The Impacts of Phosphorus-Containing Compounds on Soil Microorganisms of Rice Rhizosphere Contaminated by Lead
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site and Experimental Set-Up
2.2. Basic Physical and Chemical Properties of Soil
2.3. Root Box Experiment
2.4. Phospholipid Fatty Acid (PLFA) Analysis
2.5. Nomenclature of Phosphate Fatty Acids
2.6. Data Analysis
3. Results and Discussion
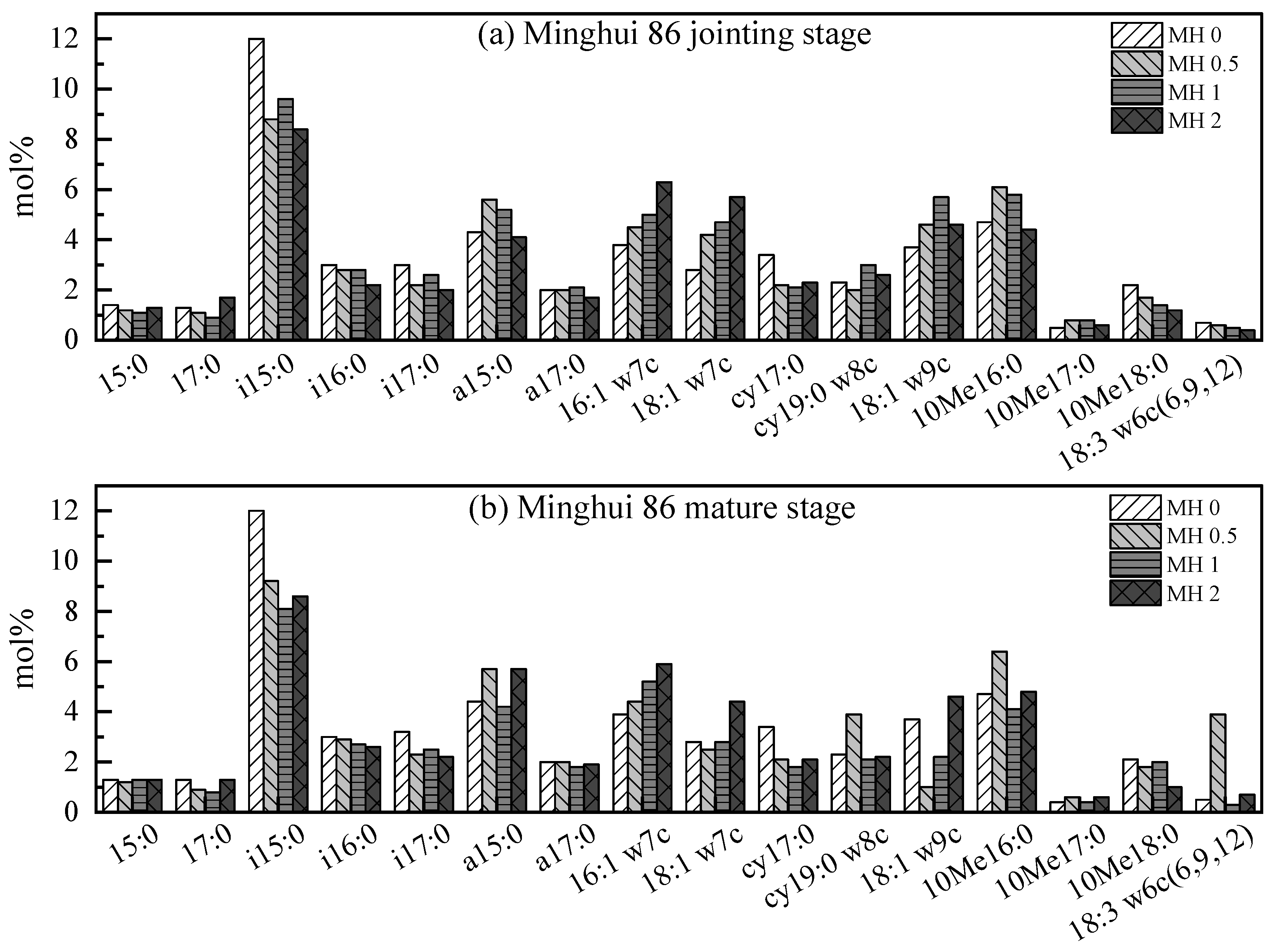
3.1. PLFA Distribution of Rice in Different Rice Stages
3.2. PLFA Distribution of Different Concentrations of Exogenous Phosphorus
3.2.1. The PLFA Distribution of YD at Given Concentrations
3.2.2. The PLFA Distribution of MH at Given Concentrations
3.3. Variation in Microbial Community Structure at Jointing Stage
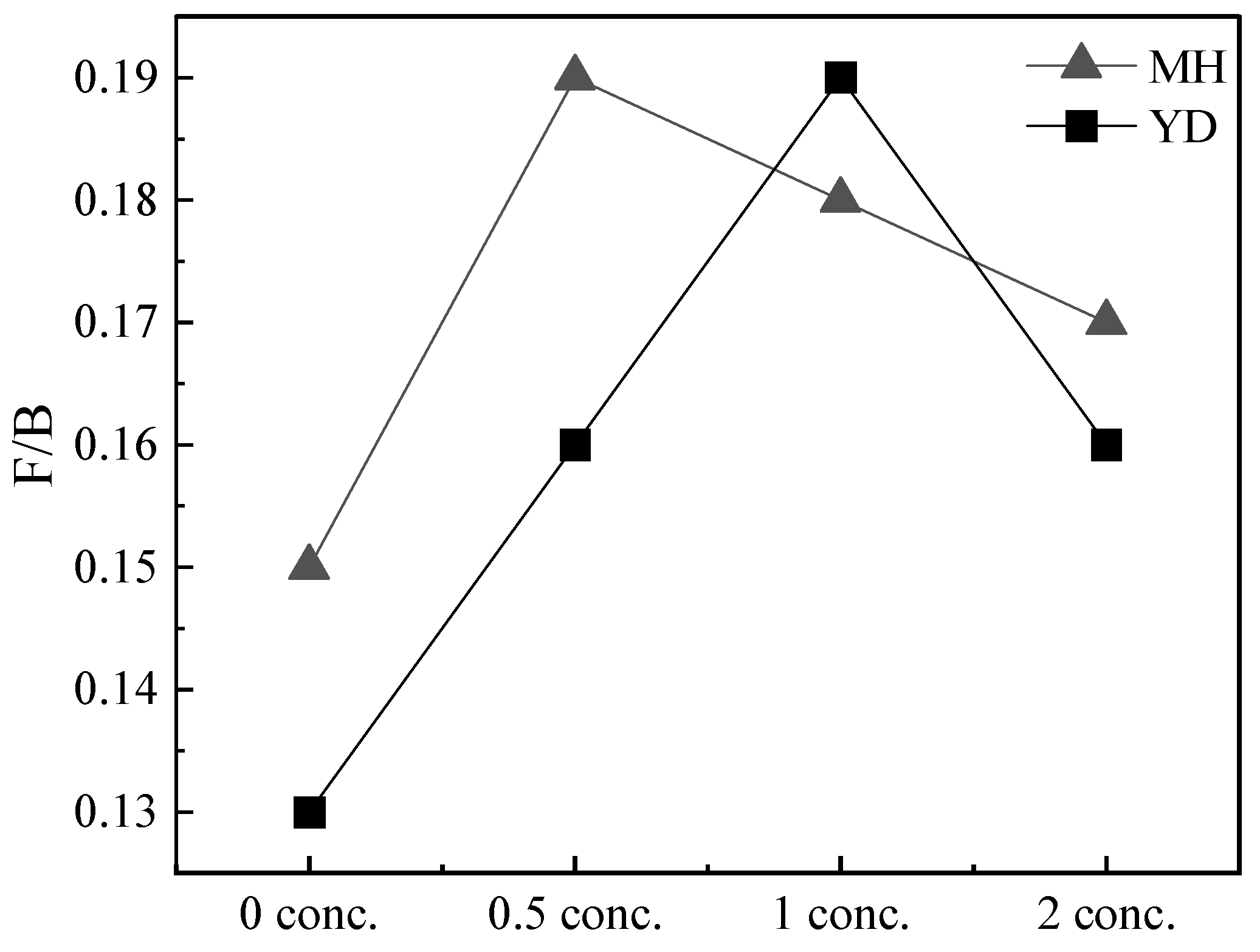
3.4. The Molar Fraction Ratio at Mature Stage
3.5. The absolute contents of the two kinds of rice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Lennon, J.T.; Aanderud, Z.T.; Lehmkuhl, B.K.; Schoolmaster, D.R., Jr. Mapping the niche space of soil microorganisms using taxonomy and traits. Ecology 2012, 93, 1867–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.; Hu, J.; Jousset, A. More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Schwalb, S.A.; Heinze, S.; Joergensen, R.G.; Wichern, F. Functions of elements in soil microorganisms. Microbiol. Res. 2021, 252, 126832. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lu, M.; Tu, N.; Li, Y. Phosphate-modified ferric-based material remediates lead and arsenic co-contaminated soil and enhances maize seedling growth. Environ. Sci. Pollut. Res. 2019, 27, 7234–7243. [Google Scholar] [CrossRef] [PubMed]
- Bonkowski, M. Protozoa and plant growth: The microbial loop in soil revisited. New Phytol. 2004, 162, 617–631. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Freeman, J.L. Environmental cleanup using plants: Biotechnological advances and ecological considerations. Front. Ecol. Environ. 2006, 4, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Hedrich, R. Ion Channels in Plants. Physiol. Rev. 2012, 92, 1777–1811. [Google Scholar] [CrossRef]
