Bagworms in Indonesian Plantation Forests: Species Composition, Pest Status, and Factors That Contribute to Outbreaks
Abstract
:1. Introduction
2. Species Composition of Bagworms in Indonesia
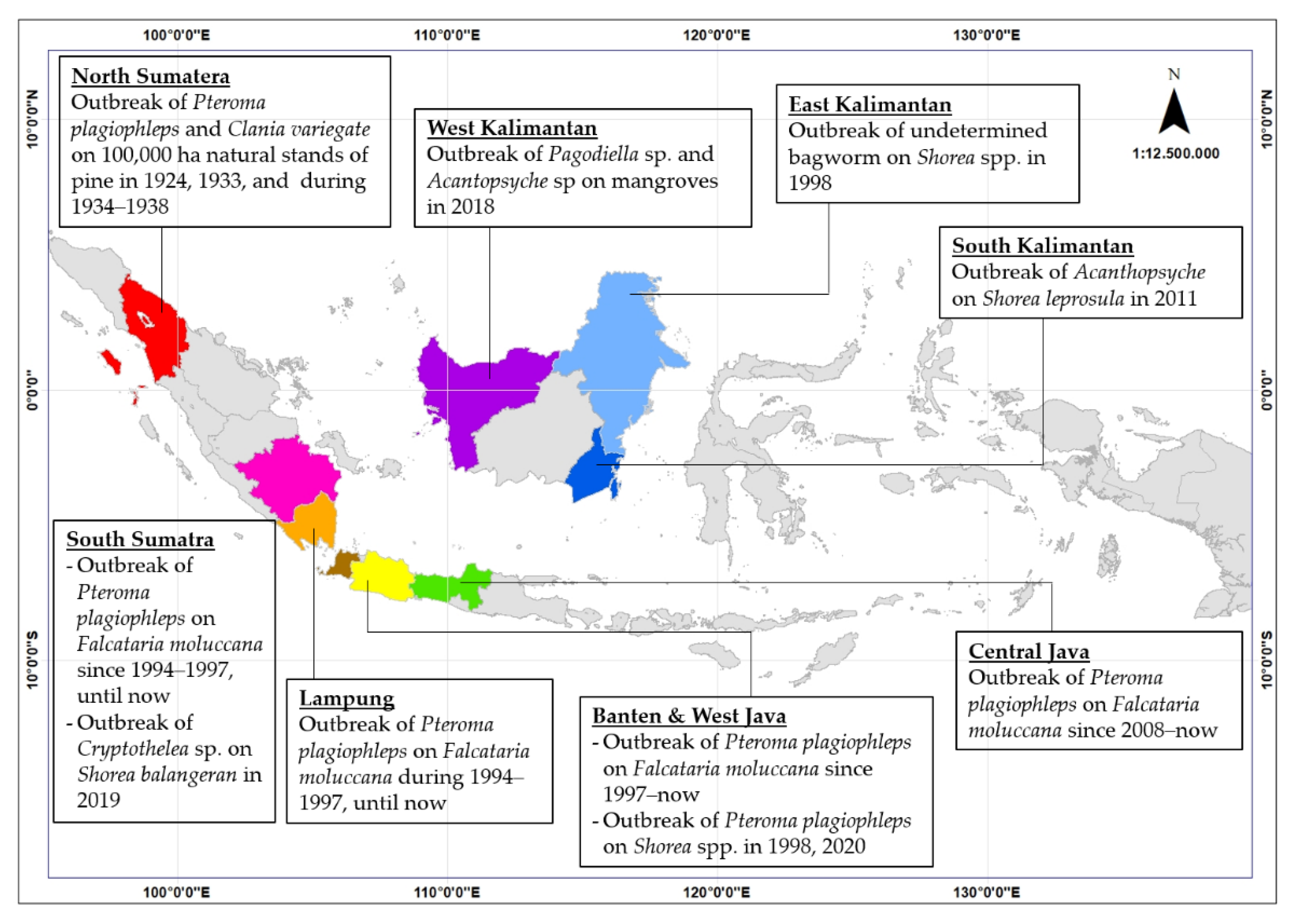
3. Pest Status of Bagworms in Indonesian Plantation Forests
| Bagworm Species | Host Plant | Host Stage | Level of Damage | Location | Reference |
|---|---|---|---|---|---|
| Pteroma plagiophleps | Falcataria moluccana | seedling, tree | minor–severe | Java, Sumatra | [8,25,33,56,74] |
| Acacia mangium | tree | minor | Java, Sumatra | [37,62,63] | |
| Rhizophora sp. | tree | severe | Sumatra | [75] | |
| Soneratia caseolaris | tree | minor | Sumatra | [76] | |
| Shorea leprosula | seedling, tree | minor–severe | Java, Kalimantan, Sumatra | [58,59] | |
| Shorea balangeran | tree | minor | Sumatra | [57] | |
| Shorea macrophylla | seedling | minor | Java | [60] | |
| Shorea stenoptera | seedling | minor | Java | [60] | |
| Anisoptera sp. | seedling | minor | Java | [60] | |
| Azadirachta excelsa | seedling, tree | minor | Sumatra | [26,58] | |
| Neolamarckia cadamba | tree | minor | Sumatra | [56] | |
| Maesopsis eminii | tree | minor | Sumatra | [56] | |
| Pinus merkusii | tree | severe | Sumatra | [10,30,31] | |
| Acanthopsyche sp. | Avicennia alba, Bruguiera parvifolia | tree | minor–moderate | Kalimantan | [29] |
| Shorea leprosula | tree | moderate–severe | Kalimantan | [61] | |
| Clania sp. | Falcataria moluccana | Seedling, tree | minor–severe | Java, Kalimantan | [25,77,78] |
| Cryptothelea sp. | Falcataria moluccana | Seedling, tree | minor–severe | Java | [8,25,77,78] |
| Pinus merkusii | tree | severe | Sumatra | [10] | |
| Shorea selanica | tree | minor | Kalimantan | [79] | |
| Avicenia sp. | tree | n.a. | Sumatra | [69] | |
| Bruguiera sp. | tree | n.a. | Sumatra | [69] | |
| Shorea balangeran | seedling, tree | minor | Sumatra | [27,57] | |
| Mahasena corbeti | Neolamarckia cadamba | tree | moderate | Sumatra | [44] |
| Metisa plana | Shorea balangeran | seedling, tree | minor | Sumatra | [27,57] |
| Anisoptera marginata | seedling | minor | Sumatra | [80] | |
| Pagodiella sp. | Avicennia alba, Bruguiera parviflora | tree | minor–moderate | Kalimantan | [29] |
| Rhizophora apiculata | seedling | minor | Sumatra, Sulawesi | [27,70] | |
| Neolamarckia cadamba | tree | minor | Sumatra | [72] | |
| Azadirachta excelsa | tree | minor | Sumatra | [72] | |
| Michelia champaca | tree | minor | Sumatra | [72] | |
| Amatissa sp. | Falcataria moluccana | seedling, tree | minor–severe | Java, Kalimantan | [8,37,77,78] |
| Chalia javana | Falcataria moluccana | tree | minor | Java | [25] |
| Kophenecuprea | Falcataria moluccana | tree | minor | Java | [25] |
| Psyche sp. | Falcataria moluccana | tree | severe | Java | [37] |
| Acacia mangium | tree | n.a. | Java | [37] |
4. The Key Factors That Lead to Bagworm Outbreaks in Indonesian Plantation Forests
4.1. Reproductive Strategy and Mode of Dispersal
4.2. Climate
4.3. Monoculture Plantation
4.4. Cultivation Practices
4.5. Natural Enemies
5. Bagworm Outbreak in Falcataria moluccana Plantation: Case in Java
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kementerian Lingkungan Hidup Dan Kehutanan. Kawasan Hutan Indonesia. Available online: http://pktl.menlhk.go.id/?pg=j2540i2525c2620h2525h2615k2620y2610g2525b2620v2545k2555m2565x2615 (accessed on 28 September 2021).
- BPS-Statistics Indonesia. Statistics of Timber Culture Establishment 2019; BPS: Jakarta, Indonesia, 2020. [Google Scholar]
- Balai Pemantapan Kawasan Hutan XI Jawa-Madura. Strategi Pengembangan Pengelolaan dan Arahan Kebijakan Hutan Rakyat di Pulau Jawa; Balai Pemantapan Kawasan Hutan XI Jawa-Madura: Yogyakarta, Indonesia, 2009. [Google Scholar]
- Badan Pusat Statistik. Sensus Pertanian 2013. Jumlah Tanaman Kehutanan yang Diusahakan Menurut Wilayah dan Jenis Tanaman. Available online: https://st2013.bps.go.id/dev2/index.php/site/tabel?tid=65&wid=0 (accessed on 28 September 2021).
- Asosiasi Pengusaha Hutan Indonesia. Roadmap Hutan Produksi Tahun 2016–2045; Asosiasi Pengusaha Hutan Indonesia: Jakarta, Indonesia, 2016. [Google Scholar]
- Wagner, D.L. Moth. In Encyclopedia of Biodiversity; Levin, S., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 384–403. [Google Scholar]
- Ciesla, W.M. Forest Entomology: A Global Perspective; John Wiley & Sons Ltd.: Chichester, West Sussex, UK, 2011; pp. 108–111. [Google Scholar]
- Gullan, P.J.; Cranston, P.S. The Insects: An Outline of Entomology; John Wiley & Sons Ltd.: Chichester, UK, 2014; pp. 6–10. [Google Scholar]
