Shallow-Water Benthic Communities on Soft Bottoms of a Sub-Arctic Fjord (Southern Barents Sea, Russia) along a Gradient of Ecological Factors
Abstract
:1. Introduction
2. Materials and Methods
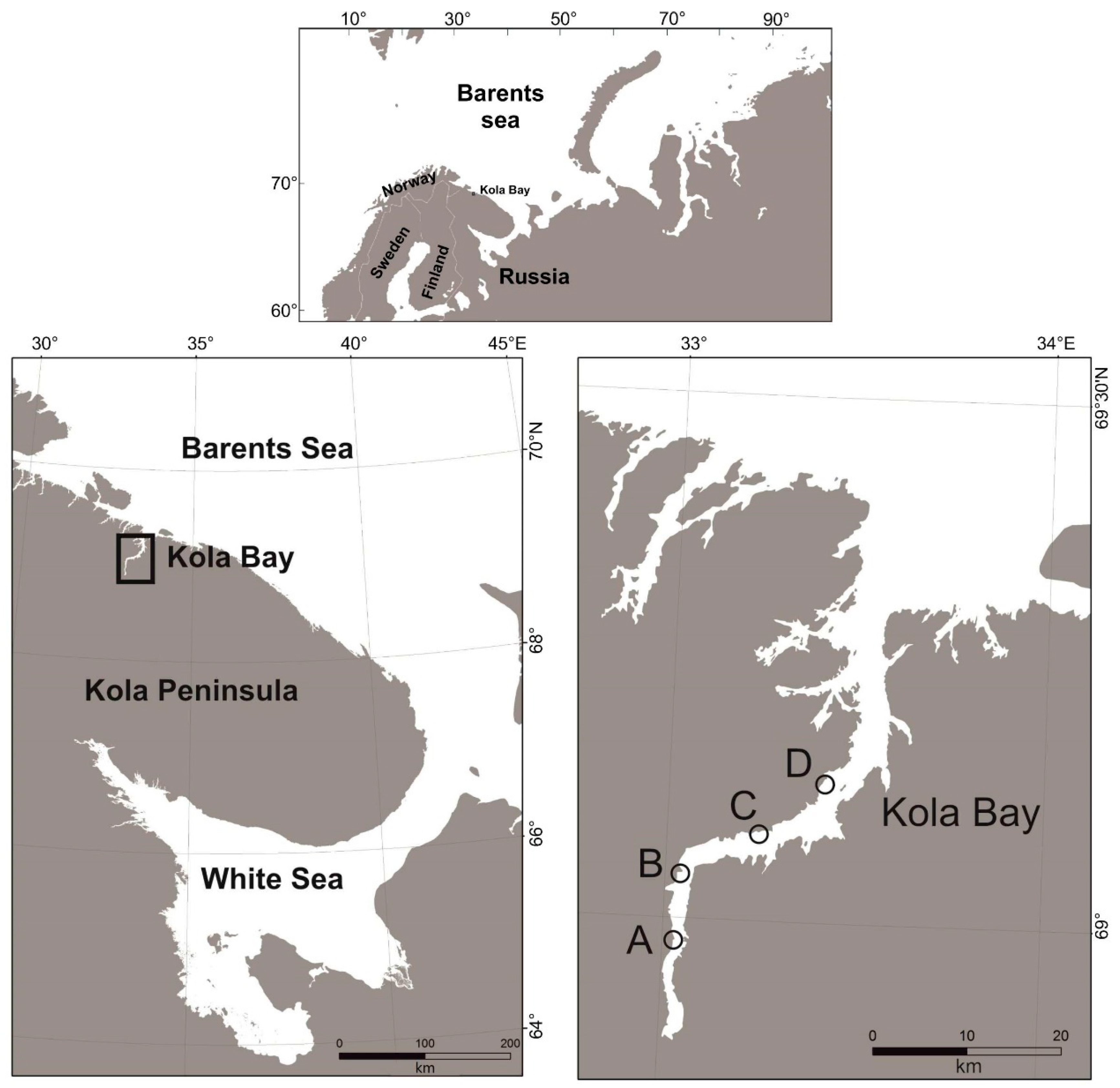
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Statistical Analysis
3. Results
3.1. Environmental Conditions
3.2. Benthic Community Structure
3.3. Environmental Control of Benthic Communities
4. Discussion
4.1. Environmental Conditions
4.2. Benthic Community Structure
4.3. Environmental Control of Benthic Communities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakobsen, T.; Ozhigin, V.K. (Eds.) The Barents Sea: Ecosystem, Resources, Management: Half a Century of Russian-Norwegian Co-operation; Tapir Academic Press: Trondheim, Norway, 2011. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Ecology and distribution of red king crab larvae in the Barents Sea: A review. Water 2022, 14, 2328. [Google Scholar] [CrossRef]
- Wassmann, P.; Reigstad, M.; Haug, T.; Rudels, B.; Carroll, M.L.; Hop, H.; Gabrielsen, G.W.; Falk-Petersen, S.; Denisenko, S.G.; Arashkevich, E.; et al. Food webs and carbon flux in the Barents Sea. Prog. Oceanogr. 2006, 71, 232–287. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Structure of mesozooplankton community in the Barents Sea and adjacent waters in August 2009. J. Nat. Hist. 2013, 47, 2095–2114. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Estimated copepod production rate and structure of mesozooplankton communities in the coastal Barents Sea during summer–autumn 2007. Polar Biol. 2012, 35, 1321–1342. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Coastal mesozooplankton assemblages during spring bloom in the eastern Barents Sea. Biology 2022, 11, 204. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Commercial fish and shellfish in the Barents Sea: Have introduced crab species affected the population trajectories of commercial fish? Rev. Fish Biol. Fish. 2015, 25, 297–322. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Red king crab (Paralithodes camtschaticus) fisheries in Russian waters: Historical review and present status. Rev. Fish Biol. Fish. 2018, 28, 331–353. [Google Scholar] [CrossRef]
- Kraan, C.; van der Meer, J.; Dekinga, A.; Piersma, T. Patchiness of macrobenthic invertebrates in homogenized intertidal habitats: Hidden spatial structure at a landscape scale. Mar. Ecol. Prog. Ser. 2009, 383, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Munari, C.; Borja, A.; Corinaldesi, C.; Rastelli, E.; Lo Martire, M.; Pitacco, V.; Mistri, M. First application of the ambi index to the macrobenthic soft-bottom community of Terra Nova Bay (Ross Sea, Southern Ocean). Water 2022, 14, 2994. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, T.; Shan, X.; Jin, X.; Teng, G.; Wei, C. Stable isotope analysis of food web structure and the contribution of carbon sources in the sea adjacent to the Miaodao Archipelago (China). Fishes 2022, 7, 32. [Google Scholar] [CrossRef]
- Dauvin, J.-C.; Pezy, J.-P.; Poizot, E.; Lozach, S.; Trentesaux, A. A multidisciplinary approach for a better knowledge of the benthic habitat and community distribution in the central and western English Channel. J. Mar. Sci. Eng. 2022, 10, 1112. [Google Scholar] [CrossRef]
- Denisenko, S.G.; Denisenko, N.V.; Lehtonen, K.K.; Andersin, A.B.; Laine, A.O. Macrozoobenthos of the Pechora Sea (SE Barents Sea): Community structure and spatial distribution in relation to environmental conditions. Mar. Ecol. Prog. Ser. 2003, 258, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, S.K.; Denisenko, S.G.; Renaud, P.E.; Emblow, C.S.; Ambrose Jr, W.G.; Ellingsen, I.H.; Skarðhamar, J. Benthic macrofauna and productivity regimes in the Barents Sea—Ecological implications in a changing Arctic. J. Sea Res. 2009, 61, 222–233. [Google Scholar] [CrossRef]
- Hunt, C.D. Variability in the benthic Mn flux in coastal marine ecosystems resulting from temperature and primary production. Limnol. Oceanogr. 1983, 28, 913–923. [Google Scholar] [CrossRef]
- McLusky, D.S.; Elliott, M. The Estuarine Ecosystem: Ecology, Threats and Management, 3rd ed.; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Degen, R.; Jørgensen, L.L.; Ljubin, P.; Ellingsen, I.H.; Pehlke, H.; Brey, T. Patterns and drivers of megabenthic secondary production on the Barents Sea shelf. Mar. Ecol. Prog. Ser. 2016, 546, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Aller, R.C.; Cochran, J.K. The critical role of bioturbation for particle dynamics, priming potential, and organic C remineralization in marine sediments: Local and basin scales. Front. Earth Sci. 2019, 7, 157. [Google Scholar] [CrossRef] [Green Version]
