic flora. Bibl. Genet. 1922, 3, 1–62. [Google Scholar]
- Wise, M.J.; Coffey, L.E.; Abrahamson, W.G. Nutrient stress and gall flies interact to affect floral-sex ratio in gynomonoecious Solidago altissima (Asteraceae). Am. J. Bot. 2008, 95, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Torices, R.; Anderberg, A.A. Phylogenetic analysis of sexual systems in Inuleae (Asteraceae). Am. J. Bot. 2009, 96, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Mamut, J.; Xiong, Y.Z.; Tan, D.Y.; Huang, S.Q. Pistillate flowers experience more pollen limitation and less geitonogamy than perfect flowers in a gynomonoecious herb. New Phytol. 2014, 201, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, B.; Charlesworth, D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 1978, 112, 975–997. [Google Scholar] [CrossRef]
- Chang, S.M. Gender-specific inbreeding depression in a gynodioecious plant, Geranium maculatum (Geraniaceae). Am. J. Bot. 2007, 94, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Méndez, M.; Munzinger, J. Planchonella, first record of gynomonoecy for the family Sapotaceae. Plant Syst. Evol. 2010, 287, 65–73. [Google Scholar] [CrossRef]
- Charlesworth, D. Gender and Sexual Dimorphism in Flowering Plants; Geber, M.A., Dawson, T.E., Delph, L.F., Eds.; Springer: Berlin, Germany, 1999; pp. 33–60. [Google Scholar]
- Charlesworth, D. Evolution of plant breeding systems. Curr. Biol. 2006, 16, R726–R735. [Google Scholar] [CrossRef] [Green Version]
- Barrett, S.C.H. The evolution of plant sexual diversity. Nat. Rev. Genet. 2002, 3, 274–284. [Google Scholar] [CrossRef]
- Hesse, E.; Pannell, J.R. Sexual dimorphism in a dioecious population of the wind-pollinated herb Mercurialis annua: The interactive effects of resource availability and competition. Ann. Bot. 2011, 107, 1039–1045. [Google Scholar] [CrossRef]
- Barrett, S.C.H.; Hough, J. Sexual dimorphism in flowering plants. J. Exp. Bot. 2013, 64, 67–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertin, R.I.; Kerwin, M. Floral sex ratios and gynomonoecy in Aster (Asteraceae). Am. J. Bot. 1998, 85, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Grombone-Guaratini, M.T.; Nascimento, A.A.D.; Santos-Gonçalves, A.P. Flowering and fruiting of Aulonemia aristulata: A gynomonoecious woody bamboo species from Atlantic Forest in Brazil. Rev. Bras. Bot. 2011, 34, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Huang, S.Q. Adaptive advantages of gynomonoecious species. Acta Phytotaxon. Sin. 2006, 44, 231–239, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Charnov, E.L.; Bull, J. When is sex environmentally determined? Nature 1977, 266, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.G. Parental strategies in angiosperms. N. Zeal. J. Bot. 1979, 17, 595–606. [Google Scholar] [CrossRef]
- Mamut, J.; Xiong, Y.Z.; Tan, D.Y.; Huang, S.Q. Flexibility of resource allocation in a hermaphroditic-gynomonoecious herb through deployment of female and male resources in perfect flowers. Am. J. Bot. 2017, 104, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Marshall, D.F.; Abbott, R.J. Polymorphism for outcrossing frequency at the ray floret locus in Senecio vulgaris L. III. Causes. Heredity 1984, 53, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Bertin, R.I.; Connors, D.B.; Kleinman, H.M. Differential herbivory on disk and ray flowers of gynomonoecious asters and goldenrods (Asteraceae). Biol. J. Linn. Soc. 2010, 101, 544–552. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.F.; Xie, T.P.; Du, G.Z. Variation in floral sex allocation, female success, and seed predation within racemiform synflorescence in the gynomonoecious Ligularia virgaurea (Asteraceae). J. Plant Res. 2012, 125, 527–538. [Google Scholar] [CrossRef]
- Collin, C.L.; Shykoff, J.A. Flowering phenology and female fitness: Impact of a pre-dispersal seed predator on a sexually polymorphic species. Plant Ecol. 2010, 206, 1–13. [Google Scholar] [CrossRef]
- Lloyd, D.G. Breeding systems in Cotula L. (Compositae, Anthemideae). I. The array of monoclinous and diclinous systems. New Phytol. 1972, 71, 1181–1194. [Google Scholar] [CrossRef]
- Álvarez, I.; Agudo, A.B.; Herrero, A.; Torices, R. The Mendelian inheritance of gynomonoecy: Insights from Anacyclus hybridizing species. Am. J. Bot. 2020, 107, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertin, R.I.; Gwisc, G.M. Floral sex ratios and gynomonoecy in Solidago (Asteraceae). Biol. J. Linn. Soc. 2002, 77, 413–422. [Google Scholar] [CrossRef]
