Understanding Extra-Pair Mating Behaviour: A Case Study of Socially Monogamous European Pied Flycatcher (Ficedula hypoleuca) in Western Siberia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Paternity Analysis
2.3. Overlapping in Fertility Periods
2.4. Nest Site Quality
2.5. Classification of Individuals
- —Males that did not gain EPP, and did not lose paternity in their own nest, EPY-neutral, mostly monogamous in our opinion (but see [8]).
- —Males that gained EPP, but could have also lost paternity in their own nest, EPY-positive males.
- —Males that lost paternity in their own nest, but did not gain EPP elsewhere, EPY-negative males.
- —Females not obtained EPP and mated with EPY-neutral males, EPY-neutral, mostly monogamous females.
- —Females obtained EPP, EPY-positive females.
- —Females breeding with EPY-positive males.
- —Within-pair offspring, the genetic descendants of the EPY-neutral males and EPY-neutral females.
- —maternal (within-pair) half-siblings of extra-pair offspring.
- —Extra-pair offspring.
- —Paternal (within-pair) half-siblings of extra-pair offspring.
2.6. Fitness Estimates
2.7. Statistical Analysis
3. Results
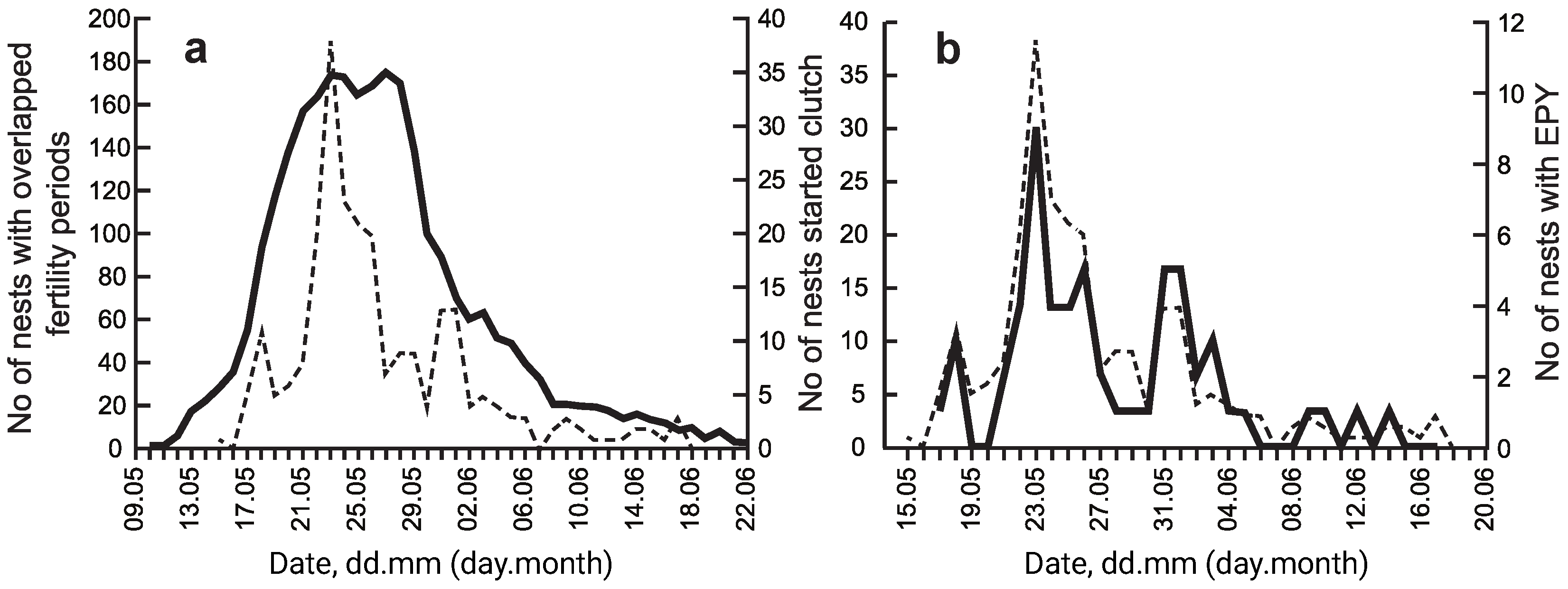
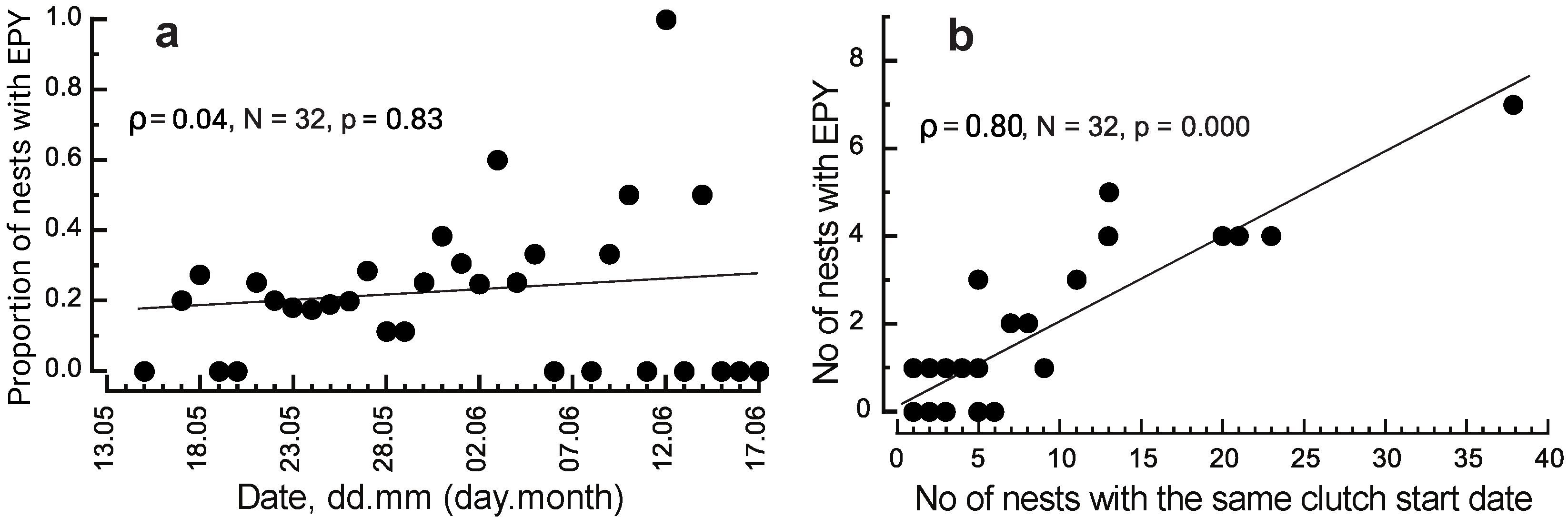
3.1. Breeding Density and Breeding Time
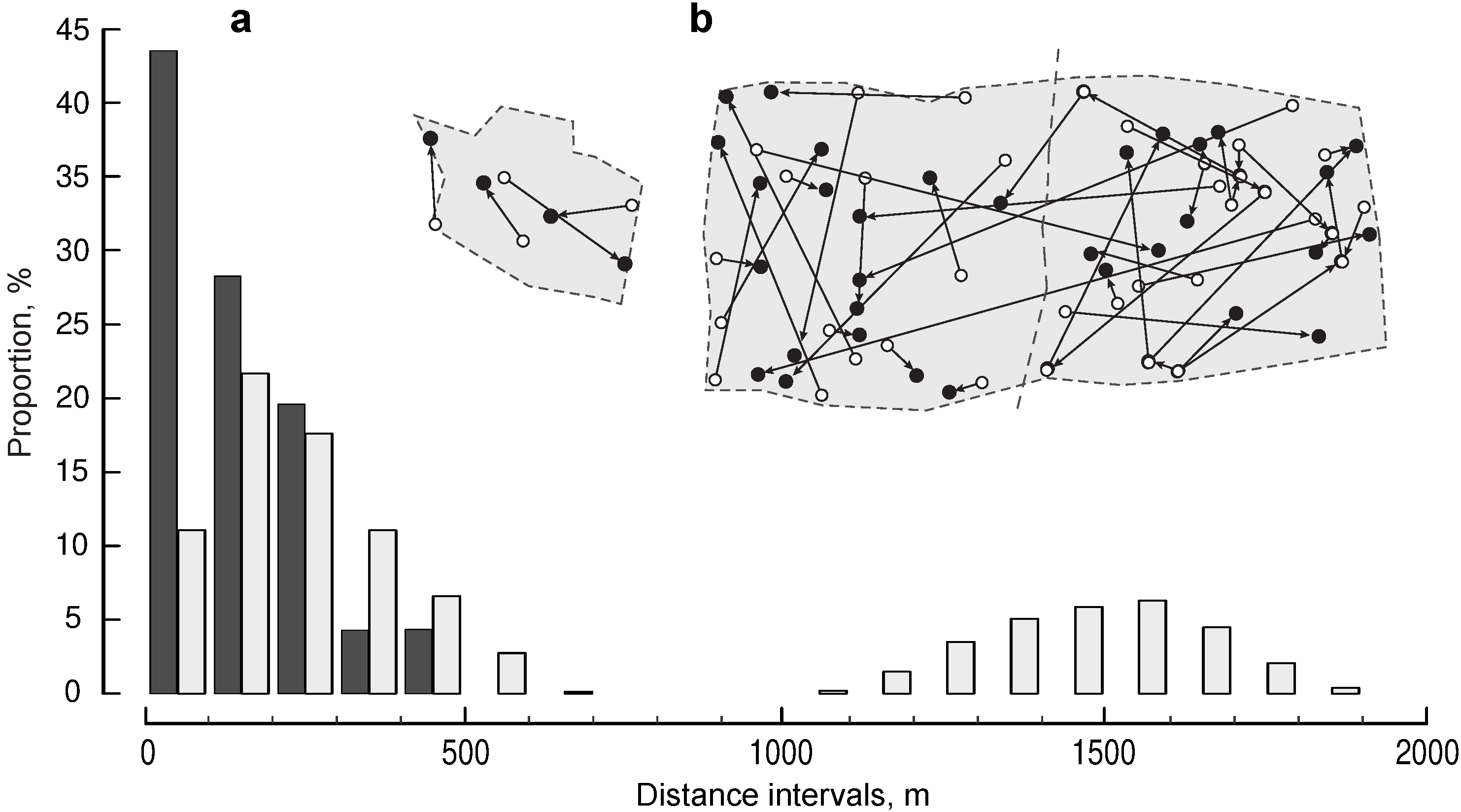
3.2. Distance between Extra-Pair Mates
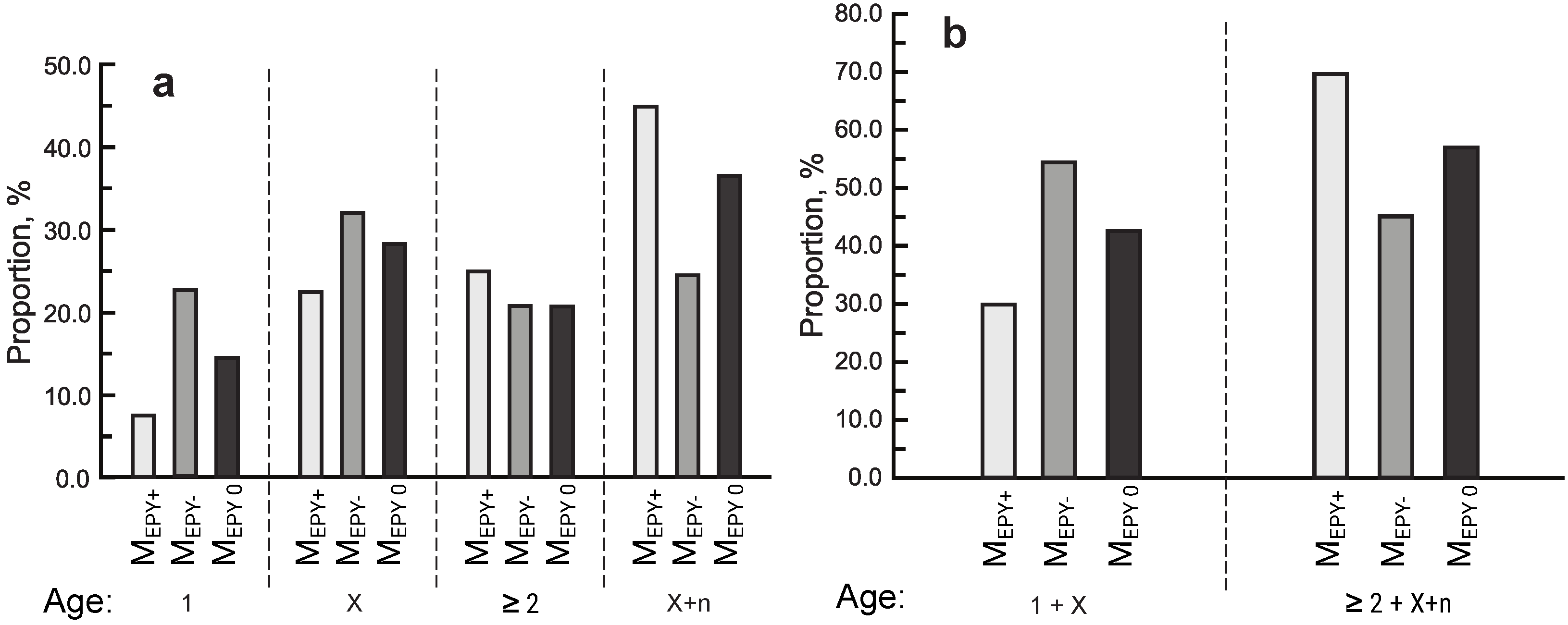
3.3. Age of Birds
3.4. Morphological Characters
3.5. Territory (Nest Site) Quality
3.6. Fecundity of Adults, Fledglings Fitness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lack, D. Ecological Adaptations for Breeding in Birds, 1st ed.; Methuen and Co. Ltd.: London, UK, 1968. [Google Scholar]
- Erritzoe, J.; Kampp, K.; Winker, K.; Frith, C.B. The Ornithologist’s Dictionary: Or Ornithological and Related Technical Terms for Layman and Expert; Lynx: Barcelona, Spain, 2007. [Google Scholar]
- Wittenberger, J.F. The Evolution of Mating Systems in Birds and Mammals. In Social Behavior and Communication; Marler, P., Vandenbergh, J.G., Eds.; Springer: Boston, MA, USA, 1979; pp. 271–349. [Google Scholar] [CrossRef]
- Griffith, S.C.; Owens, I.P.F.; Thuman, K.A. Extra pair paternity in birds: A review of interspecific variation and adaptive function. Mol. Ecol. 2002, 11, 2195–2212. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, L.; Griffith, S.C. Extra-pair paternity in birds. Mol. Ecol. 2019, 28, 4864–4882. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.; Nolan, V. Polygyny in Indigo Buntings: A Hypothesis Tested. Science 1975, 190, 1296–1297. [Google Scholar] [CrossRef]
- Ford, N.L. Variation in Mate Fidelity in Monogamous Birds. In Current Ornithology; Johnston, R.F., Ed.; Plenum Press: New York, NY, USA, 1983; pp. 329–356. [Google Scholar] [CrossRef]
- Grinkov, V.G.; Bauer, A.; Gashkov, S.I.; Sternberg, H.; Wink, M. Diversity of social-genetic relationships in the socially monogamous pied flycatcher (Ficedula hypoleuca) breeding in Western Siberia. PeerJ 2018, 6, e6059. [Google Scholar] [CrossRef]
- Lifjeld, J.T.; Gohli, J.; Albrecht, T.; Garcia-del Rey, E.; Johannessen, L.E.; Kleven, O.; Marki, P.Z.; Omotoriogun, T.C.; Rowe, M.; Johnsen, A. Evolution of female promiscuity in Passerides songbirds. BMC Evol. Biol. 2019, 19, 169. [Google Scholar] [CrossRef]