- Coma, R.; Ribes, M.; Gili, J.M.; Zabala, M. Seasonality in coastal benthic ecosystems. Trends Ecol. Evol. 2000, 15, 448–453. [Google Scholar] [CrossRef]
- Thomson, D.H. Marine benthos in the eastern Canadian High Arctic: Multivariate analyses of standing crop and community structure. Arctic 1982, 35, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Dayton, P.K.; Mordida, B.J.; Bacon, F. Polar marine communities. Am. Zool. 1994, 34, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C.; Ríos, C.; Gerdes, D.; Brey, T. Trophic structure of shallow-water benthic communities in the sub-Antarctic Strait of Magellan. Polar Biol. 2016, 39, 2281–2297. [Google Scholar] [CrossRef]
- Lyubina, O.S.; Zimina, O.L.; Frolova, E.A.; Lyubin, P.A.; Frolov, A.A.; Dikaeva, D.R.; Ahmetchina, O.Y.; Garbul, E.A. Features of the benthic communities distribution in the fjords of West Spitsbergen. Probl. Arctic Antarctic 2011, 1, 28–40. (In Russian) [Google Scholar]
- Meyer, K.S.; Sweetman, A.K.; Young, C.M.; Renaud, P.E. Environmental factors structuring Arctic megabenthos—A case study from a shelf and two fjords. Front. Mar. Sci. 2015, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Dunlop, K.; Renaud, P.E.; Berge, J.; Jones, D.O.B.; Harbour, R.P.; Tandberg, A.H.S.; Sweetman, A.K. Benthic scavenger community composition and carrion removal in Arctic and Subarctic fjords. Polar Biol. 2021, 44, 31–43. [Google Scholar] [CrossRef]
- Kröger, K.; Rowden, A.A. Polychaete assemblages of the northwestern Ross Sea shelf: Worming out the environmental drivers of Antarctic macrobenthic assemblage composition. Polar Biol. 2008, 31, 971–989. [Google Scholar] [CrossRef]
- Roy, V.; Iken, K.; Archambault, P. Environmental drivers of the Canadian Arctic megabenthic communities. PloS ONE 2014, 9, e100900. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, W.G., Jr.; Renaud, P.E.; Cochrane, S.K.; Denisenko, S.G.; Skarðhamar, J. Polychaete diversity patterns on two Arctic shelves: Impacts of ice and primary production? Zoosymposia 2009, 2, 457–485. [Google Scholar] [CrossRef] [Green Version]
- Molina, È.J.; Silberberger, M.J.; Kokarev, V.; Reiss, H. Environmental drivers of benthic community structure in a deep sub-arctic fjord system. Estuar. Coast. Shelf Sci. 2019, 225, 106239. [Google Scholar] [CrossRef]
- Gutt, J.; Arndt, J.; Kraan, C.; Dorschel, B.; Schröder, M.; Bracher, A.; Piepenburg, D. Benthic communities and their drivers: A spatial analysis off the Antarctic Peninsula. Limnol. Oceanogr. 2019, 64, 2341–2357. [Google Scholar] [CrossRef] [Green Version]
- Waga, H.; Hirawake, T.; Grebmeier, J.M. Recent change in benthic macrofaunal community composition in relation to physical forcing in the Pacific Arctic. Polar Biol. 2020, 43, 285–294. [Google Scholar] [CrossRef]
- Evseeva, O.Y.; Ishkulova, T.G.; Dvoretsky, A.G. Environmental drivers of an intertidal bryozoan community in the Barents Sea: A case study. Animals 2022, 12, 552. [Google Scholar] [CrossRef]
- Wlodarska-Kovalczuk, M.; Pearson, T. Soft-bottom faunal associations and factors affecting species distributions in an Arctic glacial fjord (Kongsfjord, Spitsbergen). Polar Biol. 2004, 27, 155–167. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Wlodarska-Kowalczuk, M.; Legezinska, J.; Zajaczkowsky, M. Shallow sublittoral macrozoobenthos in Kongsfjord, West Spitsbergen, Svalbard. Pol. Polar Res. 2005, 26, 137–155. [Google Scholar]
- Pecuchet, L.; Blanchet, M.-A.; Frainer, A.; Husson, B.; Jørgensen, L.L.; Kortsch, S.; Primicerio, R. Novel feeding interactions amplify the impact of species redistribution on an Arctic food web. Glob. Change Biol. 2020, 26, 4894–4906. [Google Scholar] [CrossRef] [PubMed]
