Assessing the Effect of Full Protection on the Biomass of Ericaria amentacea and Understory Assemblages: Evidence from Two Mediterranean Marine Protected Areas
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area and Sampling Design
2.2. Statistical Analysis
3. Results
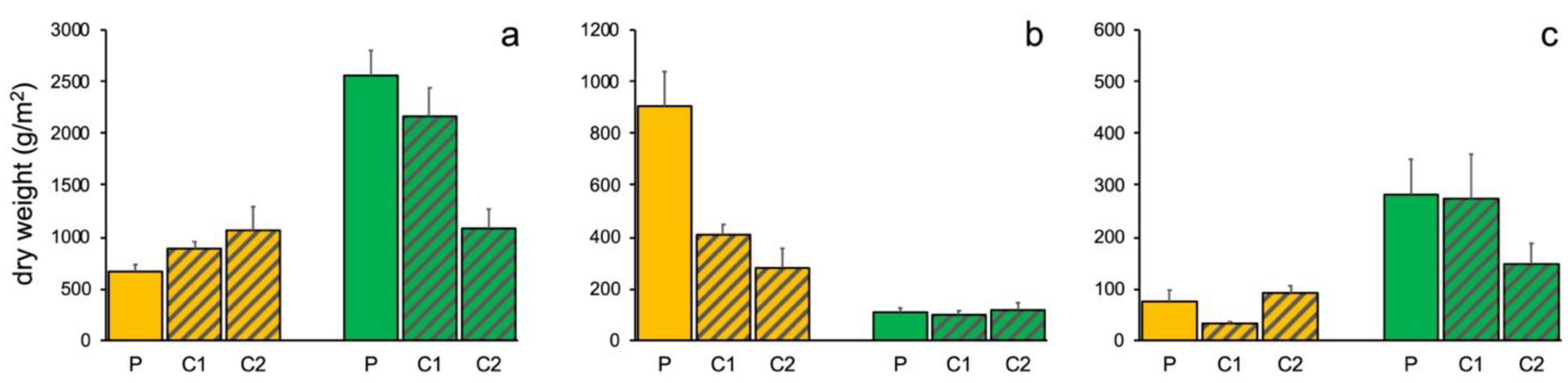
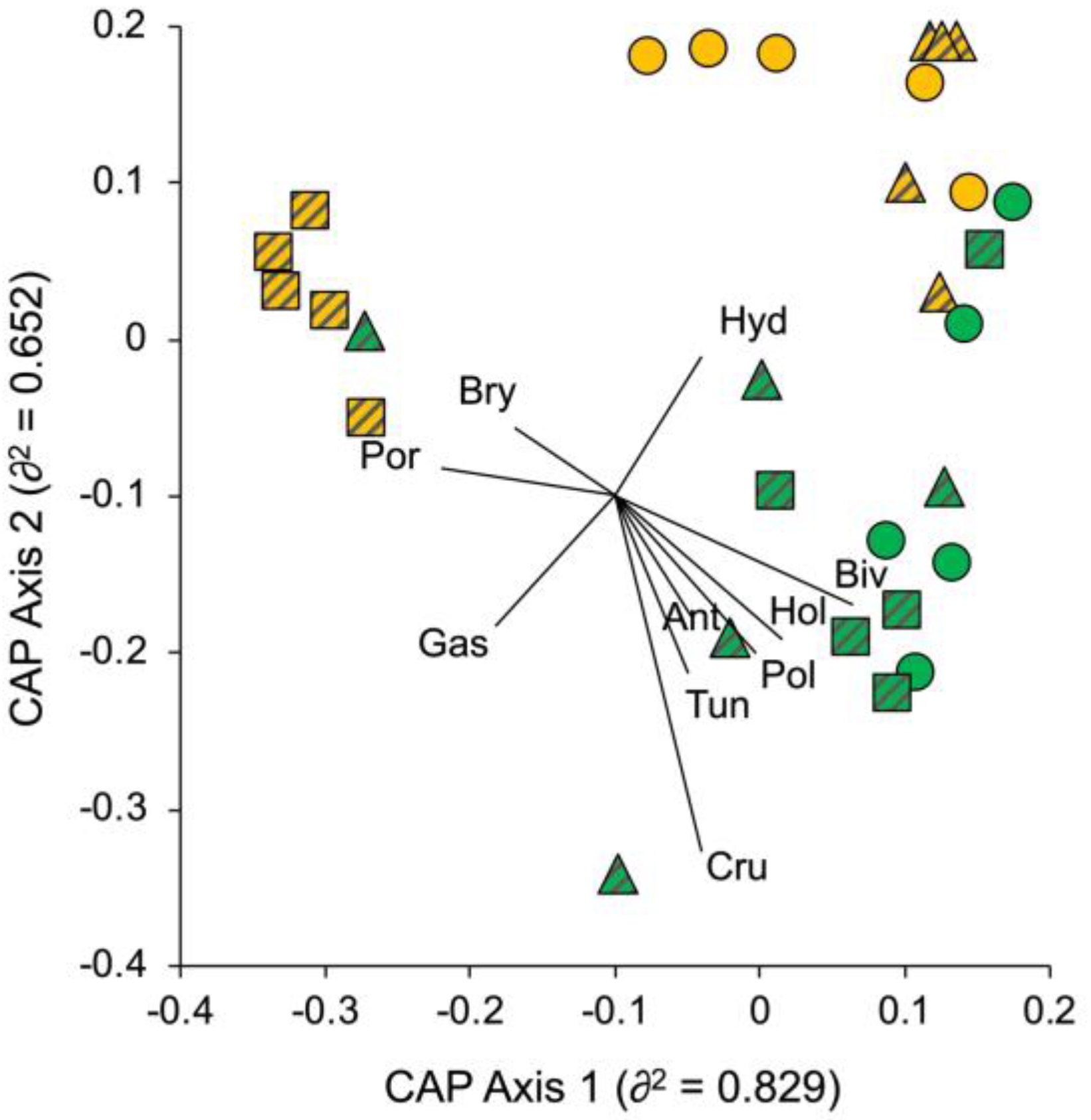
3.1. Portofino MPA
3.2. Isole Ciclopi MPA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, C.M.; Gattuso, J.-P.; Hancke, K.; Gundersen, H.; Filbee-Dexter, K.; Pedersen, M.F.; Middelburg, J.J.; Burrows, M.T.; Krumhansl, K.A.; Wernberg, T.; et al. Global estimates of the extent and production of macroalgal forests. Glob. Ecol. Biogeogr. 2022, 31, 1422–1439. [Google Scholar] [CrossRef]
- Pitacco, V.; Orlando-Bonaca, M.; Mavrič, B.; Popovič, A.; Lipej, L. Mollusc fauna associated with the Cystoseira algal associations in the Gulf of Trieste (Northern Adriatic Sea). Medit. Mar. Sci. 2014, 15, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Smale, D.; Wernberg, T. Extreme climatic event drives range contraction of a habitat- forming species. Proc. R. Soc. B 2013, 280, 20122829. [Google Scholar] [CrossRef] [PubMed]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Thibaut, T. The fate of Cystoseira crinita, a forest-forming Fucale (Phaeophyceae, Stramenopiles), in France (North Western Mediterranean Sea). Estuar. Coast. Shelf Sci. 2016, 181, 196–208. [Google Scholar] [CrossRef]