- Jeffreys, A.J. Highly variable minisatellites and DNA fingerprints. Biochem. Soc. Trans. 1987, 15, 309–317. [Google Scholar] [CrossRef]
- Wink, M.; Dyrcz, A. Mating systems in birds: A review of molecular studies. Acta Ornithol. 1999, 34, 91–109. [Google Scholar]
- Westneat, D.F.; Stewart, I.R. Extra-pair paternity in birds: Causes, correlates, and conflict. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 365–396. [Google Scholar] [CrossRef]
- Neudorf, D.L.H. Extrapair paternity in birds: Understanding variation among species. Auk 2004, 121, 302–307. [Google Scholar] [CrossRef]
- Forstmeier, W.; Nakagawa, S.; Griffith, S.C.; Kempenaers, B. Female extra-pair mating: Adaptation or genetic constraint? Trends Ecol. Evol. 2014, 29, 456–464. [Google Scholar] [CrossRef]
- McLeod, D.V.; Day, T. Sexually transmitted infection and the evolution of serial monogamy. Proceed. Biol. Sci. 2014, 281, 20141726. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.P. On the evolution of sexually transmitted diseases in birds. J. Avian Biol. 1998, 29, 314. [Google Scholar] [CrossRef]
- Sheldon, B.C. Sexually transmitted disease in birds: Occurrence and evolutionary significance. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1993, 339, 491–497. [Google Scholar] [CrossRef]
- Davies, N.B. Dunnock Behaviour and Social Evolution; Oxford Series in Ecology and Evolution; Oxford University Press: Oxford, UK, 1992; Volume 3. [Google Scholar]
- Kempenaers, B.; Verheyen, G.R.; van den Broeck, M.; Burke, T.; van Broeckhoven, C.; Dhondt, A. Extra-pair paternity results from female preference for high-quality males in the blue tit. Nature 1992, 357, 494–496. [Google Scholar] [CrossRef]
- Sheldon, B.C. Sperm competition in the chaffinch: The role of the female. Anim. Behav. 1994, 47, 163–173. [Google Scholar] [CrossRef]
- Westneat, D.F.; Gray, E.M. Breeding synchrony and extrapair fertilizations in two populations of red-winged blackbirds. Behav. Ecol. 1998, 9, 456–464. [Google Scholar] [CrossRef]
- Brekke, P.; Cassey, P.; Ariani, C.; Ewen, J.G. Evolution of extreme-mating behaviour: Patterns of extrapair paternity in a species with forced extrapair copulation. Behav. Ecol. Sociobiol. 2013, 67, 963–972. [Google Scholar] [CrossRef]
- Girndt, A.; Chng, C.W.T.; Burke, T.; Schroeder, J. Male age is associated with extra-pair paternity, but not with extra-pair mating behaviour. Sci. Rep. 2018, 8, 8378. [Google Scholar] [CrossRef]
- Eliassen, S.; Jørgensen, C. Extra-pair mating and evolution of cooperative neighbourhoods. PLoS ONE 2014, 9, e99878. [Google Scholar] [CrossRef]
- Albrecht, T.; Kreisinger, J.; Piálek, J. The strength of direct selection against female promiscuity is associated with rates of extrapair fertilizations in socially monogamous songbirds. Am. Nat. 2006, 167, 739–744. [Google Scholar] [CrossRef]
- Arnqvist, G.; Kirkpatrick, M. The evolution of infidelity in socially monogamous passerines revisited: A reply to Griffith. Am. Nat. 2007, 169, 282–283. [Google Scholar] [CrossRef]
- Weatherhead, P.J.; Robertson, R.J. Offspring quality and the polygyny threshold: “The sexy son hypothesis”. Am. Nat. 1979, 113, 201–208. [Google Scholar] [CrossRef]