- Yang, Q.W.; Shu, W.S.; Qiu, J.W.; Wang, H.B.; Lan, C.Y. Lead in paddy soils and rice plants Lechang and its potential health risk around lead/zinc Mine, Guangdong, China. Environ. Int. 2004, 30, 883–889. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Wang, W.; Li, T.; He, Z.; Yang, X. Current status of agricultural soil pollution by heavy metals in China: A meta-analysis. Sci. Total Environ. 2019, 651, 3034–3042. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, X.; Peng, Y.; Zheng, Z.; Gao, X.; Ma, Y.; Chen, S.; Cui, S.; Fan, B.; Chen, Q. Effects of phosphorus-containing material application on soil cadmium bioavailability: A meta-analysis. Environ. Sci. Pollut. Res. 2022, 29, 42372–42383. [Google Scholar] [CrossRef] [PubMed]
- Maenpaa, K.A.; Kukkonen, J.V.K.; Lydy, M.J. Remediation of heavy metal-contaminated soils using phosphorus: Evaluation of bioavailability using an earthworm bioassay. Arch. Environ. Contam. Toxicol. 2002, 43, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, F.; Zou, B.; Chen, Y.; Zhao, J.; Mo, Q.; Li, Y.; Li, X.; Xia, H. Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biol. Fertil. Soils 2015, 51, 207–215. [Google Scholar] [CrossRef]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.-M.; Guhr, A.; Borer, E.T.; Seabloom, E.W.; Spohn, M. Effects of nitrogen and phosphorus addition on microbial community composition and element cycling in a grassland soil. Soil Biol. Biochem. 2020, 151. [Google Scholar] [CrossRef]
- Lechevalier, M.P. Lipids in bacterial taxonomy—A taxonomist’s view. CRC Crit. Rev. Microbiol. 1977, 5, 109–210. [Google Scholar] [CrossRef]
- Petersen, S.O.; Klug, M.J. Effects of sieving, storage, and incubation temperature on the phospholipid Fatty Acid profile of a soil microbial community. Appl. Environ. Microbiol. 1994, 60, 2421–2430. [Google Scholar] [CrossRef] [Green Version]
- Green, C.T.; Scow, K.M. Analysis of phospholipid fatty acids (PLFA) to characterize microbial communities in aquifers. Hydrogeol. J. 2000, 8, 126–141. [Google Scholar] [CrossRef]
- Roslev, P.; Iversen, N.; Henriksen, K. Direct fingerprinting of metabolically active bacteria in environmental samples by substrate specific radiolabelling and lipid analysis. J. Microbiol. Methods 1998, 31, 99–111. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Germida, J.J. Biolog analysis and fatty acid methyl ester profiles indicate that pseudomonad inoculants that promote phytoremediation alter the root-associated microbial community of Bromus biebersteinii. Soil Biol. Biochem. 