Molecular and Morphological Analyses Support Different Taxonomic Units for Asian and Australo-Pacific Forms of Ischnura aurora (Odonata, Coenagrionidae)
Abstract
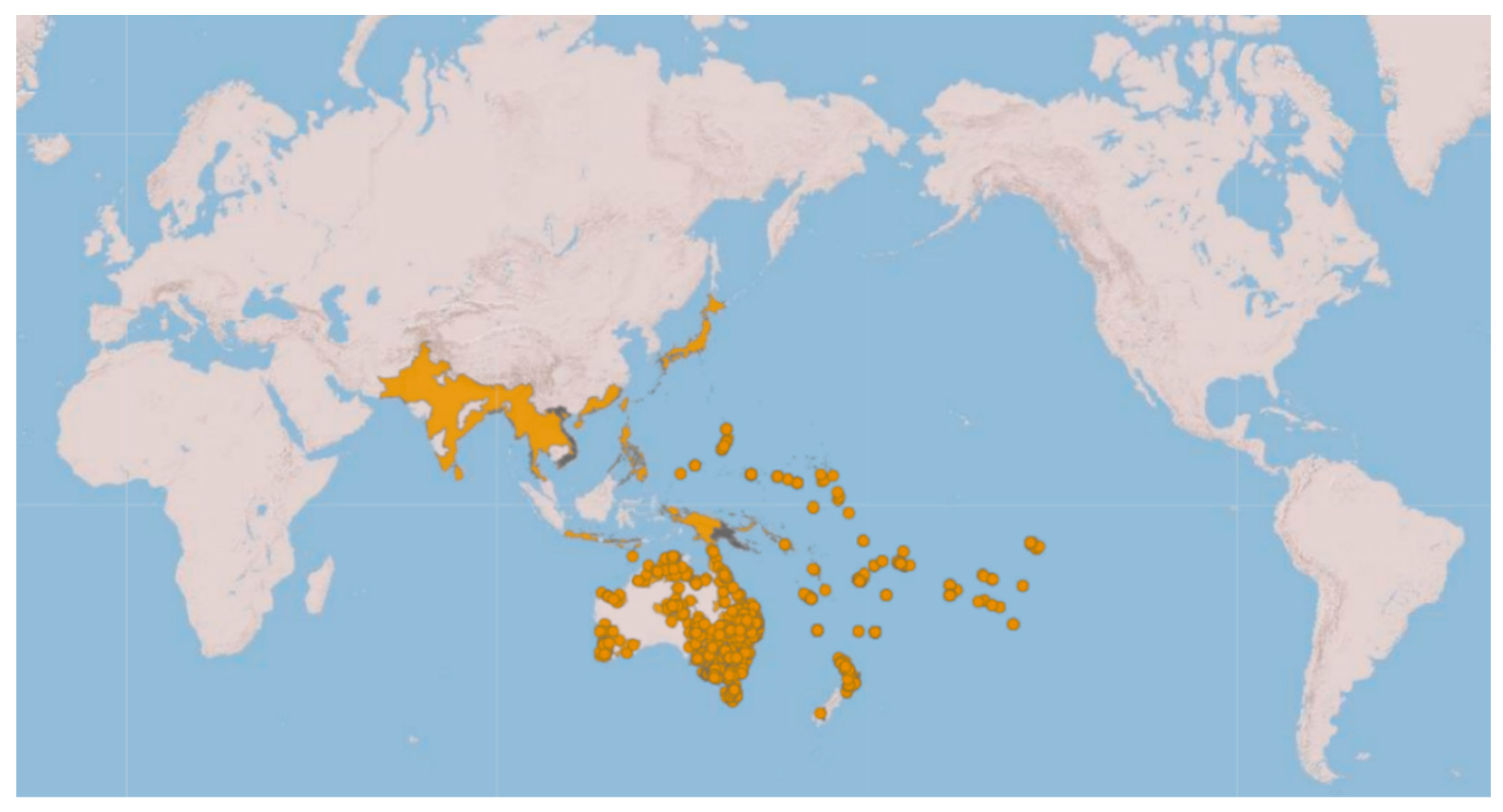
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection, DNA Extraction and Sequencing
2.2. Genetic Analyses
2.3. Morphological Analyses
3. Results
3.1. Genetic Analyses
| Taxa | Data Source | GenBank No. | Marker | Reason for Exclusion |
|---|---|---|---|---|
| Ischnura aurora | Nolan et al. [44] | EU219876 | COI | Sequence corresponds to the 3′ end of the COI gene |
| I. aurora | Nolan et al [44] | EU219877 | COI | Sequence corresponds to the 3′ end of the COI gene |
| I. aurora | Mehmood et al. (unpublished) | LC198680 | COI | Sequence corresponds to the 3′ end of the COI gene |
| I. aurora | Ramage et al. [45] | KX053527 | COI | Ambiguity-coded bases in sequence |
| I. aurora | Ramage et al. [45] | KX053531 | COI | Ambiguity-coded bases in sequence |
| I. aurora | Dumont et al. [46] | FN356100 | ITS | Specimen misidentification and/or misplacement? |
| Ischnura delicata | Ashfaq et al. (unpublished) | KY832433 | COI | Ambiguity-coded bases in sequence |
| I. delicata | Ashfaq et al. (unpublished) | KY838304 | COI | Ambiguity-coded bases (insertion of 3 “Ns”) in sequence |
| I. delicata | Ashfaq et al. (unpublished) | KY844428 | COI | Ambiguity-coded bases in sequence |
| Ischnura rubilio | Pavithran et al. (unpublished) | MW143324 | COI | Both sequences are identical. No stop codons, but sequences are odd compared to references. Similar to several marine sponge genera. “COI-like/COI-numt” sequence? |
| I. rubilio | Dumont [23] | MH449981 | COI | |
| I. rubilio | Dumont [23] | MH449992 | COI | Specimen misidentification and/or misplacement? |
| I. rubilio | Dumont [23] | MH447434 | ITS | Specimen misidentification and/or misplacement? |
| I. rubilio | This study | OM964923 | COI | Ambiguity codes in sequence. Similar to Nesobasis spp. Annotated as “COI-like/COI-numt” sequences |
| I. rubilio | This study | OM964924 | COI | |
| I. rubilio | This study | OM964925 | COI | |
| I. rubilio | This study | OM964926 | COI |
3.2. Morphological Analyses
4. Discussion
5. Conclusions
- Genetic analyses have showed that specimens currently under the names of Ischnura rubilio and I. delicata belong to a clade that also includes the I. aurora found within the Asian distribution area of this species.
- All the I. aurora found within the Australo-Pacific distribution area cluster together in a separate clade. Species delimitation analyses have identified these two clades as different taxonomic units.
- Concordant with the results of the genetic analyses, the morphology of the I. aurora collected in China is closer to I. rubilio than to I. aurora from Australia and Fiji.
- Given these results, we confirm the status of I. rubilio as a valid species and provide an identification key for its separation from I. aurora.
- Genetic analyses point also to the existence of at least a third taxonomic unit within the aurora clade, which stress the need to revise all available material belonging to the numerous subspecies of I. aurora that have been described.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Quality Control (QC) and Curation of GenBank Sequence Data