- Yezerinac, S.M.; Weatherhead, P.J. Reproductive synchrony and extra-pair mating strategy in a socially monogamous bird, Dendroica petechia. Anim. Behav. 1997, 54, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Jennions, M.D.; Petrie, M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000, 75, 21–64. [Google Scholar] [CrossRef]
- Arct, A.; Drobniak, S.M.; Cichoń, M. Genetic similarity between mates predicts extrapair paternity—A meta-analysis of bird studies. Behav. Ecol. 2015, 26, 959–968. [Google Scholar] [CrossRef]
- Cramer, E.R.A.; Greig, E.I.; Kaiser, S.A. Strong sexual selection despite spatial constraints on extrapair paternity. Behav. Ecol. 2020, 31, 618–626. [Google Scholar] [CrossRef]
- Arnqvist, G.; Kirkpatrick, M. The evolution of infidelity in socially monogamous passerines: The strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 2005, 165, 26–37. [Google Scholar] [CrossRef]
- Sheldon, B.C. Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1994, 257, 25–30. [Google Scholar] [CrossRef]
- Arnqvist, G.; Nilsson, T. The evolution of polyandry: Multiple mating and female fitness in insects. Anim. Behav. 2000, 60, 145–164. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Hromada, M. Do males of the great grey shrike, Lanius excubitor, trade food for extrapair copulations? Anim. Behav. 2005, 69, 529–533. [Google Scholar] [CrossRef]
- Ledwoń, M.; Neubauer, G. True deception during extra-pair courtship feeding: Cheating whiskered tern Chlidonias hybrida females perform better. J. Avian Biol. 2018, 49, e01503. [Google Scholar] [CrossRef]
- Stacey, P.B. Female promiscuity and male reproductive success in social birds and mammals. Am. Nat. 1982, 120, 51–64. [Google Scholar] [CrossRef]
- Davies, N.B.; Harley, I.R.; Hatchwell, B.J.; Langmore, N.E. Female control of copulations to maximize male help: A comparison of polygynandrous alpine accentors, Prunella collaris, and dunnocks, P. modularis. Anim. Behav. 1996, 51, 27–47. [Google Scholar] [CrossRef]
- Krams, I.A.; Mennerat, A.; Krama, T.; Krams, R.; Jõers, P.; Elferts, D.; Luoto, S.; Rantala, M.J.; Eliassen, S. Extra-pair paternity explains cooperation in a bird species. Proc. Natl. Acad. Sci. USA 2022, 119, e2112004119. [Google Scholar] [CrossRef]
- Halliday, T.; Arnold, S.J. Multiple mating by females: A perspective from quantitative genetics. Anim. Behav. 1987, 35, 939–941. [Google Scholar] [CrossRef]
- Forstmeier, W.; Martin, K.; Bolund, E.; Schielzeth, H.; Kempenaers, B. Female extrapair mating behavior can evolve via indirect selection on males. Proc. Natl. Acad. Sci. USA 2011, 108, 10608–10613. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Schroeder, J.; Winney, I.; Burke, T.; Nakagawa, S. Are extra-pair males different from cuckolded males? A case study and a meta-analytic examination. Mol. Ecol. 2015, 24, 1558–1571. [Google Scholar] [CrossRef]
- Reid, J.M.; Wolak, M.E. Is there indirect selection on female extra-pair reproduction through cross-sex genetic correlations with male reproductive fitness? Evol. Lett. 2018, 2, 159–168. [Google Scholar] [CrossRef]
