Very High Food Plant Diversity among Ethnic Groups in Northern Thailand
Abstract
:1. Introduction
2. Materials and Methods
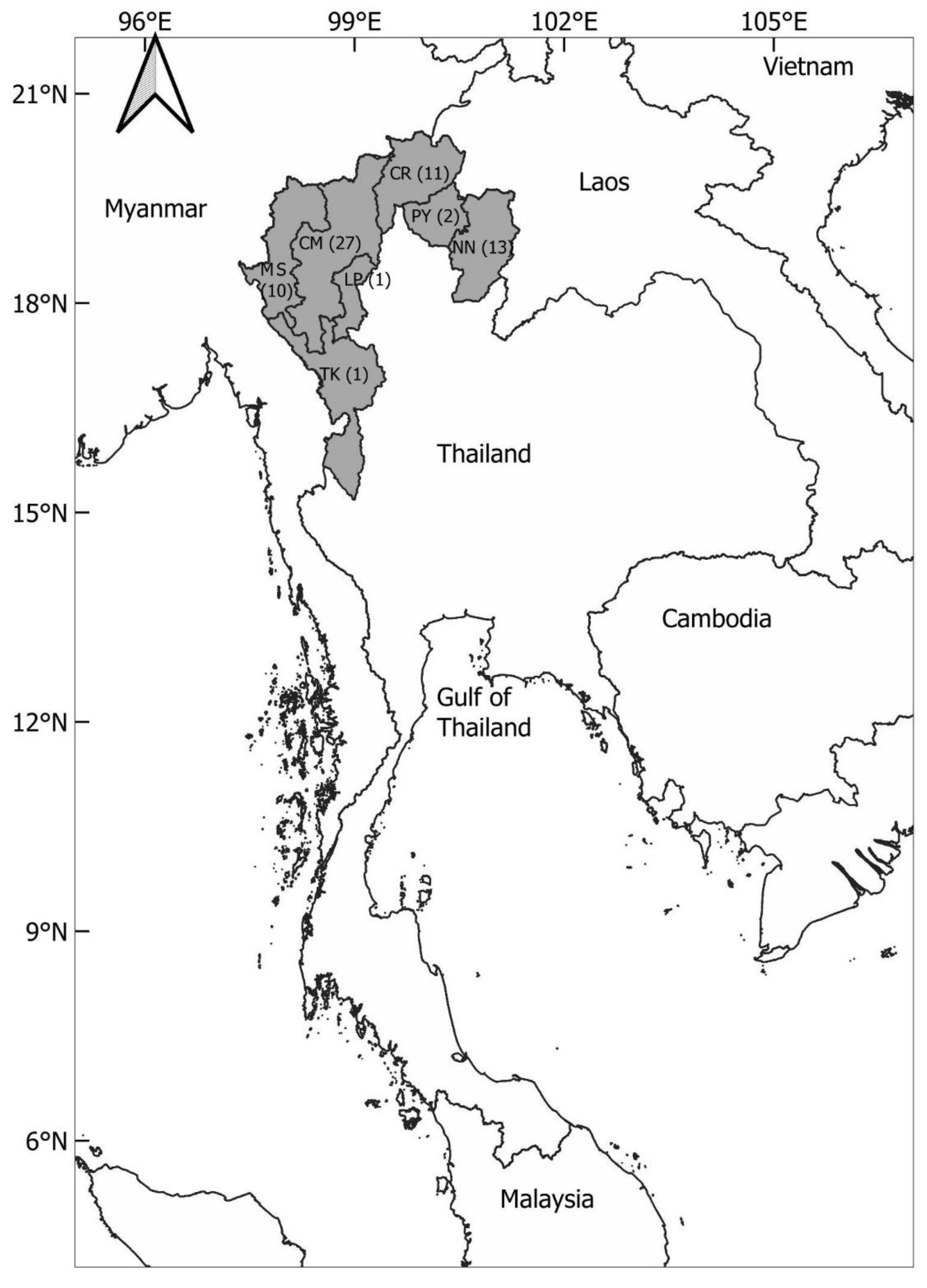
2.1. Study Area
2.2. Data Source
2.3. Data Management
2.4. Data Analyses
3. Results
3.1. Plants Used as Food
3.2. Ethnicity and Food Plants
3.3. Plant Prevalence, Category, and Origin
3.4. Uses of Rare Species
4. Discussion
4.1. Food Plant Diversity
4.2. Use of Rare Species
4.3. Exotic Species in Traditional Knowledge
4.4. Plants and Ethnicities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sabaté, J.; Soret, S. Sustainability of plant-based diets: Back to the future. Am. J. Clin. Nutr. 2014, 100, 476S–482S. [Google Scholar] [CrossRef] [Green Version]
- Leff, B.; Ramankutty, N.; Foley, J.A. Geographic distribution of major crops across the world. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Warren, J. The Nature of Crops: How We Came to Eat the Plants We Do; CABI: Oxfordshire, UK, 2015. [Google Scholar]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S.; et al. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Baldi, A.; Bruschi, P.; Campeggi, S.; Egea, T.; Rivera, D.; Obón, C.; Lenzi, A. The Renaissance of Wild Food Plants: Insights from Tuscany (Italy). Foods 2022, 11, 300. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Pieroni, A. Nutritional Ethnobotany in Europe: From Emergency Foods to Healthy Folk Cuisines and Contemporary Foraging Trends. In Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Sánchez-Mata, M.d.C., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 33–56. [Google Scholar] [CrossRef]
- Schulp, C.J.E.; Thuiller, W.; Verburg, P.H. Wild food in Europe: A synthesis of knowledge and data of terrestrial wild food as an ecosystem service. Ecol. Econ. 2014, 105, 292–305. [Google Scholar] [CrossRef]
- Hadjichambis, A.C.H.; Paraskeva-Hadjichambi, D.; Della, A.; Elena Giusti, M.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-de-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Nedelcheva, A. An ethnobotanical study of wild edible plants in Bulgaria. Eurasia J Biosci 2013, 7, 77–94. [Google Scholar] [CrossRef]
- Kalle, R.; Sõukand, R. Historical ethnobotanical review of wild edible plants of Estonia (1770s–1960s). Acta Soc. Bot. Pol. 2012, 81, 271–281. [Google Scholar] [CrossRef]
- Simkova, K.; Polesny, Z. Ethnobotanical review of wild edible plants used in the Czech Republic. J. Appl. Bot. Food Qual. 2015, 88, 49–67. [Google Scholar] [CrossRef]