- Lelana, N.E.; Anggraeni, I. An outbreak of bagworm on Falcataria molluccana: A case study in Central Java. In Proceedings of the International Conference on The Impacts of Climate Change to Forest Pest and Diseases in The Tropics, Yogyakarta, Indonesia, 10–12 October 2012; Mohammed, C., Beadle, C., Roux, J., Rahayu, S., Eds.; Faculty of Forestry, Universitas Gadjah Mada: Yogyakarta, Indonesia, 2012; pp. 99–103. [Google Scholar]
- Morgan, F.D.; Suratmo, F.G. Host Preferences of Hypsipyla robusta (Moore) (Lepidoptera: Pyralidae) in West Java. Aust. For. 1976, 39, 103–112. [Google Scholar] [CrossRef]
- Nair, K.S.S. Insect pests and diseases for major plantation species. In Insect Pests and Diseases in Indonesia Forests: An Assessment of the Major Threat, Research Efforts and Literature; Nair, K.S.S., Ed.; Centre for International Forestry Research: Bogor, Indonesia, 2000. [Google Scholar] [CrossRef]
- Rhainds, M.; Sadof, C. Control of bagworms (Lepidoptera: Psychidae) using contact and soil-applied systemic insecticides. J. Econ. Entomol. 2009, 102, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Usha, A.U.; Jose, J. Bag morphology of commonly occurring bagworms (Lepidoptera: Psychidae) in Kerala—A taxonomic tool. In Faunal Diversity and Recent Trends in Animal Taxonomy; Christ College: Irinjalakkuda, India, 2018; pp. 118–121. [Google Scholar]
- Sugiura, S. Bagworm bags as portable armour against invertebrate predators. Peer. J. 2016, 2016, e1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.D.K. Bagworm decorations are an anti-predatory structure. Ecol. Entomol. 2020, 45, 924–928. [Google Scholar] [CrossRef]
- Rivers, D.B.; Antonelli, A.L.; Yoder, J.A. Bags of bagworm Thyridopteryx ephemeraeformis (Lepidoptera: Psychidae) protect diapausing eggs from water loss and chilling injury. Ann. Entomol. Soc. Am. 2002, 95, 481–486. [Google Scholar] [CrossRef]
- Sobczyk, T. World Catalogue of Insects Vol. 10, (Psychidae: Lepidoptera); Nuss, M., Ed.; Apollo Books Aps: Stenstrup, Denmark, 2011. [Google Scholar]
- Yoshioka, T.; Tsubota, T.; Tashiro, K.; Jouraku, A.; Kameda, T. A study of the extraordinarily strong and tough silk produced by bagworms. Nat. Commun. 2019, 10, 1469. [Google Scholar] [CrossRef]
- Rhainds, M.; Davis, D.R.; Price, P.W. Bionomics of bagworms (Lepidoptera: Psychidae). Annu. Rev. Entomol. 2008, 54, 209–226. [Google Scholar] [CrossRef]
- Davis, D.R.; Robinson, G.S. Tineoidea. In Handbook of Zoology IV: Lepidoptera, Moths and Butterflies Volume 1: Evolution, Systematics, and Biogeography; Kristensen, N.P., Ed.; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1999; pp. 91–107. [Google Scholar]
- Davis, D.R.; Quintero, D.A.; Cambra, R.A.T.; Aiello, A. Biology of a new panamanian bagworm moth (Lepidoptera: Psychidae) with predatory larvae and eggs individually wrapped in setal cases. Annu. Entomol. Soc. Am. 2008, 101, 689–702. [Google Scholar] [CrossRef]
- Villanuera, R.T.; Rodrigues, J.C.V.; Childers, C.C. Larval Cryptothelea gloverii (Lepidoptera: Psychidae) an arthropod predator and herbivore on Florida citrus. Exp. Appl. Acarol. 2005, 36, 83–92. [Google Scholar] [CrossRef]
- Pierce, N.E. Predatory and parasitic Lepidoptera: Carnivores living on plants. J. Lepid. Soc. 1995, 49, 412–453. [Google Scholar]
- Davis, D.R. Bagworm moths of the Western Hemisphere. Bull. US Natl. Mus. 1964, 244, 1–233. [Google Scholar] [CrossRef]
- Darmawan, U.W.; Triwidodo, H.; Hidayat, P.; Haneda, N.F.; Lelana, N.E. Bagworms and their natural enemies associated with albizia (Falcataria moluccana (Miq.) Barneby & J.W. Grimes plantation. J. Penelit. Hutan Tanam. 2020, 17, 296–307. [Google Scholar] [CrossRef]
- Utami, S.; Anggraeni, I. Serangan Hama Ulat Kantong (Pteroma plagiophleps) pada Tanaman Kayu Bawang (Dysoxylum Mollissimun Blume) di Persemaian dan Lapangan. In Peran dan Tantangan Entomologi di Era Global, Proceedings of the Kongres VIII Dan Seminar Nasional Perhimpunan Entomologi Indonesia, Bogor, Indonesia, 24–25 January 2012; Pudjianto, D., Laba, I.W., Eds.; Perhimpunan Entomologi Indonesia: Bogor, Indonesia, 2014; pp. 207–216. [Google Scholar]
- Asmaliyah, A.; Anggraeni, I. Uji aplikasi beberapa bioinsektisida dan kombinasinya terhadap serangan hama ulat kantong Pagodiella sp. pada bibit Rhizophora apiculata di persemaian. J. Penelit. Hutan Tanam. 2009, 6, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Asmaliyah, A.; Hadi, E.E.W.; Irianto, S.B.; Imanullah, A.; Bastoni, B.; Siahaan, H.; Purwanto, P. Bagworm infestation on Shorea balangeran in the degraded peatland restoration plot. IOP Conf. Ser. Earth Environ. Sci. 2020, 533, 12041. [Google Scholar] [CrossRef]