- Mauck, R.A.; Marschall, E.A.; Parker, P.G. Adult survival and imperfect assessment of parentage: Effects on male parenting decisions. Am. Nat. 1999, 154, 99–109. [Google Scholar] [CrossRef]
- Arnold, K.E.; Owens, I.P.F. Extra-pair paternity and egg dumping in birds: Life history, parental care and the risk of retaliation. Proceed. Biol. Sci. 2002, 269, 1263–1269. [Google Scholar] [CrossRef]
- Valcu, C.M.; Valcu, M.; Kempenaers, B. The macroecology of extra-pair paternity in birds. Mol. Ecol. 2021, 30, 4884–4898. [Google Scholar] [CrossRef] [PubMed]
- Kuranov, B.D. Nesting biology of the pied flycacther (Ficedula hypoleuca, Passeriformes, Muscicapidae) in the southeastern part of its distribution area. Zool. Zhurnal 2018, 97, 321–336. [Google Scholar] [CrossRef]
- Lundberg, A.; Alatalo, R.V. The Pied Flycatcher; T ‘I&’ AD Poyser Ltd.: London, UK, 1992. [Google Scholar]
- Artemev, A.V. Populyatsionnaya Ekologiya Muholovki-Pestrushki v Severnoy Zone Areala; Nauka: Moscow, Russia, 2008. [Google Scholar]
- Sternberg, H. Pied flycatcher. In Lifetime Reproduction in Birds; Newton, I., Ed.; Academic Press Ltd.: London, UK, 1989; pp. 55–74. [Google Scholar]
- Sternberg, H.; Grinkov, V.G.; Ivankina, E.V.; Ilyina, T.A.; Kerimov, A.B.; Schwarz, A. Evaluation of the size and composition of nonbreeding surplus in a pied flycatcher Ficedula hypoleuca population: Removal experiments in Germany and Russia. Ardea 2002, 90, 461–470. [Google Scholar]
- Both, C.; Burger, C.; Ouwehand, J.; Samplonius, J.M.; Ubels, R.; Bijlsma, R.G. Delayed age at first breeding and experimental removals show large non-breeding surplus in Pied Flycatchers. Ardea 2017, 105, 43–60. [Google Scholar] [CrossRef]
- Grinkov, V.G.; Sternberg, H. Delayed start of first-time breeding and non-breeders surplus in the Western Siberian population of the European Pied Flycatcher. bioRxiv 2018, 387829. [Google Scholar] [CrossRef]
- Lifjeld, J.T.; Slagsvold, T.; Lampe, H.M. Low frequency of extra-pair paternity in pied flycatchers revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 1991, 29, 95–101. [Google Scholar] [CrossRef]
- Rätti, O.; Hovi, M.; Lundberg, A.; Tegelström, H.; Alatalo, R.V. Extra-pair paternity and male characteristics in the pied flycatcher. Behav. Ecol. Sociobiol. 1995, 37, 419–425. [Google Scholar] [CrossRef]
- Lubjuhn, T.; Winkel, W.; Epplen, J.T.; Brün, J. Reproductive success of monogamous and polygynous pied flycatchers (Ficedula hypoleuca). Behav. Ecol. Sociobiol. 2000, 48, 12–17. [Google Scholar] [CrossRef]
- Slagsvold, T.; Johnsen, A.; Lampe, H.M.; Lifjeld, J.T. Do female pied flycatchers seek extrapair copulations with familiar males? A test of the incomplete knowledge hypothesis. Behav. Ecol. 2001, 12, 412–418. [Google Scholar] [CrossRef]
- Lehtonen, P.K.; Primmer, C.R.; Laaksonen, T. Different traits affect gain of extrapair paternity and loss of paternity in the pied flycatcher, Ficedula hypoleuca. Anim. Behav. 2009, 77, 1103–1110. [Google Scholar] [CrossRef]
- Moreno, J.; Martínez, J.G.; Morales, J.; Lobato, E.; Merino, S.; Tomás, G.; Vásquez, R.A.; Möstl, E.; Osorno, J.L. Paternity loss in relation to male age, territorial behaviour and stress in the pied flycatcher. Ethology 2010, 116, 76–84. [Google Scholar] [CrossRef]