- Maurice, S. Gynomonoecy in Silene italica (Caryophyllaceae): Sexual phenotypes in natural populations. Plant Biol. 1999, 1, 346–350. [Google Scholar] [CrossRef]
- Swenson, U.; Bremer, K. Patterns of floral evolution of four Asteraceae genera (Senecioneae, Blennospermatinae) and the origin of white flowers in New Zealand. Syst. Biol. 1997, 46, 407–425. [Google Scholar] [CrossRef]
- Abbott, R.J.; Schmitt, J. Effect of environment on percentage female ray florets per capitulum and outcrossing potential in a self-compatible composite (Senecio vulgaris L. var. hibernicus Syme). New Phytol. 1985, 101, 219–229. [Google Scholar] [CrossRef]
- Collin, C.L.; Pennings, P.S.; Rueffler, C.; Widmer, A.; Shykoff, J.A. Natural enemies and sex: How seed predators and pathogens contribute to sex-differential reproductive success in a gynodioecious plant. Oecologia 2002, 131, 94–102. [Google Scholar] [CrossRef]
- Lande, R.S.; Schemske, D.W. The evolution of selffertilization and inbreeding depression in plants. I. Genetic models. Evolution 1985, 39, 24–40. [Google Scholar]
- Charlesworth, D.; Morgan, M.T.; Charlesworth, B. Inbreeding depression, genetic load, and the evolution of outcrossing rates in a multilocus system with no linkage. Evolution 1990, 44, 1469–1489. [Google Scholar] [CrossRef]
- Uyenoyama, M.K.; Holsinger, K.E.; Waller, D.M. Ecological and genetic factors directing the evolution of selffertilization. Oxford Surv. Evol. Biol. 1993, 9, 327–381. [Google Scholar]
- Charlesworth, D.; Charlesworth, B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 1987, 18, 237–268. [Google Scholar] [CrossRef]
- Hokanson, K.; Hancock, J. Early-acting inbreeding depression in three species of Vaccinium (Ericaceae). Sex. Plant Reprod. 2000, 13, 145–150. [Google Scholar] [CrossRef]
- Keller, L.F.; Waller, D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002, 17, 231–241. [Google Scholar] [CrossRef]
- Goodwillie, C.; Kalisz, S.; Eckert, C.G. The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and empirical evidence. Annu. Rev. Ecol. Evol. 2005, 36, 47–79. [Google Scholar] [CrossRef] [Green Version]
- Ashman, T.L. The relative importance of inbreeding and maternal sex in determining progeny fitness in Sidalcea oregana ssp. spicata, a gynodioecious plant. Evolution 1992, 46, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, J.; Schmid, B.; Matthies, D.; Albrecht, M. Inbreeding depression under drought stress in the rare endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands. PLoS ONE 2012, 7, e47415. [Google Scholar] [CrossRef] [Green Version]
- Delph, L.F.; Mutikainen, P. Testing why the sex of the maternal parent affects seedling survival in a gynodioecious species. Evolution 2003, 57, 231–239. [Google Scholar] [CrossRef]
- Dufay, M.; Lahiani, E.; Brachi, B. Gender variation and inbreeding depression in gynodioecious-gynomonoecious Silene nutans (Caryophyllaceae). Int. J. Plant. Sci. 2010, 171, 53–62. [Google Scholar] [CrossRef]
- Chen, X.Q.; Liang, S.Y.; Xu, J.M.; Tamura, M.N. Liliaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MO, USA, 2000; Volume 24, pp. 87–88. [Google Scholar]
- Shi, X.; Wang, J.C.; Zhang, D.Y.; Gaskin, J.F.; Pan, B.R. Pollination ecology of the rare desert species Eremosparton songoricum (Fabaceae). Aust. J Bot. 2010, 58, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Tang, H. Patterns of ephemeral plant communities and their adaptations to temperature and precipitation regimes in Junggar Desert, Xinjiang. Biol. Sci. 2010, 18, 346–354, (In Chinese with English Abstract). [Google Scholar]
- Mamut, J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Intermediate complex morphophysiological dormancy in seeds of the cold desert sand dune geophyte Eremurus anisopterus (Xanthorrhoeaceae; Liliaceae s.l.). Ann. Bot. 2014, 114, 991–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.G.; van Iersel, M.W. Nutrient solution concentration affects shoot: Root ratio, leaf area ratio, and growth of subirrigated salvia (Salvia splendens). Hortscience 2004, 39, 49–54. [Google Scholar] [CrossRef]
- Husband, B.C.; Schemske, D.W. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 1996, 50, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. Inbreeding depression and the cost of inbreeding on seed germination. Seed Sci. Res. 2015, 25, 355–385. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; Debroy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1–140. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 12 May 2019).