- Makarevich, P.R.; Vodopianova, V.V.; Bulavina, A.S. Dynamics of the spatial chlorophyll-a distribution at the Polar Front in the marginal ice zone of the Barents Sea during spring. Water 2022, 14, 101. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Population dynamics of the invasive lithodid crab, Paralithodes camtschaticus, in a typical bay of the Barents Sea. ICES J. Mar. Sci. 2013, 70, 1255–1262. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Effects of environmental factors on the abundance, biomass, and individual weight of juvenile red king crabs in the Barents Sea. Front. Mar. Sci. 2020, 7, 726. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. New echinoderm-crab epibiotic associations from the coastal Barents Sea. Animals 2021, 11, 917. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibiotic communities of common crab species in the coastal Barents Sea: Biodiversity and infestation patterns. Diversity 2022, 14, 6. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Renewal of the recreational red king crab fishery in Russian waters of the Barents Sea: Potential benefits and costs. Mar. Policy 2022, 136, 104916. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibionts of an introduced king crab in the Barents Sea: A second five-year study. Diversity 2023, 15, 29. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Inter-annual dynamics of the Barents Sea red king crab (Paralithodes camtschaticus) stock indices in relation to environmental factors. Polar Sci. 2016, 10, 541–552. [Google Scholar] [CrossRef]
- Martínez, M.L.; Intralawan, A.; Vázquez, G.; Pérez-Maqueo, O.; Sutton, P.; Landgrave, R. The coasts of our world: Ecological, economic and social importance. Ecol. Econ. 2007, 63, 254–272. [Google Scholar] [CrossRef]
- Mangan, S.; Bulmer, R.H.; Greenfield, B.L.; Hailes, S.F.; Carter, K.; Hewitt, J.E.; Lohrer, A.M. Resilience and species accumulation across seafloor habitat transitions in a northern New Zealand harbour. Diversity 2022, 14, 998. [Google Scholar] [CrossRef]
- Sampaio, L.; Moreira, J.; Rubal, M.; Guerrero-Meseguer, L.; Veiga, P. A Review of coastal anthropogenic impacts on mytilid mussel beds: Effects on mussels and their associated assemblages. Diversity 2022, 14, 409. [Google Scholar] [CrossRef]
- Coccia, C.; Vega, C.; Fierro, P. Macroinvertebrate-based biomonitoring of coastal wetlands in Mediterranean Chile: Testing potential metrics able to detect anthropogenic impacts. Water 2022, 14, 3449. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Cucumaria in Russian waters of the Barents Sea: Biological aspects and aquaculture potential. Front. Mar. Sci. 2021, 8, 613453. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Winter zooplankton in a small arctic lake: Abundance and vertical distribution. Water 2021, 13, 912. [Google Scholar] [CrossRef]
- Deryugin, K.M. Fauna of the Kola Fjord and the conditions of its existence. Trans. Imper. Acad. Sci. St. Petersburg 1915, 34, 1–929. (In Russian) [Google Scholar]
- Zenkevitch, L.A. Biology of the Seas of the USSR; Wiley-Interscience Publishers: New York, NY, USA, 1963. [Google Scholar]
- Antipova, T.V. Some data on the current state of benthos in the Kola Bay. In Benthos of the Barents Sea: Distribution, Ecology and Population Structure; Matishov, G.G., Ed.; KSC RAS Press: Apatity, Russia, 1984; pp. 44–47. (In Russian) [Google Scholar]
- Frolova, E.A. Ecological condition of benthos of the Kola Bay. In Ecological Situation and Protection of Flora and Fauna of the Barents Sea; Matishov, G.G., Ed.; KSC RAS Press: Apatity, Russia, 1991; pp. 121–125. (In Russian) [Google Scholar]
- Frolova, E.A.; Mitina, E.G.; Gudimov, A.V.; Sikorsky, A.V. Bottom fauna of sublitoral. In The Kola Bay: Oceanography, Biology, Ecosystems, Pollutants; Matishov, G.G., Ed.; KSC RAS Press: Apatity, Russia, 1997; pp. 101–123. (In Russian) [Google Scholar]
- Pavlova, L.V.; Akhmetchina, O.Y.; Garbul, E.A.; Dikaeva, D.R.; Zimina, O.L.; Noskovich, A.E.; Frolov, A.A.; Frolova, E.A. The new data on the benthos condition of the Kola Bay (Barents Sea). Trans. Kola Sci. Cent. RAS 2019, 6, 35–75. (In Russian) [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Life cycle of Oithona similis (Copepoda, Cyclopoida) in Kola Bay (Barents Sea). Mar. Biol. 2009, 156, 1433–1446. [Google Scholar] [CrossRef]
- Dzhenyuk, S.L.; Moiseev, D.V.; Borovaya, L.I.; Ipatov, A.N. Oceanological characteristics and processes. In Kola Bay: Development and Rational Use of Natural Resources; Matishov, G.G., Ed.; Nauka: Moscow, Russia, 2009; pp. 20–45. (In Russian) [Google Scholar]
- Ilyin, G.V.; Karnatov, A.N. Physico-geographical characteristics of Kola Bay. In Kola Bay and oil: Biota, Vulnerability Maps, Pollution; Shavykin, A.A., Ed.; Renome RAS: Saint-Petersburg, Russia, 2018; pp. 52–70. (In Russian) [Google Scholar]