- Kang, Y.; Łuczaj, Ł.; Kang, J.; Zhang, S. Wild food plants and wild edible fungi in two valleys of the Qinling Mountains (Shaanxi, central China). J. Ethnobiol. Ethnomed. 2013, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Łuczaj, Ł.; Kang, J.; Wang, F.; Hou, J.; Guo, Q. Wild food plants used by the Tibetans of Gongba Valley (Zhouqu county, Gansu, China). J. Ethnobiol. Ethnomed. 2014, 10, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Kang, Y.; Ji, X.; Guo, Q.; Jacques, G.; Pietras, M.; Łuczaj, N.; Li, D.; Łuczaj, Ł. Wild food plants and fungi used in the mycophilous Tibetan community of Zhagana (Tewo County, Gansu, China). J. Ethnobiol. Ethnomed. 2016, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Łuczaj, Ł.; Ye, S.; Zhang, S.; Kang, J. Wild food plants and wild edible fungi of Heihe valley (Qinling Mountains, Shaanxi, cetral China): Herbophilia and indifference to fruits and mushroom. Acta Soc. Bot. Pol. 2012, 81, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, A.; Langenberger, G.; Sauerborn, J. A comparison of the wild food plant use knowledge of ethnic minorities in Naban River Watershed National Nature Reserve, Yunnan, SW China. J. Ethnobiol. Ethnomed. 2012, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Qiu, Z. Consumers’ Attitudes towards Edible Wild Plants: A Case Study of Noto Peninsula, Ishikawa Prefecture, Japan. Int. J. For. Res. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.M.; Khan, M.A.; Khan, N.; Shah, M.H. Ethnobotanical survey of medicinally important wild edible fruits species used by tribal communities of Lesser Himalayas-Pakistan. J. Ethnopharmacol. 2013, 148, 528–536. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Khan, M.A.; Shah, M.H.; Shah, M.M.; Pervez, A.; Ahmad, M. Ethnobotanical appraisal and cultural values of medicinally important wild edible vegetables of Lesser Himalayas-Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.H.; Yadav, S.; Takahashi, T.; Łuczaj, Ł.; D’Cruz, L.; Okada, K. Consumption patterns of wild edibles by the Vasavas: A case study from Gujarat, India. J. Ethnobiol. Ethnomed. 2018, 14, 57. [Google Scholar] [CrossRef]
- Maxwell, J. A synopsis of the vegetation of Thailand. Trop. Nat. Hist. 2004, 4, 19–29. [Google Scholar]
- van Welzen, P.C.; Madern, A.; Raes, N.; Parnell, J.; Simpson, D.; Byrne, C.; Curtis, T.; Macklin, J.; Trias-Blasi, A.; Prajaksood, A. The current and future status of floristic provinces in Thailand. In Land Use, Climate Change and Biodiversity Modeling: Perspectives and Applications; IGI Global: Hershey, PA, USA, 2011; pp. 219–247. [Google Scholar]