- Canal, D.; Jovani, R.; Potti, J. Multiple mating opportunities boost protandry in a pied flycatcher population. Behav. Ecol. Sociobiol. 2012, 66, 67–76. [Google Scholar] [CrossRef]
- Canal, D.; Jovani, R.; Potti, J. Male decisions or female accessibility? Spatiotemporal patterns of extra pair paternity in a songbird. Behav. Ecol. 2012, 23, 1146–1153. [Google Scholar] [CrossRef]
- González-Braojos, S.; Ruiz de Castańeda, R.; Cantarero, A.; Sánchez-Tójar, A.; Martínez, J.G.; Moreno, J. Extra-pair matings, context-dependence and offspring quality: A brood manipulation experiment in pied flycatchers. Behaviour 2013, 150, 359–380. [Google Scholar] [CrossRef]
- de la Hera, I.; Reed, T.E.; Pulido, F.; Visser, M.E. Feather mass and winter moult extent are heritable but not associated with fitness-related traits in a long-distance migratory bird. Evol. Ecol. 2013, 27, 1199–1216. [Google Scholar] [CrossRef]
- Moreno, J.; Martínez, J.G.; González-Braojos, S.; Cantarero, A.; Ruizde-Castañeda, R.; Precioso, M.; López-Arrabé, J. Extra-pair paternity declines with female age and wing length in the pied flycatcher. Ethology 2015, 121, 501–512. [Google Scholar] [CrossRef]
- Tomotani, B.M.; Caglar, E.; de La Hera, I.; Mateman, A.C.; Visser, M.E. Early arrival is not associated with more extra-pair fertilizations in a long-distance migratory bird. J. Avian Biol. 2017, 48, 854–861. [Google Scholar] [CrossRef]
- Lifjeld, J.T.; Slagsvold, T.; Dale, S.; Ellegren, H. A sexually selected paradox in the pied flycatcher: Attractive males are cuckolded. Auk 1997, 114, 112–115. [Google Scholar] [CrossRef]
- Canal, D.; Dávila, J.; Potti, J. Male phenotype predicts extra-pair paternity in pied flycatchers. Behaviour 2011, 148, 691–712. [Google Scholar] [CrossRef]
- Møller, A.P.; Brohede, J.; Cuervo, J.J.; de Lope, F.; Primmer, C. Extrapair paternity in relation to sexual ornamentation, arrival date, and condition in a migratory bird. Behav. Ecol. 2003, 14, 707–712. [Google Scholar] [CrossRef]
- Grinkov, V.G.; Bauer, A.; Sternberg, H.; Wink, M. Genetic background of social interactions in a Siberian population of Pied Flycatcher. Ornithol. Sci. 2014, 13, 1–2. [Google Scholar]
- Plaza, M.; Cantarero, A.; Gil, D.; Moreno, J. Experimentally flight-impaired females show higher levels of extra-pair paternity in the pied flycatcher Ficedula hypoleuca. Biol. Lett. 2019, 15, 20190360. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Velando, A.; González-Braojos, S.; Ruiz-de Castañeda, R.; Cantarero, A. Females paired with more attractive males show reduced oxidative damage: Possible direct benefits of mate choice in pied flycatchers. Ethology 2013, 119, 727–737. [Google Scholar] [CrossRef]
- Bushuev, A.V.; Husby, A.; Sternberg, H.; Grinkov, V.G. Quantitative genetics of basal metabolic rate and body mass in free-living pied flycatchers. J. Zool. 2012, 288, 245–251. [Google Scholar] [CrossRef]
- Drost, R. Ueber das Brutkleid männlicher Trauerfliegenfänger, Muscicapa hypoleuca. Vogelzug 1936, 6, 179–186. [Google Scholar]
- Grinkov, V.G.; Bauer, A.; Sternberg, H.; Wink, M. Heritability of the extra-pair mating behaviour of the pied flycatcher in Western Siberia. PeerJ 2020, 8, e9571. [Google Scholar] [CrossRef]
