Evolutionary Ecology of Fixed Alternative Male Mating Strategies in the Ruff (Calidris pugnax)
Abstract
:1. Introduction
2. Biology of Ruffs
2.1. Systematics and Taxonomy
2.2. Adult Description
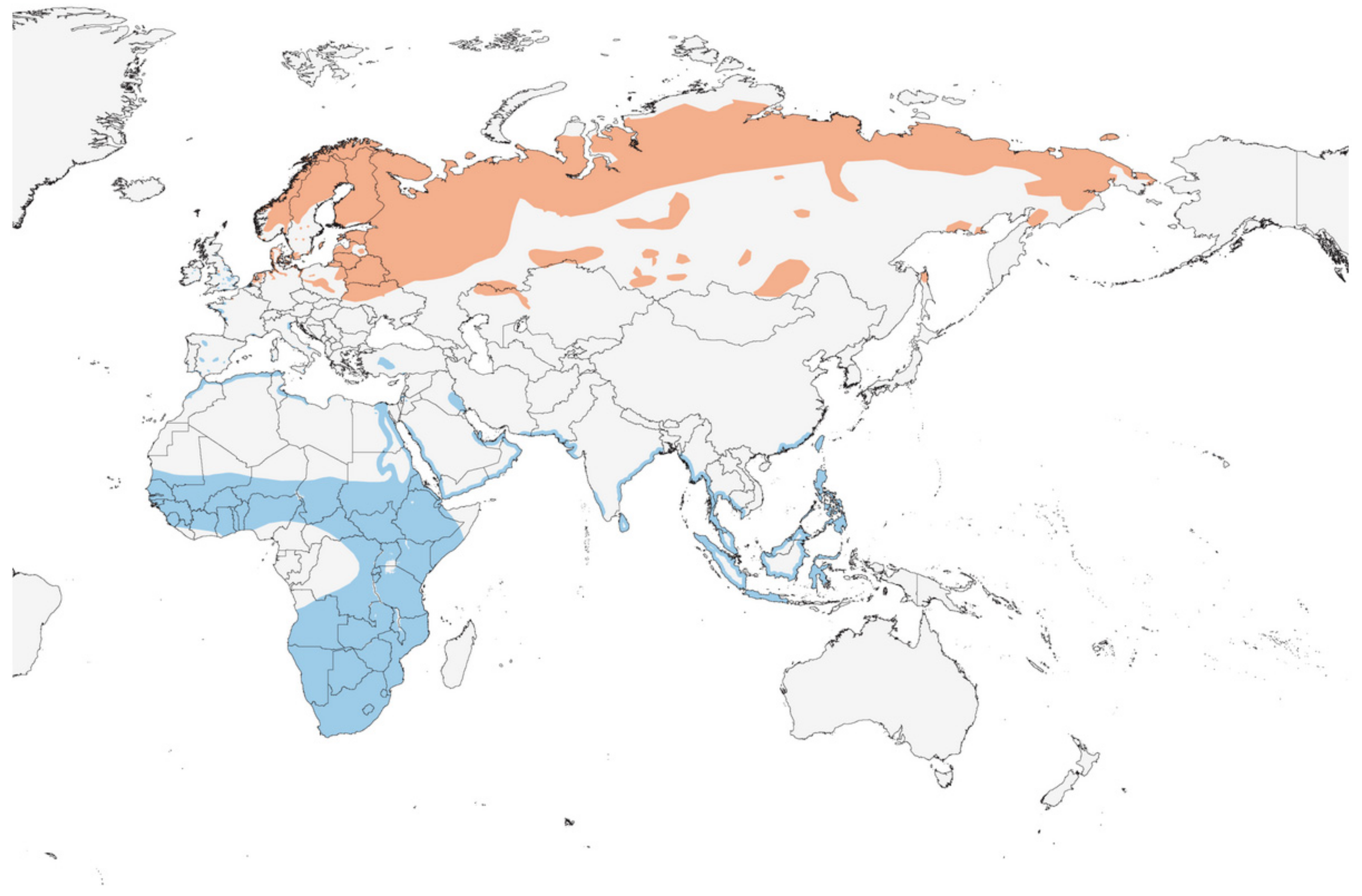
2.3. Distribution and Habitats
2.4. Food and Foraging
2.5. Demography
3. Behavioral Ecology of Breeding Ruffs
3.1. Male Strategies on Leks
3.2. Male-Female Interactions on Leks
4. Genetics and Genomics of Male Alternative Strategies in Ruffs
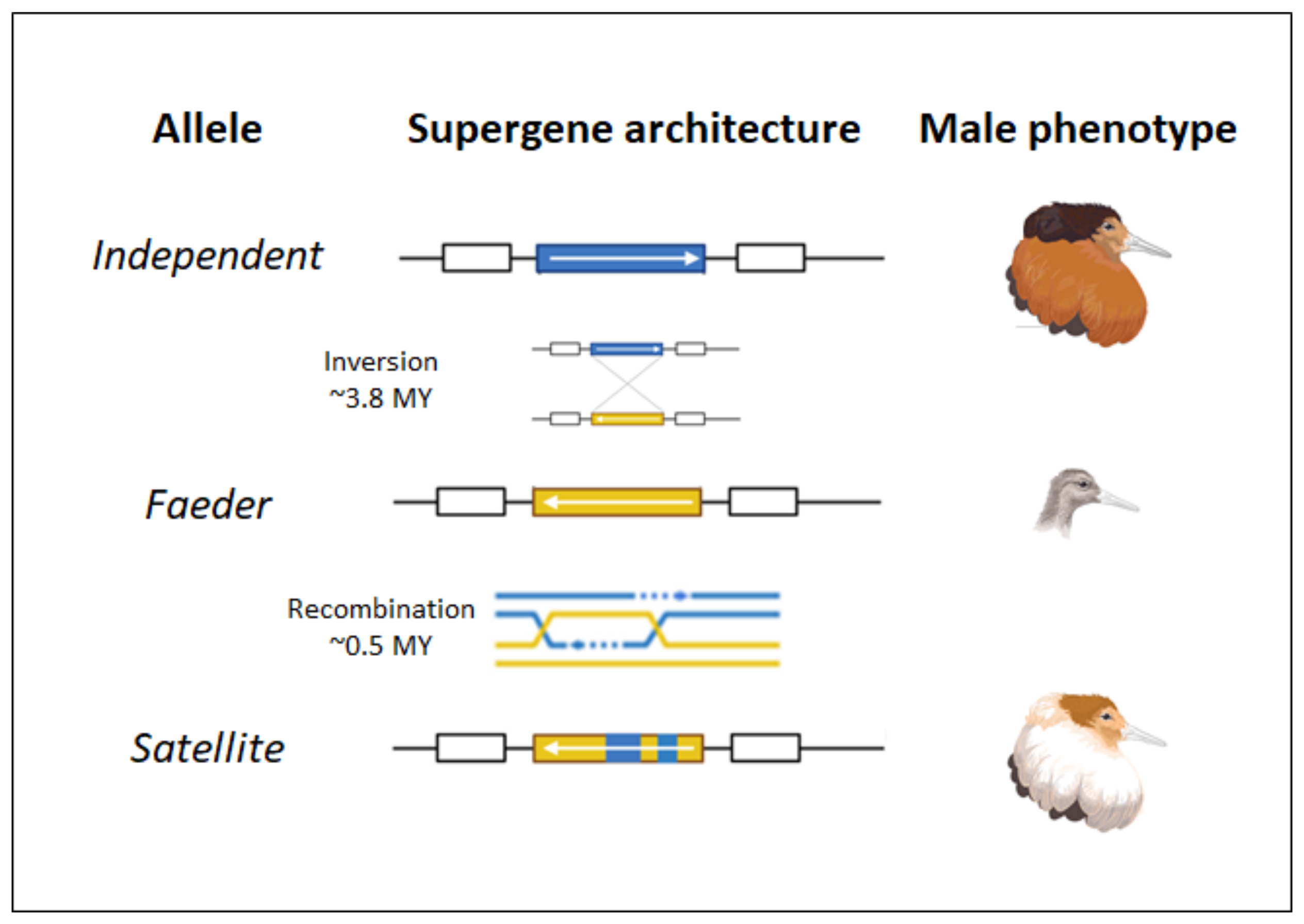
4.1. Structural Genomics
4.2. Functional Genomics
5. Evolution and Maintenance of Male Alternative Strategies in Ruffs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waltz, E.C. Alternative mating tactics and the law of diminishing returns: The satellite threshold model. Behav. Ecol. Sociobiol. 1982, 10, 75–83. [Google Scholar] [CrossRef]
- Gross, M.R. Alternative reproductive strategies and tactics: Diversity within sexes. Trends Ecol. Evol. 1996, 11, 92–98. [Google Scholar] [CrossRef]
- Sinervo, B.; Lively, C.M. The rock-paper-scissors game and the evolution of alternative male strategies. Nature 1996, 380, 240–243. [Google Scholar] [CrossRef]
- Shuster, S.M. Alternative mating strategies. In Evolutionary Behavioral Ecology; Fox, C., Westneats, D.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 434–450. [Google Scholar]
- Taborsky, M.; Brockmann, H.J. Alternative reproductive tactics and life history phenotypes. In Animal Behaviour: Evolution and Mechanisms; Kappeler, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 537–586. [Google Scholar]