1998, 30, 1717–1723. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Barillot, C.D.C.; Sarde, C.-O.; Bert, V.; Tarnaud, E.; Cochet, N. A standardized method for the sampling of rhizosphere and rhizoplan soil bacteria associated to a herbaceous root system. Ann. Microbiol. 2013, 63, 471–476. [Google Scholar] [CrossRef]
- Macnaughton, S.J.; Stephen, J.R.; Venosa, A.D.; Davis, G.A.; Chang, Y.J.; White, D.C. Microbial population changes during bioremediation of an experimental oil spill. Appl. Environ. Microbiol. 1999, 65, 3566–3574. [Google Scholar] [CrossRef] [Green Version]
- Macnaughton, S.; Stephen, J.R.; Chang, Y.J.; Peacock, A.; Flemming, C.A.; Leung, K.T.; White, D.C. Characterization of metal-resistant soil eubacteria by polymerase chain reaction--denaturing gradient gel electrophoresis with isolation of resistant strains. Can. J. Microbiol. 1999, 45, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Grayston, S.J.; Campbell, C.D.; Bardgett, R.D.; Mawdsley, J.L.; Clegg, C.D.; Ritz, K.; Griffiths, B.S.; Rodwell, J.S.; Edwards, S.J.; Davies, W.J.; et al. Assessing shifts in microbial community structure across a range of grasslands of differing management intensity using CLPP, PLFA and community DNA techniques. Appl. Soil Ecol. 2004, 25, 63–84. [Google Scholar] [CrossRef]
- Qi, G.F.; Ma, G.Q.; Chen, S.; Lin, C.C.; Zhao, X.Y. Microbial Network and Soil Properties Are Changed in Bacterial Wilt-Susceptible Soil. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germida, J.J.; Janzen, H.H. Factors affecting the oxidation of elemental sulfur in soils. Fertil. Res. 1993, 35, 101–114. [Google Scholar] [CrossRef]
- Dong, W.Y.; Zhang, X.Y.; Liu, X.Y.; Fu, X.L.; Chen, F.S.; Wang, H.M.; Sun, X.M.; Wen, X.F. Responses of soil microbial communities and enzyme activities to nitrogen and phosphorus additions in Chinese fir plantations of subtropical China. Biogeosciences 2015, 12, 5537–5546. [Google Scholar] [CrossRef] [Green Version]
- Jensen, H.L. THE FUNGUS FLORA OF THE SOIL. Soil Sci. 1931, 31, 123. [Google Scholar] [CrossRef]