- Ellegren, H. Polymerase-chain-reaction (PCR) analysis of microsatellites: A new approach to studies of genetic relationships in birds. Auk 1992, 109, 886–895. [Google Scholar] [CrossRef]
- Primmer, C.R.; Moller, A.P.; Ellegren, H. New microsatellites from the pied flycatcher Ficedula hypoleuca and the swallow Hirundo rustica genomes. Hereditas 1996, 124, 281–283. [Google Scholar] [CrossRef]
- Leder, E.H.; Karaiskou, N.; Primmer, C.R. Seventy new microsatellites for the pied flycatcher, Ficedula hypoleuca and amplification in other passerine birds. Mol. Ecol. Resour. 2008, 8, 874–880. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Dunn, P.O.; Lifjeld, J.T. Can extra-pair copulations be used to predict extra-pair paternity in birds? Anim. Behav. 1994, 47, 983–985. [Google Scholar] [CrossRef][Green Version]
- Griffith, S. The evolution of infidelity in socially monogamous passerines: Neglected components of direct and indirect selection. Am. Nat. 2007, 169, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Birkhead, T.R.; Briskie, J.V.; Slagsvold, T. Breeding-cycle patterns of sperm storage in the pied flycatcher (Ficedula hypoleuca). Auk 1997, 114, 792–796. [Google Scholar] [CrossRef]
- von Haartman, L. Territory in the pied flycatcher Muscicapa hypolueca. Ibis 1956, 98, 460–475. [Google Scholar] [CrossRef]
- Lifjeld, T.J.; Slagsvold, T.; Ellegren, H. Experimental mate switching in pied flycatchers: Male copulatory access and fertilization success. Anim. Behav. 1997, 53, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Silverin, B. Reproductive organs and breeding behaviour of the male pied flycatcher Ficedula hypoleuca (Pallas). Ornis Scand. 1975, 6, 15. [Google Scholar] [CrossRef]
- Chek, A.A.; Lifjeld, J.T.; Robertson, R.J. Lack of Mate-guarding in a Territorial Passerine Bird with a Low Intensity of Sperm Competition, the Pied Flycatcher; (Ficedula hypoleuca). Ethology 1996, 102, 134–145. [Google Scholar] [CrossRef]
- Cormack, R.M. Estimates of survival from the sighting of marked animals. Biometrika 1964, 51, 429–438. [Google Scholar] [CrossRef]
- Jolly, G.M. Explicit estimates from capture-recapture data with both death and immigration-stochastic model. Biometrika 1965, 52, 225–248. [Google Scholar] [CrossRef]
- Seber, G.A.F. A note on the multiple-recapture census. Biometrika 1965, 52, 249–260. [Google Scholar] [CrossRef]
- Lebreton, J.D.; Burnham, K.P.; Clobert, J.; Anderson, D.R. Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol. Monogr. 1992, 62, 67–118. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, S120–S139. [Google Scholar] [CrossRef]
- Laake, J.L. RMark: An R Interface for Analysis of Capture-Recapture Data with MARK; AFSC Processed Rep. 2013-01; Alaska Fisheries Science Center, NOAA, National Marine Fisheries Service: Seattle, WA, USA, 2013. [Google Scholar]
- Brommer, J.E.; Merilä, J.; Kokko, H. Reproductive timing and individual fitness. Ecol. Lett. 2002, 5, 802–810. [Google Scholar] [CrossRef]
- McGraw, J.B.; Caswell, H. Estimation of individual fitness from life-history data. Am. Nat. 1996, 147, 47–64. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021. [Google Scholar]