- Zuyev, Y.A. The Upper Sublitoral Megabenthos of Kola Bay (Barents Sea). PhD Thesis, Murmansk Marine Biological Institute RAS (MMBI RAS), Murmansk, Russia, 2012. (In Russian). [Google Scholar]
- Pavlova, L.V.; Dvoretsky, G. Prey selectivity in juvenile red king crabs from the coastal Barents Sea. Diversity 2022, 14, 568. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Zuyev, Y.A. The red king crab (Paralithodes camtschaticus) in Kola bay: Distribution, population and size/age structure changes from 2006 to 2008. Rybn Khoz. 2010, 6, 66–69. (In Russian) [Google Scholar]
- GOST 12536-2014; Methods of Laboratory Granulometric (Grain-Size) and Microaggregate Distribution. Interstate Council for Standardization, Metrology and Certification, STI: Moscow, Russia, 2015. (In Russian)
- Romanovsky, S.I. Sedimentological Foundations of Lithology; Nedra: Saint Petersburg, Russia, 1977. [Google Scholar]
- GOST 23740-2016; Soils. Methods of Laboratory Determination of Organic Composition. Interstate Council for Standardization, Metrology and Certification, Standartinform: Moscow, Russia, 2017. (In Russian)
- Macdonald, T.A.; Burd, B.J.; Macdonald, V.I.; van Roodselaar, A. Taxonomic and feeding guild classification for the marine benthic macroinvertebrates of the Strait of Georgia, British Columbia. Can. Tech. Rep. Fish. Aquat. Sci. 2010, 2874, 1–63. [Google Scholar]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef] [Green Version]
- Gagaev, S.Y. Polychaetes (Annelida: Polychaeta) of the Kara Sea. Proc. Zool. Inst. RAS 2021, 325, 183–196. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; p. 256. [Google Scholar]
- Word, J.Q. The Infaunal Trophic Index; Annual Report 1978; Coastal Water Research Project: El Segundo, CA, USA, 1978. [Google Scholar]
- Denisenko, S.G.; Barbashova, M.A.; Skvortsov, V.V.; Belyakov, V.P.; Kurashov, E.A. The results of assessment of the ecological state of zoobenthos communities according to the “Difference of Evenness” Index (DE′). Inland Water Biol. 2013, 6, 39–47. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- McGovern, M.; Poste, A.E.; Oug, E.; Renaud, P.E.; Trannum, H.C. Riverine impacts on benthic biodiversity and functional traits: A comparison of two sub-Arctic fjords. Estuar. Coast. Shelf Sci. 2020, 240, 106774. [Google Scholar] [CrossRef]
- Pavlidis, M.A. Lithology of modern sediments in the eastern Barents Sea. Ber. Polarforsch. 1996, 212, 126–135. [Google Scholar]
- Potanin, V.A.; Larin, B.V. Dynamics of waters of the southern part of the Kola Bay. Prir. Hoz. Sev. 1989, 17, 66–71. (In Russian) [Google Scholar]
- Mityaev, M.V.; Gerasimova, M.V. Suspended Matter in the Southern and Middle Part of the Bay. Kola Bay: Development and Rational Nature Management; Nauka: Moscow, Russia, 2009; pp. 52–55. (In Russian) [Google Scholar]
- Ishkulova, T.G.; Pastukhov, I.A. The current hydrochemical state of the Kola Bay water area in autumn. Trans. Kola Sci. Cent. RAS 2019, 6, 13–22. (In Russian) [Google Scholar]
- Dale, J.E.; Aitken, A.E.; Gilbert, R.; Risk, M.J. Macrofauna of Canadian Arctic fjords. Mar. Geol. 1989, 85, 331–358. [Google Scholar] [CrossRef]
- Hop, H.; Pearson, T.; Hegseth, E.N.; Kovacs, K.M.; Wiencke, C.; Kwasniewski, S.; Eiane, K.; Mehlum, F.; Gulliksen, B.; Wlodarska-Kowalczuk, M.; et al. The marine ecosystem of Kongsfjorden, Svalbard. Polar Res. 2002, 21, 167–208. [Google Scholar] [CrossRef]
- Holte, B.; Oug, E. Soft-bottom macrofauna and responses to organic enrichment in the subarctic waters of Tromso, Northern Norway. J. Sea Res. 1996, 36, 227–237. [Google Scholar] [CrossRef]
- Węsławski, J.M.; Kendall, M.A.; Włodarska-Kowalczuk, M.; Iken, K.; Kędra, M.; Legezynska, J.; Sejr, M.K. Climate change effects on Arctic fjord and coastal macrobenthic diversity–observations and predictions. Mar. Biodiv. 2011, 41, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Mikac, B.; Licciano, M.; Jaklin, A.; Iveša, L.; Giangrande, A.; Musco, L. Diversity and distribution patterns of hard bottom polychaete assemblages in the north Adriatic Sea (Mediterranean). Diversity 2020, 12, 408. [Google Scholar] [CrossRef]
- Gallucci, F.; Christofoletti, R.A.; Fonseca, G.; Dias, G.M. The effects of habitat heterogeneity at distinct spatial scales on hard-bottom-associated communities. Diversity 2020, 12, 39. [Google Scholar] [CrossRef] [Green Version]