Appendix A.1. ITS Dataset

Appendix A.2. COI Dataset





Appendix B
| Species | Clade | Collection Locality Data | GenBank Acc. Nos | |
|---|---|---|---|---|
| COI | ITS | |||
| Aciagrion migratum | - | Yamashiro, Kyoto, Japan | AB708460 | AB706565 |
| Aciagrion pallidum | - | Thailand | MH881302 | FN356029 |
| Ceriagrion auranticum | - | Ibusuki, Kagoshima, Japan | AB708472 | AB706577 |
| Ceriagrion cerinorubellum | - | Malaysia | LC366789 | LC366195 |
| Ceriagrion melanurum | - | Kugunu, Gifu, Japan | AB708477 | AB706582 |
| Ceriagrion nipponicum | - | Suita, Osaka, Japan | AB708479 | AB706584 |
| Coenagrion ecornutum | - | Onbetsu, Hokkaido, Japan | AB708481 | AB706586 |
| Coenagrion hylas | - | Otofuke, Hokkaido, Japan | AB708483 | AB706588 |
| Coenagrion lanceolatum | - | Matsumoto, Nagano, Japan | AB708485 | AB706590 |
| C. lanceolatum | - | Abashiri, Hokkaido, Japan | AB708489 | AB706594 |
| Coenagrion terue | - | Murakami, Niigata, Japan | AB708490 | AB706595 |
| Enallagma circulatum | - | Tobetsu, Hokkaido, Japan | AB708491 | AB706596 |
| Enallagma cyathigerum | - | Bastemosen, Bornholm, Germany | MN934768 | MN963489 |
| E. cyathigerum | - | Slesvig-Holstein, Germany | MN934790 | MN963513 |
| Erythromma humerale | - | Ikeda, Hokkaido, Japan | AB708496 | AB706601 |
| Ischnura abyssinica | - | Ambo, Ethiopia | MH450002 | MH447433 |
| Ischnura asiatica | - | Fuchu, Toyama, Japan | AB708497 | AB706602 |
| I. asiatica | - | Tsukuba, Ibaraki, Japan | LC366722 | LC366128 |
| Ischnura elegans | - | Ikeda, Hokkaido, Japan | AB708504 | AB706609 |
| Ischnura evansi | - | Sarbaz Gorge, Iran | MH450005 | MH447425 |
| Ischnura ezoin | - | Anijima, Ogasawara, Tokyo, Japan | AB708467 | AB706572 |
| I. ezoin | - | Otoutojima, Ogasawara, Tokyo, Japan | AB708468 | AB706573 |
| I. ezoin | - | Mukojima, Ogasawara, Tokyo, Japan | LC366905 | LC366311 |
| I. ezoin | - | Mujkojima, Ogasawara, Tokyo, Japan | LC366906 | LC366312 |
| Ischnura heterosticta | - | Fiji | AB708507 | MH447432 |
| Ischnura nursei | - | Jaipur, India | MH449984 | MH447413 |
| Ischnura praematura | - | Yunnan, China | MZ514810 | MZ514811 |
| I. praematura | - | Yunnan, China | MZ514812 | MZ514813 |
| I. praematura | - | Yunnan, China | MZ514814 | MZ514815 |
| I. praematura | - | Yunnan, China | MZ514816 | MZ514817 |
| Ischnura pumilio | - | n.a. | MK818664 | FN356107 |
| I. pumilio | - | n.a. | MN939053 | KC430228 |
| I. pumilio | - | n.a. | MT680681 | KC430231 |
| I. pumilio | - | n.a. | NC021617 | MH447407 |
| Ischnura rufostigma | - | Thailand | AB708508 | AB706613 |
| Ischnura saharensis | - | n.a. | MK818648 | MH447432 |
| Ischnura senegalensis | - | Yonaguni, Okinawa, Japan | AB708511 | AB706616 |
| Ischnura sp. | - | Yunnan, China | AB708512 | AB706617 |
| Ischnura sp. | - | Yunnan, China | AB708513 | AB706618 |
| Mortonagrion hirosei | - | Ishinomaki, Miyagi, Japan | AB708515 | AB706620 |
| M. selenion | - | Yamada, Toyama, Japan | AB708517 | AB706622 |
| Pseudagrion microcephalum | - | Yonaguni, Okinawa, Japan | AB708538 | AB706643 |
| Ischnura aurora | Australo-Pacific | New South Wales, Australia | KF369414 | n.a. |
| Ischnura aurora | Australo-Pacific | Queensland, Australia | JF839452 | n.a. |