- Hidayati, S.; Franco, F.M.; Bussmann, R.W. Ready for phase 5-current status of ethnobiology in Southeast Asia. J. Ethnobiol. Ethnomed. 2015, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.F. Ethnobotany of hill tribes of northern Thailand. I. medicinal plants of Akha. Econ. Bot. 1986, 40, 38–53. [Google Scholar] [CrossRef]
- Anderson, E.F. Ethnobotany of hill tribes of northern Thailand. II. Lahu medicinal plants. Econ. Bot. 1986, 40, 442–450. [Google Scholar] [CrossRef]
- Use, H.; Leeratiwong, C.; Maneenoon, K.; Sawangchote, P. Ethnobotanical study in Ko Hong Hill, Songkhla Province. Thai J. Bot. 2016, 8, 157–180. [Google Scholar]
- Pholhiamhan, R.; Saensouk, S.; Saensouk, P. Ethnobotany of Phu Thai ethnic group in Nakhon Phanom province, Thailand. Walailak J. Sci. Technol. 2018, 15, 679–699. [Google Scholar] [CrossRef]
- Srisanga, P.; Wongpakam, S.; Kamkuan, W.; Pekthong, T.; Tovaranonte, J.; Yaso, T.; Nontachaiyapoom, S. Ethnobotany of Akha in Huay Yuak Pa So village, Mae Fah Luang district and Ban Mai Patthana village, Mae Suai district, Chiang Rai province. Thai J. Bot. 2011, 3, 93–114. [Google Scholar]
- Panyadee, P.; Balslev, H.; Wangpakapattanawong, P.; Inta, A. Woody Plant Diversity in Urban Homegardens in Northern Thailand. Econ. Bot. 2016, 70, 285–302. [Google Scholar] [CrossRef]
- Panyadee, P.; Balslev, H.; Wangpakapattanawong, P.; Inta, A. Karen Homegardens: Characteristics, Functions, and Species Diversity. Econ. Bot. 2018, 72, 1–19. [Google Scholar] [CrossRef]
- Phumthum, M.; Srithi, K.; Inta, A.; Junsongduang, A.; Tangjitman, K.; Pongamornkul, W.; Trisonthi, C.; Balslev, H. Ethnomedicinal plant diversity in Thailand. J. Ethnopharmacol. 2018, 214, 90–98. [Google Scholar] [CrossRef]
- Smitinand, T. The genus Dipterocarpus Gaertn. f. in Thailand. Thai Forest Bull. 1958, 4, 1–64. [Google Scholar]
- Cook, F.E.M. Economic Botany Data Collection Standard; Whitstable Litho: Kent, UK, 1995. [Google Scholar]
- POWO. Plant of the World Online. Available online: http://www.plantsoftheworldonline.org/ (accessed on 20 June 2022).
- Lindsay, S.; Middleton, D.J. Ferns of Thailand, Laos and Cambodia. Available online: http://rbg-web2.rbge.org.uk/thaiferns (accessed on 11 April 2021).
- Pooma, R.; Suddee, S. Thai Plant Names Tem Smitinand Revised Edition 2014; Office of the Forest Herbarium, Department of National Park, Wildlife and Plant Conservation: Bangkok, Thailand, 2014.