- Baddeley, A.; Turner, R. Spatstat: An R Package for analyzing spatial point patterns. J. Stat. Softw. 2005, 12, 1–42. [Google Scholar] [CrossRef]
- Sætre, G.P.; Dale, S.; Slagsvold, T. Female pied flycatchers prefer brightly coloured males. Anim. Behav. 1994, 48, 1407–1416. [Google Scholar] [CrossRef]
- Saetre, G.P.; Moum, T.; Bures, S.; Kral, M.; Adamjan, M.; Moreno, J. A sexually selected character displacement in flycatchers reinforces premating isolation. Nature 1997, 387, 589–592. [Google Scholar] [CrossRef]
- Alatalo, R.V.; Lundberg, A.; Glynn, C. Female pied flycatchers choose territory quality and not male characteristics. Nature 1986, 323, 152–153. [Google Scholar] [CrossRef]
- Grinkov, V.G.; Palko, I.V.; Sternberg, H. Character displacement within the breeding area questions reinforcement in Ficedula flycatchers. bioRxiv 2019, 515916. [Google Scholar] [CrossRef]
- Huhta, E.; Alatalo, R.V. Plumage colour and male-male interactions in the pied flycatcher. Anim. Behav. 1993, 45, 511–518. [Google Scholar] [CrossRef]
- Dale, S.; Slagsvold, T. Plumage coloration and conspicuousness in birds: Experiments with the pied flycatcher. Auk 1996, 113, 849–857. [Google Scholar] [CrossRef][Green Version]
- Cameron, E.; Day, T.; Rowe, L. Sexual conflict and indirect benefits. J. Evol. Biol. 2003, 16, 1055–1060. [Google Scholar] [CrossRef]
- Chapman, T.; Arnqvist, G.; Bangham, J.; Rowe, L. Sexual conflict. Trends Ecol. Evol. 2003, 18, 41–47. [Google Scholar] [CrossRef]
- Kokko, H.; Brooks, R.; Jennions, M.D.; Morley, J. The evolution of mate choice and mating biases. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Shy, M.M. Interspecific feeding among birds: A review. J. Field Ornithol. 1982, 53, 370–393. [Google Scholar]
- Zubkova, O.A. Records of nest valence and visits of nest by stranger in the Pied Flycatcher. Ornithologia 2013, 38, 122–124. (In Russian) [Google Scholar]
- Severtsov, A.S.; Kreslavsky, A.G.; Cherdantsev, V.G. Three mechanisms of evolution. In Contemporary Problems of Evolutionary Theory; Tatarinov, L.P., Ed.; Nauka: Moscow, Russia, 1993; pp. 17–42. [Google Scholar]
- Cherdantsev, V.G.; Kreslavsky, A.G.; Severtsov, A.S. Episelective evolution. Evoutionary Theory 1996, 11, 69–87. [Google Scholar]
- Moczek, A.P. On the origins of novelty in development and evolution. BioEssays 2008, 30, 432–447. [Google Scholar] [CrossRef]
- Moczek, A.P. The nature of nurture and the future of evodevo: Toward a theory of developmental evolution. Integr. Comp. Biol. 2012, 52, 108–119. [Google Scholar] [CrossRef]
- Gilbert, S.F.; Bosch, T.C.G.; Ledón-Rettig, C. Eco-Evo-Devo: Developmental symbiosis and developmental plasticity as evolutionary agents. Nat. Rev. Genet. 2015, 16, 611–622. [Google Scholar] [CrossRef]
- Emlen, S.; Oring, L. Ecology, sexual selection, and the evolution of mating systems. Science 1977, 197, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Westneat, D.F.; Sherman, P.W.; Morton, M.L. The ecology and evolution of extra-pair copulations in birds. In Current Ornithology; Power, D.M., Ed.; Current Ornithology, Plenum Press: New York, NY, USA, 1990; pp. 331–369. [Google Scholar]