| I. aurora | Australo-Pacific | Adelaide, Australia | MH449987 | MH447412 |
| I. aurora | Australo-Pacific | Perth, Australia | MH449988 | MH447411 |
| I. aurora | Australo-Pacific | Australia | MT680686 | n.a. |
| I. aurora | Australo-Pacific | Japan | MT680687 | n.a. |
| I. aurora | Australo-Pacific | American Samoa | MK818649 | n.a. |
| I. aurora | Australo-Pacific | American Samoa | MT680690 | n.a. |
| I. aurora | Australo-Pacific | Tonga | MT680689 | n.a. |
| I. aurora | Australo-Pacific | French Polynesia | MT680688 | n.a. |
| I. aurora | Australo-Pacific | French Polynesia | MT680691 | n.a. |
| I. aurora | Australo-Pacific | Fiji | AB708502 | AB706607 |
| I. aurora | Australo-Pacific | Fiji | AB708503 | AB706608 |
| I. aurora | Australo-Pacific | Maroe Bay, Huahine island, French Polynesia | KX053530 | n.a. |
| I. aurora | Australo-Pacific | Afareaitu, Moorea island, French Polynesia | KX053529 | n.a. |
| I. aurora | Australo-Pacific | Paopao river, Moorea island, French Polynesia | KX053532 | n.a. |
| I. aurora | Australo-Pacific | Paopao river, Moorea island, French Polynesia | KX053524 | n.a. |
| I. aurora | Australo-Pacific | Pihaena, Moorea island, French Polynesia | KX053528 | n.a. |
| I. aurora | Australo-Pacific | Mount Mauru, Tahiti island, French Polynesia | KX053526 | n.a. |
| I. aurora | Australo-Pacific | Mount Mauru, Tahiti island, French Polynesia | KX053525 | n.a. |
| I. aurora | Australo-Pacific | Wallis and Futuna | n.a. | MH447410 |
| I. aurora | Australo-Pacific | Guam | AB708500 | AB706605 |
| I. aurora | Australo-Pacific | Guam | AB708501 | AB706606 |
| I. aurora | Australo-Pacific | Iojima, Ogasawara, Tokyo, Japan | AB708498 | AB706603 |
| I. aurora | Australo-Pacific | Iojima, Ogasawara, Tokyo, Japan | AB708499 | AB706604 |
| I. aurora | Australo-Pacific | Baliem valley New Guinea | MH449995 | n.a. |
| I. aurora | Australo-Pacific | Malappuram, Kerala, India | KR149808 | n.a. |
| I. aurora | Asian | Ugani Sahib, Rajpura, Patial (Punjab), India | MG517558 | n.a. |
| I. aurora | Asian | Nakhon Sawan, Thailand | MH450006 | MH447414 |
| I. aurora | Asian | Thailand | KT957479 | n.a. |
| I. aurora | Asian | Thailand | KT957480 | n.a. |
| I. aurora | Asian | Thailand | KT957481 | n.a. |
| I. aurora | Asian | Thailand | KT957482 | n.a. |
| I. aurora | Asian | Thailand | KT957483 | n.a. |
| I. aurora | Asian | Thailand | KT957484 | n.a. |
| I. aurora | Asian | Thailand | KT957485 | n.a. |
| I. aurora | Asian | Thailand | KT957486 | n.a. |
| I. aurora | Asian | Thailand | KT957487 | n.a. |
| I. aurora | Asian | Thailand | KT957488 | n.a. |
| I. aurora | Asian | Thailand | KT957489 | n.a. |
| I. aurora | Asian | Thailand | KT957490 | n.a. |
| I. aurora | Asian | Thailand | KT957491 | n.a. |
| I. aurora | Asian | Thailand | KT957492 | n.a. |
| I. aurora | Asian | Thailand | KT957493 | n.a. |
| Ischnura delicata | Asian | Islamabad, Pakistan | KY843451 | n.a. |
| Ischnura rubilio | Asian | India | MN850442 | n.a. |
| I. aurora | 3rd taxonomic unit in ASAP | Baliem Valley New Guinea | MH449994 | n.a. |
| I. aurora | Asian? 4th taxonomic unit in ASAP | India | MT511656 | n.a. |
| Voucher ID | Sex | Collection Date | Collection Locality |
|---|---|---|---|
| ACR-00738 | male | 01/12/2013 | Long Swamp; Nelson, Victoria, Australia. ACR leg and det. |