- Chamchumroon, V.; Suphuntee, N.; Tetsana, N.; Poopath, M.; Tanikkool, S. Threatened Plants in Thailand; Office of the Forest Herbarium, Forest and Plant Conservation Research Office: Bangkok, Thailand, 2017. [Google Scholar]
- Cámara-Leret, R.; Dennehy, Z. Indigenous Knowledge of New Guinea’s Useful Plants: A Review1. Econ. Bot. 2019, 73, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Rossato, S.C.; De LeitãO-Filho, H.F.; Begossi, A. Ethnobotany of caiçaras of the Atlantic Forest coast (Brazil). Econ. Bot. 1999, 53, 387–395. [Google Scholar] [CrossRef]
- Panyadee, P. Comparison of Plants Composition and Structure of Homegardens of Ethnic Groups in Chiang Mai Province, Thailand. Doctoral Dissertation, Chiang Mai University, Chiang Mai, Thailand, 2017. [Google Scholar]
- Albuquerque, U.P.; Lucena, R.F.; Monteiro, J.M.; Florentino, A.T.; Cecília de Fátima, C. Evaluating two quantitative ethnobotanical techniques. Ethnobot. Res. Appl. 2006, 4, 51–60. [Google Scholar] [CrossRef]
- Punchay, K.; Inta, A.; Tiansawat, P.; Balslev, H.; Wangpakapattanawong, P. Traditional knowledge of wild food plants of Thai Karen and Lawa (Thailand). Genet. Resour. Crop Evol. 2020, 67, 1277–1299. [Google Scholar] [CrossRef]
- Cruz-Garcia, G.S.; Price, L.L. Ethnobotanical investigation of ’wild’ food plants used by rice farmers in Kalasin, Northeast Thailand. J. Ethnobiol. Ethnomed. 2011, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Khamfachuea, K.; Trisonthi, P.; Trisonthi, C. Ethnobotany of the Karen at Ban Chan and Chaem Luang subdistricts, Mae Chaem district, Chiang Mai province. Thai J. Bot. 2010, 2, 275–297. [Google Scholar]
- Sutjaritjai, N.; Wangpakapattanawong, P.; Balslev, H.; Inta, A. Traditional Uses of Leguminosae among the Karen in Thailand. Plants 2019, 8, 600. [Google Scholar] [CrossRef] [Green Version]
- Phumthum, M.; Balslev, H.; Barfod, A.S. Important Medicinal Plant Families in Thailand. Front. Pharmacol. 2019, 10, 1125. [Google Scholar] [CrossRef] [Green Version]
- Zenderland, J.; Hart, R.; Bussmann, R.W.; Paniagua Zambrana, N.Y.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Khutsishvili, M.; Batsatsashvili, K. The Use of “Use Value”: Quantifying Importance in Ethnobotany. Econ. Bot. 2019, 73, 293–303. [Google Scholar] [CrossRef]
- Aryal, K.P.; Poudel, S.; Chaudhary, R.P.; Chettri, N.; Chaudhary, P.; Ning, W.; Kotru, R. Diversity and use of wild and non-cultivated edible plants in the Western Himalaya. J. Ethnobiol. Ethnomed. 2018, 14, 10. [Google Scholar] [CrossRef]
- Łuczaj, Ł. Archival data on wild food plants used in Poland in 1948. J. Ethnobiol. Ethnomed. 2008, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Boesi, A. Traditional knowledge of wild food plants in a few Tibetan communities. J. Ethnobiol. Ethnomed. 2014, 10, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kujawska, M.; Łuczaj, Ł. Wild Edible Plants Used by the Polish Community in Misiones, Argentina. Hum. Ecol. 2015, 43, 855–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sõukand, R.; Kalle, R. Perceiving the Biodiversity of Food at Chest-height: Use of the Fleshy Fruits of Wild Trees and Shrubs in Saaremaa, Estonia. Hum. Ecol. 2016, 44, 265–272. [Google Scholar] [CrossRef]
- Ahmad, K.; Pieroni, A. Folk knowledge of wild food plants among the tribal communities of Thakht-e-Sulaiman Hills, North-West Pakistan. J. Ethnobiol. Ethnomed. 2016, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Uprety, Y.; Poudel, R.C.; Shrestha, K.K.; Rajbhandary, S.; Tiwari, N.N.; Shrestha, U.B.; Asselin, H. Diversity of use and local knowledge of wild edible plant resources in Nepal. J. Ethnobiol. Ethnomed. 2012, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Balemie, K.; Kebebew, F. Ethnobotanical study of wild edible plants in Derashe and Kucha Districts, South Ethiopia. J. Ethnobiol. Ethnomed. 2006, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, B.; Waghmode, A. Role of wild edible fruits as a food resource: Traditional knowledge. Int. J. Pharm. Life Sci. 2011, 2, 919–924. [Google Scholar]