- Holte, B.; Oug, E.; Cochrane, S. Depth-related benthic macrofaunal biodiversity patterns in three undisturbed north Norwegian fjords. Sarsia 2004, 89, 91–101. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Britayev, T.A.; Rzhavsky, A.V. Benthos elimination by juvenile red king crabs Paralithodes camtschaticus (Tilesius, 1815) in the Barents Sea coastal zone: Experimental data. Dokl. Biol. Sci. 2007, 414, 231–234. [Google Scholar] [CrossRef]
- Pavlova, L.V. Effect of juvenile red king crabs on zoobenthos in Kola Bay (Barents Sea). Dokl. Biol. Sci. 2008, 422, 312–315. [Google Scholar] [CrossRef]
- Pavlova, L.V. The red king crab Paralithodes camchaticus (Tilesius, 1815) (Decapoda: Anomura): The use of species equality indicators to assess the influence on the benthos of the Barents Sea. Russ. J. Mar. Biol. 2021, 47, 508–514. [Google Scholar] [CrossRef]
- Kuznetsov, A.P. Ecology of Bottom Communities in the World Ocean (Trophic Structure of Bottom Fauna); Nauka Press: Moscow, Russia, 1980. (In Russian) [Google Scholar]
- Commito, J.A.; Ambrose, W.G., Jr. Multiple trophic levels in soft-bottom communities. Mar. Ecol. Prog. Ser. 1985, 26, 289–293. [Google Scholar] [CrossRef]
- Calizza, E.; Careddu, G.; Sporta Caputi, S.; Rossi, L.; Costantini, M.L. Time-and depth-wise trophic niche shifts in Antarctic benthos. PLoS ONE 2018, 13, e0194796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.W.; Bergen, M.; Weisberg, S.B.; Cadien, D.; Dalkey, A.; Montagne, D.; Stull, J.K.; Velarde, R.G. Benthic response index for assessing infaunal communities on the southern California mainland shelf. Ecol. Appl. 2001, 11, 1073–1087. [Google Scholar] [CrossRef]
- Pearson, T.H.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Ann. Rev. 1978, 16, 229–311. [Google Scholar]
- Fleischack, P.C.; de Freitas, A.J. Physical parameters influencing the zonation of surf zone benthos. Estuar. Coast. Shelf Sci. 1989, 28, 517–530. [Google Scholar] [CrossRef]
- Rosenberg, R. Benthic marine fauna structured by hydrodynamic processes and food availability. Neth. J. Sea Res. 1995, 34, 303–317. [Google Scholar] [CrossRef]
- Kim, S.-L.; Yu, O.-H. Understanding the spatial and temporal distribution and environmental characteristics of polychaete assemblages in the coastal waters of ulleungdo, East Sea of Korea. J. Mar. Sci. Eng. 2021, 9, 1310. [Google Scholar] [CrossRef]
- Boutoumit, S.; Bououarour, O.; El Kamcha, R.; Pouzet, P.; Zourarah, B.; Benhoussa, A.; Maanan, M.; Bazairi, H. Spatial patterns of macrozoobenthos assemblages in a sentinel coastal lagoon: Biodiversity and environmental drivers. J. Mar. Sci. Eng. 2021, 9, 461. [Google Scholar] [CrossRef]
- Tine, M.; Diop, P.; Diadhiou, H.D. Benthic fauna assessment along the navigation channel from the mouth of the casamance estuary to Ziguinchor city. Conservation 2022, 2, 367–387. [Google Scholar] [CrossRef]
- Chan, F.T.; Stanislawczyk, K.; Sneekes, A.C.; Dvoretsky, A.; Gollasch, S.; Minchin, D.; David, M.; Jelmert, A.; Albretsen, J.; Bailey, S.A. Climate change opens new frontiers for marine species in the Arctic: Current trends and future invasion risks. Glob. Change Biol. 2019, 25, 25–38. [Google Scholar] [CrossRef]
- Gray, J.S. Animal-sediment relationships. Oceanogr. Mar. Biol. Ann. Rev. 1974, 12, 223–261. [Google Scholar]
- Herman, P.M.; Middelburg, J.J.; Heip, C.H. Benthic community structure and sediment processes on an intertidal flat: Results from the ECOFLAT project. Cont. Shelf Res. 2001, 21, 2055–2071. [Google Scholar] [CrossRef]
- Shull, D.H.; Yasuda, M. Size-selective downward particle transport by cirratulid polychaetes. J. Mar. Res. 2001, 59, 453–473. [Google Scholar] [CrossRef] [Green Version]
- Kolyuchkina, G.A.; Syomin, V.L.; Grigorenko, K.S.; Basin, A.B.; Lyubimov, I.V. The role of abiotic factors in the vertical distribution of macrozoobenthos on the northeastern Black Sea coast. Zool. Zhurnal 2020, 99, 784–800. (In Russian) [Google Scholar] [CrossRef]
- Moore, P.G. Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanogr. Mar. Biol. Ann. Rev. 1977, 15, 225–363. [Google Scholar]
- Anisimova, N.; Berenboim, B.; Gerasimova, O.; Manushin, I.; Pinchukov, M. On the effect of red king crab on some components of the Barents Sea ecosystem. Ecosystem dynamics and optimal long-term harvest in the Barents Sea fisheries. IMR/PINRO Jt. Rep. Ser. 2005, 2005/2, 298–306. [Google Scholar]
- Britayev, T.A.; Rzhavsky, A.V.; Pavlova, L.V.; Dvoretskij, A.G. Studies on impact of the alien Red King Crab (Paralithodes camtschaticus) on the shallow water benthic communities of the Barents Sea. J. Appl. Ichthyol. 2010, 26 (Suppl. 2), 66–73. [Google Scholar] [CrossRef]