- Nihranz, C.T.; Walker, W.S.; Brown, S.J.; Mescher, M.C.; De Moraes, C.M.; Stephenson, A.G. Transgenerational impacts of herbivory and inbreeding on reproductive output in Solanum carolinense. Am. J. Bot. 2020, 107, 286–297. [Google Scholar] [CrossRef] [Green Version]
- de Vries, F.T.; Manning, P.; Tallowin, J.R.B.; Mortimer, S.R.; Pilgrim, E.S.; Harrison, K.A.; Hobbs, P.J.; Quirk, H.; Shipley, B.; Cornelissen, J.H.C.; et al. Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecol. Lett. 2012, 15, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.7.2. 2012. Available online: http://CRAN.R-project.org/package=MuMIn (accessed on 7 March 2012).
- Kalisz, S. Fitness consequences of mating system, seed weight, and emergence date in a winter annual, Collinsia verna. Evolution 1989, 43, 1263–1272. [Google Scholar] [CrossRef]
- Ruane, L.G.; Dickens, M.E.; Wall, M.E. Fitness consequences of short- and long-distance pollinations in Phlox hirsuta, an endangered species. Am. J. Bot. 2016, 102, 1659–1665. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.L.; Lovell, P.H.; Moore, K.G. The shapes and sizes of seeds. Annu. Rev. Ecol. Syst. 1970, 1, 327–356. [Google Scholar] [CrossRef]
- Seiwa, K. Effects of seed size and emergence time on tree seedling establishment: Importance of developmental constraints. Oecologia 2000, 123, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Koelewijn, H.P.; van Damme, J.M.M. Effects of seed size, inbreeding and maternal sex on offspring fitness in gynodioecious Plantago coronopus. J. Ecol. 2005, 93, 373–383. [Google Scholar] [CrossRef]
- Bu, H.Y.; Chen, X.L.; Xu, X.L.; Liu, K.; Jia, P.; Du, G.Z. Seed mass and germination in an alpine meadow on the eastern Tsinghai–Tibet plateau. Plant Ecol. 2007, 191, 127–149. [Google Scholar] [CrossRef]
- Dufay, M.; Billard, E. How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Ann. Bot. 2012, 109, 505–519. [Google Scholar] [PubMed] [Green Version]
- Collin, C.L.; Shykoff, J.A. Outcrossing rates in the gynomonoecious-gynodioecious species Dianthus sylvestris (Caryophyllaceae). Am. J. Bot. 2003, 90, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.C.; Noland, T.L.; Morneault, A.E. The effects of seed mass on germination, seedling emergence, and early seedling growth of eastern white pine (Pinus strobus L.). New Forest. 2006, 32, 33–49. [Google Scholar] [CrossRef]
- Arnaud, M.; Jean-Philippe, B.; José, E.; Grégory, M.; Carlos, G.D.L. Rapid plant invasion in distinct climates involves different sources of phenotypic variation. PLoS ONE 2013, 8, e55627. [Google Scholar]
- Zhang, D.Y.; Jiang, X.H. Maiting system evolution, resource allocation, and genetic diversity in plants. Acta. Phytoecol. Sin. 2001, 25, 130–143, (In Chinese with English Abstract). [Google Scholar]
- Webb, C.J. Test of a model predicting equilibrium frequencies of females in populations of gynodioecious angiosperms. Heredity 1981, 46, 397–405. [Google Scholar] [CrossRef]