- Haneda, N.F.; Suheri, M. Mangrove pests at Batu Ampar, Kubu Raya, West Kalimantan. J. Silvikultur Trop. 2019, 9, 16–23. [Google Scholar] [CrossRef]
- Kalshoven, L.G.E. Important Outbreak of Insect Pest in the Forest of Indonesia. In Proceedings of the Transactions of the 9th International Congress of Entomology, Amsterdam, The Netherlands, 17–24 August 1951; Junk, W., Ed.; Hague, The Netherlands, 1953; Volume 2, pp. 229–234. [Google Scholar]
- Nair, K.S.S. Tropical Forest Insect Pest, Ecology, Impact, and Management; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Pillai, S.R.M.; Gopi, K.C. The bagworm Pteroma plagiophleps Hamp. (Lepidoptera: Psychidae) attack on Acacia nilotica (Linn.) Wild. ex del. Indian For. 1990, 116, 581–583. [Google Scholar]
- Nair, K.S.S.; Mathew, G. Biology, Infestation characteristics and impact of the bagworm, Pteroma plagiophleps Hamps in forest plantations of Paraserianthes falcataria. Entomon 1992, 17, 1–13. [Google Scholar]
- Kamarudin, N.; Robinson, G.S.; Wahid, M.B. Common bagworm pests (Lepidoptera: Psychidae) of oil palm in Malaysia with notes on related South east Asian species. Malay. Nat. J. 1994, 48, 93–123. [Google Scholar]
- Hättenschwiler, P.; Dewhurst, C.; Nyaure, S.; Bonneau, L. New bagworms (Lepidoptera, Psychidae) from oil palm plantations in Papua New Guinea. Mitt. Schweiz. Entomol. Ges. 2013, 86, 253–260. [Google Scholar]
- Sobczyk, T. Contribution to the knowledge of Oiketicinae from the Indo-Australian region with focus on New Guinea (Lepidoptera: Psychidae). SUGAPA 2020, 12, 124–156. [Google Scholar] [CrossRef]
- Suharti, M.; Sitepu, I.R.; Darwiati, W.; Anggraeni, I. Uji efikasi beberapa agens pengendali biologi, nabati dan kimia terhadap hama ulat kantong. Bull. Penelit. Hutan 2000, 624, 11–28. [Google Scholar]
- Sankaran, T. The oil palm bagworms of Sabah and the possibilities of their biological control. PANS Pest Artic. News Summ. 1970, 16, 43–55. [Google Scholar] [CrossRef]
- Wood, B.J.; Kamarudin, N. Bagworm (Lepidoptera: Psychidae) infestation in Centennial of the Malaysian oil palm industry—A review of causes and control. J. Oil Palm Res. 2019, 31, 364–380. [Google Scholar] [CrossRef]
- Tuck, H.C.; Ibrahim, Y.; Chong, K.K. Infestation by the bagworms Metisa plana and Pteroma pendula for the period 1986–2000 in major oil palm estates managed by golden hope plantation berhad in Peninsular Malaysia. J. Oil Palm Res. 2011, 23, 1040–1050. [Google Scholar]
- Halim, M.; Aman-Zuki, A.; Ahmad, S.Z.S.; Din, A.M.M.; Rahim, A.A.; Masri, M.M.M.; Md Zain, B.N.; Yaakop, S. Exploring the abundance and DNA barcode information of eight parasitoid waps species (Hymenoptera), the natural enemies of important pest of oil palm, bagworms, Metisa plana (Lepidoptera: Psychidae) toward the biocontrol approach and its application in Malaysia. J. Asia-Pac. Entomol. 2018, 21, 1359–1365. [Google Scholar] [CrossRef]
- Halim, M.; Ahmad, S.Z.S.; Din, A.M.M.; Yaakop, S. The diversity and abundance of potential Hymenopteran parasitoids assemblage associated with Metisa plana (Lepidoptera: Psychidae) in three infested oil palm plantations in Peninsular Malaysia. AIP Conf. Proceeding 2019, 2111, 060024. [Google Scholar] [CrossRef]
- Priwiratama, H.; Rozziansha, T.A.P.; Prasetyo, A.E.; Susanto, A. Effect of bagworm Pteroma pendula Joannis attack on the decrease in oil palm productivity. J. Hama Dan Penyakit Tumbuh. Trop. 2019, 19, 101–108. [Google Scholar] [CrossRef]
- Sudarsono, H.; Purnomo, P.; Hariri, A.M. Population assessment and appropriate spraying technique to control the bagworm (Metisa plana Walker) in North Sumatra and Lampung. Agrivita 2011, 33, 188–198. [Google Scholar]
- Safitri, D.Y.; Indriyanto, I.; Hariri, A.M. Pest attacked level on jabon plantation (Anthocephalus cadamba Miq) at Negara Ratu II Village Natar Districts of South Lampung Regency. J. Silva Lestari 2017, 5, 77–86. [Google Scholar]
- Murgianto, F.; Edyson, E.; Setyawan, Y.P.; Tamba, L.M.; Ardiyanto, A.; Siregar, A.H. First report of an ice cream cone bagworm Manatha Conglacia Haettenschwiler (Lepidoptera: Psychidae) in oil palm plantations of Central Kalimantan, Indonesia. In Proceedings of the International Conference on Tropical Agrifood, Feed and Fuel (ICTAFF 2021), virtual. 7 September 2021; Suhardi, S.A., Ismanto, A., Masangkay, P., Eds.; Advances in Biological Sciences Research. Atlantis Press: Dordrecht, The Netherlands, 2022; Volume 17, pp. 1–4. [Google Scholar]