- Mannino, A.M.; Micheli, C. Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Giaccone, G. Note sistematiche ed osservazioni fitosociologiche sulle Laminariales del mediterraneo occidentale. G. Bot. Ita. 1969, 103, 457–474. [Google Scholar] [CrossRef]
- Ballesteros, E.; Sala, E.; Garrabou, J.; Zabala, M. Community structure and frond size distribution of a deep water stand of Cystoseira spinosa (Phaeophyta) in the Northwestern Mediterranean. Eur. J. Phycol. 1998, 33, 121–128. [Google Scholar] [CrossRef]
- Gozler, A.; Kopuz, U.; Agirbas, E. Seasonal changes of invertebrate fauna associated with Cystoseira barbata facies of Southeastern Black Sea coast. Afr. J. Biotechnol. 2010, 9, 8852–8859. [Google Scholar]
- Cheminée, A.; Sala, E.; Pastor, J.; Bodilis, P.; Thiriet, P.; Mangialajo, L.; Cottalorda, J.M.; Francour, P. Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J. Exp. Mar. Biol. Ecol. 2013, 442, 70–79. [Google Scholar] [CrossRef]
- Barcelona Convention Protocol concerning Specially Protected Areas and Biological Diversity in the Mediterranean. Annex II: Endangered or threatened species. Convention for the Protection of the Marine Environment and the Coastal Region of the Mediterranean, 1995. Available online: http://www.rac-spa.org (accessed on 10 December 2022).