- Fuhrmann, M.M.; Pedersen, T.; Ramasco, V.; Nilssen, E.M. Macrobenthic biomass and production in a heterogenic subarctic fjord after invasion by the red king crab. J. Sea Res. 2015, 106, 1–13. [Google Scholar] [CrossRef]
- Oug, E.; Sundet, J.H.; Cochrane, S.K.J. Structural and functional changes of soft-bottom ecosystems in northern fjords invaded by the red king crab (Paralithodes camtschaticus). J. Mar. Syst. 2018, 180, 255–264. [Google Scholar] [CrossRef]
- Pavlova, L.V. Ration of the red king crab on coastal shoals of the Barents Sea. Dokl. Biol. Sci. 2015, 463, 200–204. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Size-at-age of juvenile red king crab (Paralithodes camtschaticus) in the coastal Barents Sea. Cah. Biol. Mar. 2014, 55, 43–48. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Size at maturity of female red king crab, Paralithodes camtschaticus, from the costal zone of Kola Peninsula (southern Barents Sea). Cah. Biol. Mar. 2015, 56, 49–54. [Google Scholar]
- Hixon, M.A.; Menge, B.A. Species diversity: Prey refuges modify the interactive effects of predation and competition. Theor. Popul. Biol. 1991, 39, 178–200. [Google Scholar] [CrossRef]
- Diehl, S. Fish predation and benthic community structure: The role of omnivory and habitat complexity. Ecology 1992, 73, 646–1661. [Google Scholar] [CrossRef]
- Ellis, R.D. Red grouper (Epinephelus morio) shape faunal communities via multiple ecological pathways. Diversity 2019, 11, 89. [Google Scholar] [CrossRef]
- Kvitek, R.G.; Oliver, J.S.; DeGange, A.R.; Anderson, B.S. Changes in Alaskan soft-bottom prey communities along a gradient in sea otter predation. Ecology 1992, 73, 413–428. [Google Scholar] [CrossRef]
- Oug, E.; Cochrane, S.K.J.; Sundet, J.H.; Norling, K.; Nilsson, H.C. Effects of the invasive red king crab (Paralithodes camtschaticus) on soft-bottom fauna in Varangerfjorden, northern Norway. Mar. Biodiv. 2011, 41, 467–479. [Google Scholar] [CrossRef]





| Parameter | Station | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | C1 | C2 | D1 | D2 | ||
| Latitude | 69.97364 | 68.97344 | 69.03966 | 69.03957 | 69.07886 | 69.07823 | 69.11787 | 69.11787 | |
| Longitude | 33.0292 | 33.03041 | 33.03225 | 33.03443 | 33.20043 | 33.20071 | 33.39318 | 33.39404 | |
| Distance from the fjord head, km | 10 | 10 | 17 | 17 | 25 | 25 | 34 | 34 | |
| Abiotic variables | |||||||||
| Depth, m | 7 | 11 | 7 | 10 | 8 | 11 | 7 | 15 | |
| Bottom temperature, °C | 7 | 7 | 7 | 7 | 8 | 8 | 8 | 8 | |
| Bottom salinity, psu | 32.4 | 33.2 | 34.2 | 34.4 | 33.1 | 34.0 | 33.3 | 34.6 | |
| Seasonal temperature amplitude, °C * | 5.3 | 5.9 | 5.6 | 4.7 | 7.3 | 7.0 | 6.0 | 5.6 | |
| Seasonal salinity amplitude, psu * | 5.7 | 1.0 | 3.5 | 0.9 | 3.0 | 2.4 | 3.3 | 0.5 | |
| Sediments | Silt | Silt | Silty sand | Silty sand | Silty sand | Silty sand | Sand, gravel | Sand, gravel, pebbles | |
| Size categories of sediment grains: | |||||||||
| Pebble | >10 mm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.0 |
| Gravel | >5 mm | 0 | 0 | 5.6 | 0 | 0 | 0 | 1.11 | 30.54 |
| 5–2 mm | 0.05 | 0 | 16.73 | 1.01 | 1.86 | 0.35 | 4.66 | 21.85 | |
| 2–1 mm | 0.10 | 0.00 | 10.43 | 0.86 | 1.28 | 0.60 | 13.96 | 4.63 | |
| Sand | 1–0.5 mm | 0.61 | 0.05 | 9.74 | 2.07 | 1.86 | 1.20 | 32.28 | 3.47 |
| 0.5–0.25 mm | 1.52 | 0.30 | 9.70 | 4.45 | 8.77 | 4.77 | 29.88 | 6.84 | |
| 0.25–0.1 mm | 6.79 | 2.22 | 24.52 | 13.94 | 57.58 | 27.80 | 11.28 | 13.72 | |
| Aleurite | 0.1–0.05 mm | 2.65 | 0.25 | 19.29 | 20.70 | 24.48 | 33.33 | 6.13 | 6.90 |
| 0.05–0.01 mm | 51.56 | 50.70 | 0.77 | 27.16 | 1.21 | 15.24 | 0.11 | 0.21 | |
| Pelite | 0.01–0.005 mm | 12.50 | 16.90 | 0.13 | 9.94 | 0.54 | 4.42 | 0.14 | 0.17 |
| 0.005–0.001 mm | 3.91 | 7.61 | 1.03 | 2.65 | 0.27 | 2.95 | 0.45 | 0.67 | |
| <0.001 mm | 20.31 | 21.97 | 2.06 | 17.22 | 2.15 | 9.34 | 0.00 | 0.00 | |
| Sediment sorting coefficients | 0.62 | 0.62 | 0.83 | 0.82 | 0.55 | 0.75 | 0.72 | 0.79 | |
| Organic matter content, % | 9.53 | 8.90 | 2.71 | 3.01 | 1.40 | 2.34 | 0.49 | 2.84 | |
| Biotic variables | |||||||||
| Red king crab density, ind 1000 m−2 ** | 0 | 0 | 2 | 2 | 2 | 2 | 34 | 130 | |
| Red king crab biomass, g 1000 m−2 ** | 0 | 0 | 71.2 | 71.2 | 60 | 60 | 465.8 | 3406 | |
| Parameter | Station | |||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | C1 | C2 | D1 | D2 | |
| Number of phyla | 5 | 5 | 6 | 4 | 5 | 4 | 4 | 4 |