| ACR-00776 | female | 02/12/2013 | Ming Ming Swamp, Grampians National Park, Victoria, Australia. ACR leg and det. |
| ACR-00793 | female | 04/12/2013 | Ming Ming Swamp, Grampians National Park, Victoria, Australia. ACR leg and det. |
| ACR-00794 | female | 04/12/2013 | Ming Ming Swamp, Grampians National Park, Victoria, Australia. ACR leg and det. |
| ACR-00795 | female | 04/12/2013 | Ming Ming Swamp, Grampians National Park, Victoria, Australia. ACR leg and det. |
| ACR-00818 | male | 09/12/2013 | pond at Bandiana, Wodonga, Victoria, Australia. ACR leg and det. |
| ACR-00819 | male | 09/12/2013 | pond at Bandiana, Wodonga, Victoria, Australia. ACR leg and det. |
| ACR-02338 | male | 08/05/2015 | River at Si Fang Jing, Yunnan, China. ISV leg and det. |
| ACR-02379 | male | 13/05/2015 | River at Xi Meng, Yunnan, China. ISV leg and det. |
| ACR-02880 | male | 19/06/2015 | Meng Ding, Yunnan China. ISV leg and det. |
| ACR-02956 | male | 26/06/2015 | Na Bang, Yunnan, China. ISV leg and det. |
| ACR-02957 | female | 26/06/2015 | Na Bang, Yunnan, China. ISV leg and det. |
| ACR-03503 | male | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03504 | male | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03505 | male | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03506 | male | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03507 | female | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03508 | female | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03509 | female | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03510 | female | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03511 | female | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03513 | female | 02/07/2015 | Rice field at Huaping. Yunnan, China. ISV leg and det. |
| ACR-03888 | male | 10/06/2016 | Pond in agricultural area. Mengding, Yunnan, China. ACR leg and det. |
| ACR-03917 | female | 11/06/2016 | Pond in agricultural area. Mengding, Yunnan, China. HZ leg and det. |
| ACR-03998 | female | 19/06/2016 | River at Meng Lun, Yunnan, China. ACR leg and det. |
| ACR-04067 | male | 24/06/2016 | Stream at Meng Lung, Yunnan, China. ACR leg and det. |
| ACR-05007 | male | 06/06/2018 | Somosomo damm, Chakaudrove, Taveuni, Fiji. |
| ACR-05008 | female | 06/06/2018 | Somosomo damm, Chakaudrove, Taveuni, Fiji. |
| ACR-05009 | male | 06/06/2018 | Somosomo damm, Chakaudrove, Taveuni, Fiji. |
| ACR-05010 | male | 06/06/2018 | Somosomo damm, Chakaudrove, Taveuni, Fiji. |
| ACR-05091 | female | 11/06/2018 | Korovuli, Seqaqa, Labasa, Vanua Levu, Fiji. |
| Australo-Pacific aurora | Asian aurora | I. delicata | I. rubilio | I. aurora MH449994 | I. aurora MT511656 | I. abyssinica | I. asiatica | I. elegans | I. evansi | I. ezoin | I. heterosticta | I. nursei | I. praematura | I. pumilio | I. rufostigma | I. saharensis | I. senegalensis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Australo-Pacific aurora | 0.020 | n.a. | 0.017 | n.a. | n.a. | 0.081 | 0.094 | 0.088 | 0.101 | 0.082 | 0.095 | 0.116 | 0.069 | 0.083 | 0.087 | 0.091 | 0.109 | |
| Asian aurora | 0.031 | n.a. | 0.001 | n.a. | n.a. | 0.084 | 0.094 | 0.090 | 0.104 | 0.079 | 0.094 | 0.110 | 0.070 | 0.081 | 0.081 | 0.097 | 0.106 | |