- Austad, S.N. Classification of alternative reproductive behaviors and methods for field-testing ESS models. Am. Zool. 1984, 24, 309–319. [Google Scholar] [CrossRef]
- Plaistow, S.J.; Johnstone, R.J.A.; Colegrave, N.; Spencer, M. Evolution of alternative mating tactics: Conditional versus mixed strategies. Behav. Ecol. 2004, 15, 534–542. [Google Scholar] [CrossRef]
- Küpper, C.; Stocks, M.; Risse, J.E.; dos Remedios, N.; Farrell, L.L.; McRae, S.B.; Morgan, T.C.; Karlionova, N.; Pinchuk, P.; Verkuil, Y.I.; et al. A supergene determines highly divergent male reproductive morphs in the ruff. Nat. Genet. 2016, 48, 79. [Google Scholar] [CrossRef] [Green Version]
- Lamichhaney, S.; Fan, G.; Widemo, F.; Gunnarsson, U.; Thalmann, D.S.; Hoeppner, M.P.; Kerje, S.; Gustafson, U.; Shi, C.; Zhang, H.; et al. Structural genomic changes underlie alternative reproductive strategies in the ruff (Philomachus pugnax). Nat. Genet. 2016, 48, 84. [Google Scholar] [CrossRef] [Green Version]
- Piersma, T.; van Gils, J.; Wiersma, P. Family Scolopacidae (sandpipers, snipes and phalaropes). In Handbook of the Birds of the World, Vol. 3. Hoatzin to Auks; del Hoyo, J., Elliott, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 1996; pp. 444–533. [Google Scholar]
- Colwell, M.A. Shorebirds Ecology, Conservation and Management; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 2010; 328p. [Google Scholar]