- Falace, A.; Alongi, G.; Cormaci, M.; Furnari, G.; Curielc, D. Changes in the benthic algae along the Adriatic Sea in the last three decades. Chem. Ecol. 2010, 26, 77–90. [Google Scholar] [CrossRef]
- Ballesteros, E.; Torras, X.; Pinedo, S.; García, M.; Mangialajo, L.; De Torres, M. A new methodology based on littoral community cartography for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Pannacciulli, F.; Bulleri, F.; Moschella, P.S.; Airoldi, L.; Relini, G.; Cinelli, F. Predicting the consequences of anthropogenic disturbance: Large-scale effects of loss of canopy algae on rocky shores. Mar. Ecol. Progr. Ser. 2001, 214, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Thibaut, T.; Blanfuné, A.; Markovic, L.; Verlaque, M.; Boudouresque, C.F.; Perret-Boudouresque, M.; Maćic, V.; Bottin, L. Unexpected abundance and long-term relative stability of the brown alga Cystoseira amentacea, hitherto regarded as a threatened species, in the north-western Mediterranean Sea. Mar. Pollut. Bull. 2014, 89, 305–323. [Google Scholar] [CrossRef]
- Arévalo, R.; Pinedo, S.; Ballesteros, E. Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: Descriptive study and test of proposed methods to assess water quality regarding macroalgae. Mar. Pollut. Bull. 2007, 55, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Mangialajo, L.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Progr. Ser. 2008, 358, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Strain, E.M.A.; Thomson, R.J.; Micheli, F.; Mancuso, F.P.; Airoldi, L. Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat- forming algae in marine ecosystems. Glob. Chang. Biol. 2014, 20, 3300–3312. [Google Scholar] [CrossRef]
- Mineur, F.; Arenas, F.; Assis, J.; Davies, A.J.; Engelen, A.H.; Fernandes, F.; Malta, E.; Thibaut, T.; Van Nguyen, T.; Vaz-Pinto, F.; et al. European seaweeds under pressure: Consequences for communities and ecosystem functioning. J. Sea Res. 2015, 98, 91–108. [Google Scholar] [CrossRef]
- Mancuso, F.P.P.; Strain, E.M.A.; Piccioni, E.; De Clerck, O.; Sarà, G.; Airoldi, L. Status of vulnerable Cystoseira populations along the Italian infralittoral fringe, and relationships with environmental and anthropogenic variables. Mar. Pollut. Bull. 2018, 129, 762–771. [Google Scholar] [CrossRef]
- Blanfuné, A.; Boudouresque, C.F.; Verlaque, M.; Thibaut, T. The ups and downs of a canopy-forming seaweed over a span of more than one century. Sci. Rep. 2019, 9, 5250. [Google Scholar] [CrossRef] [Green Version]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- Colletti, A.; Savinelli, B.; Di Muzio, G.; Rizzo, L.; Tamburello, L.; Fraschetti, S.; Musco, L.; Danovaro, R. The date mussel Lithophaga lithophaga: Biology, ecology and the multiple impacts of its illegal fishery. Sci. Tot. Environ. 2020, 744, 140866. [Google Scholar] [CrossRef] [PubMed]
- Belegratis, M.R.; Bitis, I.; Economou-Amilli, A.; Ott, J.A. Epiphytic patterns of macroalgal assemblages on Cystoseira species (Fucales, Phaeophyta) in the east coast of Attica (Aegean Sea, Greece). Hydrobiologia 1999, 412, 67–80. [Google Scholar] [CrossRef]
- Chiarore, A.; Bertocci, I.; Fioretti, S.; Meccariello, A.; Saccone, G.; Crocetta, F.; Patti, F.P. Syntopic Cystoseira taxa support different molluscan assemblages in the Gulf of Naples (southern Tyrrhenian Sea). Mar. Freshw. Res 2019, 70, 1561–1575. [Google Scholar] [CrossRef]
- Tamburello, L.; Chiarore, A.; Fabbrizzi, E.; Colletti, A.; Franzitta, G.; Grech, D.; Rindi, F.; Rizzo, L.; Savinelli, B.; Fraschetti, S. Can we preserve and restore overlooked macroalgal forests? Sci. Total Environ. 2022, 806, 150855. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Cusson, M.; Bulleri, F.; Davoult, D.; Arenas, F.; Aspden, R.; Benedetti–Cecchi, L.; Bevilacqua, S.; Davidson, I.; Defew, E.; et al. Large–scale variation in combined impacts of canopy loss and disturbance on community structure and ecosystem functioning. PLoS ONE 2013, 8, e66238. [Google Scholar] [CrossRef] [Green Version]