| I. delicata | 0.031 | 0.000 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| I. rubilio | 0.031 | 0.000 | 0.000 | n.a. | n.a. | 0.041 | 0.052 | 0.047 | 0.052 | 0.047 | 0.052 | 0.064 | 0.050 | 0.052 | 0.041 | 0.047 | 0.058 | |
| I. aurora MH449994 | 0.024 | 0.027 | 0.027 | 0.027 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
| I. aurora MT511656 | 0.022 | 0.013 | 0.013 | 0.013 | 0.022 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
| I. abyssinica | 0.135 | 0.126 | 0.126 | 0.126 | 0.131 | 0.131 | 0.079 | 0.036 | 0.045 | 0.062 | 0.057 | 0.074 | 0.054 | 0.069 | 0.042 | 0.042 | 0.066 | |
| I. asiatica | 0.132 | 0.128 | 0.127 | 0.127 | 0.132 | 0.123 | 0.150 | 0.079 | 0.094 | 0.044 | 0.084 | 0.103 | 0.042 | 0.040 | 0.075 | 0.083 | 0.101 | |
| I. elegans | 0.138 | 0.129 | 0.129 | 0.129 | 0.133 | 0.124 | 0.064 | 0.145 | 0.036 | 0.066 | 0.043 | 0.068 | 0.058 | 0.065 | 0.053 | 0.014 | 0.055 | |
| I. evansi | 0.131 | 0.126 | 0.126 | 0.126 | 0.131 | 0.124 | 0.058 | 0.141 | 0.058 | 0.079 | 0.034 | 0.073 | 0.065 | 0.080 | 0.051 | 0.038 | 0.057 | |
| I. ezoin | 0.130 | 0.125 | 0.125 | 0.125 | 0.136 | 0.116 | 0.149 | 0.089 | 0.147 | 0.145 | 0.077 | 0.086 | 0.026 | 0.033 | 0.064 | 0.076 | 0.090 | |
| I. heterosticta | 0.129 | 0.120 | 0.120 | 0.120 | 0.122 | 0.120 | 0.058 | 0.145 | 0.069 | 0.064 | 0.132 | 0.074 | 0.068 | 0.079 | 0.060 | 0.045 | 0.040 | |
| I. nursei | 0.134 | 0.130 | 0.130 | 0.130 | 0.132 | 0.128 | 0.087 | 0.158 | 0.078 | 0.070 | 0.159 | 0.083 | 0.080 | 0.092 | 0.076 | 0.075 | 0.083 | |
| I. praematura | 0.148 | 0.144 | 0.144 | 0.144 | 0.144 | 0.144 | 0.140 | 0.112 | 0.156 | 0.142 | 0.118 | 0.144 | 0.153 | 0.030 | 0.055 | 0.064 | 0.083 | |
| I. pumilio | 0.150 | 0.154 | 0.154 | 0.154 | 0.161 | 0.154 | 0.160 | 0.125 | 0.147 | 0.148 | 0.115 | 0.154 | 0.148 | 0.129 | 0.070 | 0.075 | 0.092 | |
| I. rufostigma | 0.122 | 0.113 | 0.113 | 0.113 | 0.118 | 0.109 | 0.078 | 0.127 | 0.069 | 0.071 | 0.136 | 0.078 | 0.090 | 0.138 | 0.154 | 0.056 | 0.073 | |
| I. saharensis | 0.134 | 0.137 | 0.137 | 0.137 | 0.137 | 0.137 | 0.069 | 0.154 | 0.035 | 0.062 | 0.155 | 0.080 | 0.094 | 0.149 | 0.147 | 0.075 | 0.061 | |
| I. senegalensis | 0.131 | 0.133 | 0.133 | 0.133 | 0.129 | 0.133 | 0.069 | 0.145 | 0.084 | 0.078 | 0.154 | 0.051 | 0.096 | 0.136 | 0.174 | 0.082 | 0.086 |

References
- Wheeler, Q. Taxonomic triage and the poverty of phylogeny. Philos. Trans. R. Soc. Lond. B 2004, 359, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O. Taxonomy as a fundamental discipline. Philos. Trans. R. Soc. Lond. B 2004, 359, 739. [Google Scholar] [CrossRef] [PubMed]
- Padial, J.M.; Miralles, A.; De la Riva, I.; Vences, M. The integrative future of taxonomy. Front. Zool. 2010, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 2005, 85, 407–415. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; Dewaard, J. Biological identification through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Tautz, D.; Arctander, P.; Minelli, A.; Thomas, R.H.; Vogler, A.P. A plea for DNA taxonomy. Trends in Ecology and Evolution 2003, 18, 70–74. [Google Scholar] [CrossRef]
- Baker, S.; Dalebout, M.L.; Lavery, S.; Ross, H.A. www.DNA-surveillance: Applied molecular taxonomy for species conservation and discovery. Trends Ecol. Evol. 2003, 18, 271–272. [Google Scholar] [CrossRef]