- Motlhanka, D.M.; Makhabu, S.W. Medicinal and edible wild fruit plants of Botswana as emerging new crop opportunities. J. Med. Plants Res 2011, 5, 1836–1842. [Google Scholar]
- Phumthum, M.; Balslev, H. Use of Medicinal Plants Among Thai Ethnic Groups: A Comparison. Econ. Bot. 2019, 73, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. “We Became Rich and We Lost Everything”: Ethnobotany of Remote Mountain Villages of Abruzzo and Molise, Central Italy. Hum. Ecol. 2021, 49, 217–224. [Google Scholar] [CrossRef]
- Aparicio Aparicio, J.C.; Voeks, R.A.; Silveira Funch, L. Are Mixtec Forgetting Their Plants? Intracultural Variation of Ethnobotanical Knowledge in Oaxaca, Mexico. Econ. Bot. 2021, 75, 215–233. [Google Scholar] [CrossRef]
- Wester, L. Knowledge of traditional food plants in northeast Thailand. Trop. For. 21st Century 1997, 96, 1–15. [Google Scholar]
- Nguanchoo, V.; Wangpakapattanawong, P.; Balslev, H.; Inta, A. Exotic Plants Used by the Hmong in Thailand. Plants 2019, 8, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanijajiva, O.; Kadereit, J.W. Morphological and molecular evidence for interspecific hybridisation in the introduced African genus Crassocephalum (Asteraceae: Senecioneae) in Asia. Syst. Biodivers. 2009, 7, 269–276. [Google Scholar] [CrossRef]
- Adjatin, A.; Dansi, A.; Eze, C.S.; Assogba, P.; Dossou-Aminon, I.; Akpagana, K.; Akoègninou, A.; Sanni, A. Ethnobotanical investigation and diversity of Gbolo (Crassocephalum rubens (Juss. ex Jacq.) S. Moore and Crassocephalum crepidioides (Benth.) S. Moore), a traditional leafy vegetable under domestication in Benin. Genet. Resour. Crop Evol. 2012, 59, 1867–1881. [Google Scholar] [CrossRef]
- Hart, G.; Gaoue, O.G.; de la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M.J.; Balslev, H.; León-Yánez, S.; Jørgensen, P.; Duffy, D.C. Availability, diversification and versatility explain human selection of introduced plants in Ecuadorian traditional medicine. PLoS ONE 2017, 12, e0184369. [Google Scholar] [CrossRef] [PubMed]
- Srithi, K. Comparative Ethnobotany in Nan Province, Thailand. Doctoral Dissertion, Chiang Mai University, Chiang Mai, Thailand, 2012. [Google Scholar]
- Ruokolainen, L.; Blanchet, G. Introduction to Ecological Multivariate Analysis; University of Helsinki: Helsinki, Finland, 2014. [Google Scholar]
- Junsongduang, A.; Balslev, H.; Inta, A.; Jampeetong, A.; Wangpakapattanawong, P. Karen and Lawa medicinal plant use: Uniformity or ethnic divergence? J. Ethnopharmacol. 2014, 151, 517–527. [Google Scholar] [CrossRef]
- Quave, C.L.; Pieroni, A. A reservoir of ethnobotanical knowledge informs resilient food security and health strategies in the Balkans. Nat. Plants 2015, 1, 14021. [Google Scholar] [CrossRef]
- Bunsongthae, A.; Chaiwong, C. Conservation and utilization of ethnic plants in some areas of Maehongson province. North. Reg. J. Sci. Technol. 2010, 3, 22–43. [Google Scholar]
- Reid, A. A History of Southeast Asia: Critical Crossroads; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Wilson, J.E.; Siemonsma, J.S. Colocasia esculenta (L.) Schott. In Plant Resources of South-Eas Asia No 9. Plant Yielding Non-Seed Carbohydrate; Flach, M., Rumawas, F., Eds.; Backuys Publishers: Leiden, Netherlands, 1996; pp. 69–72. [Google Scholar]
- Gerrano, A.S.; Jansen Van Rensburg, W.S.; Adebola, P.O.; Manjeru, P.; Bairu, M.W.; Venter, S.L. Evaluation and selection of taro [Colocasia esculentra (L.) Schott] accessions under dryland conditions in South Africa. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 219–227. [Google Scholar] [CrossRef]