- Pujiastuti, Y. Biodiversity of Bagworm (Lepidoptera: Psychidae) on Ornamental Plant in South Sumatera, Indonesia. In Multidisciplinary Scientist as Indonesia Resources Toward Society Welfare Equality and Sustainability, Proceedings of the 8th Hokkaido Indonesia Student Association Scientific Meeting, Japan, 6 February 2010; Awaludin., A., Rayadin, Y., Eds.; Indonesia Student Association in Hokkaido (PPI Hokkaido): Sapporo, Japan, 2010; pp. 1–7. [Google Scholar]
- Okore, O.O.; Ekedo, C.M.; Ibediugha, B.N.; Ozua, G.O. Pest status of Thyridopteryx ephemeraeformis (Lepidoptera: Psychidae) (Bagworms) on Delonix regia (Fabales: Fabaceae), an ornamental tree. Int. J. Agric. Earth Sci. 2019, 2, 43–51. [Google Scholar] [CrossRef]
- Pravitasari, N.R. Pengamatan ulat kantung (Lepidoptera: Psychidae) pada beberapa pertanaman jambu biji (Psidium guajava L.) di daerah Bogor. Skripsi. Bachelor’s Thesis, Fakultas Pertanian, Institut Pertanian Bogor, Bogor, Indonesia, 2009. [Google Scholar]
- Taufik, E. Serangan hama ulat kantong pada tanaman kemiri sunan. InfoTek Perkeb. 2017, 9, 3. [Google Scholar]
- Akbar, A. Identifikasi jenis-jenis hama dan penyakit pada meranti merah (Shorea leprosula Miq) di Pulau Laut, Kalimantan Selatan. In Proceedings of the Dukungan BPK Banjarbaru Dalam Pembangunan Kehutanan di Kalimantan, Prosiding Ekspose Hasil Penelitian Balai Penelitian Kehutanan Banjarbaru, Banjarbaru, Indonesia, 25–26 October 2011; Balai Penelitian Kehutanan Banjarbaru: Banjarbaru, Indonesia, 2011; pp. 213–223. [Google Scholar]
- Chung, G.F. Effect of pests and diseases on oil palm yield. In Palm Oil: Production, Processing, Characterization, and Uses; Lai, O.M., Tan, C.P., Akoh, C.C., Eds.; AOCS Press: Urbana, OH, USA, 2012; pp. 163–210. [Google Scholar]
- Appleton, M.R.; van Staden, J. Bagworms (Lepidoptera: Psychidae): Potential pest of guayule (Parthenium argentatum Gray) plantations in southern Africa. S. Afr. J. Plant Soil 1992, 9, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Frank, S.D. A survey of key arthropod pests on common southeastern street trees. Arboric Urban For. 2019, 45, 155–166. [Google Scholar] [CrossRef]
- Zulfiyah, A. Pest problems and threat in industrial plantation forests at PT Musi Hutan Persada, South Sumatra. In Proceedings of the Workshop Permasalahan dan Strategi Pengelolaan Hama di Areal Hutan Tanaman, Central Java, Indonesia, 17–19 June 1997; Suratmo, F.G., Hadi, S., Husaeni, E.A., Rahmatsjah, O., Kasno Nuhamara, S.T., Haneda, N.F., Eds.; Fakultas Kehutanan dan Departemen Kehutanan: West Java, Indonesia, 1998. [Google Scholar]
- Surachman, I.F.; Indriyanto, I.; Hariri, A.M. Inventarisasi hama persemaian di hutan tanaman rakyat desa Ngambur Kecamatan Bengkunat Belimbing Kabupaten Lampung Barat. J. Sylva Lestari 2004, 2, 7–16. [Google Scholar] [CrossRef]
- Budiman, I.; Bastoni, B.; Sari, E.N.; Hadi, E.E.; Asmalyah, A.; Siahaam, H.; Januar, R.; Hapsari, R.D. Progress of paludiculture projects in supporting peatland ecosystem restoration in Indonesia. Glob. Ecol. Conserv. 2020, 23, e01084. [Google Scholar] [CrossRef]
- Priatna, D.; Utami, S. Beberapa jenis hama yang menyerang bibit tanaman hutan di persemaian. In Proceedings of the Serangga Untuk Pertanian Berkelanjutan Dan Kesehatan Lebih Baik, Prosiding Seminar Nasional PEI Cabang Palembang, Palembang, Indonesia, 12–13 July 2018; Herlinda, S., Ed.; Perhimpunan Entomologi Indonesia: Bogor, Indonesia, 2018; pp. 203–211. [Google Scholar]
- Abi Oramahi, H.; Wulandari, S.R. Identifikasi morfologi serangga berpotensi sebagai hama dan tingkat kerusakan pada bibit meranti merah (Shorea leprosula). J. Hutan Lestari. 2017, 5, 644–652. [Google Scholar]
- Darwiati, W.; Anggraeni, I.; Intari, S.E. Serangan ulat kantong pada bibit meranti di persemaian. Info. Hutan. 2005, 2, 345–351. [Google Scholar]
- Utami, S.; Kurniawan, A. Potensi hama pada pola agroforestri kayu bawang di Provinsi Bengkulu. In Agroforestry Untuk Pangan dan Lingkungan yang Lebih Baik, Prosiding Seminar Nasional Agroforestri, Malang, Indonesia, 21 May 2013; Kuswantoro, D.P., Widyaningsih, T.S., Fauziyah, E., Rachmawati, R., Eds.; Kerjasama Balai Penelitian Teknologi Agroforestry, Fakultas Pertanian Universitas Brawijaya, World Agroforestry Centre (ICRAF), dan Masyarakat Agroforestri Indonesia: Malang, Indonesia, 2013; pp. 197–303. [Google Scholar]