- Santos-Medellín, C.; Edwards, J.; Liechty, Z.; Nguyen, B.; Sundaresan, V. Drought Stress Results in a Compartment-Specific Restructuring of the Rice Root-Associated Microbiomes. MBio 2017, 8, e00764-17. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, M.; Lin, H.; Gao, P.; Yang, Z.; Wang, D.; Sun, X.; Li, B.; Wang, Q.; Sun, W. Response of soil protozoa to acid mine drainage in a contaminated terrace. J. Hazard. Mater. 2022, 421, 126790. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-P.; Zhang, L.-M.; Guo, J.-F.; Ray, J.L.; He, J.-Z. Impact of long-term fertilization practices on the abundance and composition of soil bacterial communities in Northeast China. Appl. Soil Ecol. 2010, 46, 119–124. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Effects of exogenous rare earth elements on phosphorus adsorption and desorption in different types of soils. Chemosphere 2014, 103, 148–155. [Google Scholar] [CrossRef]
- Mexal, J.; Reid, C.P.P. The growth of selected mycorrhizal fungi in response to induced water stress. Can. J. Bot. 1973, 51, 1579–1588. [Google Scholar] [CrossRef]
- Ventosa, A.; Nieto, J.J.; Oren, A. Biology of Moderately Halophilic Aerobic Bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 504–544. [Google Scholar] [CrossRef] [Green Version]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Pennanen, T. Microbial communities in boreal coniferous forest humus exposed to heavy metals and changes in soil pH—A summary of the use of phospholipid fatty acids, Biolog® and 3H-thymidine incorporation methods in field studies. Geoderma 2001, 100, 91–126. [Google Scholar] [CrossRef]
- Polyanskaya, L.P.; Zvyagintsev, D.G. The content and composition of microbial biomass as an index of the ecological status of soil. Eurasian Soil Sci. 2005, 38, 625–633. [Google Scholar]
- Moore, J.C. Impact of agricultural practices on soil food web structure: Theory and application. Agric. Ecosyst. Environ. 1994, 51, 239–247. [Google Scholar] [CrossRef]
- Wu, J.; Li, R.; Lu, Y.; Bai, Z. Sustainable management of cadmium-contaminated soils as affected by exogenous application of nutrients: A review. J. Environ. Manag. 2021, 295, 113081. [Google Scholar] [CrossRef]
- Syers, K.; Bekunda, M.; Cordell, D.; Corman, J.; Johnston, J.; Rosemarin, A.; Salcedo, I.; Lougheed, T.J.U.y.b. Phosphorus and food production. In UNEP Year Book; UNEP: Nairobi, Kenya, 2011; pp. 34–45. [Google Scholar]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: Insights from meta-analysis and theoretical models. Ecol. Lett. 