- Panyadee, P.; Balslev, H.; Wangpakapattanawong, P.; Inta, A. Medicinal plants in homegardens of four ethnic groups in Thailand. J. Ethnopharmacol. 2019, 239, 111927. [Google Scholar] [CrossRef] [PubMed]
- Junsongduang, A. Roles and importance of sacred forest in biodiversity conservation in Mae Chaem district, Chiang Mai Province. Doctoral Dissertation, Chiang Mai University, Chiang Mai, Thailand, 2014. [Google Scholar]
- Yaso, T. Ethnobotany of the H’tin and Lua in Phuphaa Subdistrict, Baugleua District, Nan Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2000. [Google Scholar]
- Boonkorn, P. Ethnobotanical Study of the Lisu at Sarm-Kula Village, Wiang Papao, Chiang Rai. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 1997. [Google Scholar]
- Borikut, M. Ethnobotany of Lua in Ban Num Phae Bo Kluea District, Nan Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2019. [Google Scholar]
- Chanklom, W. Ethnobotany and Food Security of Lahu Community at Ban Mai Pattana, Chiang Dao District, Chiang Mai Province. Master’s Thesis, Kasetsart University, Bangkok, Thailand, 2006. [Google Scholar]
- Hutasing, P. Ethnobotany of Akha in Mae La-ngong Village, Phrao District, Chiang Mai Province; Special Project; Chiang Mai University: Chiang Mai, Thailand, 2015. [Google Scholar]
- Inta, A. Ethnobotany and crop diversity of Tai Lue and Akha communities in the upper northern Thailand and the Xishuangbanna Dai autonomous prefecture, China. Ph.D. Thesis, Chiang Mai University, Chiang Mai, Thailand, 2008. [Google Scholar]
- Johnson, N.; Grivetti, L.E. Environmental change in Northern Thailand: Impact on wild edible plant availability. Ecol. Food Nutr. 2002, 41, 373–399. [Google Scholar] [CrossRef]
- Kaewsangsai, S. Ethnobotany of Karen in Khun Tuen Noi Village, Mae Tuen Sub-district, Omkoi District, Chiang Mai Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2016. [Google Scholar]
- Kamwong, K. Ethnobotany of Karens at Ban Mai Sawan and Ban Huay Pu Ling, Ban Luang Sub-District, Chom Thong District, Chiang Mai Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2009. [Google Scholar]
- Kantasrila, R. Ethnobotany of Karen at Ban Wa Do Kro, Mae Song Sub-District, Tha Song Yang District, Tak Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2016. [Google Scholar]
- Khamfachuea, K. Ethnobotany of the Karen at Ban Chan and Chaem Luang Subdistricts, Mae Chaem District, Chiang Mai Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2008. [Google Scholar]
- Klamwaewwong, C. Ethnobotanical study of H’tin hill tribe at Wangsao village, Nan. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 1996. [Google Scholar]
- Krueasan, D. Plant Diversity and Utilization by Local People in Ban Toon Sub-district, Mueang District, Phayao Province; Special Project; Chiang Mai University: Chiang Mai, Thailand, 1998. [Google Scholar]
- Krueasan, D. Management, Conservation and Utilization of Plant Species by Hmong of Pah Poo Chom Village, Mae Taeng District, Chiang Mai Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2000. [Google Scholar]
- Moonjai, J.; Inta, A. Ethnobotany of Lawa in La-Oob Village, Mae La Noi District, Mae Hong Son Province. Thai J. Bot. 2016, 8, 181–199. [Google Scholar]
- Muangyen, N. Ethnobotany of Tai Lue and Tai Yuan in Samoeng District, Chiang Mai Province. Master’s Thesis, Chiang Mai Universiy, Chiang Mai, Thailand, 2013. [Google Scholar]
- Nguanchoo, V.; Srisanga, P.; Swangpol, S.; Prathanturarug, S.; Jenjittikul, T. Food Plants in Hmong Cuisine in Northern Thailand. Thai J. Bot. 2014, 6, 131–146. [Google Scholar] [CrossRef]
- Noitana, P.; Saipara, S.; Khoomput, K. Ethnobotany of Hmong at Nanoi district, Nan province. Naresuan Phayao J. 2013, 6, 213–219. [Google Scholar]