- Nair, K.S.S. Pest Outbreak in Tropical Forest Plantations. Is There a Greater Risk for Exotic Tree Species; Center for International Forestry Research: Jakarta, Indonesia, 2001. [Google Scholar]
- Tiffani, N.A.; Ramdan, H.; Dungani, D. The characteristics of Acacia mangium stands at site 23B, RPH Maribaya, BKPH Parung Panjang, KPH Bogor, which is attacked by pest and diseases. IOP Conf. Ser. Earth Environ. Sci. 2020, 528, 12043. [Google Scholar] [CrossRef]
- Tripathy, M.K.; Parida, G.; Behera, M.C. Diversity of insect pests and their natural enemies infesting sal (Shorea robusta Garten f.) in Odisha. J. Entomol. Zool. Stud. 2020, 8, 1812–1822. [Google Scholar]
- Santhakumaran, L.N.; Remadevi, O.K.; Sivaramakrishnan, V.R. A new record of the insect defoliator Pteroma plagiophleps Hamp. (Lepidoptera: Psychidae) from mangrove along the Goa coast (India). Indian For. 1995, 12, 153–155. [Google Scholar]
- Basri, M.W. Life History, Ecology and Economic Impact of the Bagworm, Metisa plana Walker (Lepidoptera: Psychidae), on the Oil Palm, Elaeis guineensis Jacquin (Palmae), in Malaysia. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 1993; p. 231. [Google Scholar]
- Cheong, Y.L.; Sajap, A.S.; Noor, H.M.; Omar, D.; Abood, F. Demography of the bagworm Pteroma pendula Joannis on an exotic tree Acacia mangium wild in Malaysia. Malaysian For. 2010, 73, 77–85. [Google Scholar]
- Cheong, Y.L.; Sajap, A.S.; Hafidzi, M.N.; Omar, D.; Abood, F. Outbreaks of bagworm and their natural enemies in an oil palm, Elaeis guineensis, Plantation at Hutan Melintang Perak, Malaysia. J. Entomol. 2010, 7, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Hardi, T.W.; Siringoringo, H.H. Identifikasi hama mangrove dan pengendaliannya dengan bakteri. Bull. Penelit. Hutan 2000, 621, 55–64. [Google Scholar]
- Wahid, A. Efikasi bioinsektisida dan kombinasinya pada bibit mangrove Rhizophora spp. di persemaian. Agroland 2010, 17, 162–168. [Google Scholar]
- Ong, S.P.; Cheng, S.; Chong, V.C.; Tan, Y.S. Pest of Planted Mangroves in Peninsular Malaysia; Toh, A.N., Ed.; Forest Research Institute Malaysia: Selangor, Malaysia, 2010. [Google Scholar]
- Utami, S.; Kurniawan, A. Hama Penting pada Tegakan Hutan dan Letak Pengendaliannya di KHDTK Kemampo Sumetera Selatan. In Serangga untuk Pertanian Berkelanjutan dan Kesehatan Lebih Baik, Proceedings of the Seminar Nasional PEI Cabang Palembang, Palembang, Indonesia, 12–13 July 2018; Herlinda, S., Ed.; Perhimpunan Entomologi Indonesia: Bogor, Indonesia, 2018; pp. 404–414. [Google Scholar]
- Lee, C.Y. Urban Forest insect pests and their management in Malaysia. Formosan Entomol. 2014, 33, 207–2014. [Google Scholar]
- Utami, S.; Haneda, N.F. Bioaktivitas ekstrak umbi gadung dan minyak nyamplung sebagai pengendali hama ulat kantong (Pteroma plagiophleps Hampsin). J. Penelit. Hutan Tanam. 2012, 9, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Dewiyanti, I.; Yunita, Y. Identifikasi dan kelimpahan hama penyebab ketidakberhasilan rehabilitasi ekosistem mangrove. Ilmu Kelaut. 2013, 18, 150–156. [Google Scholar]
- Utami, S.; Lelana, N.E.; Kunarso, K.; Kurniawan, A.; Haneda, N.F. Pests of Sonneratia caseolaris seedlings in the mangrove restoration area nursery of Berbak-Sembilang National Park and its damage. In Proceedings of the 6th International Conference of Indonesia Forestry Researchers (INAFOR): Managing Forest and Natural Resources Meeting Sustainable and Friendly Use, Bogor, Indonesia, 7−8 September 2021; Earth and Environmental Science: Bristol, UK; Volume 914, p. 12019. [Google Scholar] [CrossRef]
- Anggraeni, I.; Ismanto, A. Keanekaragaman jenis ulat kantong yang menyerang di berbagai pertanaman sengon (Paraserianthes falcataria (L.) Nielson) di Pulau Jawa. J. Sains Nat. Univ. Nusa Bangsa 2013, 3, 184–192. [Google Scholar] [CrossRef]
- Haerumi, W.; Suryantini, R.; Herawatiningsih, R. Identifikasi dan tingkat kerusakan oleh serangga perusak pada bibit sengon (Falcataria moluccana) di persemaian permanen Balai Pengelolaan Daerah Aliran Sungai dan Hutan Lindung Kapuas Pontianak. J. Hutan Lestari 2019, 7, 349–362. [Google Scholar]
- Manya, M. Inventarisasi serangan hama anakan meranti merah (Shorea selanica) di lokasi CIMTROP Universitas Palangka Raya, Kalimantan Tengah. Agrisilvika 2017, 1, 6–13. [Google Scholar]
- Zeni, S.A.; Rachmawati, N.; Fitriani, A. Frekuensi dan intensitas serangan hama penyakit pada bibit mersawa (Anisoptera marginata Korth) di persemaian BP2LHK Banjarbaru Kalimantan Selatan. J. Sylva Sci. 2021, 4, 339–345. [Google Scholar] [CrossRef]