| α-diversity (species per station) | 31 | 46 | 21 | 41 | 30 | 40 | 52 | 47 |
| Taxonomic composition | An 65% Nm 3% Ar 6% Mo 23% Ed 3% | An 70% Nm 4% Ar 4% Mo 20% Ed 2% | Cn 2% An 75% Nm 2% Ar 6% Mo 14% Ed 2% | An 76% Ar 7% Mo 15% Ed 2% | An 67% Nm 3% Ar 3% Mo 23% Ed 3% | An 72% Ar 13% Mo 13% Ed 3% | An 40% Ar 15% Mo 38% Ed 6% | An 46% Ar 9% Mo 43% Ed 2% |
| Mean abundance (SE), ind. m−2 | 13,790 (6100) | 40,830 (17,480) | 5150 (510) | 7780 (450) | 3906 (510) | 1850 (1400) | 21,936 (2905) | 2300 (450) |
| Mean biomass (SE), g m−2 | 34(19) | 25(15) | 28(4) | 76(60) | 24(6) | 8(7) | 40(6) | 5(1) |
| Dominant by abundance | CP (77%) | CP (81%) | CS (22%) | CS (25%) | CP (32%) | CH (17%) | NG (39%) | NG (37%) |
| Dominant by biomass | CC (54%) | CP (37%) | LC (57%) | AS (61%) | ME (39%) | LA (30%) | BC (52%) | ME (20%) |
| H’ | 1.56 | 1.34 | 3.88 | 3.35 | 2.76 | 4.18 | 3.04 | 3.77 |
| J’ | 0.32 | 0.24 | 0.69 | 0.63 | 0.56 | 0.79 | 0.53 | 0.68 |
| D | 0.60 | 0.66 | 0.11 | 0.15 | 0.24 | 0.08 | 0.21 | 0.17 |
| ITI | 6.5 | 4.5 | 45.4 | 36.2 | 17.1 | 16.2 | 35.0 | 38.1 |
| DE | 0.17 | 0.32 | –0.24 | –0.29 | 0.07 | –0.19 | –0.03 | 0.05 |
| Epifauna:infauna ratio by biomass | 1:49 | 1:12 | 1:9 | 1:99 | 1:1.3 | 1:2.3 | 2.3:1 | 1:1 |
| Dominant trophic group by abundance | SSDF (83%) | SSDF (85%) | CAR (35%) | CAR (35%) | CAR (42%) SSDF (42%) | SSDF (44%) | SF (32%) | OMN (38%) |
| Dominant trophic group by biomass | SF (58%) | SSDF (45%) | SDF (73%) | SF (62%) | SF (49%) | SSDF (35%) | SF (78%) | SF (52%) |
| Group | Taxon | Station | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | C1 | C2 | D1 | D2 | ||
| Abundance, ind. m−2 | |||||||||
| Nmt | Nematoda g.sp. | 0 | 133 | 0 | 16 | 0 | 21 | 8640 | 860 |
| Art | Balanus crenatus | 0 | 0 | 0 | 0 | 6 | 6 | 176 | 0 |
| Art | Verruca stroemia | 0 | 0 | 0 | 0 | 0 | 181 | 0 | 213 |
| Pol | Alitta virens | 0 | 0 | 59 | 80 | 12 | 74 | 0 | 0 |
| Pol | Capitella capitata | 650 | 1440 | 300 | 672 | 188 | 128 | 80 | 0 |
| Pol | Capitella sp. | 128 | 0 | 0 | 0 | 0 | 0 | 0 | 60 |
| Pol | Chaetozone setosa | 690 | 1160 | 1120 | 1920 | 363 | 80 | 16 | 42 |
| Pol | Chone sp. | 104 | 91 | 230 | 112 | 0 | 5 | 1408 | 0 |
| Pol | Cistenides hyperborea | 8 | 37 | 155 | 123 | 175 | 310 | 3285 | 112 |
| Pol | Cossura pygodactylata | 10,060 | 33,140 | 475 | 1160 | 1260 | 150 | 0 | 5 |
| Pol | Eteone flava | 104 | 171 | 464 | 272 | 106 | 107 | 96 | 37 |
| Pol | Galathowenia oculata | 64 | 240 | 48 | 48 | 0 | 11 | 0 | 0 |
| Pol | Glycera capitata | 0 | 5 | 10 | 0 | 6 | 16 | 75 | 60 |
| Pol | Laonice cirrata | 40 | 101 | 293 | 181 | 6 | 53 | 0 | 0 |
| Pol | Leitoscoloplos acutus | 0 | 0 | 59 | 11 | 0 | 213 | 138 | 85 |
| Pol | Micronephthys neotena | 616 | 1760 | 860 | 1560 | 1363 | 123 | 0 | 0 |
| Pol | Parougia caeca | 40 | 42 | 123 | 224 | 18 | 5 | 0 | 0 |
| Pol | Pholoe assimilis | 32 | 192 | 379 | 880 | 50 | 96 | 1160 | 190 |
| Pol | Pholoe baltica | 0 | 16 | 0 | 0 | 6 | 0 | 160 | 0 |
| Pol | Phyllodoce maculata | 0 | 37 | 11 | 0 | 31 | 16 | 80 | 0 |
| Biv | Hiatella arctica | 0 | 0 | 0 | 0 | 0 | 0 | 2280 | 5 |
| Biv | Macoma calcarea | 360 | 1400 | 11 | 69 | 56 | 43 | 624 | 60 |
| Biv | Mya arenaria | 16 | 0 | 60 | 53 | 25 | 0 | 293 | 0 |
| Biv | Mytilus edulis | 112 | 202 | 16 | 0 | 19 | 0 | 2357 | 117 |
| Biv | Parvicardium pinnulatum | 24 | 21 | 11 | 16 | 25 | 0 | 11 | 60 |
| Oph | Ophiura robusta | 8 | 11 | 11 | 16 | 70 | 11 | 5 | 11 |
| Biomass, g m−2 | |||||||||
| Nem | Nemertea g. sp. | 0.63 | 0 | 0.14 | 0 | 0 | 0 | 0 | 0 |
| Art | Balanus balanus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.18 |
| Art | Balanus crenatus | 0 | 0 | 0 | 0 | 0.04 | 0.01 | 20.64 | 0 |
| Art | Rostroculodes borealis | 0 | 0 | 0 | 0 | 0 | 0 | 0.51 | 0 |
| Pol | Alitta virens | 0 | 0 | 1.23 | 0.63 | 0.05 | 0.79 | 0 | 0 |
| Pol | Capitella capitata | 0.41 | 1.07 | 0.09 | 0.12 | 0.24 | 0.03 | 0.04 | 0 |
| Pol | Chaetozone setosa | 3.86 | 5.05 | 3.77 | 6.97 | 2.00 | 0.30 | 0.01 | 0.01 |
| Pol | Cistenides hyperborea | 0.01 | 0.40 | 0.14 | 0.09 | 2.76 | 0.39 | 3.24 | 0 |
| Pol | Cistenides granulata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.48 |
| Pol | Cossura pygodactylata | 3.10 | 9.51 | 0.09 | 0.48 | 0.33 | 0.02 | 0 | 0.00 |
| Pol | Eteone flava | 0.53 | 0.63 | 1.37 | 0.42 | 0.43 | 0.14 | 0.07 | 0.01 |
| Pol | Glycera capitata | 0 | 0.77 | 0.70 | 0 | 0.03 | 1.02 | 0.15 | 0.25 |