- Nuammee, A. Ethnobotany of Hmong in Ban Pang Chang, Tambon Pong, Amphoe Santisuk, Changwat Nan. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 2012. [Google Scholar]
- Panta, K. Ethnobotany of Lisu in Khun Chae village, Phrao district, Chiang Mai Province; Special Project; Chiang Mai University: Chiang Mai, Thailand, 2015. [Google Scholar]
- Panyadee, P. Plant Utilization and Communitiy Forest Management in Sri Bua Ban Village, Sir Bua Ban Sub-District, Mueang District, Lamphun Province; Special Project; Chiang Mai University: Chiang Mai, Thailand, 2010. [Google Scholar]
- Panyadee, P. Plant diversity in Homegardens of Tai Yai Communities in Pang Mapha District, Mae Hong Son province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2012. [Google Scholar]
- Penpanassak, T.; Inta, A. Use of local vegetables in Phayao province. Thai J. Bot. 2018, 10, 93–108. [Google Scholar]
- Phongloy, T. Biodiversity and Utilization of Plants from Protected and Utilized Forests by Tai Yai Communities in Chiang Mai and Mae Hong Son Province. Ph.D. Thesis, Chiang Mai University, Chiang Mai, Thailand, 2015. [Google Scholar]
- Pongamornkul, W. An Ethnobotanical study of the Karen at Ban Yang Pu Toh and Ban Yang Thung Pong, Chiang Dao district, Chiang Mai Province; Independent Research; Chiang Mai University: Chiang Mai, Thailand, 2003. [Google Scholar]
- Pongamornkul, W. An Ethnobotanical Study of Lua in Royal Project Areas, Mae Hong Son Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2006. [Google Scholar]
- Pongamornkul, W.; Muangyen, N. Ethnobotany of Tai Yai in Khun Yuam District, Mae Hong Son Province; The Botanical Garden Organization: Chiang Mai, Thailand, 2012. [Google Scholar]
- Pongamornkul, W.; Muangyen, N. Ethnobotany of Karen in Sop Moei District, Mae Hong Son Province; The Botanical Garden Organization: Chiang Mai, Thailand, 2013. [Google Scholar]
- Pongamornkul, W.; Kumpetch, P.; Panya, R. Ethnobotanical studies of Karen in Mae Lid Luang, Mae Hor subdistrict, Mae Sariang District, Mae Hong Son province; The Botanical Garden Organization: Chiang Mai, Thailand, 2002. [Google Scholar]
- Pongsattayapipat, R. Ethnobotany of the White Hmong at Chang Kian village, Chiang Mai; Special Project; Chiang Mai University: Chiang Mai, Thailand, 1992. [Google Scholar]
- Pongsattayapipat, R. Survey and Collection of Cereals, Legumes and Earth Crops Consumed by the Ninorities in Some Areas of Doi Mae Salong in Chiang Rai Province. Master’s Thesis, Chaiang Mai University, Chiang Mai, Thailand, 1999. [Google Scholar]
- Ponpim, Y. Ethnobotany of the hill tribes in Kaenoy’s and Nongkheuw’s Royal Project in Chiang Mai. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 1996. [Google Scholar]
- Sellers, H.A. A Linguistic Approach to Ethnobotanical Plant Name Classification in Southern Lisu. Ph.D. Thesis, La Trobe University, Bundoora, Australia, 2015. [Google Scholar]
- Songsangchun, A. Plants Usages of Khon Muang and Lawa in Phu Fah Subdistrict, Bo Klua District, Nan Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2015. [Google Scholar]
- Sriboonpoun, N. Traditional Food Plants of Tai Yai in Laktaeng Village, Wiang Haeng District, Chiang Mai Province; Special Project; Chiang Mai University: Chiang Mai, Thailand, 2011. [Google Scholar]
- Srisanga, P. Ethnobotanical study of the Hmong Lai at Mae Sa Mai village, Chiang Mai; Special Project; Chiang Mai University: Chiang Mai, Thailand, 1993. [Google Scholar]
- Srithi, K.; Balslev, H.; Tanming, W.; Trisonthi, C. Weed Diversity and Uses: A Case Study from Tea Plantations in Northern Thailand. Econ. Bot. 2017, 71, 147–159. [Google Scholar] [CrossRef]
- Supawimolpan, W. Ethnobotany of Khamu in Ban Huai Sa Taeng, Thung Chang district, Nan Province; Special Project; Chiang Mai University: Chiang Mai, Thailand, 2011. [Google Scholar]