- Rahayu, S.; Subyanto, S.; Kuswanto, K. The occurrence of pest and disease of Shorea spp.: A preliminary study in Wanariset and Bukit Soeharto Forest Area in East Kalimantan, Indonesia. In Proceedings of the Seminar on Ecological Approach for Productivity and Sustainability of Dipterocarp Forests, Yogyakarta, Indonesia, 7–8 July 1998; Sabarnudin, H.M.S., Suhardi, S., Okimori, Y., Eds.; Gajah Mada University and Kansai Environmental Engineering Center: Yogyakarta, Indonesia, 1998; pp. 53–57. [Google Scholar]
- Wood, B.; Corley, R.; Goh, K. Studies on the effect of pest damage on oil palm yield. In Advance in Oil Palm Cultivation; Wastie, R.L., Earp, D.A., Eds.; The Incorporated Society of Planters: Kuala Lumpur, Malaysia, 1973; pp. 360–379. [Google Scholar]
- Basri, M.W.; Kevan, P.G. Life History and feeding behavior of the oil palm bagworm Metisa plana Walker (Lepidoptera: Psychidae). Elaeis 1995, 7, 18–34. [Google Scholar]
- Kamarudin, N.; Wahid, M.B. Interactions of the bag worm, Pteroma pendula (Lepidoptera: Psychidae) and its natural enemies in an oil palm plantation in perak. J. Oil Palm Res. 2010, 22, 758–764. [Google Scholar]
- Cheong, Y.L.; Tey, C.C. Enviromental Factors which Influence Bagworm Outbreak. In Proceedings of the 5th MPOB dan IOPRI International Seminar: Sustainable Management of Pests and Disease in Oil Palm—The Way Forward, Kuala Lumpur, Malaysia, 22–23 November 2013; Malaysian Palm Oil Board: Selangor, Malaysia, 2013. [Google Scholar]
- Wood, B.J.; Kamarudin, N. A review of developments in integrated pest management (IPM) of bagworm (Lepidoptera: Psychidae) infestation in oil palms in Malaysia. J. Oil Palm Res. 2019, 31, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.A.; Walter, A.D.; Tooker, J.; Ginzel, M.; Reagel, P.F.; Lacey, E.S.; Bennett, A.; Grossman, E.M.; Hanks, L. Conservation biological control in urban landscapes: Manipulating parasitoids of bagworm (Lepidoptera: Psychidae) with flowering forbs. Biol. Control 2005, 34, 99–107. [Google Scholar] [CrossRef]
- Rhainds, M.; Gries, G.; Ho, C.T.; Chew, P.S. Dispersal by bagworm larvae, Metisa plana: Effects of population density, larval sex, and host plant attributes. Ecol. Entomol. 2002, 27, 204–212. [Google Scholar] [CrossRef]
- Denno, R.F.; Peterson, M.A. Density-dependent dispersal and its consequences for population dynamics. In Population Dynamics: New Approaches and Synthesis; Cappuccino, N., Price, P.W., Eds.; Academic Press: San Diego, CA, USA, 1995; p. 113130. [Google Scholar]
- Badrulisham, A.S.; Kageyama, D.; Halim, M.; Aman-Zuki, A.; Masri, M.M.; Ahmad, S.N.; Md-Zain, B.M.; Yaakop, S. New insights into the phylogeography of the oil palm pest, Metisa plana towards its management control. J. Oil Palm Res. 2021. [Google Scholar] [CrossRef]
- Skendžic, S.; Zovko, M.; Živkovic, I.P.; Lešic, V.; Lemic, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Barbosa, P.; Waldvogel, M.G.; Breisch, N.L. Temperature modification by bags of the bagworm Thyridopteryx ephemeraeformis (Haworth) (Lepidoptera: Psychidae). Can. Entomol. 1983, 115, 855–858. [Google Scholar] [CrossRef]
- Smith, M.P.; Barrows, E.D. Effects of larval case size and host plant species on case internal temperature in the bagworm, Thyridopteryx ephemeraeformis (Haworth) (Lepidoptera: Psychidae). Proc. Entomol. Soc. Wash. 1991, 93, 834–838. [Google Scholar]
- Ibrahim, Y.; Tuck, H.C.; Chong, K.K. Effects of temperature on the development and survival of the bagworms Pteroma pendula and Metisa plana (Lepidoptera: Psychidae). J. Oil Palm Res 2013, 25, 1–8. [Google Scholar]
- Ho, C.T. Ecological Studies of Pteroma Pendula Joannis and Metisa Plana Walker (Lepidoptera: Psycidae) towards Improved Integrated Management of Infestation Oil Palm. Ph.D. Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2002. [Google Scholar]
- Kamarudin, N.; Arshad, O. Diversity and activity of insect natural enemies of the bagworm (Lepidoptera: Psychidae) within an oil palm plantation in perak, Malaysia. J. Oil Palm 2016, 28, 296–307. [Google Scholar] [CrossRef]
- Sanudin, S.; Fauziah, E. Characteristic of private forest based on its management orientation: Case study in Sukamaju Village, Ciamis District and Kiarajangkung Village, Tasikmalaya District, West Java. Pros. Sem. Nas. Masy. Biodiv. Indon. 2015, 1, 696–701. [Google Scholar]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Staab, M.; Schuldt, A. The influence of tree diversity on natural enemies-a Review of the “enemies” hypothesis in Forests. Curr. For. Rep. 2020, 6, 243–259. [Google Scholar] [CrossRef]