- Bulleri, F.; Benedetti-Cecchi, L.; Acunto, S.; Cinelli, F.; Hawkins, S.J. The influence of canopy algae on vertical patterns of distribution of low-shore assemblages on rocky coasts in the northwest Mediterranean. J. Exp. Mar. Biol. Ecol. 2002, 267, 89–106. [Google Scholar] [CrossRef]
- Bianchelli, S.; Danovaro, R. Impairment of microbial and meiofaunal ecosystem functions linked to algal forest loss. Sci. Rep. 2020, 10, 19970. [Google Scholar] [CrossRef]
- Fabbrizzi, E.; Scardi, M.; Ballesteros, E.; Benedetti-Cecchi, L.; Cebrian, E.; Ceccherelli, G.; De Leo, F.; Deidun, A.; Guarnieri, G.; Falace, A.; et al. Modeling Macroalgal Forest Distribution at Mediterranean Scale: Present Status, Drivers of Changes and Insights for Conservation and Management. Front. Mar. Sci. 2020, 7, 20. [Google Scholar] [CrossRef]
- Wilson, S.K.; Fisher, R.; Pratchett, M.S.; Graham, N.A.J.; Dulvy, N.K.; Turner, R.A.; Cakacaka, A.; Polunin, N.V.C.; Rushton, S.P. Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob. Chang. Biol. 2008, 14, 2796–2800. [Google Scholar] [CrossRef]
- Schiel, D.R.; Lilley, S.A. Impacts and negative feedbacks in community recovery over eight years following removal of habitat-forming macroalgal. J. Exp. Mar. Biol. Ecol. 2011, 407, 108–115. [Google Scholar] [CrossRef]
- Milazzo, M.; Badalamenti, F.; Riggio, S.; Chemello, R. Patterns of algal recovery and small-scale effects of canopy removal as a result of human trampling on a Mediterranean rocky shallow community. Biol. Conserv. 2004, 117, 191–202. [Google Scholar] [CrossRef]
- Fraschetti, S.; Bevilacqua, S.; Guarnieri, G.; Terlizzi, A. Idiosyncratic effects of protection in a remote marine reserve. Mar. Ecol. Progr. Ser. 2012, 466, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Ballesteros, E.; Francour, P.; Guidetti, P.; Meisnesz, A.; Thibaut, T.; Mangialajo, L. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of marine protected areas. Adv. Oceanogr. Limnol. 2013, 4, 83–101. [Google Scholar] [CrossRef]
- Aller, E.A.; Jiddawi, N.S.; Eklöf, J.S. Marine protected areas increase temporal stability of community structure, but not density or diversity, of tropical seagrass fish communities. PLoS ONE 2017, 12, e0183999. [Google Scholar] [CrossRef] [Green Version]
- Shears, N.T.; Babcock, R.B. Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 2002, 132, 131–142. [Google Scholar] [CrossRef]
- Guidetti, P. Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol. Appl. 2006, 16, 963–976. [Google Scholar] [CrossRef]
- Sala, E.; Ballesteros, E.; Dendrinos, P.; Di Franco, A.; Ferretti, F.; Foley, D.; Fraschetti, S.; Friedlander, A.; Garrabou, J.; Güçlüsoy, H.; et al. The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 2012, 7, e32742. [Google Scholar] [CrossRef] [Green Version]
- Medrano, A.; Hereu, B.; Mariani, S.; Neiva, J.; Paulino, C.; Rovira, G.; Serrão, E.A.; Linares, C. Ecological traits, genetic diversity and regional distribution of the macroalga Treptacantha elegans along the Catalan coast (NW Mediterranean Sea). Sci. Rep. 2020, 10, 19219. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Vellani, V.; Fabbrizio, P.; Falace, A.; Ciriaco, S.; Segarich, M.; Spoto, M. Multidecadal monitoring highlighted long-term stability of protected assemblages within a Mediterranean marine reserve. Estuar. Coast. Shelf Sci. 2022, 274, 107946. [Google Scholar] [CrossRef]
- Fraschetti, S.; Guarnieri, G.; Bevilacqua, S.; Terlizzi, A.; Boero, F. Protection enhances community and habitat stability: Evidence from a mediterranean marine protected area. PLoS ONE 2013, 8, e81838. [Google Scholar] [CrossRef]