- Li, J.M.; Koski, M.H.; Ashman, T.L. Functional characterization of gynodioecy in Fragaria vesca ssp. bracteata (Rosaceae). Ann. Bot. 2012, 109, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, D.G. Self- and cross-fertilization in plants. II. The selection of self-fertilization. Int. J. Plant Sci. 1992, 153, 370–380. [Google Scholar] [CrossRef]
- Ramsey, M.; Vaughton, G. Effect of environment on the magnitude of inbreeding depression in seed germination in a partially self-fertile perennial herb (Blandfordia grandiflora, Liliaceae). Int. J. Plant Sci. 1998, 159, 98–104. [Google Scholar] [CrossRef]
- Barringer, B.C.; Geber, M.A. Mating system and ploidy influence levels of inbreeding depression in Clarkia (Onagraceae). Evolution 2008, 62, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.L.; Penet, L.; Shykoff, J.A. Early inbreeding depression in the sexually polymorphic plant Dianthus sylvestris (Caryophyllaceae): Effects of selfing and biparental inbreeding among sex morphs. Am. J. Bot. 2009, 96, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Leppik, E.E. The evolution of capitulum types of the Compositae in the light of insect flower interaction. In The Biology and Chemistry of the Compositae; Heywood, V.H., Harbone, J.B., Turner, B.L., Eds.; Academic Press: London, UK, 1977; pp. 61–89. [Google Scholar]



| Source of Variation | d.f. | F | p |
|---|---|---|---|
| Seed set | |||
| Plant type (PT) | 1, 19 | 20.799 | 0.0002 |
| Flower type (FT) | 1, 133 | 86.986 | <0.0001 |
| Pollination type (PT′) | 2, 133 | 11.448 | <0.0001 |
| FT * PT′ | 2, 133 | 10.258 | 0.0001 |
| PT * PT′ | 2, 133 | 0.0241 | 0.976 |
| Seed mass | |||
| Plant type (PT) | 1, 29 | 2.215 | 0.147 |
| Flower type (FT) | 1, 203 | 44.407 | <0.0001 |
| Pollination type (PT′) | 2, 203 | 32.104 | <0.0001 |
| FT * PT′ | 2, 203 | 0.967 | 0.382 |
| PT* PT′ | 2, 203 | 1.359 | 0.259 |
| Initial E:S ratio | |||
| Plant type (PT) | 1, 218 | 0.620 | 0.433 |
| Flower type (FT) | 1, 218 | 0.300 | 0.255 |
| Pollination type (PT′) | 2, 218 | 0.130 | 0.324 |
| FT * PT′ | 2, 218 | 0.010 | 0.987 |
| Final E:S ratio | |||
| Plant type (PT) | 1, 218 | 1.230 | 0.268 |
| Flower type (FT) | 1, 218 | 5.110 | 0.024 |
| Pollination type (PT′) | 2, 218 | 1.580 | 0.209 |
| FT * PT′ | 2, 218 | 0.920 | 0.401 |
| Seed germination | |||
| Plant type (PT) | 1, 3 | 9.320 | 0.055 |
| Flower type (FT) | 1, 21 | 25.329 | 0.0001 |
| Pollination type (PT′) | 2, 21 | 24.533 | <0.0001 |
| FT * PT′ | 2, 21 | 1.4868 | 0.249 |
| PT* PT′ | 2, 21 | 0.8807 | 0.429 |
| Source of Variation | Survival | Biomass | ||||
|---|---|---|---|---|---|---|
| d.f. | F | p | d.f. | F | p | |
| Plant type (PT) | 1, 2 | 4.939 | 0.1563 | 1, 19 | 44.918 | <0.0001 |
| Flower type (FT) | 1, 86 | 10.623 | 0.0016 | 1, 817 | 131.391 | <0.0001 |
| Pollination type (PT′) | 2, 86 | 26.427 | <0.0001 | 2, 817 | 126.087 | <0.0001 |
| Stress treatment (ST) | 4, 86 | 124.124 | <0.0001 | 4, 817 | 333.552 | <0.0001 |
| FT * PT′ | 2, 86 | 1.213 | 0.3023 | 2, 817 | 5.477 | 0.0043 |