- Jehl, J.R., Jr. It’s Calidridine. Wader Study Group Bull. 2010, 117, 195. [Google Scholar]
- Van Gils, J.P.; Wiersma, G.M. Kirwan. Ruff (Calidris pugnax), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, A., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Gibson, R.; Baker, A. Multiple gene sequences resolve phylogenetic relationships in the shorebird suborder Scolopaci (Aves: Charadriiformes). Mol. Phyl. Evol. 2012, 64, 66–72. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Zhang, F.; Wang, G.-H.; Zhang, M.-Y.; Liang, T.; Lu, C.-H. Complete mitochondrial genome of the Ruff, Calidris pugnax (Aves, Scolopacidae). Mitochondrial DNA Part B 2020, 5, 1246–1247. [Google Scholar] [CrossRef] [Green Version]
- Banks, R.C. Classification and nomenclature of the sandpipers (Aves: Arenariinae). Zootaxa 2012, 3513, 86–88. [Google Scholar] [CrossRef]
- Chesser, R.T.; Banks, R.C.; Barker, F.K.; Cicero, C.; Dunn, J.L.; Kratter, A.W.; Lovette, I.J.; Rasmussen, P.C.; Remsen, J.V., Jr.; Rising, J.D.; et al. Fifty-Fourth Supplement to the American Ornithologists’ Union Check-list of North American Birds. The Auk 2013, 130, 558–571. [Google Scholar] [CrossRef] [Green Version]
- Crochet, P.-A.; Barthel, P.H.; Bauer, H.-G.; van den Berg, A.B.; Bezzel, E.; Collinson, J.M.; Dubois, P.J.; Fromholtz, J.; Helbig, A.J.; Jiguet, F.; et al. AERC TAC’s Taxonomic Recommendations: 2015 Report. 2015. Available online: http://www.aerc.eu/tac.html (accessed on 21 January 2022).