- Moritz, C.; Cicero, C. DNA barcoding: Promise and pitfalls. PLoS Biol. 2004, 2, e354. [Google Scholar] [CrossRef] [Green Version]
- Bachtrog, D.; Andolfatto, P. Selection, recombination and demographic history in Drosophila miranda. Genetics 2006, 174, 2045–2059. [Google Scholar] [CrossRef] [Green Version]
- Rubinoff, D. Utility of mitochondrial DNA barcodes in species conservation. Conserv. Biol. 2006, 20, 1026–1033. [Google Scholar] [CrossRef]
- Song, H.; Buhay, J.E.; Whiting, M.F.; Crandall, K.A. Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc. Natl. Acad. Sci. USA 2008, 105, 13486–13491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papakostas, S.; Michaloudi, E.; Proios, K.; Brehm, M.; Verhage, L.; Rota, J.; Peña, C.; Stamou, G.; Pritchard, V.L.; Fontaneto, D.; et al. Integrative taxonomy recognizes evolutionary units despite widespread mitonuclear discordance: Evidence from a rotifer cryptic species complex. Syst. Biol. 2016, 65, 508–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ožana, S.; Dolný, A.; Pánek, T. Nuclear copies of mitochondrial DNA as a potential problem for phylogenetic and population genetic studies of Odonata. Syst. Entomol. 2022, 1–12. [Google Scholar] [CrossRef]
- Goulding, T.C.; Dayrat, B. Integrative taxonomy: Ten years of practice and looking into the future. Arch. Zool. Mus. Lomonosov Mosc. State Univ. 2016, 54, 116–133. [Google Scholar]
- Carvalho, F.G.; Duarte, L.S.; Seger, G.D.S.; Nakamura, G.; Guillermo-Ferreira, R.; Cordero-Rivera, A.; Juen, L. Detecting Darwinian shortfalls in the Amazonian Odonata. Neotrop. Entomol. 2022, in press. [CrossRef]
- Sanmartín-Villar, I.; Zhang, H.; Cordero-Rivera, A. Colour polymorphism and ontogenetic colour changes in Ischnura rufostigma (Odonata: Coenagrionidae). Odonatologica 2016, 45, 77–86. [Google Scholar] [CrossRef]
- Sanmartín-Villar, I.; Lorenzo-Carballa, M.O.; Zhang, H.; Cordero-Rivera, A. Ischnura praematura sp. nov. (Odonata: Zygoptera: Coenagrionidae): A species from Yunnan (China) whose females mate in the teneral state. Zootaxa 2022, 5087, 59–74. [Google Scholar] [CrossRef]
- Sánchez-Guillén, R.; Ceccarelli, S.F.; Villalobos, F.; Neupane, S.; Rivas-Torres, A.; Sanmartín-Villar, I.; Wellenreuther, M.; Bybee, S.M.; Velásquez-Vélez, M.I.; Realpe, E.; et al. The evolutionary history of colour polymorphism in Ischnura damselflies (Odonata: Coenagrionidae). Odonatologica 2020, 49, 333–370. [Google Scholar] [CrossRef]
- Blow, R.; Willink, B.; Svensson, E.I. A molecular phylogeny of forktail damselflies (genus Ischnura) reveals a dynamic macroevolutionary history of female colour polymorphisms. Mol. Phylogenetics Evol. 2021, 160, 107–134. [Google Scholar] [CrossRef]
- O’Farrell, A.F. Odonata. In The Insects of Australia; Mackerras, I.M., Ed.; Melbourne University Press: Melbourne, Australia, 1973; pp. 241–261. [Google Scholar]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata; Harley Books: Colchester, UK, 1999; p. 882. ISBN 0-946-58964-X. [Google Scholar]
- Dow, R.A.; Rowe, R.; Marinov, M. Ischnura aurora. The IUCN Red List of Threatened Species 2020: E.T167375A83371053. 2020. Available online: https://www.iucnredlist.org/species/167375/83371053 (accessed on 2 November 2021).