| Pol | Harmothoe imbricata | 0 | 0 | 0.01 | 0 | 0.14 | 1.07 | 0.12 | 0 |
| Pol | Laonice cirrata | 3.00 | 0.90 | 16.21 | 16.81 | 1.64 | 1.47 | 0 | 0 |
| Pol | Leitoscoloplos acutus | 0.02 | 0 | 0.12 | 0.19 | 0 | 2.54 | 0.13 | 0.06 |
| Pol | Micronephthys neotena | 0.63 | 1.67 | 0.66 | 0.71 | 1.28 | 0.08 | 0 | 0 |
| Pol | Pholoe assimilis | 0.01 | 0.23 | 0.37 | 0.35 | 0.04 | 0.05 | 0.40 | 0.04 |
| Pol | Praxillella praetermissa | 0.37 | 0.39 | 0.01 | 0.02 | 0 | 0.01 | 0 | 0 |
| Pol | Scoletoma fragilis | 0 | 0 | 0.94 | 1.46 | 1.59 | 0.10 | 0 | 0.12 |
| Pol | Spio limicola | 0 | 0 | 0.30 | 0.04 | 0.09 | 0.02 | 0 | 0 |
| Gas | Cryptonatica affinis | 0 | 0 | 0 | 0 | 0 | 0 | 1.35 | 0 |
| Gas | Lepeta coeca | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.23 |
| Gas | Margarites groenlandicus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.18 |
| Biv | Arctica islandica | 0 | 0 | 0.09 | 46.51 | 0 | 0 | 0.17 | 0 |
| Biv | Ciliatocardium ciliatum | 18.55 | 0.3 | 0 | 0 | 0 | 0 | 0.03 | 0 |
| Biv | Crenella decussata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.13 |
| Biv | Heteranomia squamula | 0 | 0 | 0 | 0 | 0.01 | 0.01 | 0.95 | 0.02 |
| Biv | Hiatella arctica | 0 | 0 | 0 | 0 | 0 | 0 | 1.48 | 0.01 |
| Biv | Macoma calcarea | 1.14 | 2.90 | 0.04 | 0.22 | 0.18 | 0.08 | 1.10 | 0.04 |
| Biv | Mya arenaria | 0.61 | 0 | 1.11 | 0.51 | 0.68 | 0 | 3.21 | 0 |
| Biv | Mya truncata | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0.75 |
| Biv | Mytilus edulis | 0.09 | 0.10 | 0.01 | 0 | 9.30 | 0 | 2.21 | 0.87 |
| Biv | Palliolum tigerinum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.22 |
| Biv | Parvicardium pinnulatum | 0.20 | 0.15 | 0.07 | 0.10 | 1.59 | 0 | 1.81 | 0.07 |
| Species | Average Dissimilarity = 76.58% | |||
|---|---|---|---|---|
| Av. Diss | SD | Contrib | Cum | |
| Arctica islandica | 14.71 | 0.65 | 19.2 | 19.2 |
| Laonice cirrata | 13.62 | 1.29 | 17.78 | 36.99 |
| Ciliatocardium ciliatum | 13.34 | 0.88 | 17.42 | 54.41 |
| Cossura pygodactylata | 9.53 | 1.37 | 12.45 | 66.86 |
| Mytilus edulis | 5.86 | 0.66 | 7.65 | 74.51 |
| Macoma calcarea | 2.95 | 1.48 | 3.86 | 78.37 |
| Chaetozone setosa | 2.78 | 1.39 | 3.63 | 82.00 |
| Scoletoma fragilis | 2.00 | 2.51 | 2.62 | 84.61 |
| Cistenides hyperborea | 1.77 | 0.76 | 2.31 | 86.92 |
| Parvicardium pinnulatum | 0.97 | 0.74 | 1.27 | 88.19 |
| Alitta virens | 0.94 | 0.98 | 1.22 | 89.42 |
| Capitella capitata | 0.92 | 1.33 | 1.2 | 90.62 |
| Variable | Abundance | Biomass | |||||
| EV | F | P | Variable | EV | F | P | |
| Crab-D | 40 | 3.93 | 0.001 | T | 26 | 2.09 | 0.026 |
| OM | 20 | 2.98 | 0.038 | OM | 19 | 2.09 | 0.074 |
| Crab-B | 14 | 1.58 | 0.213 | S | 19 | 1.75 | 0.101 |
| T | 12 | 2.72 | 0.076 | dS | 12 | 1.53 | 0.286 |
| H | 7 | 2.24 | 0.120 | H | 9 | 1.67 | 0.363 |
| S | 5 | 2.42 | 0.337 | Sand | 9 | 1.06 | 0.420 |
| Gravel | 2 | 0 | 1.000 | Gravel | 6 | 0 | 1.000 |
| Variable | Diversity | Functional and Trophic Structure | |||||
| EV | F | P | Variable | EV | F | P | |
| T | 36 | 22.25 | 0.007 | H | 7 | 43.22 | 0.048 |
| dS | 28 | 2.39 | 0.180 | Aleurite | 26 | 3.29 | 0.080 |
| H | 17 | 1.67 | 0.247 | Crab-D | 14 | 3.24 | 0.085 |
| S | 14 | 1.13 | 0.318 | Crab-B | 34 | 3.14 | 0.103 |
| Gravel | 4 | 14.84 | 0.068 | Gravel | 7 | 1.99 | 0.210 |
| OM | 1 | 5.60 | 0.175 | Pelite | 12 | 1.63 | 0.234 |
| Sand | 0 | 0 | 1.000 | Sand | 0 | 0 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, L.V.; Zuyev, Y.A.; Dvoretsky, A.G. Shallow-Water Benthic Communities on Soft Bottoms of a Sub-Arctic Fjord (Southern Barents Sea, Russia) along a Gradient of Ecological Factors. Diversity 2023, 15, 84. https://doi.org/10.3390/d15010084
Pavlova LV, Zuyev YA, Dvoretsky AG. Shallow-Water Benthic Communities on Soft Bottoms of a Sub-Arctic Fjord (Southern Barents Sea, Russia) along a Gradient of Ecological Factors. Diversity. 2023; 15(1):84. https://doi.org/10.3390/d15010084
Chicago/Turabian StylePavlova, Lyudmila V., Yury A. Zuyev, and Alexander G. Dvoretsky. 2023. "Shallow-Water Benthic Communities on Soft Bottoms of a Sub-Arctic Fjord (Southern Barents Sea, Russia) along a Gradient of Ecological Factors" Diversity 15, no. 1: 84. https://doi.org/10.3390/d15010084
APA StylePavlova, L. V., Zuyev, Y. A., & Dvoretsky, A. G. (2023). Shallow-Water Benthic Communities on Soft Bottoms of a Sub-Arctic Fjord (Southern Barents Sea, Russia) along a Gradient of Ecological Factors. Diversity, 15(1), 84. https://doi.org/10.3390/d15010084