- Van Rhijn, J.G. The Ruff; T. & A.D. Poyser: London, UK, 1991; 209p. [Google Scholar]
- Hogan-Warburg, A.J. Social behavior of the Ruff (Philomachus pugnax (L.). Ardea 1966, 54, 109–229. [Google Scholar] [CrossRef] [Green Version]
- Andersen, F. Contributions to the breeding biology of the Ruff (Philomachus pugnax (L.)): II. Dan. Ornithol Tidssk 1948, 42, 125–148. [Google Scholar]
- Widemo, F. Alternative reproductive strategies in the ruff, Philomachus pugnax: A mixed ESS? Anim. Behav. 1998, 56, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Lank, D.B.; Dale, J. Visual signals for individual identification. The silent “song” of ruffs. Auk 2001, 118, 759–765. [Google Scholar]
- Jukema, J.; Piersma, T. Permanent female mimics in a lekking shorebird. Biol. Lett. 2006, 2, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Lank, D.B.; Smith, C.M.; Hanotte, O.; Burke, T.; Cooke, F. Genetic polymorphism for alternative mating behaviour in lekking male ruff Philomachus pugnax. Nature 1995, 378, 59–62. [Google Scholar] [CrossRef]
- Cramp, S.; Simmons, K.E.L. The Birds of the Western Palearctic; Volume III. Waders to Gulls; Oxford University Press: Oxford, UK; New York, NY, USA, 1983; 913p. [Google Scholar]
- Jehl, J.R.; Murray, B.G. The evolution of normal and reverse sexual size dimorphism in shorebirds and other birds. In Current Ornithology; Johnston, R.F., Ed.; Plenum Press: New York, NY, USA, 1986; pp. 1–86. [Google Scholar]
- Tinbergen, N. Ruff. Brit. Birds 1959, 52, 302–306. [Google Scholar]
- Van Rhijn, J.G. Behavioural dimorphism in male ruffs, Philomachus pugnax (L.). Behaviour 1973, 47, 153–227. [Google Scholar] [CrossRef] [Green Version]
- Van Rhijn, J.G. A scenario for the evolution of social organization in ruffs Philomachus pugnax and other Charadriiform species. Ardea 1985, 73, 25–37. [Google Scholar]
- Höglund, J.; Lundberg, A. Plumage color correlates with body size in the ruff (Philomachus pugnax). Auk 1989, 106, 336–338. [Google Scholar]
- Zwarts, L.; Bijlsma, R.; van der Kamp, J.; Wymenga, E. Living on the Edge. Wetlands and Birds in a Changing Sahel; KNNV Publishing: Zeist, The Netherlands, 2009; 564p. [Google Scholar]
- Verkuil, Y.I. The Ephemeral Shorebird. Population History of Ruffs. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2010; 192p. [Google Scholar]
- Shorebird, R. Encyclopedia of Evolutionary Psychological Science; Shackelford, T.K., Weekes-Shackelford, V.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–4. [Google Scholar]
- Schmaltz, L.E.; Loonstra, A.H.J.; Wymenga, E.; Hobson, K.A.; Piersma, T. Quantifying the non-breeding provenance of staging Ruffs, Philomachus pugnax, using stable isotope analysis of different tissues. J. Ornithol. 2018, 159, 191–203. [Google Scholar] [CrossRef]
- Gill, J.A.; Clark, J.; Clark, N.; Sutherland, W.J. Sex differences in the migration, moult and wintering areas of British-ringed Ruff. Ringing Migr. 1995, 16, 159–167. [Google Scholar] [CrossRef]
- Kokko, H.; Gunnarsson, T.G.; Morrell, L.J.; Gill, J.A. Why do female migratory birds arrive later than males? J. Anim. Ecol. 2006, 75, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Schmaltz, L.E.; Juillet, C.; Tinbergen, J.M.; Verkuil, Y.I.; Hooijmeijer, J.C.E.W.; Piersma, T. Apparent annual survival of staging ruffs during a period of population decline: Insights from sex and site-use related differences. Popul. Ecol. 2015, 57, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Andersen, F. Contributions to the breeding biology of the Ruff (Philomachus pugnax). Dan. Ornithol Tidssk 1944, 38, 26–30. [Google Scholar]