- Dumont, H.J. Phylogeny of the genus Ischnura, with emphasis on the old world taxa (Zygoptera: Coenagrionidae). Odonatologica 2013, 42, 301–308. [Google Scholar]
- Hagen, H.A. Synopsis der Neuroptera Ceylons [Pars I]. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 1858, 8, 471–488. [Google Scholar]
- Sélys-Longchamps, M.E. Synopsis des Agrionines, 5me légion: Agrion (suite). Bulletins de l’Académie Royale des Sciences, des Lettres et des Beaux-Arts de Belgique 1876, 41, 247–322. [Google Scholar]
- Brauer, F. Dritter Bericht über die auf der Weltfahrt der kais. Fregatte Novara gesammelten Libellulinen. Verhandlungen der K. K. Zoologisch-Botanischen Gesellschaft 1865, 15, 501–512. [Google Scholar]
- Papazian, M.; Dumont, H.J.; Mary-Sasal, N.J. The Odonata of the Pacific Ocean Islands of Wallis and Futuna, with special reference to speciation in Ischnura aurora (Brauer). Odonatologica 2007, 36, 53–62. [Google Scholar]
- Kalkman, V.J.; Babu, R.; Bedjanič, M.; Connif, K.; Gyeltshen, T.; Khan, M.K.; Subramanian, K.A.; Zia, A.; Orr, A.G. Checklist of the dragonflies and damselflies (Insecta: Odonata) of Bangladesh, Bhutan, India, Nepal, Pakistan and Sri Lanka. Zootaxa 2020, 4849, 1–84. [Google Scholar] [CrossRef]
- Rowe, R.J. Ischnura aurora (Brauer 1865) (Zygoptera: Coenagrionidae), an Australo-Pacific species. N. Z. J. Zool. 2010, 37, 189–192. [Google Scholar] [CrossRef]
- Futahashi, R.; Okude, G.; Sugimura, M.; Ugai, S. Interspecific hybrids in Japanese Odonata. Tombo 2018, 60, 1–49. [Google Scholar]
- Buhay, J.E. “COI-like” sequences are becoming problematic in molecular systematic and DNA barcoding studies. J. Crustacean Biol. 2009, 29, 96–110. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inkscape. Inkscape Project. 2020. Available online: https://inkscape.org (accessed on 25 June 2022).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- The GIMP Development Team. GIMP. 2019. Available online: https://www.gimp.org (accessed on 25 June 2022).
- Garrison, R.W.; von Ellenrieder, N.; Louton, J.A. Damselfly Genera of the New World; Johns Hopkins University Press: Baltimore, MD, USA, 2010; p. 490. [Google Scholar]
- Nolan, L.; Hogg, I.D.; Sutherland, D.L.; Stevens, M.I.; Schnabel, K.E. Allozyme and mitochondrial DNA variability within the New Zealand damselfly genera Xanthocnemis, Austrolestes, and Ischnura (Odonata). N. Z. J. Zool. 2007, 34, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Ramage, T.; Martins-Simoes, P.; Mialdea, G.; Allemand, R.; Duplouy, A.; Rousse, P.; Davies, N.; Roderick, G.K.; Charlat, S. A DNA barcode-based survey of terrestrial arthropods in the Society Islands of French Polynesia: Host diversity within the SymbioCode Project. Eur. J. Taxon. 2017, 272, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dumont, H.J.; Vierstraete, A.; Vanfleteren, J.R. A molecular phylogeny of the Odonata (Insecta). Syst. Entomol. 2010, 35, 6–18. [Google Scholar] [CrossRef]
- De Knijf, G.; Sparrow, D.; Dimitriou, A.; Kent, R.; Kent, H.; Siedle, K.; Lewis, J.; Crossley, L. Distribution, ecology and status of a threatened species Ischnura intermedia (Insecta: Odonata), new for Europe. Int. J. Odonatol. 2016, 19, 257–274. [Google Scholar] [CrossRef]
- Zhang, H.; Ning, X.; Yu, X.; Bu, W.-J. Integrative species delimitation based on COI, ITS, and morphological evidence illustrates a unique evolutionary history of the genus Paracercion (Odonata: Coenagrionidae). PeerJ 2021, 9, e11459. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Rivera, A. Ciclomorfosis y fenología en Ischnura graellsii Rambur, 1842 (Odonata: Coenagrionidae). Actas II Congr. Ibérico Entomol. 1998; 419–430. [Google Scholar]