2013, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Kole, S.C. Soil Organic Matter and Microbial Role in Plant Productivity and Soil Fertility. In Advances in Soil Microbiology: Recent Trends and Future Prospects: Volume 2: Soil-Microbe-Plant Interaction; Adhya, T.K., Mishra, B.B., Annapurna, K., Verma, D.K., Kumar, U., Eds.; Springer: Singapore, 2017; pp. 219–238. [Google Scholar]
- Doran, J.W.; Smith, M.S. Organic Matter Management and Utilization Of Soil and Fertilizer Nutrients. In Soil Fertility and Organic Matter as Critical Components of Production Systems; Wiley: Hoboken, NJ, USA, 1987; pp. 53–72. [Google Scholar]
- Heijboer, A.; ten Berge, H.F.M.; de Ruiter, P.C.; Jørgensen, H.B.; Kowalchuk, G.A.; Bloem, J. Plant biomass, soil microbial community structure and nitrogen cycling under different organic amendment regimes; a 15N tracer-based approach. Appl. Soil Ecol. 2016, 107, 251–260. [Google Scholar] [CrossRef]
- Requena, N.; Jimenez, I.; Toro, M.; Barea, J.M. Interactions between plant-growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in mediterranean semi-arid ecosystems. New Phytol. 1997, 136, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Schmid, M.; Tuinen, D.v.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Eskin, N.; Vessey, K.; Tian, L. Research Progress and Perspectives of Nitrogen Fixing Bacterium, Gluconacetobacter diazotrophicus, in Monocot Plants. Int. J. Agron. 2014, 2014, 208383. [Google Scholar] [CrossRef]






| Items | Contents | Chinese Soil Environmental Quality Standard ii (GB15618-1995) | |
|---|---|---|---|
| pH | 5.70 (water) | / | |
| Soil organic (g/kg) | 21.8 | / | |
| Cation exchange capacity (cmol/kg) | 9.30 | / | |
| Character | Sand (%) | 65.0 | / |
| Silt (%) | 15.5 | / | |
| Clay (%) | 19.5 | / | |
| Elemental analysis | Ca (mg/kg) | 1968 | / |
| Fe (mg/kg) | 8618 | / | |
| Al (mg/kg) | 31,650 | / | |
| Mn (mg/kg) | 1341 | / | |
| P (mg/kg) | 3.23 | / | |
| Total lead (mg/kg) | 15,258 | ≤250 | |
| Total zinc (mg/kg) | 527.5 | ≤200 | |
| Total copper (mg/kg) | 185.7 | ≤50.0 | |
| Total cadmium (mg/kg) | 3.90 | ≤0.30 | |
| Total arsenic (mg/kg) | 2790 | ≤40 | |
| Microorganism | Corresponding Fatty Acids |
|---|---|
| Bacteria | 15:0, 17:0, i15:0, i16:0, i17:0, al5:0, a17:0, 16:lω7, 18:lω5, 18:lω7, cyl7:0, cy19:0 |
| Fungi | 18:lω9, 18:2ω6, 18:3ω6, 18:3ω3 |