- Guarnieri, G.; Bevilacqua, S.; De Leo, F.; Farella, G.; Maffia, A.; Terlizzi, A.; Fraschetti, S. The Challenge of Planning Conservation Strategies in Threatened Seascapes: Understanding the Role of Fine Scale Assessments of Community Response to Cumulative Human Pressures. PLoS ONE 2016, 11, e0149253. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, G. The choice of a sieving mesh size in the quantitative assessment of marine macrobenthos: A necessary compromise between aims and constraints. Mar. Environ. Res. 1990, 30, 21–35. [Google Scholar] [CrossRef]
- Gage, J.D.; Hughes, D.J.; Gonzáles Vecino, J.L. Sieve size influence in estimating biomass, abundance and diversity in samples of deep-sea macrobenthos. Mar. Ecol. Progr. Ser. 2002, 225, 97–107. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variances; Cambridge University Press: New York, NY, USA, 1997; p. 504. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J.; Robinson, J. Generalized discriminant analysis based on distances. Aus. N. Zeal. J. Stat. 2003, 45, 301–318. [Google Scholar] [CrossRef]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Gerber, L.R.; Beger, M.; McCarthy, M.A.; Possingham, H.P. A theory for optimal monitoring of marine reserves. Ecol. Lett. 2005, 8, 829–837. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Azzola, A.; Cocito, S.; Morri, C.; Oprandi, A.; Peirano, A.; Sgorbini, S.; Montefalcone, M. Biodiversity Monitoring in Mediterranean Marine Protected Areas: Scientific and Methodological Challenges. Diversity 2022, 14, 43. [Google Scholar] [CrossRef]
- García-Charton, J.A.; Pérez-Ruzafa, A.; Sánchez-Jerez, P.; Bayle-Sempere, J.T.; Reñones, O.; Moreno, D. Multi-scale spatial heterogeneity, habitat structure, and the effect of marine reserves on Western Mediterranean rocky reef fish assemblages. Mar. Biol. 2004, 144, 161–182. [Google Scholar] [CrossRef]
- Claudet, J.; Osenberg, C.W.; Benedetti-Cecchi, L.; Domenici, P.; García-Charton, J.-A.; Pérez-Ruzafa, A.; Badalamenti, F.; Bayle-Sempere, J.; Brito, A.; Bulleri, F.; et al. Marine reserves: Size and age do matter. Ecol. Lett. 2008, 11, 481–489. [Google Scholar] [CrossRef]
- Guidetti, P.; Baiata, P.; Ballesteros, E.; Di Franco, A.; Hereu, B.; Macpherson, E.; Micheli, F.; Pais, A.; Panzalis, P.; Rosenberg, A.A.; et al. Large-scale assessment of Mediterranean Marine Protected Areas effects on fish assemblages. PLoS ONE 2014, 9, e91841. [Google Scholar] [CrossRef] [Green Version]
- Paoli, C.; Povero, P.; Burgos, E.; Dapueto, G.; Fanciulli, G.; Massa, F.; Scarpellini, P.; Vassallo, P. Natural capital and environmental flows assessment in marine protected areas: The case study of Liguria region (NW Mediterranean Sea). Ecol. Model. 2018, 368, 121–135. [Google Scholar] [CrossRef]
- McClure, E.C.; Sievers, K.T.; Abesamis, R.A.; Hoey, A.S.; Alcala, A.C.; Russ, G.R. Higher fish biomass inside than outside marine protected areas despite typhoon impacts in a complex reefscape. Biol. Conserv. 2020, 241, 108354. [Google Scholar] [CrossRef]
- Goñi, R.; Adlerstein, S.; Alvarez-Berastegui, D.; Forcada, A.; Reñones, O.; Criquet, G.; Polti, S.; Cadiou, G.; Valle, C.; Lenfant, P.; et al. Spillover from six western Mediterranean marine protected areas: Evidence from artisanal fisheries. Mar. Ecol. Prog. Ser. 2008, 366, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Linares, C.; Bianchimani, O.; Torrents, O.; Marschal, C.; Drap, P.; Garrabou, J. Marine protected areas and the conservation of long-lived invertebrates: The Mediterranean red coral. Mar. Ecol. Progr. Ser. 2010, 402, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Canessa, M.; Bavestrello, G.; Bo, M.; Enrichetti, F.; Trainito, E. Filling a Gap: A Population of Eunicella verrucosa (Pallas, 1766) (Anthozoa, Alcyonacea) in the Tavolara-Punta Coda Cavallo Marine Protected Area (NE Sardinia, Italy). Diversity 2022, 14, 405. [Google Scholar] [CrossRef]