- Jaatinen, K.; Lehikoinen, A.; Lank, D.B. Female-biased sex ratios and the proportion of cryptic male morphs of migrant juvenile ruffs (Philomachus pugnax) in Finland. Ornis Fenn. 2010, 87, 125–134. [Google Scholar]
- Höglund, J.; Alatalo, R.V. Leks; Princeton University Press: Princeton, NJ, USA, 1995; 248p. [Google Scholar]
- Höglund, J.; Lundberg, A. Sexual selection in a monomorphic lek-breeding bird: Correlates of male mating success in the great snipe Gallinago media. Behav. Ecol. Sociobiol. 1987, 21, 211–216. [Google Scholar] [CrossRef]
- Lanctot, R.B.; Scribner, K.T.; Kempenaers, B.; Weatherhead, P.J. Lekking without a paradox in the Buff-Breasted Sandpiper. Am. Nat. 1997, 149, 1051–1070. [Google Scholar] [CrossRef]
- Kempenaers, B.; Valcu, M. Breeding site sampling across the Arctic by individual males of a polygynous shorebird. Nature 2017, 541, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.; Widemo, F.; Sutherland, W.J.; Nordenfors, H. Ruffs, Philomachus pugnax, and distribution models: Can leks be regarded as patches? Oikos 1998, 82, 370–376. [Google Scholar] [CrossRef]
- Höglund, J.; Montgomerie, R.; Widemo, F. Costs and consequences of variation in the size of ruff leks. Behav. Ecol. Sociobiol. 1993, 32, 31–39. [Google Scholar] [CrossRef]
- Widemo, F. Competition for females on leks when male competitive abilities differ: Empirical test of a model. Behav. Ecol. 1998, 9, 427–430. [Google Scholar] [CrossRef]
- Vervoort, R.; Kempenaers, B. Variation in lek attendance and copulation success of independent and satellite male ruffs Calidris pugnax. Ardea 2019, 107, 303–320. [Google Scholar] [CrossRef]
- Verkuil, Y.I.; Jukema, J.; Gill, J.A.; Karlionova, N.; Melter, J.; Hooijmeijer, J.C.E.W.; Piesma, T. Non-breeding fæder ruffs Philomachus pugnax associate according to sex, not morphology. Bird Study 2008, 55, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Hugie, D.M.; Lank, D.B. The resident’s dilemma: A female choice model for the evolution of alternative mating strategies in lekking male ruffs (Philomachus pugnax). Behav. Ecol. 1997, 8, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Mustonen, J. Influence of Intraspecific Relationships on the Allocation of Temporal Resources in the Lekking Ruff, Calidris pugnax. Master’s Thesis, University of Oulu, Oulu, Finland, 2020; 52p. [Google Scholar]
- Lank, D.B.; Smith, C.M. Conditional lekking in ruff (Philomachus pugnax). Behav. Ecol. Sociobiol. 1987, 20, 137–145. [Google Scholar] [CrossRef]
- Loveland, J.L.; Lank, D.B.; Küpper, C. Gene expression modification by an autosomal inversion associated with three male mating morphs. Front. Genet. 2021, 12, 641620. [Google Scholar] [CrossRef]
- Johnson, D.D.P.; Briskie, J.V. Sperm competition and sperm length in shorebirds. Condor 1999, 101, 848–854. [Google Scholar]
- Lank, D.B.; Smith, C.M.; Hanotte, O.; Ohtonen, A.; Bailey, S.; Burke, T. High frequency of polyandry in a lek mating system. Behav. Ecol. 2002, 13, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Thuman, K.A.; Griffith, S.C. Genetic similarity and the nonrandom distribution of paternity in a genetically highly polyandrous shorebird. Anim. Behav. 2005, 69, 765–770. [Google Scholar] [CrossRef]