| Actinomycetes | 10Me16:0, 10Me17:0, 10Me18:0, 10Me19:0 |
| Gram-positive bacteria (G+) | i14:0, i15:0, a15:0, i16:0, i17:0, al7:0, 10Me16:0, 10Me17:0, 10Me18:0, 18:1ω9 |
| Gram-negative bacteria (G−) | 16:1ω5, 16:lω7t, 16:1ω9, 18:1ω5, 18:1ω7, cy17:0, cyl9:0, 17:1w8c, 19:1w11c |
| Soil Samples | Definition (Concentration of Calcium Dihydrogen Phosphate Added to the Test Soil) |
|---|---|
| 0 | The molar ratio of P to Pb was 0 (control) |
| 0.5 | The molar ratio of P to Pb was 0.5 |
| 1 | The molar ratio of P to Pb was 1 |
| 2 | The molar ratio of P to Pb was 2 |
| Exogenous Phosphorus Addition Status | 0 (control) | 0.5 | 1 | 2 | ||||
|---|---|---|---|---|---|---|---|---|
| PLFA (mol%) | MH | YD | MH | YD | MH | YD | MH | YD |
| Bacteria | 40.87 | 44.12 | 36.88 | 55.17 | 34.89 | 55.80 | 38.83 | 56.61 |
| Fungi | 6.10 | 5.90 | 6.83 | 8.86 | 6.21 | 10.72 | 6.44 | 9.04 |
| Actinobacteria | 8.34 | 6.95 | 8.76 | 9.02 | 6.81 | 7.12 | 6.31 | 7.19 |
| Total microbial PLFA | 55.31 | 56.97 | 52.47 | 73.05 | 47.91 | 73.64 | 51.58 | 72.84 |
| Fungi/Bacteria | 0.15 | 0.13 | 0.19 | 0.16 | 0.18 | 0.19 | 0.17 | 0.16 |
| Gram-negative bacteria (G−)/Gram-positive bacteria (G+) | 0.88 | 0.55 | 0.74 | 0.73 | 0.60 | 0.77 | 0.69 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Shi, W.; Feng, G.; Li, X.; Zhou, Q.; Fu, L.; Jin, M.; Wu, W. The Impacts of Phosphorus-Containing Compounds on Soil Microorganisms of Rice Rhizosphere Contaminated by Lead. Diversity 2023, 15, 69. https://doi.org/10.3390/d15010069
Pan X, Shi W, Feng G, Li X, Zhou Q, Fu L, Jin M, Wu W. The Impacts of Phosphorus-Containing Compounds on Soil Microorganisms of Rice Rhizosphere Contaminated by Lead. Diversity. 2023; 15(1):69. https://doi.org/10.3390/d15010069
Chicago/Turabian StylePan, Xingchen, Wenjun Shi, Guiping Feng, Xiaolong Li, Qingwei Zhou, Li Fu, Meiqing Jin, and Weihong Wu. 2023. "The Impacts of Phosphorus-Containing Compounds on Soil Microorganisms of Rice Rhizosphere Contaminated by Lead" Diversity 15, no. 1: 69. https://doi.org/10.3390/d15010069
APA StylePan, X., Shi, W., Feng, G., Li, X., Zhou, Q., Fu, L., Jin, M., & Wu, W. (2023). The Impacts of Phosphorus-Containing Compounds on Soil Microorganisms of Rice Rhizosphere Contaminated by Lead. Diversity, 15(1), 69. https://doi.org/10.3390/d15010069