- Halpern, B.S.; Warner, R.R. Marine reserves have rapid and lasting effects. Ecol. Lett. 2002, 5, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Bevilacqua, S.; Terlizzi, A.; Fraschetti, S.; Russo, G.F.; Boero, F. Mitigating human disturbance: Can protection influence trajectories of recovery in benthic assemblages? J. Anim. Ecol. 2006, 75, 908–920. [Google Scholar] [CrossRef]
- Babcock, R.C.; Shears, N.T.; Alcala, A.C.; Barrett, N.S.; Edgar, G.J.; Lafferty, K.D.; Russ, G.R. Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. Proc. Nat. Acad. Sci. USA. 2010, 107, 18256–18261. [Google Scholar] [CrossRef] [Green Version]
- Mannino, A.; Vaglica, V.; Oddo, E. Seasonal variation in total phenolic content of Dictyopteris polypodioides (Dictyotaceae) and Cystoseira amentacea (Sargassaceae) from the Sicilian coast. Flora Medit. 2014, 24, 39–50. [Google Scholar] [CrossRef]
- Falace, A.; Bressan, G. Seasonal variations of Cystoseira barbata (Stackhouse) C. Agardh Frond architecture. Hydrobiologia 2006, 555, 193–206. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Bertocci, I.; Micheli, F.; Maggi, E.; Fosella, T.; Vaselli, S. Implications of spatial heterogeneity for management of marine protected areas (MPAs): Examples from assemblages of rocky coasts in the northwest Mediterranean. Mar. Environ. Res. 2003, 55, 429–458. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, F.P.; Milazzo, M.; Chemello, R. Decreasing in patch-size of Cystoseira forests reduces the diversity of their associated molluscan assemblage in Mediterranean rocky reefs. Estuar. Coast. Shelf Sci. 2021, 250, 107163. [Google Scholar] [CrossRef]
- Parravicini, V.; Rovere, A.; Vassallo, P.; Micheli, F.; Montefalcone, M.; Morri, C.; Paoli, C.; Albertelli, G.; Fabiano, M.; Bianchi, C.N. Understanding relationships between conflicting human uses and coastal ecosystems status: A geospatial modeling approach. Ecol. Indic. 2012, 19, 253–263. [Google Scholar] [CrossRef]
- Venturini, S.; Massa, F.; Castellano, M.; Costa, S.; Lavarello, I.; Olivari, E.; Povero, P. Recreational boating in Ligurian Marine Protected Areas (Italy): A quantitative evaluation for a sustainable management. Environ. Manag. 2016, 57, 163–175. [Google Scholar] [CrossRef]
- Dapueto, G.; Massa, F.; Pergent-Martini, C.; Povero, P.; Rigo, I.; Vassallo, P.; Venturini, S.; Paoli, C. Sustainable management accounting model of recreational boating anchoring in Marine Protected Areas. J. Clean. Product. 2022, 342, 130905. [Google Scholar] [CrossRef]
- Parravicini, V.; Micheli, F.; Montefalcone, M.; Morri, C.; Villa, E.; Castellano, M.; Povero, P.; Bianchi, C.N. Conserving biodiversity in a human-dominated world: Degradation of marine sessile communities within a protected area with conflicting human uses. PLoS ONE 2013, 8, e75767. [Google Scholar] [CrossRef] [Green Version]
- Vassallo, P.; Bellardini, D.; Castellano, M.; Dapueto, G.; Povero, P. Structure and Functionality of the Mesozooplankton Community in a Coastal Marine Environment: Portofino Marine Protected Area (Liguria). Diversity 2022, 14, 19. [Google Scholar] [CrossRef]
- Salmona, P.; Verardi, D. The marine protected area of Portofino, Italy: A difficult balance. Ocean Coast. Manag. 2001, 44, 39–60. [Google Scholar] [CrossRef]
- Guidetti, P.; Milazzo, M.; Bussotti, S.; Molinari, A.; Molinari, A.; Murenu, M.; Pais, A.; Spano, N.; Balzano, R.; Agardy, A. Italian marine reserve effectiveness: Does enforcement matter? Biol. Conserv. 2008, 141, 699–709. [Google Scholar]