- Ledwoń, M.; Szczys, P. Extra-pair paternity in a species with frequent extra-pair courtship feedings, few extra-pair copulations, and male-biased parental care. J. Ornithol. 2021, 163, 437–444. [Google Scholar] [CrossRef]
- McKinney, F.; Evarts, S. Sexual coercion in waterfowl and other birds. Ornithol. Monogr. 1998, 49, 163–195. [Google Scholar] [CrossRef]
- Wagner, R.H.; Schug, M.D.; Morton, E.S. Condition-dependent control of paternity by female purple martins: Implications for coloniality. Behav. Ecol. Sociobiol. 1996, 38, 379–389. [Google Scholar] [CrossRef]
- Björklund, M.; Westman, B. Extra-pair copulations in the pied flycatcher (Ficedula hypoleuca). Behav. Ecol. Sociobiol. 1983, 13, 271–275. [Google Scholar] [CrossRef]
- Slagsvold, T.; Drevon, T. When and from whom do female pied flycatchers (Ficedula hypoleuca) solicit copulations. Behaviour 2005, 142, 1059–1076. [Google Scholar] [CrossRef]
- Lundberg, A.; Gottlander, K.; Alatalo, R.V. Extra-pair copulations and mate guarding in the polyterritorial pied flycatcher, Ficedula hypoleuca. Behaviour 1987, 101, 139–154. [Google Scholar] [CrossRef]

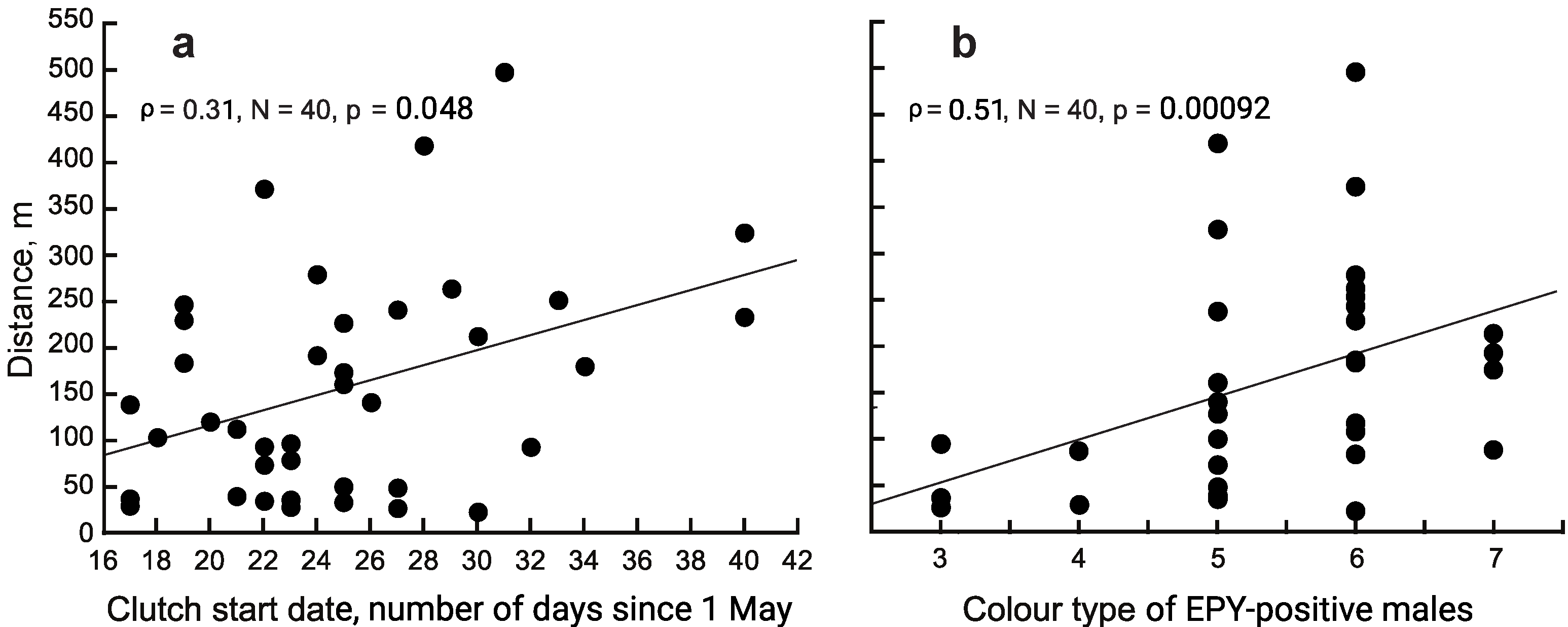

| Social and Genetic Mother | Sire | Social Father | Type | N | Recruited and Started Breeding in | Apparent Recruits | Corrected Recruits | [CI] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | N | % | N | % [CI] | ||||||
| 981 | 30 | 28 | – | 1 | 1 | 60 | 6.1% | 110 | 11.2% [9.2–13.2] | 10.3% [7.4–14.1] | ||||
| 189 | 12 | 4 | 1 | 2 | – | 19 | 10.1% | 40 | 21.2% [15.4–27.0] | 14.6% [9.9–21.1] | ||||
| 103 | 7 | 1 | – | – | – | 8 | 7.8% | 9 | 8.7% [3.3–14.1] | 10.6% [5.6–18.3] | ||||
| 212 | 5 | 4 | 1 | – | – | 10 | 4.7% | 17 | 8.0% [4.3–11.7] | 9.8% [6.1–15.2] | ||||
| Social and Genetic Mother | Sire | Social Father | Type | N | [CI] | [CI] | [CI] | [CI] |
|---|---|---|---|---|---|---|---|---|
| 981 | 0.15 [0–2.42] | 0.02 [0–0.50] | 0.28 [0–3.50] | 0.02 [0–0.50] | ||||
| 189 | 0.27 [0–3.55] | 0.04 [0–0.71] | 0.53 [0–6.30] | 0.04 [0–0.50] | ||||
| 103 | 0.24 [0–3.47] | 0.01 [0–0.00] | 0.31 [0–3.95] | 0.01 [0–0.00] | ||||
| 212 | 0.12 [0–2.14] | 0.02 [0–0.36] | 0.27 [0–3.36] | 0.02 [0–0.36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grinkov, V.G.; Bauer, A.; Sternberg, H.; Wink, M. Understanding Extra-Pair Mating Behaviour: A Case Study of Socially Monogamous European Pied Flycatcher (Ficedula hypoleuca) in Western Siberia. Diversity 2022, 14, 283. https://doi.org/10.3390/d14040283
Grinkov VG, Bauer A, Sternberg H, Wink M. Understanding Extra-Pair Mating Behaviour: A Case Study of Socially Monogamous European Pied Flycatcher (Ficedula hypoleuca) in Western Siberia. Diversity. 2022; 14(4):283. https://doi.org/10.3390/d14040283
Chicago/Turabian StyleGrinkov, Vladimir G., Andreas Bauer, Helmut Sternberg, and Michael Wink. 2022. "Understanding Extra-Pair Mating Behaviour: A Case Study of Socially Monogamous European Pied Flycatcher (Ficedula hypoleuca) in Western Siberia" Diversity 14, no. 4: 283. https://doi.org/10.3390/d14040283
APA StyleGrinkov, V. G., Bauer, A., Sternberg, H., & Wink, M. (2022). Understanding Extra-Pair Mating Behaviour: A Case Study of Socially Monogamous European Pied Flycatcher (Ficedula hypoleuca) in Western Siberia. Diversity, 14(4), 283. https://doi.org/10.3390/d14040283