- Ribeiro Leite, L.A. Mitochondrial pseudogenes in insect DNA barcoding: Differing points of view on the same issue. Biota Neotrop. 2012, 12, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Galindo-Ruiz, N.; Velasquez-Velez, M.I.; Cano-Cobos, Y.; Sánchez-Guillén, S.A.; Realpe, E. Description of a putative hybrid between Ischnura cyane and I. capreolus from Colombia (Odonata: Coenagrionidae). Not. Odonatol. 2019, 9, 144–151. [Google Scholar] [CrossRef]
- Monetti, L.; Sánchez Guillén, R.A.; Cordero-Rivera, A. Hybridization between Ischnura graellsii (Vander Linder) and I. elegans (Rambur) (Odonata: Coenagrionidae): Are they different species? Biol. J. Linn. Soc. 2002, 76, 225–235. [Google Scholar] [CrossRef]
- Rowe, R.J. Ischnura aurora (Brauer), a dragonfly with unusual mating behaviour (Zygoptera: Coenagrionidae). Odonatologica 1978, 7, 375–383. [Google Scholar]
- Sánchez-Guillén, R.A.; Wellenreuther, M.; Cordero-Rivera, A. Strong asymmetry in the relative strengths of prezygotic and postzygotic barriers between two damselfly sister species. Evolution 2012, 66, 690–707. [Google Scholar] [CrossRef]
- Lieftinck, M.A. The dragonflies (Odonata) of New Guinea and neighbouring islands. Part VII. Results of the Third Archbold expedition 1938-1939 and of the Le Roux Expedition 1939 to Netherlands New Guinea (II. Zygoptera). Nova Guin. 1949, 5, 1–271. [Google Scholar]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]








| Species | Specimen ID | Sex | Collection Locality | GenBank Acc. Nos | |
|---|---|---|---|---|---|
| COI | ITS | ||||
| Ischnura aurora | ACR819 | M | Pond at Bandiana, Wodonga, Victoria, Australia. | OM964934 | OM964914 |
| I. aurora | ACR2379 | M | Stream at Xi Meng, Yunnan, China. | OM964933 | OM964916 |
| I. aurora | ACR2880 | M | Pond at Meng Ding, Yunnan, China. | OM964932 | OM964917 |
| I. aurora | ACR2956 | M | Pond at Na Bang, Yunnan, China. | OM964931 | n.a. |
| I. aurora | ACR3503 | M | Rice fields, Huaping, Yunnan, China. | OM964930 | OM964918 |
| I. aurora | ACR3888 | M | Pond in agricultural area, Mengding, Yunnan, China. | OM964929 | OM964919 |
| I. aurora | ACR3998 | F | River at Meng Lun, Yunnan, China. | OM964928 | OM964920 |
| I. aurora | ACR4067 | M | Stream at Meng Lun, Yunnan, China. | OM964927 | OM964921 |
| I. aurora | ACR5010 | M | Somosomo Damm, Chakaudrove, Taveuni, Fiji | n.a. | OM964915 |
| Ischnura rubilio | MB-IrbKeM | M | Trivandrum, Kerala, South India. | OM964925 * | OM964922 |
| I. rubilio | MB-IrbKeF | F | OM964924 * | n.a. | |
| I. rubilio | MB-IrbTam | F | Tamil Nadu, South India. | OM964926 * | n.a. |
| I. rubilio | MB-IrbGir | M | Unknown locality, India. | OM964923 * | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Carballa, M.O.; Sanmartín-Villar, I.; Cordero-Rivera, A. Molecular and Morphological Analyses Support Different Taxonomic Units for Asian and Australo-Pacific Forms of Ischnura aurora (Odonata, Coenagrionidae). Diversity 2022, 14, 606. https://doi.org/10.3390/d14080606
Lorenzo-Carballa MO, Sanmartín-Villar I, Cordero-Rivera A. Molecular and Morphological Analyses Support Different Taxonomic Units for Asian and Australo-Pacific Forms of Ischnura aurora (Odonata, Coenagrionidae). Diversity. 2022; 14(8):606. https://doi.org/10.3390/d14080606
Chicago/Turabian StyleLorenzo-Carballa, M. Olalla, Iago Sanmartín-Villar, and Adolfo Cordero-Rivera. 2022. "Molecular and Morphological Analyses Support Different Taxonomic Units for Asian and Australo-Pacific Forms of Ischnura aurora (Odonata, Coenagrionidae)" Diversity 14, no. 8: 606. https://doi.org/10.3390/d14080606
APA StyleLorenzo-Carballa, M. O., Sanmartín-Villar, I., & Cordero-Rivera, A. (2022). Molecular and Morphological Analyses Support Different Taxonomic Units for Asian and Australo-Pacific Forms of Ischnura aurora (Odonata, Coenagrionidae). Diversity, 14(8), 606. https://doi.org/10.3390/d14080606