- Ferrante, M.; Pappalardo, A.M.; Ferrito, V.; Pulvirenti, V.; Fruciano, C.; Grasso, A.; Sciacca, S.; Tigano, C.; Copat, C. Bioaccumulation of metals and biomarkers of environmental stress in Parablennius sanguinolentus (Pallas, 1814) sampled along the Italian coast. Mar. Pollut. Bull. 2017, 122, 288–296. [Google Scholar] [CrossRef]
- Perra, P.; Pozo, K.; Guerranti, C.; Lazzeri, D.; Volpi, V.; Corsolini, S.; Focardi, S. Levels and spatial distribution of polycyclic aromatic hydrocarbons (PAHs) in superficial sediment from 15 Italian marine protected areas (MPA). Mar. Pollut. Bull. 2011, 62, 874–877. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Cottalorda, J.-M.; Hereu, B.; Susini, M.-L.; Verlaque, M. Unexpected Temporal Stability of Cystoseira and Sargassum Forests in Port-Cros, one of the Oldest Mediterranean Marine National Parks. Crypt. Algol. 2016, 37, 61–90. [Google Scholar] [CrossRef]
- Day, J.; Dudley, N.; Hockings, M.; Holmes, G.; Laffoley, D.; Stolton, S.; Wells, S.M. Guidelines for Applying the IUCN Protected Area Management Categories to Marine Protected Areas; IUCN: Gland, Switzerland, 2012. [Google Scholar]
- Ferreira, H.M.; Magris, R.A.; Floeter, S.R.; Ferreira, C.E. Drivers of ecological effectiveness of marine protected areas: A meta-analytic approach from the Southwestern Atlantic Ocean (Brazil). J. Environ. Manag. 2022, 301, 113889. [Google Scholar] [CrossRef]
- Lubchenco, J.; Palumbi, S.R.; Gaines, S.D.; Andelman, S. Plugging a hole in the ocean: The emerging science of marine reserves. Ecol. Appl. 2003, 13, S3–S7. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, S.J. Marine conservation in a rapidly changing world. Aq. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 281–287. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Fournari-Konstantinidou, I.; Sourbès, L.; Koutsoubas, D.; Katsanevakis, S. Long Term Interactions of Native and Invasive Species in a Marine Protected Area Suggest Complex Cascading Effects Challenging Conservation Outcomes. Diversity 2021, 13, 71. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Verlaque, M. Decline and local extinction of Fucales in the French Riviera: The harbinger of future extinctions? Medit. Mar. Sci. 2015, 16, 206–224. [Google Scholar] [CrossRef] [Green Version]
- Montero-Serra, I.; Garrabou, J.; Doak, D.F.; Ledoux, J.; Linares, C. Marine protected areas enhance structural complexity but do not buffer the consequences of ocean warming for an overexploited precious coral. J. Appl. Ecol. 2019, 56, 1063–1074. [Google Scholar] [CrossRef]
- Di Franco, E.; Di Franco, A.; Calò, A.; Di Lorenzo, M.; Mangialajo, L.; Bussotti, S.; Bianchi, C.N.; Guidetti, P. Inconsistent relationships among protection, benthic assemblage, habitat complexity and fish biomass in Mediterranean temperate rocky reefs. Ecol. Indic. 2021, 128, 107850. [Google Scholar] [CrossRef]
- Fraschetti, S.; Fabbrizzi, E.; Tamburello, L.; Uyarra, M.C.; Micheli, F.; Sala, E.; Pipitone, C.; Badalamenti, F.; Bevilacqua, S.; Boada, J.; et al. An integrated assessment of the Good Environmental Status of Mediterranean Marine Protected Areas. J. Environ. Manag. 2022, 305, 114370. [Google Scholar] [CrossRef]
- Sales, M.; Ballesteros, E. Shallow Cystoseira (Fucales: Ochrophyta) assemblages thriving in sheltered areas from Menorca (NW Mediterranean): Relationships with environmental factors and anthropogenic pressures. Estuar. Coast. Shelf Sci. 2009, 84, 476–482. [Google Scholar] [CrossRef]
- Sales, M.; Cebrian, E.; Tomas, F.; Ballesteros, E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar. Coast. Shelf Sci. 2011, 92, 347–357. [Google Scholar] [CrossRef]



| E. amentacea | Macroalgae | Invertebrates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | d.f. | MS | F | P | MS | F | P | MS | F | P |
| Ti | 1 | 1957.60 | 13.205 | 0.0681 | 9.64 | 2.543 | 0.2518 | 537.51 | 2.931 | |
| Si | 2 | 40.62 | 0.274 | 0.7849 | 3.34 | 0.880 | 0.5320 | 235.66 | 1.285 | |