- Fernando, I.V.S.; Jayakody, D.S. Some Aspects of the biology of Pteroma plagiophleps Hampson (Lepidoptera: Psychidae) a defoliator of Delonix regia (Boj) (Leguminosae: Caesalpiniaceae). In Proceedings of the 47th Annual Sessions of Sri Langka Association for the Advancement of Science, 1991; Sri Lanka Association for the Advancement of Science: Colombo, Sri Lanka, 1991; p. 92. [Google Scholar]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Björkman, C. Mixed forests to mitigate risk of insect outbreaks. Scand. J. For. Res. 2018, 33, 772–780. [Google Scholar] [CrossRef]
- Schuler, L.J.; Bugmann, H.; Snell, R.S. From monocultures to mixed-species forests: Is tree diversity key for providing ecosystem services at the landscape scale? Landsc. Ecol. 2016, 32, 1499–1516. [Google Scholar] [CrossRef]
- Jactel, H.; Brockerhoff, E.G. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007, 10, 835–848. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Jactel, H.; Vacher, C.; Brockerhoff, E.G.; Koricheva, J. Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J. Appl. Ecol. 2014, 51, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Guyot, V.; Castagneyrol, B.; Vialatte, A.; Deconchat, M.; Jactel, H. Tree diversity reduces pest damage in mature forests across Europe. Biol. Lett. 2016, 12, 20151037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaitaniemi, P.; Riihimäki, J.; Koricheva, J.; Vehviläinen, H. Experimental evidence for associational resistance against the European pine sawfly in mixed tree stands. Silva Fennica 2007, 41, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, W.; Kharouba, H.; Robinson, M.; Holyoak, M.; Karban, R. Variability in plant nutrients reduces insect herbivore performance. Nature 2016, 539, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Root, R.B. Organization of a plant-arthropod association in simple and diverse habitats: The fauna of Collards (Brassica Oleracea). Ecol. Monogr. 1972, 43, 95–120. [Google Scholar] [CrossRef]
- Russel, E.P. Enemies Hypothesis: A review of the effect of vegetational diversity on predatory insects and parasitoids. Environ. Entomol. 1989, 18, 590–599. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, M.E.; Petersen, M.J. Extending the ‘resource concentration hypothesis’ to the landscape-scale by considering dispersal mortality and fitness costs. Agric. Ecosyst. Environ. 2017, 249, 1–3. [Google Scholar] [CrossRef]
- Stiling, P.; Rossi, A.M.; Cattell, M.V. Associational resistance mediated by natural enemies. Ecol. Entomol. 2003, 28, 587–593. [Google Scholar] [CrossRef]
- Jactel, H.; Bauhus, J.; Boberg, J.; Bonal, D.; Castagneyrol, B.; Gardiner, B.; Gonzalez-Olabarria, J.R.; Koricheva, J.; Meurisse, N.; Brockerhoff, E.G. Tree diversity drives forest stand resistance to natural disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Pamuji, R.; Rahardjo, B.T.; Tarno, H. Populasi dan serangan hama ulat kantung Metisa plana Walker (Lepidoptera: Psychidae) serta parasitoidnya di perkebunan kelapa sawit Kabupaten Donggala, Sulawesi Tengah. J. HPT 2013, 1, 58–71. [Google Scholar]
- Fuat, S.; Adam, N.A.; Hazmi, I.R.; Yaakop, S. Interactions between Metisa plana, its hyperparasitoids and primary parasitoids from good agriculture practices (GAP) and non-gap oil palm plantations. Community Ecol. 2022. [Google Scholar] [CrossRef]
- Badrulisham, A.S.; Bakar, M.A.L.A.; Md-Zain, B.M.; Md-Nor, S.; Abd Rahman, M.R.; Mohd-Yusof, N.S.; Halim, M.; Yaakop, S. Metabarcoding of parasitic wasp, Dolichogenidea metesae (Nixon) (Hymenoptera: Braconidae) that parasitizing bagworm, Metisa plana Walker (Lepidoptera: Psychidae). Trop. Life Sci. Res. 2022, 33, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, D.S.I. Seleksi Beberapa Tanaman Inang Parasitoid dan Predator untuk Pengendalian Hayati Ulat Kantong (Metisa plana) di Perkebunan Kelapa Sawit. Master Thesis, Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Sumatera Utara, Medan, Indonesia, 2010. [Google Scholar]
- Falahudin, I. Peranan semut rangrang (Oecophylla smaragdina) dalam pengendalian biologis pada perkebunan kelapa sawit. In Proceedings of the Annual International Conference on Islamic Studies (AICIS XII), Surabaya, Indonesia, 5–8 November 2012; Nashir, A., Ed.; Annual International Conference on Islamic Studies: Surabaya, Indonesia, 2012. [Google Scholar]
- Mauludi, A.S. Dinamika Pengelolaan Huran Rakyat dan Strategi Pengembangannya di Kabupaten Bogor. Master Thesis, Sekolah Pascasarjana, Institut Pertanian Bogor, Bogor, Indonesia, 2014. [Google Scholar]