| FT * ST | 4, 86 | 3.896 | 0.0059 | 4, 817 | 2.831 | 0.0238 |
| CT * ST | 8, 86 | 3.53 | 0.0014 | 8, 817 | 1.402 | 0.1918 |
| PT * PT′ | 2, 86 | 3.049 | 0.0525 | 2, 817 | 6.697 | 0.0013 |
| PT * ST | 4, 86 | 3.721 | 0.0077 | 4, 817 | 0.074 | 0.9901 |
| FT * PT′ * ST | 8, 86 | 0.257 | 0.9778 | 8, 817 | 1.199 | 0.2967 |
| PT * PT′ * ST | 8, 86 | 0.426 | 0.9023 | 8, 817 | 0.058 | 0.9999 |
| Source of Variation | d.f. | MS | F | p |
|---|---|---|---|---|
| Pollination type | 8 | 202.272 | 3.239 | 0.011 |
| Seed mass | 1 | 49.132 | 0.787 | 0.383 |
| Error | 26 | 62.456 | ||
| R2 = 0.588 |
| Treatment | Flower Type | F | p | ||
|---|---|---|---|---|---|
| GmH | GmP | HH | |||
| Seed set | 0.188 ± 0.082 a | 0.188 ± 0.073 a | 0.164 ± 0.211 a | 0.010 | 0.990 |
| Seed mass | 0.102 ± 0.006 a | 0.165 ± 0.007 b | 0.097 ± 0.009 a | 26.219 | 0.000 |
| Initial E:S ratio | 0.024 ± 0.006 a | 0.026 ± 0.013 a | 0.021 ± 0.008 a | 0.061 | 0.941 |
| Final E:S ratio | 0.019 ± 0.002 a | 0.024 ± 0.006 a | 0.017 ± 0.004 a | 0.502 | 0.607 |
| Germination | 0.205 ± 0.031 a | 0.256 ± 0.017 b | 0.202 ± 0.046 a | 16.523 | 0.003 |
| S-C | 0.000 ± 0.000 a | 0.000 ± 0.000 a | 0.000 ± 0.000 a | — | — |
| S-T 5/2 °C | 0.086 ± 0.016 a | 0.088 ± 0.003 a | 0.062 ± 0.003 a | 2.353 | 0.176 |
| S-T 20/10 °C | 0.074 ± 0.016 a | 0.077 ± 0.021 a | 0.052 ± 0.003 a | 0.773 | 0.503 |
| S-W —0.15 Mpa | 0.129 ± 0.012 a | 0.157 ± 0.007 b | 0.125 ± 0.010 a | 13.251 | 0.001 |
| S-W —0.51 Mpa | 0.268 ± 0.016 a | 0.294 ± 0.005 b | 0.271 ± 0.030 a | 12.933 | 0.003 |
| Treatment | GmH | GmP | HH |
|---|---|---|---|
| S-C | 0.529 | 0.719 | 0.567 |
| S-T 5/2 °C | 0.604 | 0.714 | 0.597 |
| S-T 20/10 °C | 0.605 | 0.593 | 0.627 |
| S-W —0.15 Mpa | 0.566 | 0.772 | 0.464 |
| S-W —0.51 Mpa | 0.648 | 0.798 | 0.639 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamut, J.; Cheng, J.; Tan, D.; Baskin, C.C.; Baskin, J.M. Effect of Hermaphrodite–Gynomonoecious Sexual System and Pollination Mode on Fitness of Early Life History Stages of Offspring in a Cold Desert Perennial Ephemeral. Diversity 2022, 14, 268. https://doi.org/10.3390/d14040268
Mamut J, Cheng J, Tan D, Baskin CC, Baskin JM. Effect of Hermaphrodite–Gynomonoecious Sexual System and Pollination Mode on Fitness of Early Life History Stages of Offspring in a Cold Desert Perennial Ephemeral. Diversity. 2022; 14(4):268. https://doi.org/10.3390/d14040268
Chicago/Turabian StyleMamut, Jannathan, Junhui Cheng, Dunyan Tan, Carol C. Baskin, and Jerry M. Baskin. 2022. "Effect of Hermaphrodite–Gynomonoecious Sexual System and Pollination Mode on Fitness of Early Life History Stages of Offspring in a Cold Desert Perennial Ephemeral" Diversity 14, no. 4: 268. https://doi.org/10.3390/d14040268
APA StyleMamut, J., Cheng, J., Tan, D., Baskin, C. C., & Baskin, J. M. (2022). Effect of Hermaphrodite–Gynomonoecious Sexual System and Pollination Mode on Fitness of Early Life History Stages of Offspring in a Cold Desert Perennial Ephemeral. Diversity, 14(4), 268. https://doi.org/10.3390/d14040268