| P-v-Cs | 1 | 27.4 | 0.182 | 0.7109 | 0.92 | 0.674 | 0.4199 | 95.94 | 0.523 | 0.5447 |
| Cs | 1 | 54.21 | 0.215 | 0.7238 | 5.75 | 1.001 | 0.4998 | 375.38 | 1.086 | 0.4869 |
| Ti × Si | 2 | 148.24 | 2.066 | 0.1487 | 3.79 | 2.764 | 0.0831 | 183.42 | 4.322 | 0.0249 |
| Ti × P-v-Cs | 1 | 43.90 | 0.612 | 0.4418 | 1.84 | 1.343 | 0.2579 | 21.01 | 0.061 | 0.8462 |
| Ti × Cs | 1 | 252.58 | 3.131 | 0.0959 | 5.74 | 2.867 | 0.1098 | 345.82 | 8.331 | 0.0107 |
| Res | 24 | 71.76 | 1.37 | 42.44 | ||||||
| Res P | 8 | 56.94 | 0.11 | 44.30 | ||||||
| Res Cs | 16 | 80.67 | 2.00 | 41.51 | ||||||
| Transformation | log(x + 1) | log(x + 1) | None | |||||||
| Shapiro–Wilk test | W = 0.967 NS | W = 0.913 * | W = 0.969 NS | |||||||
| Cochran’s C-test | C = 0.443 NS | C = 0.753 *** | C = 0.330 NS | |||||||
| E. amentacea | Macroalgae | Invertebrates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | d.f. | MS | F | P | MS | F | P | MS | F | P |
| Ti | 1 | 2091.30 | 3.715 | 330.61 | 4.873 | 52.24 | 8.712 | 0.0982 | ||
| Si | 2 | 207.41 | 0.368 | 66.84 | 0.985 | 2.16 | 0.359 | 0.7356 | ||
| P-v-Cs | 1 | 161.37 | 0.287 | 0.6459 | 129.91 | 1.915 | 2.92 | 0.486 | 0.5577 | |
| Cs | 1 | 253.46 | 0.519 | 0.6025 | 3.76 | 0.568 | 1.39 | 0.132 | 0.7784 | |
| Ti × Si | 2 | 562.88 | 11.906 | 0.0003 | 67.85 | 12.517 | 0.0002 | 6.00 | 2.052 | 0.1505 |
| Ti × P-v-Cs | 1 | 637.72 | 1.307 | 0.4576 | 129.07 | 19.493 | 0.0002 | 1.40 | 0.480 | 0.4952 |
| Ti × Cs | 1 | 488.08 | 9.455 | 0.0073 | 6.62 | 2.571 | 0.1284 | 10.59 | 3.760 | 0.0703 |
| Res | 24 | 47.28 | 5.42 | 2.92 | ||||||
| Res P | 8 | 38.60 | 11.11 | 3.14 | ||||||
| Res Cs | 16 | 51.62 | 2.58 | 2.82 | ||||||
| Transformation | None | log(x + 1) | log(x + 1) | |||||||
| Shapiro–Wilk test | W = 0.955 NS | W = 0.989 NS | W = 0.981 NS | |||||||
| Cochran’s C-test | C = 0.329 NS | C = 0.452 NS | C = 0.306 NS | |||||||
| PFN | CIC | |||||||
|---|---|---|---|---|---|---|---|---|
| Source | d.f. | MS | Pseudo-F | P (perm) | MS | Pseudo-F | P (perm) | MSDEN |
| Ti | 1 | 6962.60 | 1.640 | 14,908.00 | 2.444 | |||
| Si | 2 | 6715.40 | 1.582 | 3396.40 | 0.557 | |||
| P-v-Cs | 1 | 9595.80 | 2.261 | 0.1694 | 3382.10 | 0.554 | 0.6978 | Ti × Si |
| Cs | 1 | 3835.20 | 0.650 | 0.6415 | 3410.70 | 0.322 | 0.8175 | Ti × Cs |
| Ti × Si | 2 | 4244.40 | 2.605 | 0.0168 | 6101.00 | 6.551 | 0.0002 | Res |
| Ti × P-v-Cs | 1 | 2592.10 | 0.440 | 0.7678 | 1623.00 | 0.153 | 0.9332 | Ti × Cs |
| Ti × Cs | 1 | 5896.70 | 3.417 | 0.0158 | 10,579.00 | 11.109 | 0.0002 | Res Cs |
| Res | 24 | 1629.50 | 931.34 | |||||
| Res P | 8 | 1437.25 | 889.38 | |||||
| Res Cs | 16 | 1725.60 | 952.29 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannarozzi, L.; Bevilacqua, S.; Alongi, G.; Asnaghi, V.; Chiantore, M.; Pagnotta, A.; Paoli, C.; Rigo, I.; Vassallo, P.; Falace, A. Assessing the Effect of Full Protection on the Biomass of Ericaria amentacea and Understory Assemblages: Evidence from Two Mediterranean Marine Protected Areas. Diversity 2023, 15, 89. https://doi.org/10.3390/d15010089
Cannarozzi L, Bevilacqua S, Alongi G, Asnaghi V, Chiantore M, Pagnotta A, Paoli C, Rigo I, Vassallo P, Falace A. Assessing the Effect of Full Protection on the Biomass of Ericaria amentacea and Understory Assemblages: Evidence from Two Mediterranean Marine Protected Areas. Diversity. 2023; 15(1):89. https://doi.org/10.3390/d15010089
Chicago/Turabian StyleCannarozzi, Laura, Stanislao Bevilacqua, Giuseppina Alongi, Valentina Asnaghi, Mariachiara Chiantore, Annachiara Pagnotta, Chiara Paoli, Ilaria Rigo, Paolo Vassallo, and Annalisa Falace. 2023. "Assessing the Effect of Full Protection on the Biomass of Ericaria amentacea and Understory Assemblages: Evidence from Two Mediterranean Marine Protected Areas" Diversity 15, no. 1: 89. https://doi.org/10.3390/d15010089