- Badan Pusat Statistik. Hasil pencacahan lengkap sensus pertanian 2013 dan survei pendapatan rumah tangga usaha pertanian 2013. Ber. Resmi Stat. 2014, 54, 7. [Google Scholar]
- Istriningsih, I.; Dewi, Y.A.; Yulianti, A.; Hanifah, V.W.; Jamal, E.; Dadang, D.; Sarwani, M.; Mardiharini, M.; Anugrah, I.S.; Darwis, V.; et al. Farmers’ knowledge and practice regarding good agricultural practices (GAP) on safe pesticide usage in Indonesia. Heliyon 2022, 8, e08708. [Google Scholar] [CrossRef] [PubMed]
- Joko, T.; Dewanti, N.A.Y.; Dangiran, H.L. Pesticide poisoning and the use of personal protective equipment (PPE) in Indonesian farmers. J. Environ. Publ. Health. 2020, 2020, 5379619. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.P.; Macfadyen, S.; Nash, M.A. Broad spectrum pesticide application alters natural enemy communities and may facilitate secondary pest outbreaks. PeerJ 2017, 5, e4179. [Google Scholar] [CrossRef] [Green Version]
- Allahyari, M.S.; Damalas, C.A.; Ebadattalab, M. Farmers’ technical knowledge about integrated pest management (IPM) in Olive production. Agriculture 2017, 7, 101. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.B. Ecological approaches and the development of ‘truly integrated’ pest management. Proc. Natl. Acad. Sci. USA 1999, 96, 5944–5951. [Google Scholar] [CrossRef] [Green Version]
- Haneda, N.F.; Hero, Y.; Rustanzi, D.F. The relatedness of community perception with the actions in forest pest management. J. Sylva Indones. 2021, 4, 24–35. [Google Scholar] [CrossRef]
- Abtew, A.; Niassy, S.; Affognon, H.; Subramanian, S.; Kreiter, S.; Garzia, G.T.; Martin, T. Farmers’ knowledge and perception of grain legume pests and their management in the Eastern province of Kenya. Crop. Prot. 2016, 87, 90–97. [Google Scholar] [CrossRef]
- Bala, K.; Sood, A.K.; Pathania, V.S.; Thakur, S. Effect of plant nutrition in insect pest management: A review. J. Pharmacogn. Phytochem. 2018, 7, 2737–2742. [Google Scholar]
- Shen, J.P.; Zhang, L.M.; Guo, J.F.; Ray, J.L.; He, J.Z. Impact of long-term fertilization practices on the abundance and composition of soil bacterial communities in Northeast China. App. Soil Ecol. 2010, 46, 119–124. [Google Scholar] [CrossRef]
- Razaq, M.; Zhang, P.; Shen, H.-I.; Salahuddin, S. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiaxin, K.; Zhaochen, Z.; Jian, Z. Classification and identification of plant species based on multi-source remote sensing data: Research progress and prospect. Biodivers. Sci. 2019, 7, 796–812. [Google Scholar] [CrossRef]
- El Sheikha, A.F. Tracing insect pests: Is there new potential in molecular techniques? Insect Mol. Biol. 2019, 28, 759–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rullan-Silva, C.D.; Olthoff, A.E.; de la Mata, J.A.D.; Pajares-Alonso, J.A. Remote monitoring of forest insect defoliation. A review. For. Syst. 2013, 22, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Carrillo, A.; Patočka, Z.; Dobrovolný, L.; Franco-Nieto, A.; Revilla-Romero, B. Monitoring Bark Beetle Forest Damage in Central Europe. A Remote Sensing Approach Validated with Field Data. Remote Sens. 2020, 12, 3634. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lelana, N.E.; Utami, S.; Darmawan, U.W.; Nuroniah, H.S.; Darwo; Asmaliyah; Haneda, N.F.; Arinana; Darwiati, W.; Anggraeni, I. Bagworms in Indonesian Plantation Forests: Species Composition, Pest Status, and Factors That Contribute to Outbreaks. Diversity 2022, 14, 471. https://doi.org/10.3390/d14060471
Lelana NE, Utami S, Darmawan UW, Nuroniah HS, Darwo, Asmaliyah, Haneda NF, Arinana, Darwiati W, Anggraeni I. Bagworms in Indonesian Plantation Forests: Species Composition, Pest Status, and Factors That Contribute to Outbreaks. Diversity. 2022; 14(6):471. https://doi.org/10.3390/d14060471
Chicago/Turabian StyleLelana, Neo Endra, Sri Utami, Ujang Wawan Darmawan, Hani Sitti Nuroniah, Darwo, Asmaliyah, Noor Farikhah Haneda, Arinana, Wida Darwiati, and Illa Anggraeni. 2022. "Bagworms in Indonesian Plantation Forests: Species Composition, Pest Status, and Factors That Contribute to Outbreaks" Diversity 14, no. 6: 471. https://doi.org/10.3390/d14060471
APA StyleLelana, N. E., Utami, S., Darmawan, U. W., Nuroniah, H. S., Darwo, Asmaliyah, Haneda, N. F., Arinana, Darwiati, W., & Anggraeni, I. (2022). Bagworms in Indonesian Plantation Forests: Species Composition, Pest Status, and Factors That Contribute to Outbreaks. Diversity, 14(6), 471. https://doi.org/10.3390/d14060471