- Sutjaritjai, N. Quantitative Ethnobotanyy of Fabaceae of Karen Communities in Chiang Mai Province, Thailand. Ph.D. Thesis, Chiang Mai University, Chiang Mai, Thailand, 2019. [Google Scholar]
- Tangtragoon, T. Ethnobotany of the Khamu, Lawa and H’tin in Some Areas of Nan Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 1998. [Google Scholar]
- Tangtragoon, T.; Tarachai, Y.; Hongwittayakorn, P.; Prokati, V.; Phreechawattanakon, P. Ethnobotany Studies in Ban Pong, Sansai District, Chiang Mai Province; Maejo University: Chiang Mai, Thailand, 2004. [Google Scholar]
- Thatsaneeyakorn, J. Ethnobotanical Study of the Akha at Doi Sa-ngo Village, Chiang Rai; Special Project; Chiang Mai University: Chiang Mai, Thailand, 1997. [Google Scholar]
- Thongsorn, P. Ethnobotany of Tin Minoriy in Dongpaya Subdistrict, Bo Klua District, Nan Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2001. [Google Scholar]
- Tovaranonte, J. Ethnobotanical study of the Lisu at Lisu Lum village, Chiang Mai; Special Project; Chiang Mai University: Chiang Mai, Thailand, 1993. [Google Scholar]
- Tovaranonte, J. Ethnobotanical Study of the Tai Lue, Hmong and Yao in Some Areas of Nan Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 1998. [Google Scholar]
- Tovaranonte, J. Ethnobotany in Surroundings Area of Mae Fah Luang University; School of Science; Mae Fah Luang University: Chiang Rai, Thailand, 2001. [Google Scholar]
- Tovaranonte, J. Ethnobotany of Tai Lue in Chiang Rai Province; School of Science, Mae Fah Luang University: Chiang Rai, Thailand, 2003. [Google Scholar]
- Trisonthi, C.; Trisonthi, P. Ethnobotanical study in Thailand, a case study in Khun Yuam District Maehongson Province. Thai J. Bot. 2009, 1, 1–23. [Google Scholar]
- Trisonthi, C.; Trisonthi, P. Ethnobotany of Lua and H ‘ tin on Doi Phukha, Nan Province. Thai J. Bot. 2011, 3, 163–185. [Google Scholar]
- Udompanid, K. Ethnobotany of Tai Yai in Naisoi Village, Mueng District, Mae Hong Son Province; Special Project; Chiang Mai University: Chiang Mai, Thailand, 2012. [Google Scholar]
- Varipo, W. Ethnobotany of Ban Pok Village, Samoeng District, Chiang Mai Province; Special Project; Chiang Mai University: Chiang Mai, Thailand, 2012. [Google Scholar]
- Winijchaiyanan, P. Ethnobotany of Karen in Chiang Mai. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 1995. [Google Scholar]
- Yaso, T. Ethnobotany of Black Lahu in Huai Pong Village, Wiang Pa Pao District, Chaiang Mai Province; Special Project; Chiang Mai University: Chiang Mai, Thailand, 1997. [Google Scholar]
- Yawutthi, A. Ethnobotany of Karen to Study Food and Material Plant in Ang Ka Noi Village and Mae Klang Luang Vilage, Chom Thong District, Chiang Mai Province; Chiang Mai University: Chiang Mai, Thailand, 2011. [Google Scholar]









| Top Ranked for UV | Native% | Exotic% |
|---|---|---|
| 10 | 55 | 45 |
| 30 | 63 | 37 |
| 50 | 69 | 31 |
| 100 | 65 | 35 |
| 200 | 69 | 31 |
| 500 | 73 | 27 |
| 1000 | 79 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panyadee, P.; Wangpakapattanawong, P.; Inta, A.; Balslev, H. Very High Food Plant Diversity among Ethnic Groups in Northern Thailand. Diversity 2023, 15, 120. https://doi.org/10.3390/d15010120
Panyadee P, Wangpakapattanawong P, Inta A, Balslev H. Very High Food Plant Diversity among Ethnic Groups in Northern Thailand. Diversity. 2023; 15(1):120. https://doi.org/10.3390/d15010120
Chicago/Turabian StylePanyadee, Prateep, Prasit Wangpakapattanawong, Angkhana Inta, and Henrik Balslev. 2023. "Very High Food Plant Diversity among Ethnic Groups in Northern Thailand" Diversity 15, no. 1: 120. https://doi.org/10.3390/d15010120
APA StylePanyadee, P., Wangpakapattanawong, P., Inta, A., & Balslev, H. (2023). Very High Food Plant Diversity among Ethnic Groups in Northern Thailand. Diversity, 15(1), 120. https://doi.org/10.3390/d15010120