- Lank, D.B.; Farrell, L.L.; Burke, T.; Piersma, T.; McRae, S.B. A dominant allele controls development into female mimic male and diminutive female ruffs. Biol. Lett. 2013, 9, 20130653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lank, D.B.; Coupe, M.; Wynne-Edwards, K. Testosterone-induced male traits in female ruffs (Philomachus pugnax): Autosomal inheritance and gender differentiation. Proc. R. Soc. Lond. Ser. B 1999, 266, 2323–2330. [Google Scholar] [CrossRef] [Green Version]
- Jiggins, C.D. A flamboyant behavioral polymorphism is controlled by a lethal supergene. Nat. Gen. 2016, 48, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Campagna, L. Avian supergenes. Science 2016, 351, 446–447. [Google Scholar] [CrossRef]
- Loveland, J.L.; Giraldo-Deck, L.M.; Lank, D.B.; Goymann, W.; Gahr, M.; Küpper, C. Functional differences in the hypothalamic-pituitary-gonadal axis are associated with alternative reproductive tactics based on an inversion polymorphism. Horm. Behav. 2021, 127, 104877. [Google Scholar] [CrossRef]
- Van Oordt, G.J.; Junge, G.C.A. Die hormonal Wirkung der Gonaden auf Sommer- und Prachtkleid III. Der Einfluss der Kastration auf männliche Kampfläufer (Philomachus pugnax). Wilhelm Roux Arch. Entwicklungsmech. Org. 1936, 134, 112–121. [Google Scholar] [CrossRef]
- Morgan, T. Hormonal Regulation of Alternative Reproductive Strategies. Master’s Thesis, University of Alaska, Fairbanks, AK, USA, 2010; 76p. [Google Scholar]
- Horton, B.M.; Michael, C.M.; Prichard, M.R.; Maney, D.L. Vasoactive intestinal peptide as a mediator of the effects of a supergene on social behaviour. Proc. R. Soc. B 2020, 287, 20200196. [Google Scholar] [CrossRef] [Green Version]
- Merritt, J.R.; Grogan, K.E.; Zinzow-Kramer, W.M.; Sun, D.; Ortlund, E.A.; Yi, S.V.; Maney, D.L. A supergene-linked estrogen receptor drives alternative phenotypes in a polymorphic songbird. Proc. Natl. Acad. Sci. USA 2020, 117, 2011347117. [Google Scholar] [CrossRef]
- Hill, W.L. Correlates of male mating success in the ruff Philomachus pugnax, a lekking shorebird. Behav. Ecol. Sociobiol. 1991, 29, 367–372. [Google Scholar] [CrossRef]
- Widemo, F.; Owens, I.P.F. Lek size, male mating skew and the evolution of lekking. Nature 1995, 373, 148–151. [Google Scholar] [CrossRef]
- Möller, A.P. Sperm competition, sperm depletion, paternal care, and relative testis size in birds. Am. Nat. 1991, 137, 882–906. [Google Scholar] [CrossRef]
- Dale, J.; Lank, D.B.; Reeve, H.K. Signaling individual identity versus quality: A model and case studies with ruffs, queleas, and house finches. Am. Nat. 2001, 158, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Deck, L.M.; Loveland, J.L.; Goymann, W.; Tschirren, B.; Burke, T.; Kempenaers, B.; Lank, D.B.; Küpper, C. Intralocus conflicts associated with a supergene. Nat. Commun. 2022, 13, 1384. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baguette, M.; Bataille, B.; Stevens, V.M. Evolutionary Ecology of Fixed Alternative Male Mating Strategies in the Ruff (Calidris pugnax). Diversity 2022, 14, 307. https://doi.org/10.3390/d14040307
Baguette M, Bataille B, Stevens VM. Evolutionary Ecology of Fixed Alternative Male Mating Strategies in the Ruff (Calidris pugnax). Diversity. 2022; 14(4):307. https://doi.org/10.3390/d14040307
Chicago/Turabian StyleBaguette, Michel, Baptiste Bataille, and Virginie M. Stevens. 2022. "Evolutionary Ecology of Fixed Alternative Male Mating Strategies in the Ruff (Calidris pugnax)" Diversity 14, no. 4: 307. https://doi.org/10.3390/d14040307
APA StyleBaguette, M., Bataille, B., & Stevens, V. M. (2022). Evolutionary Ecology of Fixed Alternative Male Mating Strategies in the Ruff (Calidris pugnax). Diversity, 14(4), 307. https://doi.org/10.3390/d14040307





